Abstract
The veA or velvet gene is necessary for biosynthesis of mycotoxins and other secondary metabolites in Aspergillus species. In addition, veA has also been demonstrated to be necessary for normal seed colonization in Aspergillus flavus and Aspergillus parasiticus. The present study shows that veA homologues are broadly distributed in fungi, particularly in Ascomycetes. The Fusarium verticillioides veA orthologue, FvVE1, is also required for the synthesis of several secondary metabolites, including fumonisin and fusarins. This study also shows that maize plants grown from seeds inoculated with FvVE1 deletion mutants did not show disease symptoms, while plants grown from seeds inoculated with the F. verticillioides wildtype and complementation strains clearly showed disease symptoms under the same experimental conditions. In this latter case, the presence of lesions coincided with accumulation of fumonisins in the plant tissues, and only these plant tissues had elevated levels of sphingoid bases and their 1-phosphate derivatives, indicating inhibition of ceramide synthase and disruption of sphingolipid metabolism. The results strongly suggest that FvVE1 is necessary for pathogenicity by F. verticillioides against maize seedlings. The conservation of veA homologues among ascomycetes suggests that veA could play a pivotal role in regulating secondary metabolism and associated pathogenicity in other fungi.
Keywords: Aspergillus spp., fumonisin, maize seedling disease, velvet gene
Introduction
The filamentous fungus Fusarium verticillioides (teleomorph Gibberella moniliformis) is commonly detected infecting maize (Zea mays), causing maize ear rot worldwide. Fusarium verticillioides produces several families of mycotoxins (Nelson et al., 1993; Rheeder et al., 2002) that often contaminate maize-based human food and animal feed (Nelson et al., 1993; Bezuidenhout et al., 1998). Fumonisins are considered the most agriculturally significant F. verticillioides mycotoxins because these compounds can cause several animal diseases, and have been epidemiologically associated with some human diseases (Marasas, 2001; Desai et al., 2002). Fumonisins are polyketide-derived metabolites that can inhibit ceramide synthase, a key enzyme in sphingolipid metabolism, and induce apoptosis (Nelson et al., 1993; Desai et al., 2002). Fumonisin B1 (FB1) is the most abundant fumonisin found in contaminated maize. Other fumonisins also found in contaminated maize are fumonisin B2 (FB2) and fumonisin B3 (FB3). Most of the regulatory mechanisms controlling fumonisin biosynthesis are still poorly understood, and only a few fumonisin regulatory genes have been investigated. Among these is the global regulatory velvet gene, FvVE1 (Myung et al., 2009). These authors previously demonstrated that FvVE1 is required for the expression of the fumonisin gene cluster and concomitant accumulation of fumonisins under laboratory conditions.
Most veA functional characterization has been carried out in Aspergillus species (Calvo, 2008). However, further study is necessary to elucidate whether veA regulatory output varies across fungal genera. The velvet homologues in Aspergillus spp. are required for the production of several secondary metabolites, including the polyketide toxins sterigmatocystin and aflatoxin in A. nidulans and in A. parasiticus and A. flavus, respectively (Kato et al., 2003; Calvo et al., 2004; Duran et al., 2007). As in the case of veA in Aspergillus spp. (Kim et al., 2002; Calvo, 2008), FvVE1 also regulates morphogenesis in F. verticillioides (Li et al., 2006), although the veA regulatory output on morphogenesis is not identical in these two fungal genera. Importantly, it was recently reported that in A. flavus veA is a factor that influences maize, peanut and cotton seed colonization (Duran et al., 2009). This current study investigates the conservation of veA in other fungi and its possible implication in pathogenicity by characterizing the role of FvVE1 in F. verticillioides systemic infection of maize plants. The results show that the broadly conserved veA orthologue, FvVE1, is necessary for the development of disease symptoms and fumonisin production in maize plants systemically infected by this important plant pathogenic fungus. This study contributes to the knowledge on the regulatory mechanisms that influence F. verticillioides fumonisin production and pathogenicity in plants.
Materials and methods
Amino acid alignment
Amino acid sequences from veA loci of 39 species were obtained from three public databases: the National Center for Biotechnology Information, Broad Institute of Harvard and MIT, and DOE Joint Genome Institute (Supplemental Table 1).
Amino acid sequences were initially aligned using ClustalW2 (Chenna et al., 2003; http://www.ebi.ac.uk/Tools/msa/clustalw2/). During the alignment search, the Gonnet matrix was selected, iteration was performed at each step of the progressive alignment (ITERATION = tree), and settings for all other parameters were defaults. Minor manual adjustments were made to preserve conserved features. Alignment of the most conserved 5´ portion of the amino acid sequences is given (Supplemental Fig. S1). The same method of alignment was used for four sequences of Fusarium (F. graminearum, GenBank accession numbers AACM01000073, DQ274058 and AACM01000074; F. oxysporum, AAXH01000669; F. solani, ACJF01000006; F. verticillioides, DQ274059).
Phylogenetic analysis
Phylogenetic reconstruction was conducted using nucleotide sequences from 39 species. The amino acid alignment was used as a template to manually place gaps in corresponding positions of the nucleotide sequences. The aligned sequence length was 906 bases. Gaps introduced by the alignment were excluded from the analysis. Because some of the sequences were highly divergent (e.g. Acremonium chrysogenum and Trichophyton rubrum) the maximum likelihood (ML) method, which is less biased by long-branch attraction artifacts, was chosen for this analysis. Tuber melanosporum was selected as the outgroup based on previous work (Ebersberger et al., 2009). ML analyses, as implemented in garli v. 0·951–1 (Zwickl, 2006), were performed using default settings (General Time Reversible model with all other parameter values estimated). A ML bootstrap analysis was also conducted with 1000 pseudoreplicates.
Strains and media
The strains used in this study were: M-3125 (MAT1-1, FvVE1); M-3120 (MAT1-2, FvVE1); M312501 (MAT1-1, ΔFvve1::HygB); M31206 (MAT1-2, ΔFvve1::HygB); M312501C1 (MAT1-1, ΔFvve1::HygB, FvVE1::GenR); M31206C5 (MAT1-2, ΔFvve1::HygB, FvVE1::GenR). MAT1-1 and MAT1-2 are the two different mating type idiomorphs (alleles) in F. verticillioides. M-3120 and M-3125 are strain designations from the Fusarium Research Center culture collection (Pennsylvania State University, PA, USA). The FvVE1 deletion strains and complementation strains were generated in both mating types as previously described by Li et al. (2006). V8 agar medium (10% V8 juice, 0·1% CaCO3, 1·5% agar) was used for production of conidia for inoculation purposes.
