Abstract
Since epithelial mucin 1 (MUC1) is associated with several adenocarcinomas at the mucosal sites, it is pertinent to test the efficacy of a mucosally targeted vaccine formulation. The B subunit of the Vibrio cholerae cholera toxin (CTB) has great potential to act as a mucosal carrier for subunit vaccines. In the present study we evaluated whether a MUC1 tandem repeat (TR) peptide chemically linked to CTB would break self-antigen tolerance in the transgenic MUC1-tolerant mouse model (MUC1.Tg) through oral or parenteral immunizations. We report that oral immunization with the CTB–MUC1 conjugate along with mucosal adjuvant, unmethylated CpG oligodeoxynucleotide (ODN) and interleukin-12 (IL-12) did not break self-antigen tolerance in MUC1.Tg mice, but induced a strong humoral response in wild-type C57BL/6 mice. However, self-antigen tolerance in the MUC1.Tg mouse model was broken after parenteral immunizations with different doses of the CTB–MUC1 conjugate protein and with the adjuvant CpG ODN co-delivered with CTB–MUC1. Importantly, mice immunized systemically with CpG ODN alone and with CTB–MUC1 exhibited decreased tumor burden when challenged with a mammary gland tumor cell line that expresses human MUC1.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0906-1) contains supplementary material, which is available to authorized users.
Keywords: Epithelial mucin 1, Cholera toxin B subunit, MUC1 transgenic mouse model, Tumor immunotherapy, Oral immunization
Introduction
The identification of tumor-associated antigens (TAAs) and their subsequent isolation has revolutionized tumor immunology. TAAs are abnormal or altered self-proteins that are potentially immunogenic. Epithelial mucin 1 (MUC1) is the test antigen of choice in this study because of its prevalence on most solid adenocarcinomas, including breast, lung, pancreas, prostate, stomach, colon and ovary [1–4]. MUC1 is a member of a group of heavily glycosylated proteins known as mucins that are localized to the apical surface of healthy epithelial cells. Aberrant glycosylation and re-orientation of MUC1 on the cell surface allows access of anti-MUC1 immune effector cells to tumor sites that are mostly excluded from normal epithelial tissues. MUC1 has been used in several clinical and preclinical studies and has shown promising results [5–18]. Since MUC1 is associated with several adenocarcinomas at the mucosal sites, we considered it pertinent to test the efficacy of a mucosally targeted vaccine formulation.
One of the most notable benefits of mucosal immunization is the fact that both systemic and mucosal immunity are triggered, which is particularly advantageous in the cases where the initial site of tumor formation is along the mucosa. Another benefit is the ease of administration of an oral vaccine, which may be used in a prophylactic setting for high risk patients. Animal studies using TAA-targeted therapies have been successful in preventing tumor formation or reducing tumor burden when used as prophylactic agents [13, 17, 19]. Disappointingly, this has not been recreated in human clinical trials, since the antigen vaccines are used as alternative therapies in advanced disease cases and not in a prophylactic setting that would allow the immune system to reach the robustness needed to target and kill the tumor cells before they acquire the mechanisms to escape immune recognition and/or killing. Therefore, targeting tumors at their primary site is pertinent. Many cancers, such as pancreatic, colorectal, cervical, lung and bladder, have primary tumors that originate at mucosal sites, which are first encountered by the mucosal immune system.
Unfortunately, most clinically relevant vaccine candidates show weak immunogenicity when delivered mucosally and poor transport across biological barriers. To bypass this weakness, we conjugated the MUC1 tandem repeat peptide to the B subunit of the Vibrio cholerae cholera toxin (CTB), which has great potential to act as a mucosal carrier for subunit vaccines [20, 21]. The efficacy of CTB as a mucosal carrier molecule relies on its strong affinity for the GM1 ganglioside receptor that is present on most cells in the body, including epithelial cells and leukocytes. Binding to the GM1 receptors on mucosal epithelial cells, specifically microfold cells (M-cells) located above the Peyer’s patch in the intestine, is thought to increase the uptake of the antigen across the mucosa and lead to an enhanced presentation of the antigen to the immune system [22–24]. CTB has immunomodulating effects by lowering the threshold concentration of the conjugated antigen required for immune cell activation and by increasing CD40 and CD86 expression on antigen-presenting cells (APCs) [25].
Our goal was to evaluate if a MUC1-specific mucosally targeted vaccine could break self-antigen tolerance and translate to a systemic response that would lead to anti-tumor immunity. The MUC1.Tg mouse model is an inbred C57BL/6 mouse strain that expresses human MUC1 in a tissue-specific fashion and is driven by its own promoter [26]. This model system allowed us to test our anti-MUC1 vaccine formulation in an appropriate immunologically tolerant host.
In the present study, we evaluated whether MUC1 tandem repeat (TR) peptide chemically linked to CTB (CTB–MUC1) would break self-antigen tolerance in MUC1.Tg mice through oral or parenteral immunizations. Oral immunizations included strong mucosal adjuvants, specifically unmethylated CpG oligodeoxynucleotides (ODNs) and recombinant mouse interleukin 12 (IL-12). We report that oral immunization with the CTB–MUC1 conjugate along with the adjuvants did not break self-antigen tolerance in MUC1.Tg mice. However, self-antigen tolerance was broken after parenteral immunizations with different doses (10 and 30 μg) of CTB–MUC1. Importantly, mice immunized with a higher dose (30 μg) of CTB–MUC1 showed decreased tumor burden after challenge with a mammary gland tumor cell line that expressed human MUC1 (C57mg.MUC1). The addition of the immunological adjuvant CpG ODN to 10 μg CTB–MUC1 augmented vaccine efficacy as demonstrated by antibody production and protection from tumor challenge.
Materials and methods
Synthesis and conjugation of CTB–MUC1
The MUC1 tandem repeat peptide with a cysteine attached to the N terminus of the peptide was synthesized at Arizona State University Department of Biochemistry (Tempe, AZ, USA). The synthetic MUC1 peptide (C-STAPPAHGVTSAPDTRPAPGSTAPPA) was then conjugated to CTB (C9903, Sigma, St. Louis, MO, USA) by Biosynthesis Incorporated (Lewisville, TX, USA). The conjugation method used m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS). For peptide conjugation, the peptide usually provides the thiol group in the form of a cysteine residue (provided by the added Cys on the MUC1 peptide), whereas the carrier provides amino groups in the form of lysine residues (CTB monomer contains 11 Lys residues per monomer). Due to the pentamer nature of CTB and the inexactness of the conjugation method, we were unable to quantify the amount of MUC1, although we attempted with a semi-quantitative anti-MUC1 ELISA. Therefore, we used a one to one ratio of CTB to MUC1 peptide for conjugation and determined the quantity of vaccine dose by the amount of CTB used for conjugation.
Mice
Wild-type (wt) and MUC1.Tg mice (both of C57BL/6 background) [26] were bred in-house at the Mayo Clinic Scottsdale Natalie Schafer Transgenic Animal Facility in pathogen-free conditions. All experimental procedures were conducted according to IACUC guidelines.
Cell line
C57 mammary gland cancer cells, derived from spontaneous mammary gland tumors of the C57BL/6 mice transfected with full-length human MUC1 gene (C57mg.MUC1 cells) [12], were maintained in DMEM with 10% fetal bovine serum (FBS), penicillin (50 U/ml) and streptomycin (50 μg/ml), supplemented with 150 μg/ml G418 and 20 ng/ml insulin (Sigma, St. Louis, MO, USA). In all experiments, 1 × 106 C57mg.MUC1 cells were injected subcutaneously.
