Abstract
Robust biofilm formation by Vibrio fischeri depends upon activation of the symbiosis polysaccharide (syp) locus, which is achieved by overexpressing the RscS sensor kinase (RscS+). Other than the Syp polysaccharide, however, little is known about V. fischeri biofilm matrix components. In other bacteria, biofilms contain polysaccharides, secreted proteins, and outer membrane vesicles (OMVs). Here, we asked whether OMVs are part of V. fischeri biofilms. Transmission electron microscopy revealed OMV-like particles between cells within colonies. In addition, OMVs could be purified from culture supernatants of both RscS+ and control cells, with the former releasing 2- to 3-fold more OMVs. The increase depended upon the presence of an intact syp locus, as an RscS+ strain deleted for sypK, which encodes a putative oligosaccharide translocase, exhibited reduced production of OMVs; it also showed a severe defect in biofilm formation. Western immunoblot analyses revealed that the RscS+ strain, but not the control strain or the RscS+ sypK mutant, produced a distinct set of nonproteinaceous molecules that could be detected in whole-cell extracts, OMV preparations, and lipopolysaccharide (LPS) extracts. Finally, deletion of degP, which in other bacteria influences OMV production, decreased OMV production and reduced the ability of the cells to form biofilms. We conclude that overexpression of RscS induces OMV production in a manner that depends on the presence of the syp locus and that OMVs produced under these conditions contain antigenically distinct molecules, possibly representing a modified form of lipopolysaccharide (LPS). Finally, our data indicate a correlation between OMV production and biofilm formation by V. fischeri.
INTRODUCTION
Biofilm formation by bacteria is often critical for environmental survival. Bacterial cells in biofilms are commonly enveloped in a self-produced extracellular polymeric matrix (13). The dense extracellular matrix and the outer layer of cells protect the interior of the community against antibacterial molecules and other conditions that are harmful to bacteria. Furthermore, biofilm formation plays an important role in successful infection and colonization of host species by pathogenic, commensal, and symbiotic bacteria.
One model system in which biofilm formation plays a key colonization role is the mutualistic association between the Hawaiian bobtail squid Euprymna scolopes and the marine bioluminescent bacterium Vibrio fischeri (for reviews of this symbiosis, see references 43 and 58). Biofilm formation on the surface of the symbiotic organ precedes and promotes subsequent entry and colonization (reviewed in reference 57). Biofilm formation and, consequently, colonization by V. fischeri depends on the presence of a polysaccharide gene cluster, syp, and a number of syp regulators, including the histidine sensor kinase RscS (59, 62). The syp cluster contains 18 genes that encode proteins involved in biosynthesis of a polysaccharide. Strains that have been disrupted with respect to rscS or syp genes exhibit a severe colonization defect. In contrast, colonization is enhanced by overexpression of RscS, which induces biofilm formation, both on the surface of the symbiotic organ and in laboratory cultures. Biofilm formation in culture is easily visualized, but only under conditions in which syp regulators are overproduced: cells in which RscS is overexpressed form wrinkled colonies on solid media and pellicles at the air-liquid interface of static cultures, whereas wild-type and vector control (VC) cells form smooth colonies and do not produce pellicles. Biofilm formation depends on the presence of an intact syp locus (62).
Under RscS-overexpressing conditions, cells produce an extracellular matrix that can be observed by both scanning and transmission electron microscopy (62). However, it remains unclear what components other than the Syp polysaccharide are present in the V. fischeri biofilm matrix. In other organisms, biofilm matrices have been shown to contain polysaccharides, proteins, nucleic acids, lipids, glycolipids, surfactants, ions such as Ca2+, and outer membrane vesicles (OMVs) (6, 13, 23).
OMVs are secreted during all phases of growth by diverse Gram-negative bacteria (3, 49), including Escherichia coli (18), Neisseria meningitidis (8), Pseudomonas aeruginosa (22), Shigella flexneri (21), Helicobacter pylori (12), Xenorhabdus nematophilus (24), Vibrio parahaemolyticus (25), and Vibrio cholerae (25). OMVs are discrete, closed outer membrane blebs that are heterogeneous in size, with diameters that range from 10 to 300 nm (3, 35, 36). OMVs contain molecules typically associated with the outer surface of bacteria, including outer membrane proteins, outer membrane lipids, and lipopolysaccharide (LPS), but also can contain periplasmic components, cytoplasmic proteins, and quorum-signaling molecules, as well as DNA and RNA (19, 27, 28, 32, 34).