Maize infection studies
In order to elucidate the role of FvVE1 on F. verticillioides maize infection, seedling assays were performed as previously described (Glenn et al., 2008). Seeds of sweet maize cultivar Silver Queen were surface sterilized with 100% commercial bleach (6·15% sodium hypochlorite) for 10 min and then rinsed twice with sterile water. The seeds were imbibed for 4 h, followed by heat shock treatment at 60°C for 5 min. Then, seeds were rinsed once more to cool them down and were placed in Petri dishes and inoculated with spore suspensions of the six strains noted above (40 seeds placed in a 100 mm diameter Petri dish and flooded with 10 mL of 1 × 104 conidia mL−1) and placed at 27°C overnight. Control seeds were mock inoculated with 10 mL sterile water. The inoculum concentration was intentionally kept low (104 conidia mL−1) so that virulence or pathogenicity factors were not potentially masked by necrotrophic fungal growth resulting from high inoculum level. For each of the seven treatments (control plus six fungal strains), three replicate pots (10 cm diameter) of sterile potting soil were each sown with 10 seeds. The pots were placed in a growth chamber (30°C day for 14 h; 20°C night for 10 h) and watered from below 2, 4 and 6 days after planting (d.a.p.). The pots were then watered from above as needed. Seedling emergence usually occurs 3–4 d.a.p. The experiment ended on the 14th d.a.p. The seedling assay, including assessment of systemic infection (see below), was conducted twice. Disease incidence for each treatment was calculated as a percentage by dividing the number of diseased seedlings per technical replicate by the total number of seedlings in that replicate. The six replicates from the two experiments were averaged to determine the mean disease incidence with standard deviation. Plants were considered diseased if they were stunted with foliar symptoms such as leaf chlorosis, atrophy, and/or necrotic lesions (Glenn et al., 2008).
The first leaf of each seedling was removed at the ligule. These leaves were pooled per replicate, lyophilized, and assessed for fumonisin content and sphingoid base accumulation as described below. As above, data from the six replicates from the two experiments were averaged to determine the mean concentrations with standard deviations. The second leaf from representative seedlings was removed at the ligule for photographing. Also, for evaluation of endophytic colonization, three seedlings from each treatment replicate were collected (total of nine seedlings per experiment), and sections (~1 cm) were taken from the mesocotyl, the stem just above the first node, and the middle of the second leaf. Sections were surface sterilized in 95% EtOH (1 min) followed by 100% commercial bleach (1 min) and a final rinse with sterile water (1 min). Sections were then placed on PDA and incubated at 27°C for 6 days. In the case of FvVe1 deletion strains, visual assessment of colony characteristics was used to determine whether the strain applied to the seed was the same as the out-growing Fusarium isolates. In all cases, the applied mutant strain was morphologically identical to isolates recovered from the plant tissues. No fungi were isolated from control seedlings. Infection frequency of seedling tissues (i.e. percentage of infected mesocotyl, stem and leaf sections) for each treatment was determined by dividing the respective number of Fusarium-infected tissue sections per experiment by nine, the number of tissue sections assayed per experiment. Mean infection frequencies and standard deviations were calculated from the two biological replicate data sets.
Fumonisin and sphingolipid analysis
The extraction and analytical procedures were as previously reported (Zitomer et al., 2008). Briefly, lyophilized maize leaf tissues were ground to a powder, spiked with D-erythro-C16-sphingosine (Matreya) and D-erythro-C17-sphingosine-1-phosphate (Avanti Polar Lipids) internal standards (10 µL each at 100 ng µL−1), and then extracted with an aliquot of 1:1 MeCN:water + 5% formic acid (1 mL per 10 mg tissue). The filtered extracts (100 µL aliquots) were diluted into the initial mobile phase (900 µL) used for reverse phase-high performance liquid chromatography (RP-HPLC). Analyses were conducted using a Finnigan Micro AS autosampler coupled to a Surveyor MS pump (Thermo-Fisher). Separation was accomplished using a Metachem Inertsil 150 × 3 mm i.d., 5 µm ODS-3 column (Metachem Technologies, Inc.). Column effluent was coupled to a Finnigan LTQ linear ion trap mass spectrometer (MS).
Statistical analysis
Statistical analysis was performed using SigmaStat software (Jandel Scientific). When many groups were compared, one-way analysis of variance was used, followed by post-hoc multiple comparisons using the Holm-Sidak method. All data were expressed as mean ± standard deviation, and differences among or between means were considered to be significant if the probability (P) was ≤ 0·05.
Results
Amino acid alignments and phylogenetic analysis
Alignments of the conserved portions of the amino acid sequences are given for the complete 39-species set (Supplemental Fig. S1). Homology with putative VeA sequences from basidiomycete species was very limited. For example, two sequences of the fungal species Ustilago maydis (GenBank acc. no. AACO01000048) and Cryptococcus neoformans (GenBank acc. no. EAK82077), presented identity ranges from 12 to 21% and from 8 to 15%, respectively (whereas all ascomycete sequences were 35–79% identical), when compared to the full length of VeA homologues in the other species. No putative homologues were found in strictly-yeast fungi, although they were found in dimorphic fungi (for example in Ajellomyces dermatitides).
A variable C-terminal region was found, while the N-terminal region was well conserved among all the amino acid sequences studied. Several consensus putative phosphorylation sites were identified (Supplemental Fig. S1), including those targeted by cyclic AMP-dependent protein kinase A (PKA) and protein kinase C (http://ca.expasy.org/prosite). No significant matches to functionally characterized domains were found, with the exception of a classical bipartite nuclear localization signal demonstrated to be functional (Stinnett et al., 2007). A predicted PEST domain was identified in the C-terminal region of VeA in A. nidulans (Kim et al., 2002); however this domain does not appear to be conserved in other fungal species.
The phylogenetic analysis showed that preliminary ML analyses of the entire conserved nucleotide regions of veA sequences produced trees that were topologically incongruent with previous well-supported phylogenies (Spatafora et al., 2006; Ebersberger et al., 2009; preliminary trees not shown). One cause of incongruence between gene trees and species trees is positive selection. To test this hypothesis, the ML analysis was performed using only third codon positions of the aligned veA sequences, which would be less affected by selection (Christin et al., 2007). ML analysis of third position sites produced a single tree with a −lnL = 10381·878 (Fig. 1). In this tree, Eurotiomycetes and Leotiomycetes were retrieved as monophyletic groups. Although the other fungal classes were not retrieved as monophyletic groups, this was largely because of short branches at deep nodes in the tree where saturation of third position substitutions might be expected to reduce phylogenetic information. This tree (Fig. 1) was largely congruent with a recently published genome-scale phylogeny (Ebersberger et al., 2009) supporting a hypothesis of selection. A further implication is that a single veA locus has evolved to generate all the veA genes identified in this study. Note that the veA tree topology was fully consistent with established fungal taxonomy.
Figure 1.