Oral immunizations
We conducted three separate oral immunizations using MUC1.Tg and C57BL/6 mice. In the first animal trial, we tested three doses of the CTB–MUC1 conjugate protein (5, 10 and 30 μg) without any adjuvant. In the second animal trial, 10 μg of CTB–MUC1 was co-delivered with the adjuvant CpG ODN (Coley Pharmaceutical Group, Canada). In the third animal trial, 10 μg of CTB–MUC1 was co-delivered with the adjuvant recombinant mouse IL-12 (rmIL-12) (PeproTech, Inc., Rocky Hill, NJ, USA). Mice were immunized by gavage (100 μl vol.) on days 0, 7, 14, 21, 28 and 35 and challenged on day 14 by subcutaneous injection of C57mg.MUC1 (1 × 106) tumor cells in the right flank region.
For the IL-12 adjuvant study, rmIL-12 was combined with DOTAP liposomes (Roche Applied Science, Indianapolis, IN, USA) to protect the cytokine from the gastric environment as reported previously [27]. Prior to immunization, mice were deprived of food for 2 h, followed by intragastric administration (100 μl) of an isotonic bicarbonate solution [8 parts HBSS and 2 parts 7.5% sodium bicarbonate]. Thirty minutes later, PBS and 10 μg CTB–MUC1 were administered by gavage. After an additional 30 min, liposomal rmIL-12 (1 μg) was administered by gavage. The rmIL-12 + 10 μg CTB–MUC1 group also received 1 μg of rmIL-12 on days 4, 11 and 17.
Parenteral immunizations
Vaccine formulations were initially tested in C57BL/6 mice using subcutaneous (s.c.) and intraperitoneal (i.p.) routes of injection and it was found that both induced similar immune responses. Since this study is a proof of principle, rather than the final design of a clinical trial and due to ease of administration, we used the intraperitonal route for all further studies. Two animal studies were conducted for the i.p. immunizations. The aim of the first trial was to test if i.p. delivery could break MUC1 tolerance in MUC1.Tg mice. The second study was to test the efficacy of the CpG ODN adjuvant when combined with the CTB–MUC1 conjugate protein. Vaccine regimen included weekly injections (100 μl vol.) on days 0, 7, 14, 21, 28 and 35. Mice were challenged on day 14 with the tumor cell line C57mg.MUC1 (1 × 106 cells).
Endpoint analysis consisted of serum analysis for anti-MUC1 IgG, IgG2a, and IgG1, 3H-thymidine proliferation assays and Mouse Th1/Th2 Cytometric Bead Array (BD Biosciences Pharmingen, San Diego, CA, USA) analysis.
Antibody responses
To detect anti-MUC1 antibodies, 96-well plates (Falcon 353911, BD Biosciences Discovery Labware, Bedford, MA, USA) were coated with 5 μg of the chemically synthesized MUC1 peptide. Serum from vaccinated animals was then serially diluted in the plates followed by secondary HRP conjugated goat anti-mouse-IgG (Pierce Biotechnology, Rockford, IL, USA), IgG1 or IgG2a (Southern Biotech, Birmingham, AL, USA). TMB peroxidase EIA substrate kit (Bio-Rad Laboratories, Hercules, CA, USA) was used for detection. Concentrations of serum anti-MUC1 were determined by linear regression from a standard curve of BC2, a mouse monoclonal antibody with epitopes in the TR domain on MUC1 [28, 29]. To confirm that antibody detection was on the MUC1 peptide and not on the cysteine within the synthesized MUC1 peptide, parallel experiments were conducted using plates coated with a MUC1 peptide without a cysteine (TAPPA).
The anti-CTB antibody ELISA was performed as the anti-MUC1 ELISA except plates were coated with ganglioside 0.5 μg/50 μl per well (Sigma–Aldrich G3018, St. Louis, MO, USA) and incubated overnight at room temperature, before adding 125 ng of CTB in 50 μl/well (Sigma–Aldrich C9903, St. Louis, MO, USA). Concentrations of serum anti-CTB were determined by linear regression from a standard curve of rabbit anti-CTB, a rabbit polyclonal antibody purified using the HiTrap Protein G HP affinity column as per manufacturer’s instructions (Amersham Biosciences, Piscataway, NJ, USA) and then quantified using the Beckman DU-600 Spectrophotometer (Beckman Instruments, Inc., Fullerton, CA, USA).
T cell proliferation assay
T cell proliferation assays were conducted to test if T cell tolerance was broken in the immunized MUC1.Tg mice. DCs were made from bone marrow as described previously [13] and pulsed with 10 μg/ml MUC1 TR peptide. Irradiated DCs (3000R) were then co-incubated with splenocytes at a 1:20 ratio for 5 days. The cells were then pulsed with 3H-thymidine to measure the proliferative activity using a Topcount liquid scintillation counter (PerkinElmer Life and Analytical Sciences, Inc., Wellesley, MA, USA).
Tumor protective immunity
Mice were challenged with C57mg.MUC1 tumor cells (1 × 106 cells) by subcutaneous injection in the right flank region. Cells were obtained from cultures, washed extensively with PBS and viability determined by trypan blue dye exclusion. Tumor burden was monitored by daily palpations. Palpable tumors were measured in cm by calipers and tumor volume was calculated according to the formula: mm3 = (length × width × height) × 10.
Immunohistochemistry and histology
To evaluate MUC1 expression, tumors were harvested from control and immunized MUC1.Tg mice at the time of killing. Tumors were fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid) followed by 70% ethanol, and paraffin embedded and step-sectioned for immunohistochemical analysis. B27.29 antibody that recognizes the human MUC1 tandem repeat sequences, but is not reactive with the mouse Muc1 protein, was used to detect MUC1 on tumor sections. Secondary antibody was a goat anti-mouse IgG conjugated to HRP (Dako, Carpinteria, CA, USA). Antibody staining was blocked with the appropriate peptides.
Flow cytometry
Pooled serum from immunized mice (n = 5/group) were incubated with 1 × 106 C57mg.MUC1 or C57mg wild-type tumor cells for 1 h at 4°C. To detect specifically bound antibodies from the serum on the tumor cells, a PE conjugated goat anti-mouse IgG secondary antibody (BD Pharmingen Franklin Lakes, NJ, USA) was used. As a positive control, the C57mg.MUC1 cell line was stained with an FITC anti-MUC1 antibody to confirm the presence of MUC1 on the cell line. The cells were then analyzed on a flow cytometer.
In vitro stimulation (IVS)
To examine if the CTB–MUC1 conjugate can stimulate MUC1.Tg and/or wild-type C57BL/6 splenocytes, two rounds of in vitro stimulation (IVS) were employed. MUC1.Tg DCs were pulsed with: (1) 50 μg MUC1 TR peptide; (2) 10 μg CTB–MUC1; (3) 25 μg CTB–MUC1; (4) 50 μg CTB–MUC1 and (5) no peptide. Splenocytes from non-treated MUC1.Tg and C57BL/6 mice were co-incubated with DCs for 5 days/round of IVS. DCs were made from bone marrow and pulsed with the specified peptides on day 5 and then 1 μg/ml LPS on day 6. On day 7, the DCs were irradiated (3000R), scraped and then co-incubated with splenocytes at a 1:20 ratio (1 × 104 cells/well DC: 2 × 105 cells/well splenocytes). For the first round of IVS, the cells were allowed to co-incubate in a 24-well flat-bottom plate for 5 days at 37°C in a 5% CO2 incubator. On day 5, the floating splenocytes were collected and re-plated on a 96-well plate with freshly prepared DCs (pulsed and irradiated in the same manner as the previous DCs) and allowed to co-incubate for an additional 5 days (day 10). On day 11, we performed a T cell proliferation assay (as described above).