OMV biogenesis occurs through a programmed process rather than by lysis or cell death, although the specific pathways leading to OMV production remain under investigation (28, 40, 60, 65). OMV production is increased under stress conditions such as an excess of protein and/or the accumulation of misfolded proteins in the periplasm. In such cases, OMV production permits excretion of waste products, thus reducing stress and promoting viability of the cells (37). Indeed, it is known that components of stress-response pathways in E. coli and other organisms impact the production of OMVs. For example, a mutation in E. coli degP, which encodes a dual-function periplasmic serine protease-chaperone, caused substantially increased OMV production (36). degP is upregulated by both the Cpx and σE stress response pathways, which are involved in the biogenesis of outer membrane proteins and the degradation of misfolded proteins (38, 45). It has been proposed that the degP mutant accumulates misfolded proteins in the periplasm; those proteins may cause increased envelope stress and/or bulging of the outer membrane and, as a result, induce OMV production (37). A similar defect was observed for degP (termed mucD) mutants of P. aeruginosa, although the impact of mucD mutation on OMV production was much less dramatic (55). A distinct mechanism by which OMV production is controlled was identified in P. aeruginosa. In this organism, OMV production is induced by the Pseudomonas quinolone signal (PQS) through an interaction of the PQS with LPS. This interaction promotes OMV production by causing increased membrane curvature, thus facilitating vesiculation (33, 55). The effect of PQS is independent of the stress response pathway, indicating that multiple pathways can lead to OMV production.
OMVs appear to be able to carry out a number of diverse functions. In addition to serving an important function in combating stress arising from misfolded proteins, OMVs can promote the removal from bacterial cells of surface-damaging agents such as phage and antibiotics (28). In addition, because OMVs provide a protected environment for a number of cellular molecules, they are able to serve as conduits for those molecules, transferring them into the environment and/or into other bacterial or eukaryotic cells. Indeed, OMVs from several Gram-negative pathogenic bacteria contain a variety of virulence factors such as protein adhesins, toxins, enzymes, and LPS and carry these molecules into host cells, thus modulating the host response (2, 27, 29, 34, 41). OMVs can also facilitate horizontal gene transfer between Gram-negative bacteria and even across species (9, 34, 61). Finally, OMVs have been found to be associated with biofilms of a number of bacteria (49), and in H. pylori, the addition of OMVs to the culture medium stimulated biofilm formation. These data indicate that OMVs can play roles in biofilm development (64).
In the present study, we investigated whether the Gram-negative bacterium V. fischeri could produce OMVs and, if so, whether their production correlated with biofilm formation. Our data indicate that V. fischeri indeed produces OMVs and that their production is increased in RscS-overexpressing (biofilm-competent) cells. Furthermore, OMVs produced by RscS-overexpressing cells are antigenically distinct from those produced by control cells, a phenotype that depends on the presence of an intact syp locus. Finally, we investigated the role of degP in OMV production and biofilm formation and found that it contributes to both phenotypes in V. fischeri, although its role in OMV production is distinct from its role in E. coli. These studies thus demonstrated a connection between OMV production and biofilm formation, identified genes involved in these processes, and revealed a role for syp in modifying the surface characteristics of OMVs produced by V. fischeri.
MATERIALS AND METHODS
Strains, plasmids, and media.
The V. fischeri strains and plasmids constructed and/or used in this study are listed in Table 1. V. fischeri strain ES114 was used as the wild-type strain in these studies. V. fischeri strains were grown in complex LB-salt (LBS) medium (16, 52) and seawater tryptone (SWT) medium (63). For most experiments investigating OMV production, we grew V. fischeri under conditions (in LBS medium overnight) in which the RscS-overexpressing (RscS+) cells remained planktonic; the only exceptions occurred when we evaluated wrinkled and smooth colonies as indicated in Results. We also used E. coli strains for the purposes of cloning and conjugation. The following antibiotics were added, as appropriate, at the indicated final concentrations: chloramphenicol (Cm) at 1 to 5 μg/ml for V. fischeri and 25 μg/ml for E. coli; tetracycline (Tc) at 5 μg/ml for V. fischeri and 15 μg/ml for E. coli; and ampicillin (Ap) at 100 μg/ml. Agar was added at a final concentration of 1.5% for solid media.