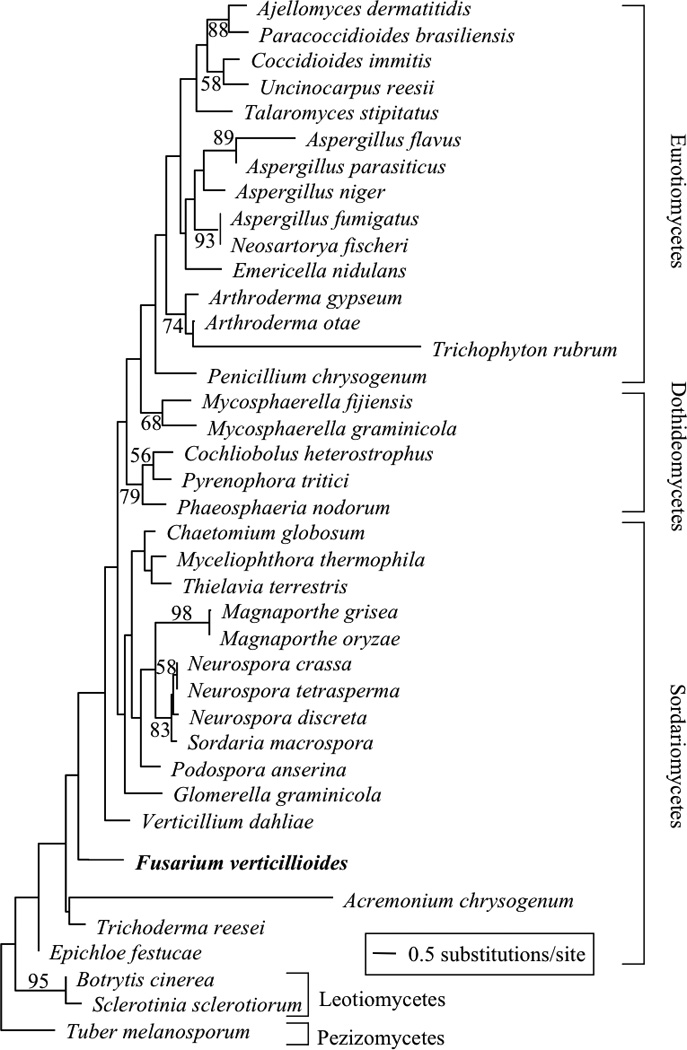
Maximum likelihood tree obtained from analysis of third codon position nucleotides in the 5' conserved region of veA. Taxonomic classes are indicated. Values along the branches are maximum likelihood bootstrap percentages. Branch lengths are proportional to the number of changes along the branch.
The alignment of VeA deduced amino acid sequences from four Fusarium species (Supplemental Fig. S2) showed significant conservation across the entire protein, with 80–99% identity range. All Fusarium VeA sequences presented putative PKA and PKC phosphorylation sites. Some of these putative sites were widely conserved across numerous fungal VeA homologous sequences (Supplemental Fig. S2).
Role of FvVE1 in pathogenicity
Disease incidence and symptoms
Disease symptoms were observed in plants grown from seeds inoculated with the F. verticillioides wildtype and complementation strains, while plants grown from seeds inoculated with the FvVE1 deletion mutants did not show disease symptoms under the same experimental conditions (Figs 2 & 3). Foliar disease development included leaf chlorosis, tissue atrophy and necrosis. No difference was observed between both mating types. Disease incidence ranged from 78% of seedlings in the M3120 treatment to 95% of seedlings in the M3125 treatment. Differences were observed with respect to maize seedling growth among the different treatments. Plants grown from seeds inoculated with wildtype and complementation strains presented stunted growth with respect to those infected with the Δfvve1 mutants or the water controls (Fig. 3A). Germination of seed did not vary among the different treatments, and germination rate was routinely > 95% (data not shown).
Figure 2.
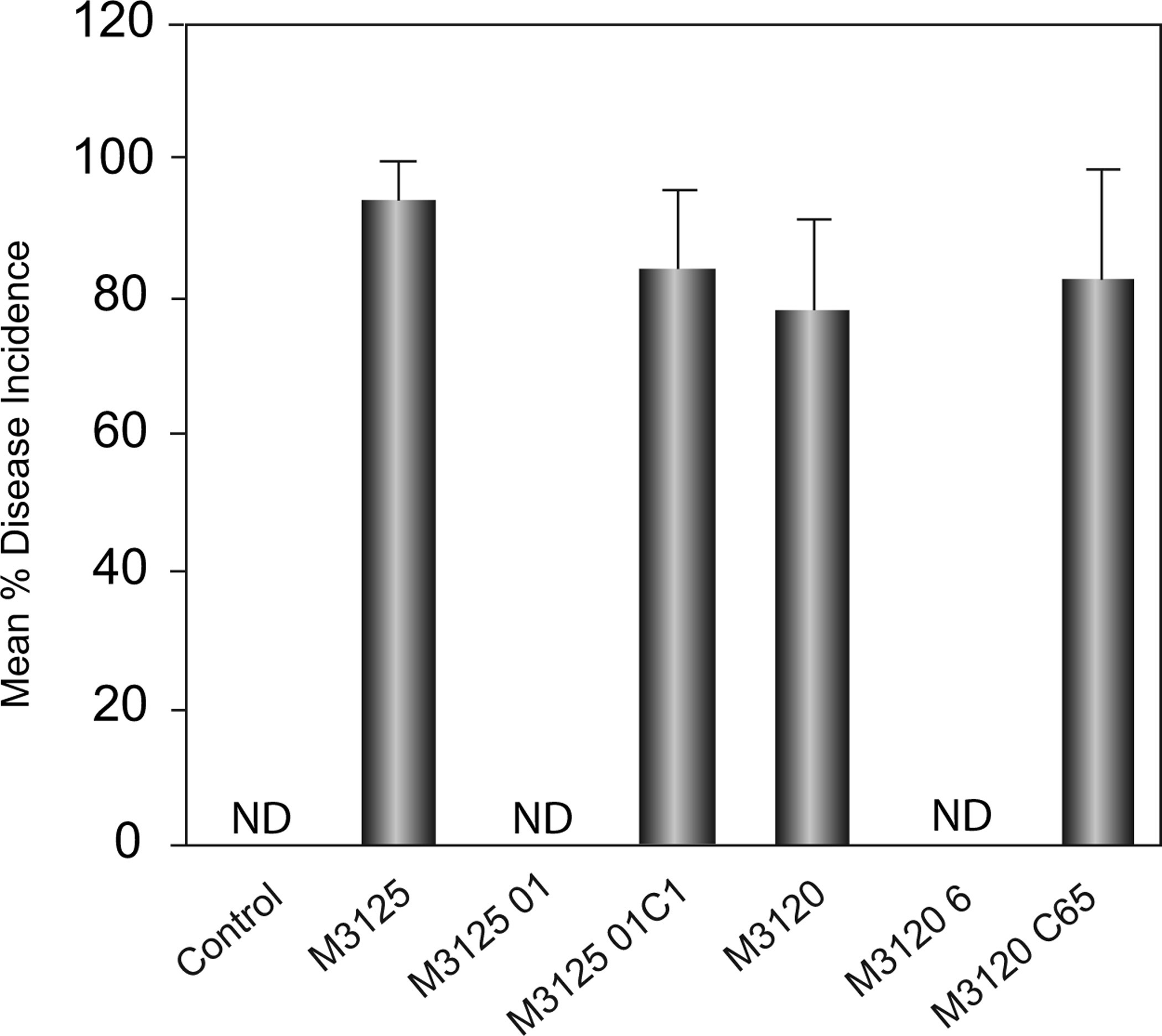
Mean disease incidence exhibited by 14-day-old Silver Queen maize seedlings. Seed were inoculated with spore suspensions of wildtype (M3125 and M3120), FvVE1 deletion (M312501 and M31206), and complementation (M312501C1 and M3120C65) strains prior to planting in sterile soil. Plants were grown at 30°C for a 14-h day and at 20°C for a 10-h night. Seedlings exhibiting disease were stunted with foliar symptoms such as chlorosis, atrophy or necrotic lesions. The experiment was conducted with a total of six technical replicates divided between two experimental replications (each technical replicate being a pot planted with 10 seed), and the data are reported as mean percent disease incidence per technical replicate. Error bar represents standard deviation. ND, no disease symptoms detected. anova indicated significant effects among treatments (P < 0·001, F = 143·9, d.f. = 6). M3125 and M312501C1 were not significantly different from each other, but were significantly different from M3120 and M3120C65 (P < 0·05).