Cytokine analysis
The Mouse Th1/Th2 Cytokine Bead Array kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to detect IL-2, IL-4, IL-5, IFN-γ and TNF-α in culture supernatant of spleen from immunized mice. Splenocytes from immunized and non-immunized mice were co-incubated with DCs pulsed with 10 μg/ml MUC1 TR peptide for 5 days, as described in T cell proliferation assay. The supernatant was then collected and placed at −80°C until further analysis. The assay was performed as directed by the manufacturer.
Statistical analysis
Statistics were analyzed with JMP 5.1.2 software (SAS Institute, Inc.). p values were generated using the two-tailed Student’s t test. Values were considered significant when p < 0.05.
Results
Oral immunizations with the CTB–MUC1 conjugate protein
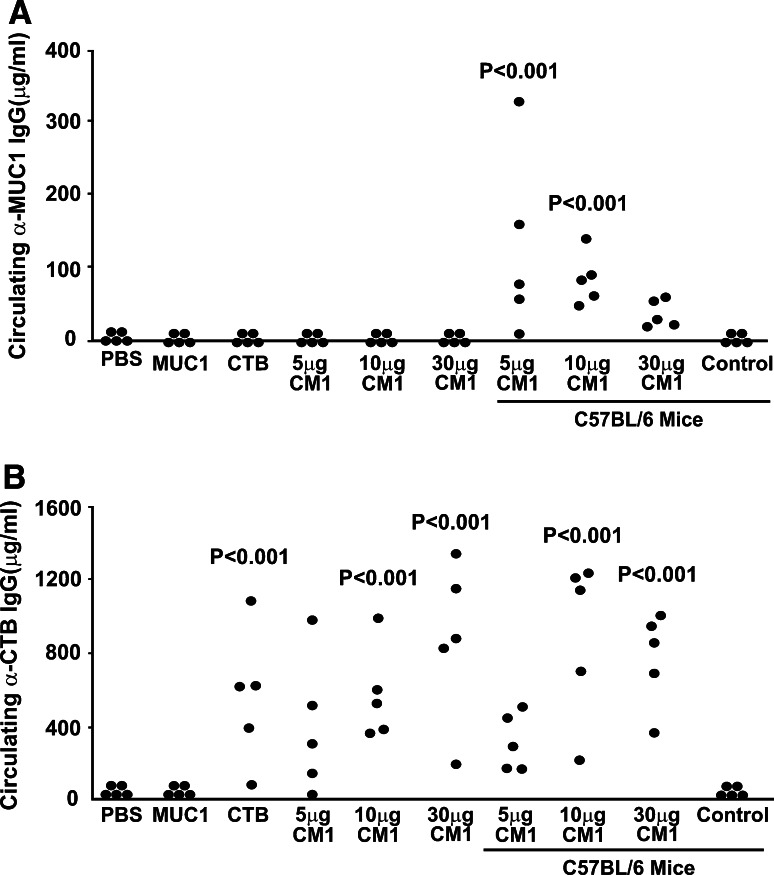
MUC1.Tg and C57BL/6 mice were orally immunized with CTB–MUC1 on a weekly basis for 6 weeks. MUC1-specific antibodies were not detected in the serum of immunized MUC1.Tg mice treated with the CTB–MUC1 conjugate protein (Fig. 1a), whereas C57BL/6 mice showed a significant increase in MUC1-specific IgG levels for the 5 and 10 μg CTB–MUC1 treatment group compared to the PBS control group (p < 0.001 and p < 0.01, respectively).
Fig. 1.
Antibodies to MUC1 were elicited in C57BL/6 mice, but not in tolerant MUC1.Tg mice following oral administration of CTB–MUC1, yet antibodies to CTB were elicited in all mice. a Circulating MUC1 IgG levels. MUC1.Tg mice treated with 5, 10 and 30 μg CTB–MUC1 (CM1) do not show any circulating MUC1 IgG, whereas C57BL/6 mice show high levels of MUC1 IgG. MUC1.Tg mice treated with 10 μg MUC1 peptide alone did not elicit antibodies to MUC1. C57BL/6 show a significant increase in MUC1 IgG levels as compared to the PBS alone group for the 5 μg CTB–MUC1 treatment group (p < 0.001) and the 10 μg CTB–MUC1 treatment group (p < 0.01). b Circulating CTB IgG levels. MUC1.Tg mice treated with 10 μg CTB–MUC1 (p < 0.01), 30 μg CTB–MUC1 (p < 0.001) and CTB alone (p < 0.01) show a significant increase compared to the PBS control group. C57BL/6 mice also gave significantly higher CTB IgG levels when treated with 10 and 30 μg CTB–MUC1 MUC1 (p < 0.001) as compared to PBS control group. Control represents PBS treated wild-type C57BL/6 mice. CM1 represents the CTB–MUC1 conjugate protein. Individual mouse data are shown (n = 5 mice/group)
Therefore, orally delivered CTB–MUC1 conjugate protein was not able to break tolerance to MUC1 in the transgenic mice. Nonetheless, in the C57BL/6 mice a strong antibody response against MUC1 was observed, suggesting that the peptide was intact in the gastric environment and it was immunogenic if it did not have to overcome self-antigen tolerance. All mice treated with CTB and CTB–MUC1 showed a strong anti-CTB IgG response (Fig. 1b). In the orally immunized C57BL/6 mice, we observed an unexpected decrease in anti-MUC1 IgG levels in the 30 μg CTB–MUC1 treatment group compared to the 5 and 10 μg CTB–MUC1 groups. At that time, we could not explain this decrease, but it may be that CTB dominated the immune response and reduced the MUC1-specific humoral response generated. Anti-CTB IgG levels showed a steady increase in antibody levels with the increased dosage of CTB–MUC1 (Fig. 1b). Again, this difference in anti-MUC1 and anti-CTB IgG levels with increasing CTB–MUC1 dosage may point to the immunodominant property of CTB with respect to the humoral immune response, which has been reported in a previous study [30]. Although oral tolerance studies have shown that high doses of antigen help induce tolerance, whereas lower doses were less likely, we are not sure if this was the case in our study since we only used micrograms of fusion protein versus milligrams of protein used in the studies described in literature. In addition, the average anti-MUC1 IgG responses between the low and high CTB–MUC1 doses are comparable in the C57BL/6 mice depicted in Fig. 1.
Oral immunizations with strong mucosal adjuvants and CTB–MUC1
Since oral immunization with the CTB–MUC1 conjugate protein did not induce a MUC1-specific immune response in the MUC1.Tg mouse model, we decided to co-deliver strong mucosal adjuvants that promote a Th1-type response along with the CTB–MUC1 conjugate. The first adjuvant study included the synthetic CpG ODN. MUC1.Tg mice treated with CpG ODN + 10 μg CTB–MUC1 did not stimulate antibodies against MUC1 (tested for IgG and IgA; data not shown).
To investigate the cellular immune response, we examined the spleen and Peyer’s patch of orally immunized MUC1.Tg mice by T cell proliferation assays and cytokine analysis. Splenocytes and Peyer’s patch cells were co-cultivated with DCs pulsed with the MUC1 TR peptide for 5 days. The supernatant was then collected and analyzed for Th1/Th2 cytokines (IL-2, IL-4, IL-5, IFN-γ and TNF-α), and cell proliferation was examined by 3H-thymidine uptake. Proliferation and cytokine levels obtained during the analysis fell within background levels (data not shown); thus, it is unlikely that T cell tolerance to MUC1 was broken with this treatment.