Table 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| CC118 l pir | Δ(ara-leu) araD Δlac74 galE galK phoA20 thi-1 rpsE rpsB argE(AM) recA1 λpir | 17 |
| DH5α l pir | endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 relAΔ(lacIZYA-argF) U169 phoA [ϕ80dlacΔ(lacZ)M15] | Fermentas |
| π3813 | lacIq thi-1 supE44 endA1 recA1 hsdR17 gyrA462 zei-298::Tn10(Tcr) thyA::(erm-pir-116) (Ermr) | 30 |
| TAM1 | mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsLendA1 nupG | Active Motif |
| V. fischeri | ||
| ES114 | Wild-type V. fischeri | 5 |
| KV1956 | pKG11/ES114 | 62 |
| KV1844 | pKV69/ES114 | 59 |
| KV5097 | ΔsypK | This study |
| KV5126 | ΔdegP | This study |
| KV5127 | pKV69/KV5097 | This study |
| KV5128 | pKG11/KV5097 | This study |
| KV5134 | pKG11/KV5126 | This study |
| KV5135 | pKV69/KV5126 | This study |
| Plasmids | ||
| pARM7 | RscS overexpression plasmid derived from pKG11 by deletion of Cmr gene; Tcr | 39 |
| pEVS104 | Conjugal helper plasmid (tra trb); Kanra | 53 |
| pJET1.2 | Commercial cloning vector; Apr | Fermentas |
| pKG11 | RscS overexpression plasmid; Cmr Tcr | 62 |
| pKV69 | Mobilizable vector; Cmr Tcr | 59 |
| pKV282 | Mobilizable vector derived from pKV69 by deletion of Cmr gene; Tcr | 39 |
| pKV363 | Mobilizable suicide vector; Cmr; pSW7848 + multiple cloning site sequences | This study |
| pKV374 | 1-kb sequences flanking sypK in pKV363; Cmr | This study |
| pSS10 | 1.3-kb sequences flanking degP in pKV363; Cmr | This study |
| pSS13 | pVSV105 containing degP; Cmr | This study |
| pSS14 | pVSV105 containing sypK; Cmr | This study |
| pSW7848 | Suicide vector; Cmr | Marie-Eve Val |
| pVSV105 | Mobilizable vector; R6Kori ori(pES213) RP4 oriT; Cmr | 10 |
Kan, kanamycin.
Construction of deletion mutants.
Plasmid pKV374, which was used to construct the sypK deletion and carried DNA flanking sypK, was constructed as follows. DNA sequences upstream and downstream of sypK were amplified from the ES114 chromosome with primer pair sypJ-F (CTCCAGAATCTAATAGAGTACC) and sypK-SOE-R (TAGGCGGCCGCACTTAGTATGCACGATACTTGTCCCATACAGCGC) and primer pair sypK-SOE-F (CATACTAAGTGCGGCCGCCTACAAACAGGAAAAACACTATGATGG) and orf1031R (TGAAATACTCAATGGCTAAAGGATG), respectively. These DNA fragments were joined using overlap extension PCR and the outside primers sypJ-F and orf1031R. The resulting PCR fragment was cloned into plasmid pJET1.2 and then subcloned into mobilizable suicide vector pKV363 by the use of XhoI and XbaI restriction enzymes and introduced into E. coli strain π3813. Plasmid pSS10, which was used to construct the degP mutant, was constructed in a similar manner, except that subcloning using the appropriate restriction enzymes was performed instead of overlap extension PCR. The primers used for generating the degP deletion construct were as follows: VF2225P1b (GCATGCGGAGTATTTCATGGCGTGGA), VF2225P2 (TAGGCGGCCGCACTTAGTATGAATAGGGGTAAGGATGGCACT), VF2225P3 (CATACTAAGTGCGGCCGCCTACGTGGTGAACACACGCTTTAT), and VF2225P4b (CTCGAGCGAAGGTTGTGGTTTGTCCT). To transfer the plasmids into V. fischeri, triparental conjugations were performed using strain π3813 carrying pEVS104 (7, 53). We then used the gene replacement protocol described by Le Roux et al. (30).
Wrinkled colony formation.
Cells were grown overnight at 28°C in tetracycline-containing LBS liquid medium. The overnight cultures were subcultured for 4 h, and then cell cultures were adjusted to an optical density at 600 nanometers (OD600) of 0.3 and 10-μl aliquots were spotted onto LBS plates containing 0.3% (vol/vol) glycerol and tetracycline. Plates were incubated at room temperature (23°C) for 18 to 48 h before the resulting colonies were photographed.
TEM.
V. fischeri strains were streaked onto dialysis membranes (Fisher Scientific) (nominal molecular weight cutoff [MWCO], 6,000 to 8,000) that were placed on LBS plates and then incubated for 3 days at room temperature. After incubation, the dialysis membranes were removed from the plates and cut to isolate individual colonies. These colonies were fixed as described previously (62). Purified OMVs were negatively stained with 2.5% (wt/vol) uranyl acetate on a Pioloform-coated copper grid. Immunoelectron microscopy was carried out using primary, biofilm-specific antibodies and, as secondary antibodies, goat anti-rabbit immunoglobulin G conjugated to 6-nm-diameter gold particles (Electron Microscopy Sciences). Samples were observed with a Hitachi H-600 transmission electron microscope (TEM) at an accelerating voltage of 75 kV.