Figure 3.
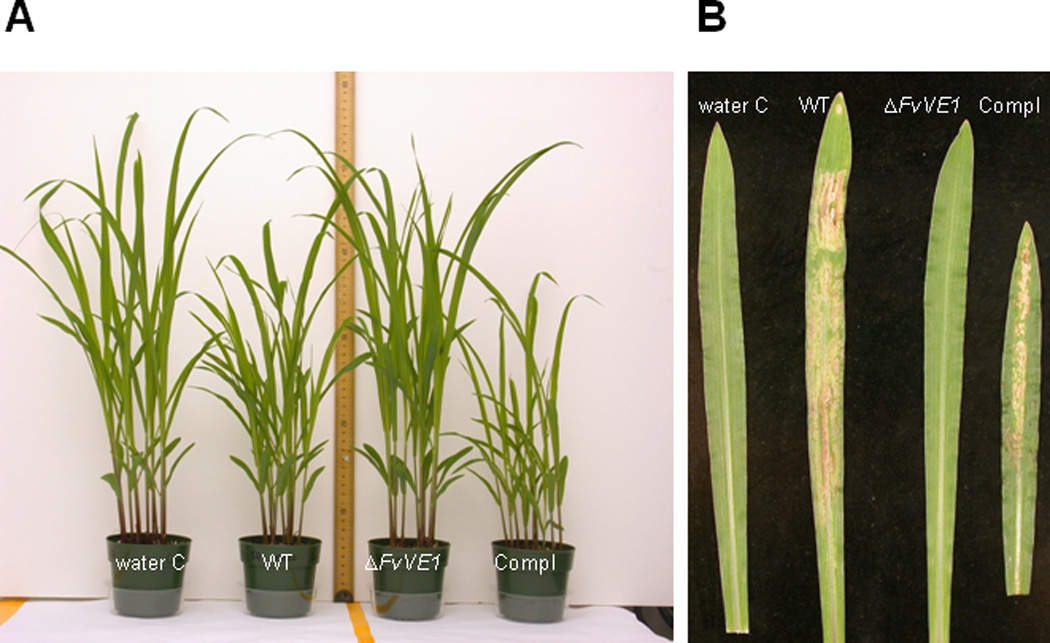
Development of symptoms exhibited by 14-day-old Silver Queen maize seedlings. Necrotic lesions, tissue atrophy and mild bleaching or chlorosis on leaves of Silver Queen seedlings grown from seed inoculated with the indicated Fusarium verticillioides strains. Water C, water control; WT, wildtype; Compl, complementation strain. Similar results were obtained with both mating types.
In planta fumonisin production
No fumonisin was detected in plants grown from seeds inoculated with FvVe1 deletion strains or plants from uninoculated seeds (Fig. 4). The studies indicate that deletion of FvVE1 results in loss of in planta fumonisin production and accumulation in maize leaves. Fumonisin analysis of the first leaves revealed that the foliage accumulated predominantly FB1, with only trace amounts of FB2 and FB3 (data not shown). The effects of FvVE1 deletion on toxin production were the same in the two separate mating types, MAT1-1 (M3125) and MAT1-2 (M3120).
Figure 4.
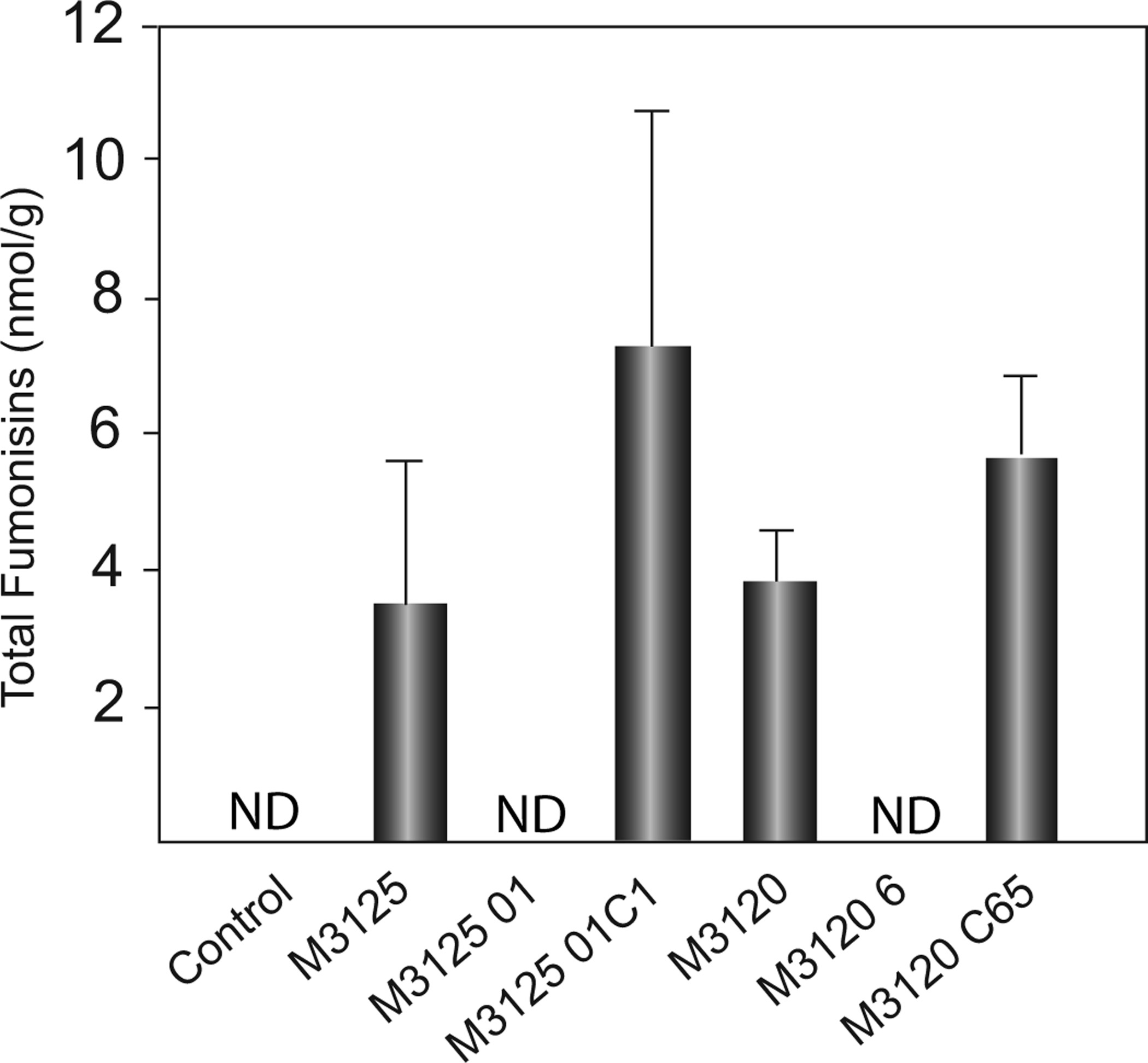
Average total content of fumonisins (FB1, FB2 and FB3) in leaf samples from 14-day-old maize seedlings. Fumonisin accumulation in the first leaf of seedlings of the sweet maize line Silver Queen inoculated with wildtype (M3125 and M3120), FvVE1 deletion (M312501 and M31206), and complementation (M312501C1 and M3120C65) strains. Each treatment had three replicates of 10 seedlings, and the first leaves from seedlings were pooled per replicate. The experiment was conducted twice, and the data are reported as mean fumonisin concentration per replicate. Error bar represents standard deviation. ND, none detected. anova indicated no significant differences among the fumonisin producing strains (P = 0·183, F = 2·065, d.f. = 11).