Since cytokines are known to play an essential role in stimulating immune responses, the second adjuvant used was recombinant mouse IL-12 (rmIL-12) that is known to drive a Th1 response by promoting IFN-γ formation by T cells and NK cells. Because of the harsh environment in the gut, rmIL-12 was encased and protected in DOTAP liposomes. MUC1.Tg mice treated with rmIL-12 + 10 μg CTB–MUC1 did not stimulate antibodies or T cells against MUC1, thus this treatment failed to break MUC1 self-antigen tolerance (data not shown).
Intraperitoneal immunizations with the CTB–MUC1 conjugate protein
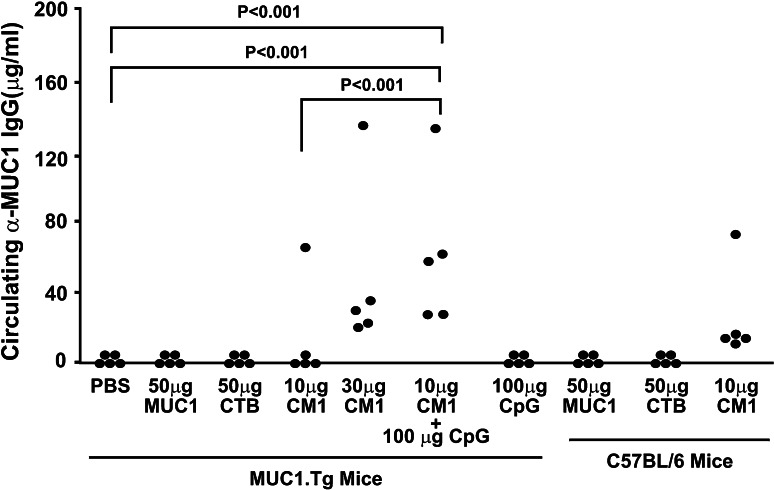
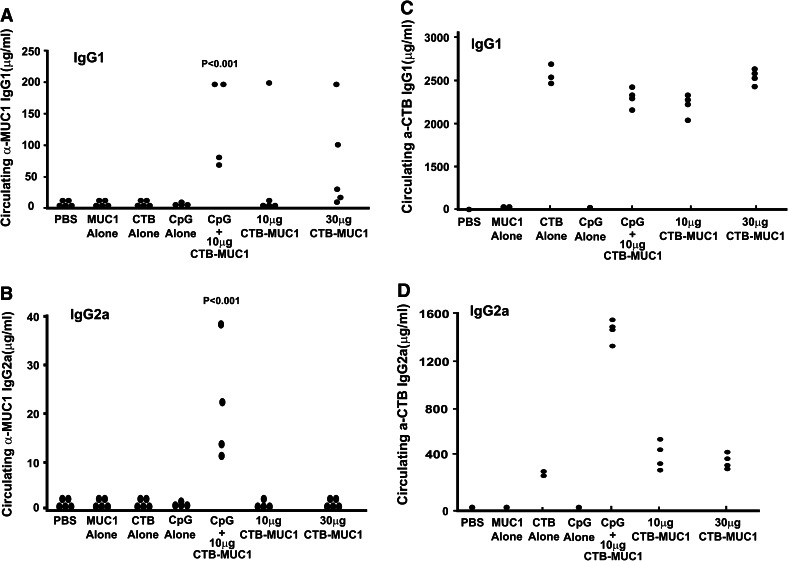
Due to the ineffectiveness of oral immunization with CTB–MUC1 to produce a MUC1-specific immune response in the MUC1.Tg mouse model, we decided to employ an alternate delivery method with the CTB–MUC1 conjugate protein along with the CpG ODN adjuvant and the proper controls. Since we did not observe any difference in the anti-MUC1 IgG levels in the mice injected with CTB–MUC1 (10 μg) + CpG ODN either i.p. or s.c., we selected i.p. injections for the future experiments (the values for anti-MUC1 IgG were 72 + 42 μg/ml for i.p. vs. 78 + 49 μg/ml for s.c. injection in n = 5 mice per group). MUC1.Tg mice were immunized i.p. on a weekly basis for 7 weeks. C57BL/6 and MUC1.Tg mice treated with MUC1 peptide alone did not generate MUC1-specific IgG, whereas immunization with the CTB–MUC1 conjugate induced a strong anti-MUC1 IgG response (Fig. 2). Moreover, the addition of the adjuvant CpG ODN (100 μg) to 10 μg CTB–MUC1 led to a significant increase (p < 0.01) in anti-MUC1 IgG levels compared to the 10 μg CTB–MUC1 group (Fig. 2). Furthermore, serum analysis for MUC1-specific IgG1 and IgG2a showed that the response was primarily T-helper type 2 (Th2) with high IgG1 titers in all the CTB–MUC1 treatment groups (Fig. 3a). However, the addition of CpG ODN (100 μg) to 10 μg CTB–MUC1 showed a strong anti-MUC1 and anti-CTB IgG2a titer (Fig. 3b, d) that was significantly higher than the PBS control group (p < 0.001), suggesting a shift from a Th2 to a Th1-type response.
Fig. 2.
Intraperitoneal (i.p.) administration of CTB–MUC1 was able to break MUC1 tolerance in MUC1.Tg mice. Circulating MUC1 IgG were detected in MUC1.Tg mice treated by i.p. injections of 10 and 30 μg CTB–MUC1 (CM1), as well as 100 μg CpG + 10 μg CTB–MUC1, but not in PBS, 50 μg MUC1 peptide and 50 μg CTB control groups. Levels of circulating MUC1 IgG levels in MUC1.Tg mice treated with 30 μg CTB–MUC1 (p < 0.01) and 100 μg CpG + 10 μg CTB–MUC1 (p < 0.001) were statistically significant compared to the PBS control. C57BL/6 mice treated with 50 μg MUC1 peptide and 50 μg CTB alone did not generate MUC1 IgG, whereas treatment with 10 μg CTB–MUC1 induced a strong MUC1 IgG response. CM1 represents the CTB–MUC1 conjugate protein. Individual mouse data are shown (n = 5 mice/group)
Fig. 3.