Isolation and purification of OMVs.
OMVs were isolated from culture supernatants by the use of a modified version of the procedure reported by Kadurugamuwa and Beveridge (22). Briefly, cells were grown in LBS medium with the appropriate antibiotics for 18 h; at that time, the optical density at 600 nm (OD600) was approximately 5.0 for all of the strains assayed. We observed no indications of biofilm formation (cell clumping) for any of the strains under these conditions. The cells were removed by centrifugation at 27,000 × g for 20 min. The supernatants were then filtered through a 0.45-μm-pore-size cellulose acetate filter. OMVs were concentrated by centrifugation at 150,000 × g for 1 h in a Beckman type 60 Ti rotor, following which pellets were suspended with 10 mM HEPES (pH 6.8) containing 0.85% NaCl (HEPES-NaCl). For some experiments, such as the TEM experiments, OMVs were further purified by using linear 10% to 50% sucrose gradient centrifugation at 100,000 × g for 16 h in a Beckman SW 41 Ti rotor, and the OMV fraction was extracted and washed with HEPES-NaCl.
OMV quantification.
The quantity of OMVs was estimated using phospholipid assays. The OMV phospholipid amount was determined using a modified ammonium ferrothiocyanate assay as reported by Stewart (54) and Tashiro et al. (55). OMV samples were subjected to a vortexing procedure using ammonium ferrothiocyanate solution (ferric chloride hexahydrate [27.03 g/liter] and ammonium thiocyanate [30.4 g/liter]) and chloroform. The absorption of the lower layer was measured in a spectrophotometer set at a wavelength of 488 nm. The values were standardized to the OD of the cell culture after cells were grown to an OD600 of 5.0. We have confirmed that there were no significant differences between the numbers of bacteria in all of the cultures at that OD and that the small amounts of contaminated cells in the OMV samples were insufficient to be measurable in our phospholipid assay. The relative amounts of OMV production were determined through comparisons with the indicated control strain.
Biofilm-specific antibodies.
To raise polyclonal antibodies, suspensions of cells from wrinkled colonies or pellicles (formed by RscS-overexpressing cells) were inoculated into rabbits by Lampire Biological Laboratories. Rabbit serum was collected and incubated with wild-type V. fischeri cells (ES114) to adsorb antibodies against common surface molecules. ES114 cells and the associated antibodies were removed by centrifugation at 27,000 × g for 20 min. The supernatant was applied to fresh ES114 cells, and the adsorption procedure was repeated. The antibodies remaining in the supernatant were then purified using a protein G Sepharose column. After the supernatant was applied, the column was washed with phosphate-buffered saline (PBS); then, the bound IgG fraction was eluted with 0.1 M glycine sulfate elution buffer (pH 2.3). Finally, antibodies were dialyzed with PBS.
Proteinase K treatment.
OMVs were resuspended in TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA) containing 0.5% (wt/vol) sodium dodecyl sulfate (SDS) and incubated for 3 h at 50°C in the absence or presence of proteinase K (final concentration, 0.1 mg/ml). Then, samples were resolved using SDS-polyacrylamide (15%) gel electrophoresis (SDS-PAGE) and analyzed using Western immunoblotting with biofilm-specific antibodies.
Isolation of lipopolysaccharide from V. fischeri cells.
Lipopolysaccharides were isolated from V. fischeri cells by the use of a modified hot phenol-water method (described by DeLoney et al. [7]). To determine the polysaccharide profile, samples were analyzed using SDS-PAGE and Western immunoblotting with biofilm-specific antibodies.
RESULTS
V. fischeri releases outer membrane vesicles.
Several recent studies have reported that many Gram-negative bacteria produce OMVs. In some cases, OMVs could be found as a biofilm matrix component (34, 49), and one study showed that OMVs induced biofilm formation (64). However, production of OMVs by the Gram-negative symbiotic bacterium V. fischeri has not yet been reported. We therefore wondered whether V. fischeri cells could release OMVs and, if so, whether OMV production contributes to biofilm formation.