Sphingolipid analysis
The first leaves of plants infected by F. verticillioides wildtype and complementation strains had elevated levels of the sphingoid bases, sphinganine and phytosphingosine, as well as the sphingoid base 1-phosphates, sphinganine 1-phosphate and phytosphingosine 1-phosphate (Fig. 5), indicating fumonisin inhibition of ceramide synthase and disruption of sphingolipid metabolism. In contrast, first leaf samples from plants infected with the FvVE1 deletion mutants (both mating types) had little if any accumulation of sphingoid bases and their 1-phosphates were comparable to control tissues.
Figure 5.
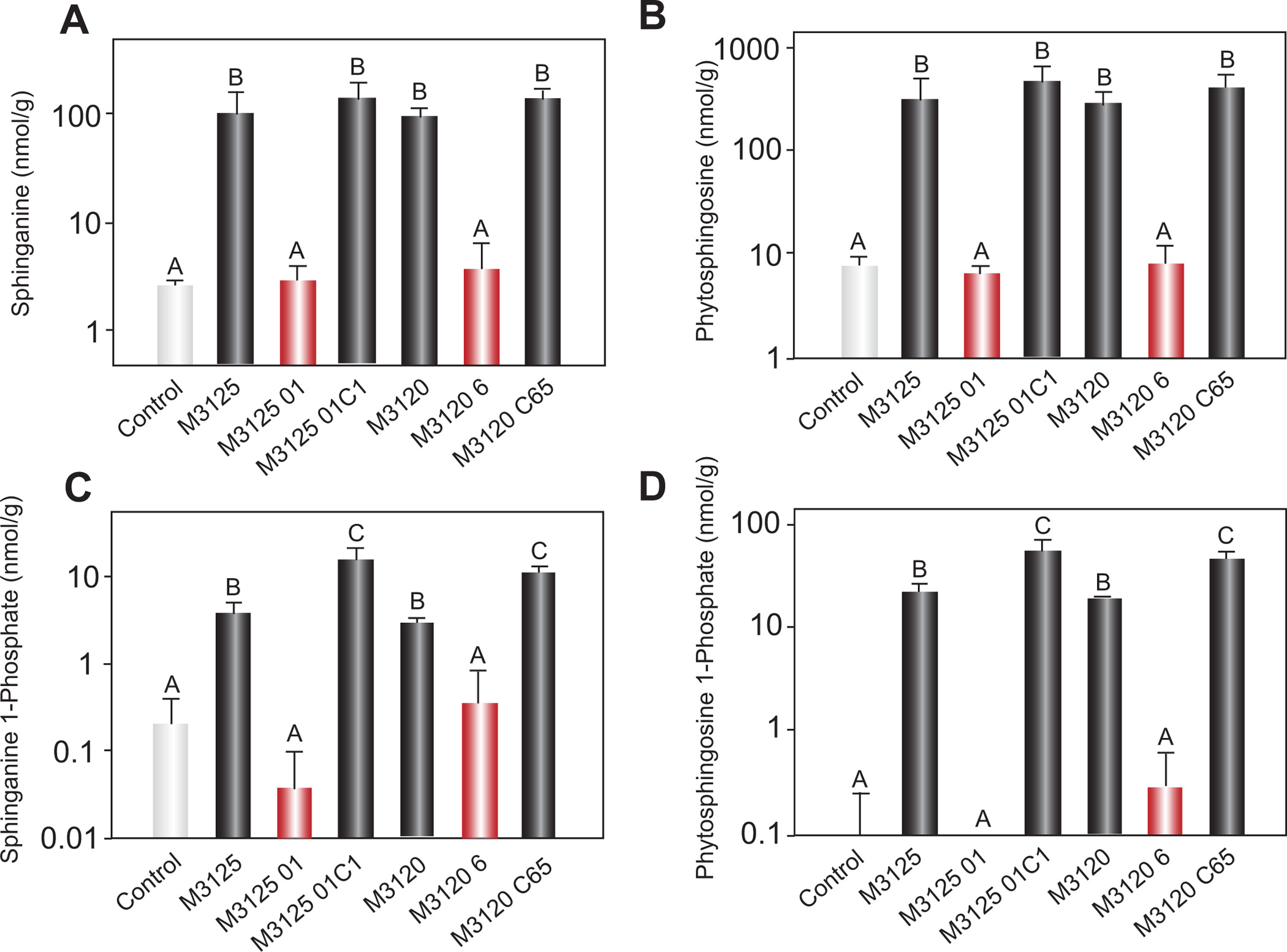
Concentrations of sphingoid bases in leaf tissue of 14-day-old Silver Queen maize seedlings. Accumulation of the sphingoid bases, sphinganine (A) and phytosphingosine (B), and the sphingoid base 1-phosphates, sphinganine 1-phosphate (C) and phytosphingosine 1-phosphate (D), in the first leaf of maize seedlings inoculated with wildtype (M3125 and M3120), FvVE1 deletion (M312501 and M31206), and complementation (M312501C1 and M3120C65) strains. Each treatment had three replicates of 10 seedlings, and the first leaves from seedlings were pooled per replicate. The experiment was conducted twice, and the data are reported as mean concentration per replicate. Error bar represents standard deviation. Different letters indicate a statistically significant difference among treatments (anova for each of the four datasets provided P < 0·001 and d.f. = 20; (A) F = 59·5, (B) F = 91·5, (C) F = 45·8, and (D) F = 111·4).
Infection frequency
The various fungal strains tested were isolated from three different tissue sections of maize seedlings grown from inoculated seeds (Fig. 6), indicating that systemic infection occurred. The frequency of tissue infection with wildtype F. verticillioides was greatest in mesocotyl and stem tissues (95 to 100% infection of each tissue type), followed by leaf tissue (varied from 22% for M3125 to 73% for M3120). The data indicated FvVE1 deletion mutants were capable of symptomless endophytic infection of the seedlings.
Figure 6.