Intraperitoneal (i.p.) administration of CTB–MUC1 induced a T helper type 2 (Th2) responses in MUC1.Tg mice. Serum analysis for anti-MUC1 IgG isotypes IgG1 (a) and IgG2a (b) and anti-CTB isotypes IgG1 (c) and IgG2a (d). MUC1.Tg mice treated with 10 and 30 μg CTB–MUC1 (CM1) as well as 100 μg CpG + 10 μg CTB–MUC1 were analyzed to determine if the response was predominately Th2 (IgG1) or Th1 (IgG2a). Circulating MUC1 IgG1 (a) and CTB IgG1 (c) are seen to be the dominant isotype in all treatments when compared with IgG2a (b and d) serum levels. Interestingly, the addition of 100 μg CpG ODN to 10 μg CTB–MUC1 increased the level of CTB IgG2a (d). MUC1.Tg mice immunized with 100 μg CpG + 10 μg CTB–MUC1 have a significantly higher level of MUC1 IgG1 (a; p < 0.01) and MUC1 IgG2a (b; p < 0.001) compared to the PBS control. Individual mouse data are shown (n = 5/treatment group)
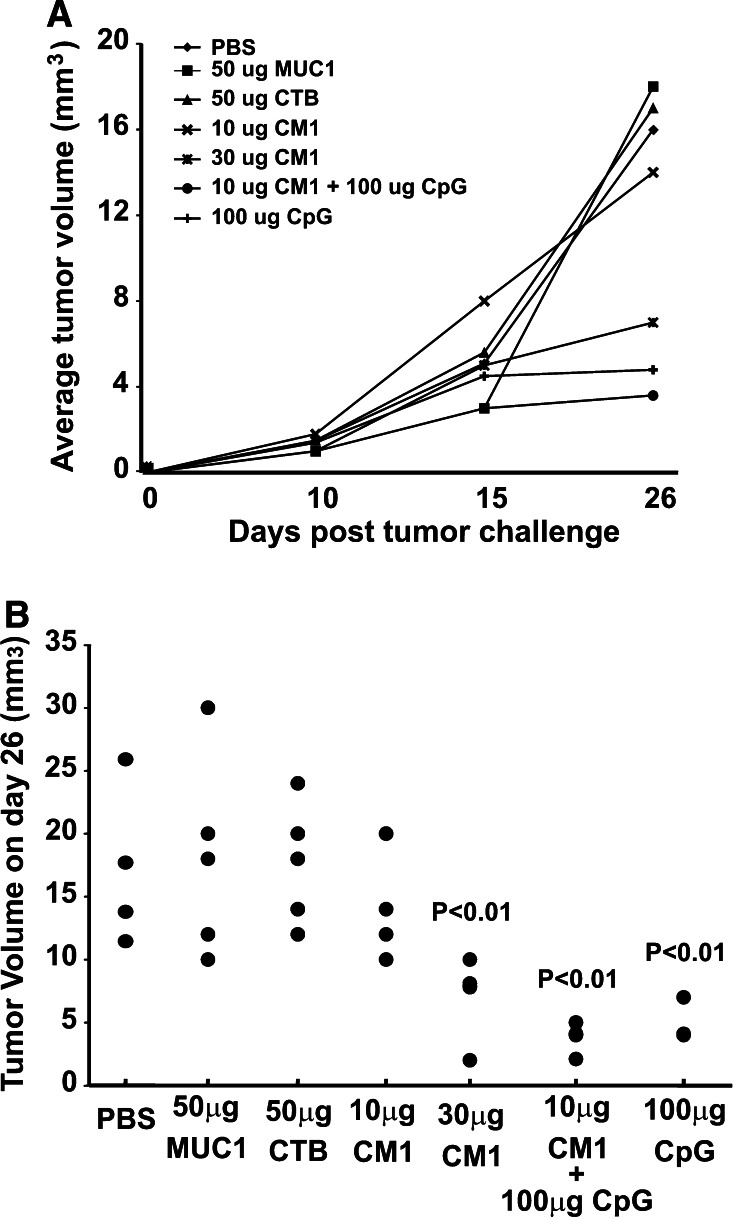
On day 14, immunized mice were challenged with a mammary gland tumor cell line that expresses human MUC1 (C57mg.MUC1) by subcutaneous injection (1 × 106 cells) into the right flank region. C57mg.MUC1 cells do not metastasize from s.c. injections and no metastasis was apparent in any treatment group. Tumor burden was determined by daily palpation after tumor challenge and continued until the day of killing (26 days post-tumor challenge). Compared to the MUC1 peptide control group, tumor burden was significantly lower (p < 0.001) in groups immunized with CpG ODN (Fig. 4b) and with higher dose (30 μg) of CTB–MUC1 conjugate protein (p < 0.01). All mice were taken down at the same time point (day 26) due to the large tumor size observed in the control groups (i.e., PBS); therefore, we cannot say for certain whether or not the tumors in the CTB–MUC1 vaccinated group would have continued to grow with time.
Fig. 4.
MUC1.Tg immunized with CTB–MUC1 and CpG ODN exhibited decreased tumor burden when challenged with a mammary gland tumor cell line that expresses human MUC1. a Tumor growth of MUC1.Tg mice challenged with the tumor cell line C57mg.MUC1. MUC1.Tg mice treated by i.p. injections of PBS, 50 μg MUC1 peptide alone, 50 μg CTB alone, 10 and 30 μg CTB–MUC1, 100 μg CpG + 10 μg CTB–MUC1, and 100 μg CpG alone were challenged with 1 × 106 C57mg.MUC1 cells by subcutaneous (s.c.) injections in the right flank region. Tumor burden was determined by daily palpation after tumor challenge on day 0 and continued until day 26. The formula used to calculate tumor mass was: mm3 = (length × width × height) × 10. b At 26 days post-challenge, tumor burden was measured. Tumor burden was significantly lower in the 100 μg CpG ODN alone (p < 0.001), 100 μg CpG ODN + 10 μg CTB–MUC1 (p < 0.001) and 30 μg CTB–MUC1 (p < 0.01) treatment groups compared to the MUC1 alone control group. CM1 represents the CTB–MUC1 conjugate protein. Individual mouse data are shown (n = 5 mice/group)
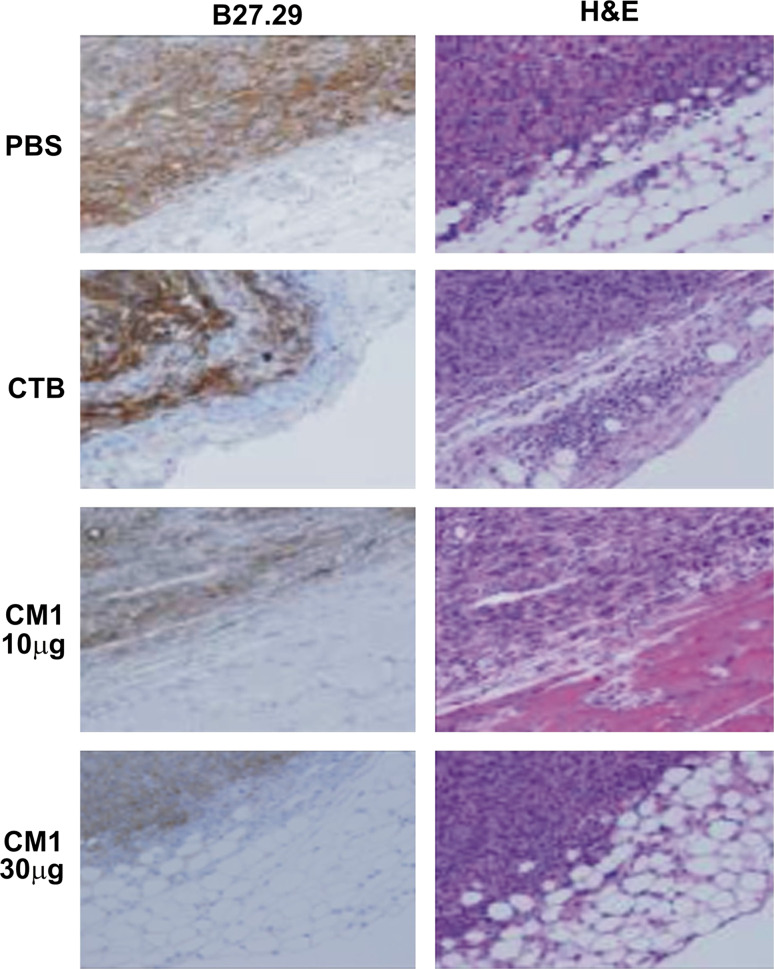
Tumor sections from MUC1.Tg mice immunized with CTB–MUC1 showed a decrease in MUC1 expression with increased CTB–MUC1 dose regimen (Fig. 5). Paraffin embedded tumor sections were stained with the B27.29 antibody that recognizes the human MUC1 tandem repeat sequences, but is not reactive with the mouse Muc1 protein. The structural integrity of the cellular mass was clearly delineated in the hematoxylin and eosin (H&E) stain. B27.29 staining visually shows a decrease in MUC1 staining with increased dose of the CTB–MUC1 regimen compared to the CTB alone control, suggesting that the decrease in tumor burden was a MUC1-specific response. Decreased MUC1 expression may be attributed to fewer tumor cells, as measured by tumor burden (Fig. 4); yet, we cannot discount the possibility that the leftover tumor cells may become resistant by decreasing MUC1 expression on their surface.