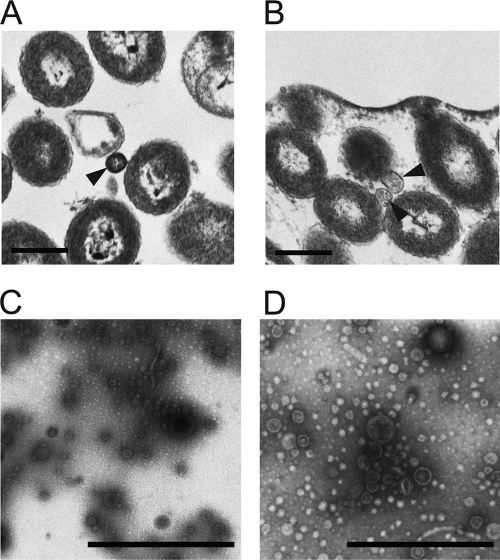
V. fischeri cells do not form significant biofilms in culture, but biofilm formation can be induced by the overexpression of the gene encoding the RscS sensor kinase (62). RscS overexpression activates transcription of the syp locus and thus matrix production, resulting in the formation of wrinkled colonies and pellicles. To observe and characterize the microstructure of the biofilm matrix, we used TEM of ultrathin sections for visual examination of the intercellular spaces of bacterial colonies (Fig. 1A and B). As reported previously, the wrinkled colonies produced by biofilm-competent (RscS-overexpressing, RscS+) V. fischeri cells contained extracellular matrices at the colony surface and between bacterial cells. In contrast, the smooth colonies of the non-biofilm-producing (vector control [VC]) strain contained only spaces of low electron density between cells (62). A careful inspection revealed that OMV-like particles were present within the spaces of low electron density between the cells in the smooth colonies formed by the VC cells and also within the extracellular matrices between cells in the wrinkled colonies formed by RscS+ cells (arrowheads in Fig. 1A and B).
Fig 1.
OMV production by V. fischeri. (A and B) Ultrathin sections of colonies of vector control wild-type cells (KV1844) (A) and RscS-overexpressing wild-type cells (KV1956) (B) are shown. Arrowheads indicate OMVs present in the spaces between the cells. (C and D) Negatively stained OMV preparations isolated from equivalent volumes of culture supernatants of the same strains, KV1844 (C) and KV1956 (D), are shown. Bars, 500 nm.
To confirm the release of OMVs from V. fischeri, the two strains were grown overnight under conditions in which the RscS+ cells remained planktonic, and then OMVs were purified from culture supernatants following centrifugation and filtration as described in Materials and Methods. The OMV samples were negatively stained with uranyl acetate and examined using TEM. We found that the supernatants contained heterogeneous populations of OMVs whose diameters ranged from 10 to 100 nm (Fig. 1C and D). The average sizes of OMVs from the VC and RscS+ cells were 29.8 ± 10.3 nm (n = 328) and 28.2 ± 13.5 nm (n = 403), respectively. These results demonstrated that, like other Gram-negative bacteria, V. fischeri is capable of producing OMVs.
OMV production is increased by RscS overexpression.
In examining the TEM preparations, we consistently observed more OMVs in samples collected from the RscS+ strain than in the VC samples (Fig. 1C and D). We therefore hypothesized that RscS overexpression caused increased OMV production. To test this hypothesis, we relied on previous studies that reported that quantification of phospholipids, which represent a major component of OMVs, is more accurate than quantification of proteins (55). We therefore used an ammonium ferrothiocyanate assay to determine phospholipid concentrations (54, 55) and thus to estimate the relative quantities of OMVs in supernatant samples. We found that OMV production by RscS+ cells had increased approximately 2.5-fold compared with the level produced by the VC cells (Fig. 2). These data supported our visual observations that OMV production by RscS+ cells was increased.
Fig 2.
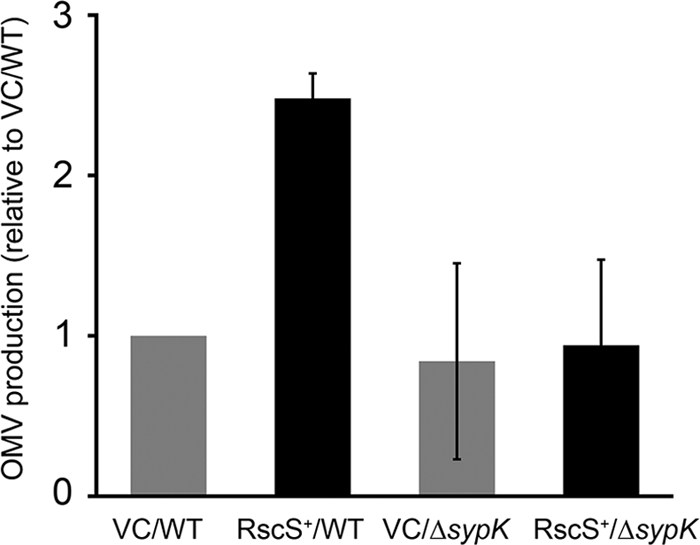
Impact of RscS overexpression and syp function on OMV production. Data represent OMV production by vector control wild-type cells (VC/WT) (KV1844), RscS-overexpressing wild-type cells (RscS+/WT) (KV1956), vector control ΔsypK cells (VC/ΔsypK) (KV5127), and RscS-overexpressing ΔsypK cells (RscS+/ΔsypK) (KV5128). Cells were grown at 23°C for 18 h. The levels of OMV production were determined by a phospholipid-based assay and standardized to the optical density of the cell culture. Relative OMV production data were determined by comparison with the vector control wild-type cell results. The data are expressed as means ± standard deviations (error bars) of the results of three independent experiments.