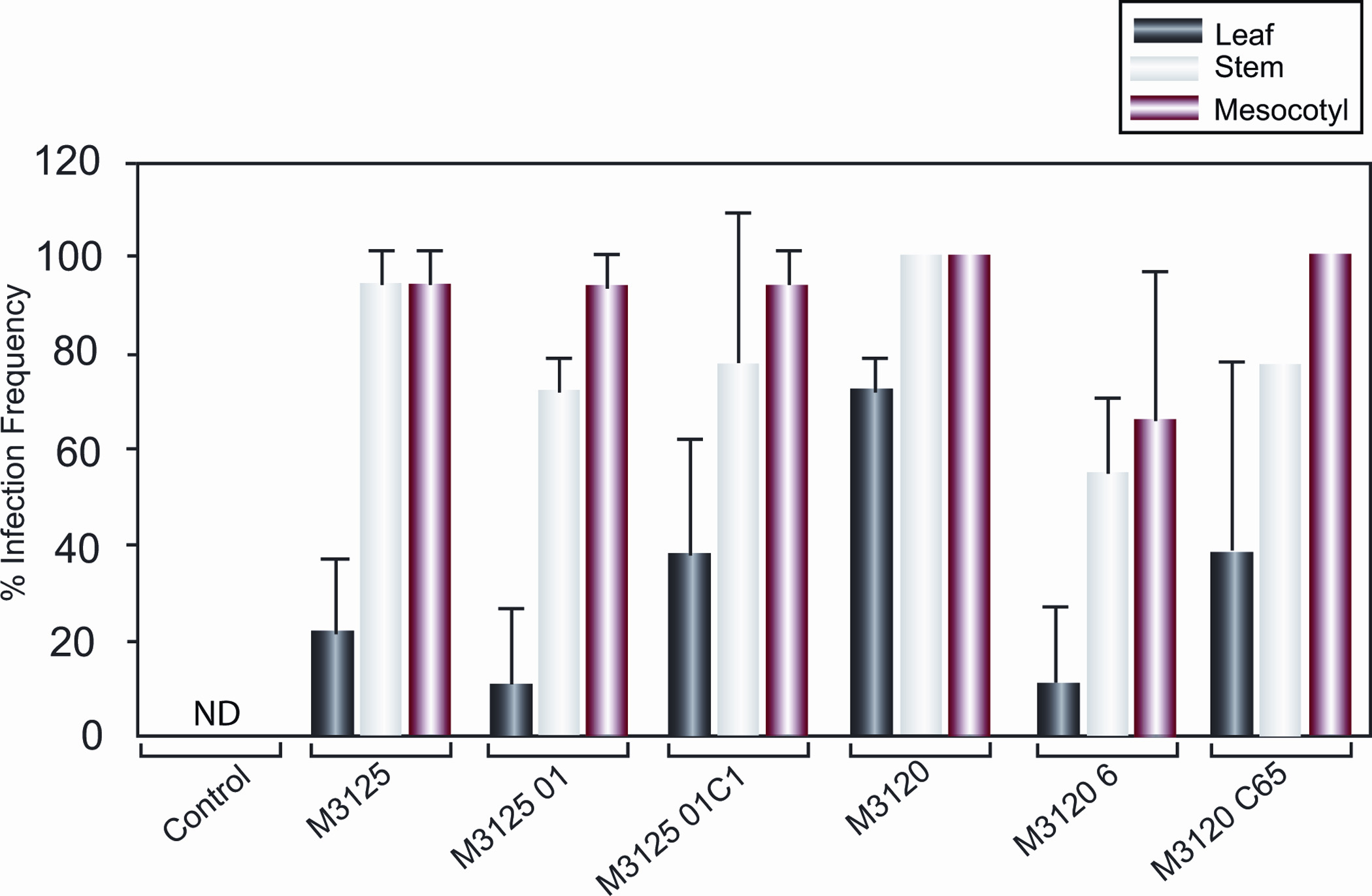
Fusarium verticillioides mean infection frequency in 14-day-old Silver Queen maize seedlings. Seedlings were grown from seed inoculated with wildtype (M3125 and M3120), FvVE1 deletion (M312501 and M31206), and complementation (M312501C1 and M3120C65) strains. Mesocotyl, stem and leaf sections (1 cm each) were taken from three seedlings from each replicate (three replicates per treatment) and surface sterilized. Sections were placed on PDA and incubated at 27°C for 6 days, at which point the percentage of Fusarium-infected sections were noted. The experiment was conducted twice, and the data are reported as mean infection frequency per experiment. Error bar represents standard deviation. ND, none detected. anova indicated no significant treatment effects within each tissue type.
Discussion
In Aspergillus species, homologues of the velvet gene, veA, are known to be involved in regulating morphogenesis, sexual and asexual development, and secondary metabolism, including the production of mycotoxins and antibiotics (Kato et al., 2003; Calvo et al., 2004; Duran et al., 2007). In this study, several additional putative veA homologues across fungal genera were identified in 39 species through an extensive search of three public databases. This group of species included plant, animal and human pathogens. The fungal species with putative veA homologues were classified in the phylum Ascomycota. It is postulated that ascomycetes diverged from basidiomycetes about 500 million years ago (Taylor et al., 1999). The low similarity found when comparing the sequences of basidiomycetes to the ascomycete VeA homologues suggests that the functions of these proteins or mechanisms of action may have diverged. In other Basidiomycota genomic databases a VeA match was not found (data not shown). The reason why VeA is more common among ascomycetes and often absent from fungal species in other phyla remains to be determined. Phylogenetic analysis of veA homologues showed a topology that was consistent with established fungal taxonomy. These results suggest that all veA are orthologues, evolving from one common ancestral gene in Ascomycota. Interestingly, veA was not found in available plant or animal genomes.
The veA gene was not found in genomes of strictly-yeast fungi (Schizosaccharomyces pombe at http://www.genedb.org/genedb/pombe/index.jsp, Saccharomyces cerevisiae at http://www.sanger.ac.uk/Projects/S_cerevisiae/, and at http://www.yeastgenome.org/) suggesting that veA function could be linked to the regulation of a filamentous growth form. Supporting this observation, the FvVE1 deletion in F. verticillioides resulted in yeast-like growth in submerged culture (Li et al., 2006). Furthermore, homologues of other velvet-like proteins, vosA and velB (Ni & Yu, 2007) have been shown to be necessary for the switch from filamentous to yeast form in Histoplasma capsulatum (Webster & Sil, 2008).
Functionality of most VeA conserved domains remains elusive, with the exception of the A. nidulans VeA bipartite NLS (Stinnet et al., 2007). It is known that VeA is transported to the nucleus (Stinnet et al., 2007) where it forms a nuclear complex (Bayram et al., 2008a; Calvo, 2008; Purschwitz et al., 2008). In the present study putative phosphorylation sites are shown to be conserved in several genera, including Fusarium. Furthermore, phosphorylation of VeA has recently been reported (Purschwitz et al., 2009), although the kinase involved in VeA phosphorylation and how phosphorylation might regulate VeA function is still unknown.
The high conservation found among VeA orthologues in ascomycetes suggests that these proteins might have similar functions in fungi of this phylum, such as regulation of morphogenesis and/or regulation of the biosynthesis of different secondary metabolites, as shown experimentally in Aspergillus spp. (Kim et al., 2002; Kato et al., 2003; Calvo et al., 2004; Duran et al., 2007; Calvo, 2008), F. verticillioides (Li et al., 2006; Myung et al., 2009), Acremonium chrysogenum (Dreyer et al., 2007) and Neurospora crassa (Bayram et al., 2008b). Interestingly, Duran et al. (2009) showed that veA is also involved in A. flavus pathogenicity when infecting different types of oil seeds. Because veA has only been found in fungi, the potential for veA and genes controlled by veA as targets to reduce fungal invasion of plants and possibly for animal/human disease control is promising.
As a further step to lay the foundation for a control strategy to reduce the impact of other fungal plant pathogens in agriculture, the role of F. verticillioides veA orthologue, FvVE1, in pathogenicity during systemic infection of maize plants was studied. The remarkable conservation of VeA orthologues in the genus Fusarium suggests that the implication of FvVE1 in pathogenicity shown in this F. verticillioides study could also be shared by other pathogenic fungal species of this genus.