Fig. 5.
Histological examination of MUC1 expression on tumors collected from immunized MUC1.Tg mice treated with higher doses of CTB–MUC1 shows a slight decrease in MUC1 staining. Methacarn fixed and paraffin-embedded sections of injected C57mg.MUC1 tumors from control and immunized MUC1.Tg mice were stained with B27.29, which recognizes the human MUC1 tandem repeat sequences but is not reactive with the mouse Muc1 protein. Sections shown were also stained with H&E. Images were collected at ×200 magnification
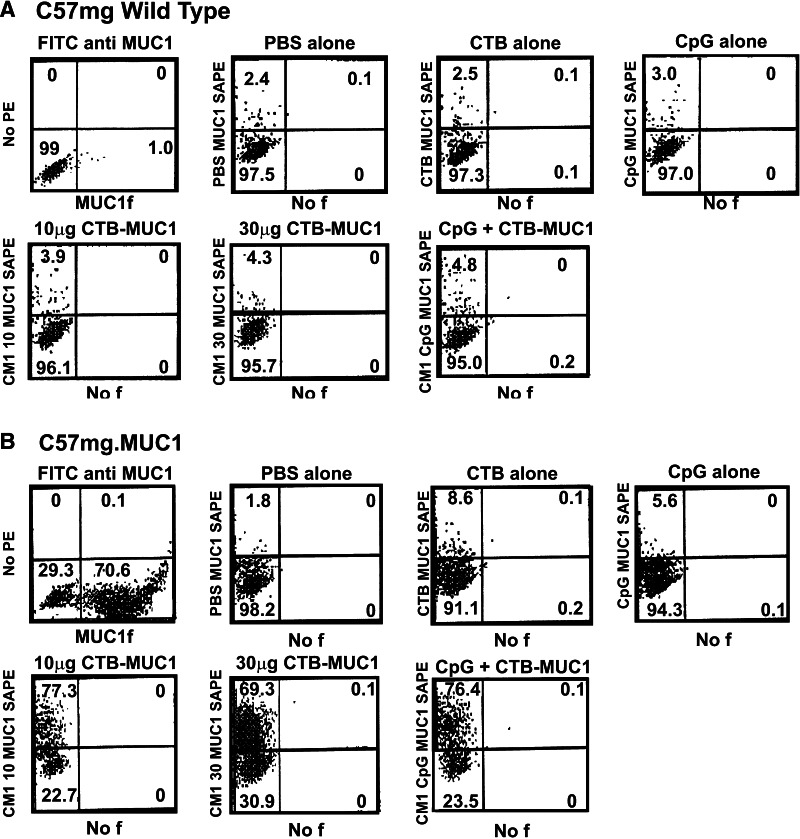
To demonstrate that MUC1.Tg mice immunized with the CTB–MUC1 conjugate protein induced antibodies that react with MUC1 as it is naturally expressed on tumor cells, we co-incubated C57mg.MUC1 cell line with the serum from immunized animals and analyzed by flow cytometry. To distinguish between MUC1-specific binding and non-specific antibody binding, we used C57mg wild-type tumor cell line that does not express MUC1. As a positive control, the tumor cell lines were stained with a commercially available FITC anti-MUC1 antibody from BD Pharmingen; C57mg.MUC1 cells showed very high MUC1 expression (80%, Fig. 6b), whereas virtually no expression was detected in the C57mg cells (1.0%, Fig. 6a). Sera from MUC1.Tg mice immunized with PBS, CTB and CpG ODN alone showed low levels of MUC1-specific binding in the C57mg.MUC1 tumor cell line and low levels of non-specific binding in the C57mg cells. The two doses of CTB–MUC1 showed MUC1-specific binding with the highest level of binding in the 10 μg dose (77.3%), followed by the 30 μg dose (69.3%). In the C57mg cells, both CTB–MUC1 dosages showed low levels of non-specific binding (2–4%). The CpG ODN + 10 μg CTB–MUC1 showed high MUC1-specific binding in the C57mg.MUC1 cells (76.4%) and low non-specific binding in the C57mg cells (4.8%). These results were obtained by combing sera from all mice in each treatment group. Due to the high variation of response we observed within each treatment group, we could not say for certain that the reactivity of one treatment was stronger than the other, but we could say that the antibodies were specific to the MUC1 expressed on the C57mg.MUC1 tumor cell line. From these results, we can conclude that our vaccine formulation does in fact induce antibodies that react with MUC1 as it is naturally expressed on tumor cells.
Fig. 6.
The vaccine formulation induced antibodies that react with MUC1 as it is naturally expressed on tumor cells. Serum from MUC1.Tg mice immunized with the 10 and 30 μg CTB–MUC1 conjugate protein and 100 μg CpG ODN + 10 μg CTB–MUC1 react with MUC1 as it is naturally expressed on the tumor cell line C57mg.MUC1 (b), but not on the wild-type control cell line C57mg (a). An FITC conjugated anti-MUC1 antibody was used as a positive control to show MUC1 expression on the C57mg.MUC1 cell line, but not on the wild-type control group. Mice immunized with PBS, 50 μg CTB and 100 μg CpG ODN alone showed low levels of reactivity in C57mg.wild-type and C57mg.MUC1 cell line. Percentage of gated populations is shown in the appropriate quadrants. SA–PE stands for secondary antibody conjugated to phycoerythrin (PE)
Discussion
In this study, we investigated if the tumor-associated antigen MUC1 conjugated to the carrier molecule CTB would break self-antigen tolerance in the MUC1.Tg mouse model via oral and i.p. delivery. We found that oral delivery of the CTB–MUC1 conjugate protein did not break MUC1 self-antigen tolerance (Fig. 1a). However, i.p. delivery of the CTB–MUC1 conjugate protein along with adjuvant CpG ODN broke MUC1 tolerance (Figs. 2, 3) and decreased tumor burden on challenge with the C57mg.MUC1 tumor cell line (Fig. 4).
Cholera toxin (CT) and the closely related Escherichia coli heat-labile toxin (LT), which bind to ganglioside receptors on all nucleated cells, have strong immuno-enhancing effects on both systemic and mucosal immune responses [31]. These toxins comprise a single A-subunit with ADP ribosyltransferase activity and five B subunits responsible for binding to cell surface gangliosides. After B subunit binding to ganglioside receptors, holotoxins are internalized and cause intracellular increases in cyclic AMP (cAMP), which results in classical watery diarrhea. Optimal immunomodulating effect is dependent on both binding and enzymatic activities of CT [32].
Recent evidence suggests that CT binds directly to antigen-presenting cells (APCs) through GM1–ganglioside interaction and induced nuclear translocation of nuclear factor (NF)-κB, leading to DC maturation and activation [33]. Therefore, CTB may not only be useful as a carrier for mucosal immunizations due to their M-cell interaction in the Peyer’s patches, but also more generally as an immunomodulating APC targeting molecule. We observed this DC-specific effect of our CTB–MUC1 conjugate protein by an in vitro stimulation assay, where DCs pulsed with MUC1 alone or CTB–MUC1 were co-cultured with MUC1.Tg splenocytes and only the CTB–MUC1 treated DCs induced blast formation and proliferation (Suppl Fig. 1).