OMVs from RscS-overexpressing cells contain antigenically distinct molecules.
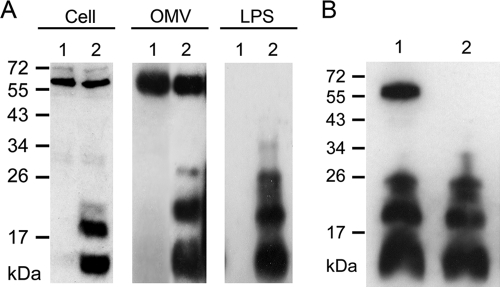
Because the increase in OMV production coincided with RscS overexpression, which is known to control the syp locus, we wondered whether those conditions also induced changes in the composition of the molecules associated with OMVs and the cell surface. To address this issue, we generated biofilm-specific antibodies as described in Materials and Methods. Briefly, RscS+ cells from biofilms (wrinkled colonies and pellicles) were inoculated into rabbits. We predicted that the resulting antisera would contain biofilm-specific antibodies as well as antibodies directed against common surface molecules such as those of flagella, pili, and outer membrane proteins. To isolate biofilm-specific antibodies, common antibodies were adsorbed through incubation with wild-type V. fischeri cells. We then used the resulting biofilm-specific antibodies in Western immunoblot experiments to identify differences in the molecules produced by RscS+ and VC cells. As controls, we used both preimmune serum and unprocessed antiserum (i.e., antiserum prior to adsorption of antibodies against common surface molecules) in Western immunoblot analyses with whole-cell extracts of RscS+ and VC cells. No molecules were detected when preimmune serum was used, whereas multiple molecules of various molecular weights were detected in both samples when unprocessed antiserum was used (data not shown). Importantly, Western immunoblotting performed using the antiserum after adsorption permitted detection of a discrete set of molecules (10 to 28 kDa in size) present in whole-cell extracts from wrinkled colonies (RscS+ cells) but not in those from smooth colonies (VC cells) (Fig. 3A [Cell]). We also detected these molecules in whole-cell extracts of planktonic RscS+ cells but not in VC cells from liquid cultures (data not shown), suggesting that planktonic cells are also capable of producing these molecules as long as RscS is overexpressed. We obtained similar profiles of bands regardless of whether we used antiserum generated from wrinkled colonies or from pellicles. Therefore, we chose to use antiserum from wrinkled colonies throughout the remainder of the experiments. Finally, in many of our experiments, we also detected a nonspecific 60-kDa molecule present in samples from both RscS+ and VC cells. It is possible that the 60-kDa molecule was an intracellular or periplasmic component; if so, then our adsorption approach would not remove antibodies against that protein. We conclude that RscS induces the production of an antigenically distinct set of molecules associated with biofilm formation.
Fig 3.
Detection of biofilm-associated molecules from RscS-overexpressing cells. (A) Western immunoblotting of whole-cell extracts (Cell) and of OMV and LPS extracts from cells of vector control wild-type cells (KV1844) (lanes 1) and RscS-overexpressing wild-type cells (KV1956) (lanes 2). (B) Proteinase K treatment of OMV extracts from RscS-overexpressing wild-type cells. Lane 1, no treatment; lane 2, OMVs treated with proteinase K. A nonspecific protein (60 kDa) was frequently detected.
We next asked whether the OMVs produced by the RscS+ cells were antigenically distinct from the OMVs from the VC cells. Indeed, the biofilm-specific antibodies permitted detection of a set of bands corresponding to OMVs from RscS+ cells that were similar to those observed with the whole-cell extracts. Importantly, these bands were not present in the OMV samples obtained from VC cells (Fig. 3A [OMV]). These data indicate that OMVs produced by RscS+ cells contain a set of biofilm-associated, antigenically distinct molecules that distinguish them from those produced by VC cells.