In previous studies it was shown that the veA orthologue, FvVE1, has a role in regulating morphogenesis (Li et al., 2006). Deletion of FvVE1 suppressed aerial hyphal growth, reduced colony surface hydrophobicity, caused alterations in hyphal polarity, and resulted in marked activation of conidiation and yeast-like growth in submerged cultures. FvVE1 deletion also notably increased the ratio of macroconidia to microconidia. These results indicated that FvVE1 plays an important role in F. verticillioides morphological differentiation.
Importantly, the results from the present study indicate that F. verticillioides FvVE1 is also necessary for pathogenicity during systemic infection of maize seedlings. This is in agreement with results from a study of F. fujikuroi infection on rice (Wiemann et al., 2010). In the present study those plants grown from seeds inoculated with F. verticillioides wildtype and complementation control strains showed lesions (Figs 2 & 3). However, plants grown from seeds inoculated with FvVE1 deletion mutants did not present disease symptoms. Additionally, the accumulation of fumonisins was evaluated during F. verticillioides systemic infection of maize seedlings. While fumonisins were detected in leaves from maize plants grown from seeds infected with the control strains, these mycotoxins were not detected in leaves from maize plants grown from seeds infected with FvVE1 deletion mutants, indicating that FvVE1 is necessary for fumonisin production during systemic infection of maize plants. These results concur with previous studies where, under laboratory conditions, FvVE1 controls the production of several secondary metabolites, including fusarins and fumonisins (Myung et al., 2009).
The fact that FvVE1 is necessary for the manifestation of disease symptoms as well as fumonisin production during systemic infection of maize seedlings strongly suggests that fumonisin production in F. verticillioides with an FvVE1 wildtype allele is required for development of the observed disease symptoms. These results further support previous work showing the requirement of FB production for the occurrence of leaf lesions on susceptible varieties of maize (Glenn et al., 2008).
In addition, the fumonisin analysis of leaf tissue from seedlings systemically infected with the F. verticillioides control strains revealed predominant accumulation of FB1, and only trace amounts of FB2 and FB3. This result is in agreement with previous studies on maize seedlings grown from seeds inoculated with F. verticillioides strain MRC 826 (Zitomer et al., 2008). These findings further support the hypothesis that maize infected with FB-producing strains may be preferentially translocating and/or accumulating FB1 at a higher rate compared to other types of fumonisin isomers.
Accumulation of fumonisins also coincided with disruption of sphingolipid metabolism, reflected by the accumulation of sphingoid bases and sphingoid base 1-phosphates (Fig. 5). It is known that fumonisins are inhibitors of ceramide synthase in plants (Abbas et al., 1994; Williams et al., 2007). In the present experiments, incidence of foliar disease symptoms such as necrotic lesions and atrophy on plants grown from seeds inoculated by F. verticillioides control strains was correlated with fumonisin production. In contrast, symptomless seedlings systemically infected with the FvVE1 deletion mutants showed no evidence of sphingolipid metabolism disruption, coinciding with an absence of fumonisins and disease symptoms. This indicates that FvVE1-dependent fumonisin production, and its effects on sphingolipid metabolism, adversely affects maize development contributing to F. verticillioides maize seedling disease.
Overall, the results indicate that FvVE1 is necessary for fumonisin biosynthesis and pathogenicity by F. verticillioides, suggesting FvVE1 as a promising target to decrease fumonisin contamination and disease in maize caused by F. verticillioides. Furthermore, the conservation of veA orthologues among ascomycetes, particularly notable among Fusarium spp., suggests that veA could play a pivotal role in regulating secondary metabolism and pathogenicity in other fungi.
Supplementary Material
Amino acid alignment of the conserved N-terminal region of VeA from 39 different fungi. Residues below the asterisks and plus symbols indicate predicted phosphorylation sites by protein kinase A (PKA) and protein kinase C (PKC), respectively. Bold type indicates a consensus PKA phosphorylation motif (K-K-[L/I]-T) present in 11 species. Abbreviations are as follows: Ach = Acremonium chrysogenum, Ade = Ajellomyces dermatitidis, Agy = Arthroderma gypseum, Aot = Arthroderma otae, Afl = Aspergillus flavus, Afu = Aspergillus fumigatus, Ani = Aspergillus niger, Apa = Aspergillus parasiticus, Bci = Botrytis cinerea, Ggl = Chaetomium globosum, Cim = Coccidioides immitis, Che = Cochliobolus heterostrophus, Eni = Emericella nidulans, Efe = Epichloe festucae, Fve = Fusarium verticillioides, Ggr = Glomerella graminicola, Mgi = Magnaporthe grisea, Mor = Magnaporthe oryzae, Mth = Myceliophthora thermophila, Mfi = Mycosphaerella fijiensis, Mga = Mycosphaerella graminicola, Nfi = Neosartorya fischeri, Ncr = Neurospora crassa, Ndi = Neurospora discreta, Nte = Neurospora tetrasperma, Pbr = Paracoccidioides brasiliensis, Pch = Penicillium chrysogenum, Pno = Phaeosphaeria nodorum, Pan = Podospora anserina, Ptr = Pyrenophora tritici, Ssc = Sclerotinia sclerotiorum, Sma = Sordaria macrospora, Tst = Talaromyces stipitatus, Tte = Thielavia terrestris, Tre = Trichoderma reesei, Tru = Trichophyton rubrum, Tme = Tuber melanosporum, Ure = Uncinocarpus reesii, Vda = Verticillium dahliae.
Complete amino acid sequences of VeA in four species of Fusarium. Sequences were aligned with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and displayed with the boxshade web server (http://www.ch.embnet.org/software/BOX_form.html). Black and grey boxes indicate identical and variable sites, respectively. Putative phosphorylation sites, which were conserved across these four species of Fusarium, are either indicated by open boxes (consensus motif for protein kinase A: [R/K] [R/K] X [S/T]) or by lightly shaded boxes (consensus motif for protein kinase C: [S/T] X [R/K]). Putative sites that were widely conserved across at least 50% of the 39 fungal VeA sequences are in heavy boxes. Fvert = F. verticilloides, Foxys = F. oxysporum, Fsola = F. solani, and Fgram = F. graminearum.
Acknowledgements
This work was supported by NIH GM074267-01A1. AMC thanks Barbara Ball for her technical support.