Since our goal was to increase the pool of effector cells to include Th1 and Th2 cells, we co-delivered CpG ODN with the CTB–MUC1 conjugate. Bacterial DNA and synthetic ODNs expressing unmethylated CpG motifs trigger an immunostimulatory cascade that culminates in the maturation, differentiation and proliferation of multiple immune cells, including DCs, macrophages, monocytes, NK cells, and B and T lymphocytes [34]. Together, these cells secrete cytokines and chemokines that create a pro-inflammatory and Th1-biased immune milieu. In our study, oral delivery of the CTB–MUC1 conjugate along with CpG ODN did not break MUC1 self-antigen tolerance in the MUC1.Tg mouse model. However, i.p. co-delivery of CpG ODN augmented the immune response of the CTB–MUC1 conjugate and shifted the response toward a Th1-type response, as demonstrated by high anti-MUC1 IgG2a levels (Fig. 3b).
Significant decrease in tumor burden was observed in CpG ODN alone (Fig. 4b; p < 0.001) and CpG ODN + 10 μg CTB–MUC1 (p < 0.001) treatment groups. Cytokine analysis of culture supernatant of splenocytes from CpG ODN and CpG ODN + 10 μg CTB–MUC1 treatment groups did not show an increase in IFN-γ levels when co-incubated with DCs pulsed with the MUC1 TR peptide (Suppl Fig. 2). The lack of response may be due to the specificity of the assay for the MUC1 TR antigen, suggesting that the immune response stimulated by CpG ODN is not specific to the MUC1 antigen. Therefore, the decrease in tumor burden in the CpG ODN alone treatment may be due to NK cell activation and the Th1-biased immune milieu that is induced by CpG ODN stimulation, which has been previously reported by Baines and Celis [35].
The current goal for cancer vaccines is to promote a CTL response that leads to tumor rejection on challenge. However, it is important to recognize the essential role antibodies play in the immune system and, if used in prophylactic settings, how they might aid in the anti-tumor immune response. We showed by flow cytometry that the antibodies induced in our vaccine formulation specifically bind to MUC1 on the C57mg.MUC1 tumor cell line (Fig. 6b). Antibodies act on tumor cells by fixing complement, facilitating antibody-dependant cellular cytotoxicity (ADCC) and/or promoting tumor uptake by APCs via opsonization. More specifically, MUC1 vaccination trials in breast cancer patients have demonstrated that the induced MUC1 antibodies can affect tumor cell killing by ADCC and that NK cells were the essential effector cells [36]. Moreover, early breast cancer patients with a natural antibody response to MUC1 have a higher probability of being free from distant disease recurrence [37]. Therefore, it may be worthwhile to formulate cancer vaccines that stimulate diverse immune effector cells at diverse sites to curtail the evasive properties of tumor cells.
Our study is the first published attempt at breaking MUC1 self-antigen tolerance using the mucosal carrier peptide CTB via alternate delivery methods. Oral delivery of the CTB–MUC1 conjugate protein induced a MUC1-specific immune response in wild-type C57BL/6 mice, but not in MUC1.Tg mice. This suggests a different requirement for induction of a mucosal immune response to a foreign antigen compared to a self-antigen. Although it is well known that oral delivery of peptides can induce immunological tolerance, we know this is not the case in our study, since the wild-type C57BL/6 mice exhibited a strong anti-MUC1 humoral response. However, breaking self-antigen tolerance and decreasing tumor burden were achieved in MUC1.Tg mice using parenteral delivery. Future studies will examine a prime boost regimen that include parenteral and mucosal delivery of the vaccine formulation to test if breaking tolerance via parenteral delivery would translate into breaking immunological tolerance in the mucosal immune system. Also, a multivalent vaccine formulation that targets multiple tumor-associated antigens would be the ideal therapy to overcome the evasive properties of tumor cells and to target a greater percentage of cancerous cells in contrast to traditional vaccines comprising a single antigen. Using the multivalent vaccine formulation along with alternate routes of administration in conjunction with carrier peptides and adjuvants is an approach that may generate a multi-faceted immune response that leads to tumor rejection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Two rounds of in vitro stimulation (IVS) using DCs pulsed with MUC1 peptide alone and CTB-MUC1 conjugate protein. Pulsed DCs were co-incubated with C57BL/6 and MUC1.Tg splenocytes (5 days per round of IVS) (A) Blast formation is clearly visible in the 10-, 25- and 50 μg CTB-MUC1 pulsed DC plates for both C57BL/6 and MUC1.Tg splenocytes. As expected, the tolerant MUC1.Tg splenocytes did not form blasts to the DCs pulsed with MUC1 peptide only. (B) Proliferation assay confirms that DCs pulsed with MUC1 alone did not induce proliferation in C57BL/6 nor MUC1.Tg splenocytes, whereas DCs pulsed with 10-, 25- and 50 μg CTB-MUC1 conjugate protein stimulated a strong T cell response in vitro. Proliferation using DCs pulsed with 10 and 50 μg CTB-MUC1 was significantly higher (p < 0.01) than MUC1 peptide pulsed Dcs. Significant difference (p < 0.001) was also observed for DCs pulsed with 25 μg CTBMUC1 (JPEG 2.52 mb)
MUC1.Tg mice treated with the 30 μg CTB-MUC1 conjugate protein showed a skewing towards a T helper type 1 response. Th1/Th2 cytokine analysis of culture supernatant of splenocytes from treated MUC1.Tg mice co-incubated with dendritic cells (DCs) pulsed with 10 μg/ml MUC1. Readings were taken after 5 days of stimulation. (A) IFN-γ levels. (B) IL-4 levels. The 30 μg CTB-MUC1 treatment group shows a high level of IFN-γ production (A; *P < 0.05, compared to PBS control), and a lower level of IL-4 production (B). Individual mouse data are shown (JPEG 835 kb)
Acknowledgments
The study was supported by CA107959-01A1 and DOD Breast Cancer Research Program (BCRP) BC022017. We would like to thank the animal facility and the histology staff at Mayo Clinic Arizona for their help with the animal study and with the histology. We would like to thank Ms. Mahnaz Sahraei for her help with the final figures.