To begin to determine the nature of the antigenically distinct molecules, we treated OMV samples with proteinase K (Materials and Methods) in the presence of SDS, which should solubilize the OMVs and release their contents, and then performed Western immunoblot assays. In the absence of proteinase K treatment, both the biofilm-associated molecules and the nonspecific 60-kDa molecule were present (Fig. 3B, lane 1). In contrast, only the small molecules were detected after proteinase K treatment (Fig. 3B, lane 2). Because the 60-kDa molecule was degraded by proteinase K treatment, we conclude that this molecule is a protein and that the enzyme was proteolytically active under our conditions. We further conclude that the other molecules are nonproteinaceous.
Because they are released from the outer membrane, OMVs generally contain surface-exposed LPS molecules (19, 27, 28, 34). We therefore wondered whether the antigenically distinct molecules could be LPS molecules. To test this hypothesis, we extracted lipopolysaccharides (LPS) from RscS+ and VC cells by a hot phenol-water method (7). Western immunoblot analysis with biofilm-specific antibodies yielded bands in the 10- to 28-kDa range from LPS extracts of RscS+ cells that were essentially indistinguishable from those from OMV preparations. These molecules were absent from extracts from VC cells (Fig. 3A [LPS]). The LPS preparations lacked the nonspecific 60-kDa protein, indicating the relative purity of these preparations. Together, these data indicate that OMVs produced by RscS+ cells (but not wild-type cells) contain a set of nonproteinaceous, biofilm-associated molecules that can be extracted with procedures that isolate LPS molecules.
Localization of biofilm-associated molecules to the OMV surface.
We hypothesized that the biofilm-associated molecules were present on the surface of OMVs. To test this hypothesis, we used the biofilm-specific antibodies in combination with immunogold labeling and electron microscopy to visualize gold particles on OMVs. We observed numerous gold particles localized to the surfaces of OMVs produced by RscS+ cells (Fig. 4A). Not all OMVs from RscS+ cells were associated with gold particles, which suggests that the biofilm-associated molecules are heterogeneously localized on OMVs. However, we also observed that many of these OMVs clustered together, which might limit access for either the primary or secondary antibodies. In contrast to the results seen with the RscS+ OMVs, gold particles were not localized to the surfaces of OMVs from VC cells (Fig. 4B). Despite the fact that a 60-kDa protein was also detected in OMV samples from VC cells by Western immunoblotting, we did not observe localization of gold particles on OMVs from VC cells, a result that further indicates that the 60-kDa protein is not a surface-exposed protein. These data thus support the conclusion that the biofilm-specific antibodies recognize molecules located on the surface of OMVs generated by RscS+ cells.