References
- Abbas HK, Tanaka T, Duke SO, et al. Fumonisin- and AAL-Toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiology. 1994;106:1085–1093. doi: 10.1104/pp.106.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram O, Krappmann S, Ni M, et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008a;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- Bayram O, Krappmann S, Seiler S, Vogt N, Braus GH. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genetics and Biology. 2008b;45:127–138. doi: 10.1016/j.fgb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Bezuidenhout SC, Gelderblom WCA, Gorst-Allman CP, et al. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. Journal of the Chemical Society, Chemical Communications. 1988;1988:743–745. [Google Scholar]
- Calvo AM. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genetics and Biology. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Calvo AM, Bok J, Brooks W, Keller NP. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Applied and Environmental Microbiology. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Hideaki K, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Research. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Salamin N, Savolainen V, Duvall MR, Besnard G. C4 Photosynthesis evolved in grasses via parallel adaptive genetic changes. Current Biology. 2007;17:1241–1247. doi: 10.1016/j.cub.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Desai K, Sullards MC, Allegood J, et al. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochimica et Biophysica Acta. 2002;1585:188–192. doi: 10.1016/s1388-1981(02)00340-2. [DOI] [PubMed] [Google Scholar]
- Dreyer J, Eichhorn H, Friedlin E, Kürnsteiner H, Kück U. A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum. Applied and Environmental Microbiology. 2007;73:3412–3422. doi: 10.1128/AEM.00129-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RM, Cary JW, Calvo AM. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Applied Microbial Biotechnology. 2007;73:1158–1168. doi: 10.1007/s00253-006-0581-5. [DOI] [PubMed] [Google Scholar]
- Duran RM, Cary JW, Calvo AM. The Role of veA on Aspergillus flavus infection of peanuts, corn and cotton. Open Mycology Journal. 2009;3:27–36. [Google Scholar]
- Ebersberger I, Gube M, Strauss S, et al. A stable backbone for the fungi. Nature Precedings. 2009 Http://hdl.handle.net/10101/npre.2009.2901.1. [Google Scholar]
- Glenn AE, Zitomer NC, Zimeri AM, Williams LD, Riley RT, Proctor RH. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Molecular Plant-Microbe Interactions. 2008;21:87–97. doi: 10.1094/MPMI-21-1-0087. [DOI] [PubMed] [Google Scholar]
- Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryotic Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Han K, Kim K, Han D, Jahng K, Chae K. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genetics and Biology. 2002;37:72–80. doi: 10.1016/s1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- Li S, Myung K, Guse D, et al. FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides. Molecular Microbiology. 2006;62:1418–1432. doi: 10.1111/j.1365-2958.2006.05447.x. [DOI] [PubMed] [Google Scholar]
- Marasas WF. Discovery and occurrence of the fumonisins: A historical perspective. Environmental Health Perspectives. 2001;109(Suppl 2):239–243. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K, Li S, Butchko RAE, et al. FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. Journal of Agricultural and Food Chemistry. 2009;57:5089–5094. doi: 10.1021/jf900783u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PE, Desjardins AE, Plattner RD. Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry, and significance. Annual Review of Phytopathology. 1993;31:233–252. doi: 10.1146/annurev.py.31.090193.001313. [DOI] [PubMed] [Google Scholar]
- Ni M, Yu JH. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One. 2007;2:e970. doi: 10.1371/journal.pone.0000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purschwitz J, Müller S, Kastner C, et al. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Current Biology. 2008;18:255–259. doi: 10.1016/j.cub.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Purschwitz J, Muller S, Fischer R. Mapping the interaction sites of Aspergillus nidulans phytochrome FphA with the global regulator VeA and the White Collar protein LreB. Molecular Genetics and Genomics. 2009;281:35–42. doi: 10.1007/s00438-008-0390-x. [DOI] [PubMed] [Google Scholar]
- Rheeder JP, Marasas WFO, Vismer HF. Production of fumonisin analogs by Fusarium species. Applied and Environmental Microbiology. 2002;68:2101–2105. doi: 10.1128/AEM.68.5.2101-2105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora JW, Sung G-H, Johnson D, et al. A five-gene phylogeny of Pezizomycotina. Mycologia. 2006;98:1018–1028. doi: 10.3852/mycologia.98.6.1018. [DOI] [PubMed] [Google Scholar]
- Stinnet S, Espeso E, Cobeno L, Arujo-Bazan L, Calvo AM. VeA subcellular localization is dependent on the importin alpha carrier and on light in the filamentous fungus Aspergillus nidulans. Molecular Microbiology. 2007;63:242–255. doi: 10.1111/j.1365-2958.2006.05506.x. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Hass H, Kerp H. The oldest fossil ascomycetes. Nature. 1999;399:648. doi: 10.1038/21349. [DOI] [PubMed] [Google Scholar]
- Webster RH, Sil A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proceedings of the National Academy of Sciences, USA. 2008;105:14573–14578. doi: 10.1073/pnas.0806221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann P, Brown DW, Kleigrewe K, et al. Molecular Microbiology. 2010;77:972–994. doi: 10.1111/j.1365-2958.2010.07263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LD, Glenn AE, Zimeri AM, Bacon CW, Smith MA, Riley RT. Fumonisin disruption of ceramide biosynthesis in maize roots and the effects on plant development and Fusarium verticillioides-induced seeding disease. Journal of Agriculture and Food Chemistry. 2007;55:2937–2946. doi: 10.1021/jf0635614. [DOI] [PubMed] [Google Scholar]
- Zitomer NC, Glenn AE, Bacon CW, Riley RTA. A single extraction method for the analysis by liquid chromatography/tandem mass spectrometry of fumonisins and biomarker of disrupted sphingolipid metabolism in tissues of maize seedlings. Analytical and Bioanalytical Chemistry. 2008;391:2257–2263. doi: 10.1007/s00216-008-2166-x. [DOI] [PubMed] [Google Scholar]
- Zwickl D. PhD thesis. garli version 0 951-1. Austin, TX, USA: University of Texas at Austin; 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. available at http://www.molecularevolution.org/mbl/software/garli/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid alignment of the conserved N-terminal region of VeA from 39 different fungi. Residues below the asterisks and plus symbols indicate predicted phosphorylation sites by protein kinase A (PKA) and protein kinase C (PKC), respectively. Bold type indicates a consensus PKA phosphorylation motif (K-K-[L/I]-T) present in 11 species. Abbreviations are as follows: Ach = Acremonium chrysogenum, Ade = Ajellomyces dermatitidis, Agy = Arthroderma gypseum, Aot = Arthroderma otae, Afl = Aspergillus flavus, Afu = Aspergillus fumigatus, Ani = Aspergillus niger, Apa = Aspergillus parasiticus, Bci = Botrytis cinerea, Ggl = Chaetomium globosum, Cim = Coccidioides immitis, Che = Cochliobolus heterostrophus, Eni = Emericella nidulans, Efe = Epichloe festucae, Fve = Fusarium verticillioides, Ggr = Glomerella graminicola, Mgi = Magnaporthe grisea, Mor = Magnaporthe oryzae, Mth = Myceliophthora thermophila, Mfi = Mycosphaerella fijiensis, Mga = Mycosphaerella graminicola, Nfi = Neosartorya fischeri, Ncr = Neurospora crassa, Ndi = Neurospora discreta, Nte = Neurospora tetrasperma, Pbr = Paracoccidioides brasiliensis, Pch = Penicillium chrysogenum, Pno = Phaeosphaeria nodorum, Pan = Podospora anserina, Ptr = Pyrenophora tritici, Ssc = Sclerotinia sclerotiorum, Sma = Sordaria macrospora, Tst = Talaromyces stipitatus, Tte = Thielavia terrestris, Tre = Trichoderma reesei, Tru = Trichophyton rubrum, Tme = Tuber melanosporum, Ure = Uncinocarpus reesii, Vda = Verticillium dahliae.
Complete amino acid sequences of VeA in four species of Fusarium. Sequences were aligned with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and displayed with the boxshade web server (http://www.ch.embnet.org/software/BOX_form.html). Black and grey boxes indicate identical and variable sites, respectively. Putative phosphorylation sites, which were conserved across these four species of Fusarium, are either indicated by open boxes (consensus motif for protein kinase A: [R/K] [R/K] X [S/T]) or by lightly shaded boxes (consensus motif for protein kinase C: [S/T] X [R/K]). Putative sites that were widely conserved across at least 50% of the 39 fungal VeA sequences are in heavy boxes. Fvert = F. verticilloides, Foxys = F. oxysporum, Fsola = F. solani, and Fgram = F. graminearum.