References
- 1.Dahiya R, Kwak KS, Byrd JC, Ho S, Yoon WH, Kim YS. Mucin synthesis and secretion in various human epithelial cancer cell lines that express the MUC-1 mucin gene. Cancer Res. 1993;53:1437–1443. [PubMed] [Google Scholar]
- 2.Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, McGuckin MA. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol. 1997;183:311–317. doi: 10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Leroy X, Zerimech F, Zini L, Copin MC, Buisine MP, Gosselin B, Aubert JP, Porchet N. MUC1 expression is correlated with nuclear grade and tumor progression in pT1 renal clear cell carcinoma. Am J Clin Pathol. 2002;118:47–51. doi: 10.1309/1F99-BPDY-7DHH-9G97. [DOI] [PubMed] [Google Scholar]
- 4.Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 5.Acres B, Apostolopoulos V, Balloul JM, Wreschner D, Xing PX, Ali-Hadji D, Bizouarne N, Kieny MP, McKenzie IF. MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–594. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acres RB, Hareuveni M, Balloul JM, Kieny MP. Vaccinia virus MUC1 immunization of mice: immune response and protection against the growth of murine tumors bearing the MUC1 antigen. J Immunother Emphasis Tumor Immunol. 1993;14:136–143. [PubMed] [Google Scholar]
- 7.Apostolopoulos V, Xing PX, McKenzie IF. Murine immune response to cells transfected with human MUC1: immunization with cellular and synthetic antigens. Cancer Res. 1994;54:5186–5193. [PubMed] [Google Scholar]
- 8.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 9.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 10.Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, Xing PX, Jamieson G, Pietersz G, Tait B, Broadbent R, Thynne G, McKenzie IF. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, Pietersz GA, Apostolopoulos V, Vaughan H, Karanikas V, Kyriakou P, McKenzie IF, Mitchell PL. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee P, Ginardi AR, Tinder TL, Sterner CJ, Gendler SJ. MUC1-specific cytotoxic T lymphocytes eradicate tumors when adoptively transferred in vivo. Clin Cancer Res. 2001;7:848s–855s. [PubMed] [Google Scholar]
- 13.Mukherjee P, Madsen CS, Ginardi AR, Tinder TL, Jacobs F, Parker J, Agrawal B, Longenecker BM, Gendler SJ. Mucin 1-specific immunotherapy in a mouse model of spontaneous breast cancer. J Immunother. 2003;26:47–62. doi: 10.1097/00002371-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P, Tinder TL, Basu GD, Pathangey LB, Chen L, Gendler SJ. Therapeutic efficacy of MUC1-specific cytotoxic T lymphocytes and CD137 co-stimulation in a spontaneous breast cancer model. Breast Dis. 2004;20:53–63. doi: 10.3233/bd-2004-20107. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Papadimitriou J, Burchell JM, Plunkett T, Graham R, Correa I, Miles D, Smith M. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–221. doi: 10.1023/A:1020360121451. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan HA, Ho DW, Karanikas VA, Ong CS, Hwang LA, Pearson JM, McKenzie IF, Pietersz GA. Induction of humoral and cellular responses in cynomolgus monkeys immunised with mannan-human MUC1 conjugates. Vaccine. 1999;17:2740–2752. doi: 10.1016/S0264-410X(98)00493-9. [DOI] [PubMed] [Google Scholar]
- 17.Xia J, Tanaka Y, Koido S, Liu C, Mukherjee P, Gendler SJ, Gong J. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol. 2003;170:1980–1986. doi: 10.4049/jimmunol.170.4.1980. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Graeber LA, Helling F, Ragupathi G, Adluri S, Lloyd KO, Livingston PO. Augmenting the immunogenicity of synthetic MUC1 peptide vaccines in mice. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 19.Pupa SM, Invernizzi AM, Forti S, Di Carlo E, Musiani P, Nanni P, Lollini PL, Meazza R, Ferrini S, Menard S. Prevention of spontaneous neu-expressing mammary tumor development in mice transgenic for rat proto-neu by DNA vaccination. Gene Ther. 2001;8:75–79. doi: 10.1038/sj.gt.3301360. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren J, Adamsson J, Anjuere F, Clemens J, Czerkinsky C, Eriksson K, Flach CF, George-Chandy A, Harandi AM, Lebens M, Lehner T, Lindblad M, Nygren E, Raghavan S, Sanchez J, Stanford M, Sun JB, Svennerholm AM, Tengvall S. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett. 2005;97:181–188. doi: 10.1016/j.imlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan E, Merritt EA, Zhang Z, Pickens JC, Roach C, Ahn M, Hol WG. Exploration of the GM1 receptor-binding site of heat-labile enterotoxin and cholera toxin by phenyl-ring-containing galactose derivatives. Acta Crystallogr D Biol Crystallogr. 2001;57:201–212. doi: 10.1107/S0907444900016814. [DOI] [PubMed] [Google Scholar]
- 23.Merritt EA, Sixma TK, Kalk KH, van Zanten BA, Hol WG. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT) Mol Microbiol. 1994;13:745–753. doi: 10.1111/j.1365-2958.1994.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, Hol WG. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli . Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 25.George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001;69:5716–5725. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 27.Marinaro M, Boyaka PN, Finkelman FD, Kiyono H, Jackson RJ, Jirillo E, McGhee JR. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)-type responses to an oral vaccine without altering mucosal IgA responses. J Exp Med. 1997;185:415–427. doi: 10.1084/jem.185.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price MR, Rye PD, Petrakou E, Murray A, Brady K, Imai S, Haga S, Kiyozuka Y, Schol D, Meulenbroek MF, Snijdewint FG, Mensdorff-Pouilly S, Verstraeten RA, Kenemans P, Blockzjil A, Nilsson K, Nilsson O, Reddish M, Suresh MR, Koganty RR, Fortier S, Baronic L, Berg A, Longenecker MB, Hilgers J, et al. Summary report on the ISOBM TD-4 Workshop: analysis of 56 monoclonal antibodies against the MUC1 mucin. San Diego, CA, USA, 17–23 November 1996. Tumour Biol. 1998;19(Suppl 1):1–20. doi: 10.1159/000056500. [DOI] [PubMed] [Google Scholar]
- 29.Xing PX, Tjandra JJ, Stacker SA, Teh JG, Thompson CH, McLaughlin PJ, McKenzie IF. Monoclonal antibodies reactive with mucin expressed in breast cancer. Immunol Cell Biol. 1989;67(Pt 3):183–195. doi: 10.1038/icb.1989.29. [DOI] [PubMed] [Google Scholar]
- 30.Matoba N, Geyer BC, Kilbourne J, Alfsen A, Bomsel M, Mor TS. Humoral immune responses by prime-boost heterologous route immunizations with CTB-MPR(649–684), a mucosal subunit HIV/AIDS vaccine candidate. Vaccine. 2006;24:5047–5055. doi: 10.1016/j.vaccine.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999;20:493–500. doi: 10.1016/S0167-5699(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 32.Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/S0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura YI, Kawashima R, Shirai Y, Kato R, Hamabata T, Yamamoto M, Furukawa K, Fujihashi K, McGhee JR, Hayashi H, Dohi T. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-kappaB translocation. Eur J Immunol. 2003;33:3205–3212. doi: 10.1002/eji.200324135. [DOI] [PubMed] [Google Scholar]
- 34.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 35.Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res. 2003;9:2693–2700. [PubMed] [Google Scholar]
- 36.Snijdewint FG, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, Verstraeten AA, Livingston PO, Hilgers J, Kenemans P. Antibody-dependent cell-mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. Int J Cancer. 2001;93:97–106. doi: 10.1002/ijc.1286. [DOI] [PubMed] [Google Scholar]
- 37.von Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FG, Kok A, Van Kamp GJ, Paul MA, Van Diest PJ, Meijer S, Hilgers J. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–583. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two rounds of in vitro stimulation (IVS) using DCs pulsed with MUC1 peptide alone and CTB-MUC1 conjugate protein. Pulsed DCs were co-incubated with C57BL/6 and MUC1.Tg splenocytes (5 days per round of IVS) (A) Blast formation is clearly visible in the 10-, 25- and 50 μg CTB-MUC1 pulsed DC plates for both C57BL/6 and MUC1.Tg splenocytes. As expected, the tolerant MUC1.Tg splenocytes did not form blasts to the DCs pulsed with MUC1 peptide only. (B) Proliferation assay confirms that DCs pulsed with MUC1 alone did not induce proliferation in C57BL/6 nor MUC1.Tg splenocytes, whereas DCs pulsed with 10-, 25- and 50 μg CTB-MUC1 conjugate protein stimulated a strong T cell response in vitro. Proliferation using DCs pulsed with 10 and 50 μg CTB-MUC1 was significantly higher (p < 0.01) than MUC1 peptide pulsed Dcs. Significant difference (p < 0.001) was also observed for DCs pulsed with 25 μg CTBMUC1 (JPEG 2.52 mb)
MUC1.Tg mice treated with the 30 μg CTB-MUC1 conjugate protein showed a skewing towards a T helper type 1 response. Th1/Th2 cytokine analysis of culture supernatant of splenocytes from treated MUC1.Tg mice co-incubated with dendritic cells (DCs) pulsed with 10 μg/ml MUC1. Readings were taken after 5 days of stimulation. (A) IFN-γ levels. (B) IL-4 levels. The 30 μg CTB-MUC1 treatment group shows a high level of IFN-γ production (A; *P < 0.05, compared to PBS control), and a lower level of IL-4 production (B). Individual mouse data are shown (JPEG 835 kb)