Fig 4.
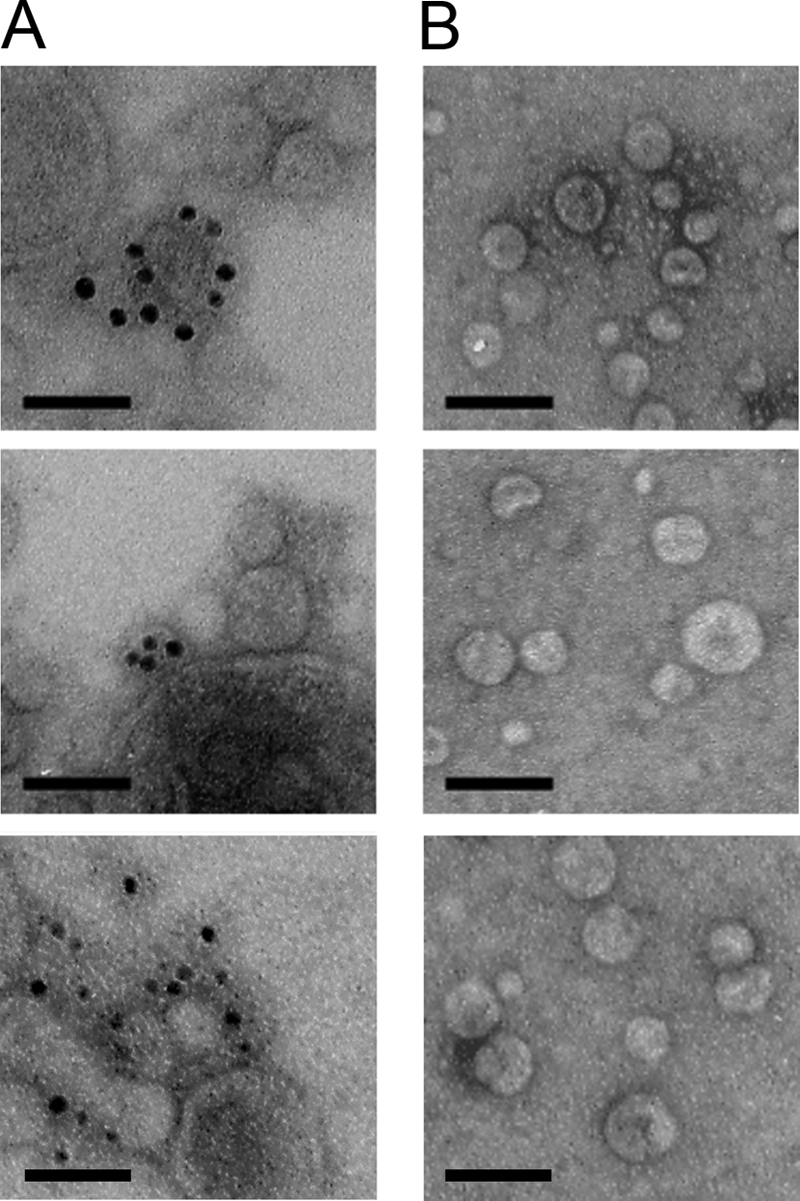
Localization of biofilm-associated molecules on OMV surfaces. Representative images from immunogold electron microscopic detection of biofilm-associated molecules on negatively stained OMVs extracted from RscS-overexpressing wild-type cells (KV1956) (A) and vector control wild-type cells (KV1844) (B) are shown. Samples were treated with biofilm-specific primary antibodies and developed with a secondary gold-conjugated anti-rabbit IgG antibody. Bars, 100 nm.
Production of the biofilm-associated molecules depends on the presence of the syp locus.
Our data thus far indicate that RscS overexpression leads to the production of biofilm-associated molecules present on the surface of OMVs. Because RscS overexpression induces transcription of the syp polysaccharide locus (20), we hypothesized that syp is responsible for generating the molecules with antigenically distinct properties under these conditions. To test this hypothesis, we constructed an unmarked nonpolar mutation in a gene predicted to be involved in Syp-polysaccharide production. In particular, we focused on the gene encoding SypK. This protein contains conserved domains that are found in the E. coli flippase protein Wzx (RfbX) (31, 63), which is responsible for the transfer of polysaccharide from the cytoplasmic to the periplasmic side of the cytoplasmic membrane. We hypothesize that a sypK mutant would not be able to transfer Syp-produced polysaccharides from the cytosolic to the periplasmic side of the membrane, thus preventing these molecules from reaching their final destination(s), including the matrix and potentially the surface of OMVs.
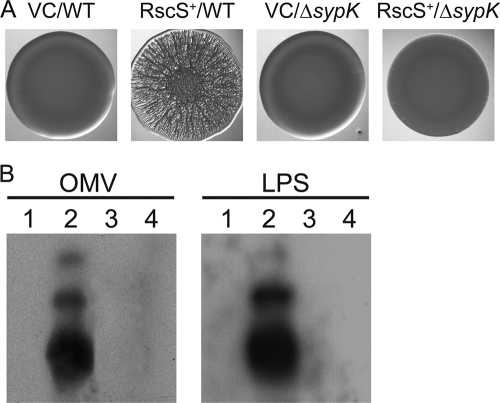
To understand the role of SypK, we first evaluated its role in biofilm formation: if SypK is important for Syp-polysaccharide production and assembly, as predicted from bioinformatics results, then mutation of sypK should disrupt the formation of biofilms that occurs upon RscS overexpression. Indeed, deletion of sypK prevented the formation of wrinkled colonies that occurs upon overexpression of RscS (RscS+/ΔsypK) (Fig. 5A): the ΔsypK mutant formed smooth colonies that were indistinguishable from those of vector-containing wild-type and ΔsypK cells (Fig. 5A). Complementation of the ΔsypK mutant with sypK carried on plasmid pSS14 restored wrinkled colony formation (data not shown). Thus, SypK is required for biofilm formation by V. fischeri.
Fig 5.
Impact of sypK deletion on biofilm-associated molecules and wrinkled colony formation. (A) Wrinkled colony morphology of vector control wild-type cells (VC/WT) (KV1844), RscS-overexpressing wild-type cells (RscS+/WT) (KV1956), vector control ΔsypK cells (VC/ΔsypK) (KV5127), and RscS-overexpressing ΔsypK cells (RscS+/ΔsypK) (KV5128). Cells were grown overnight at 28°C in tetracycline-containing LBS liquid medium. The overnight cultures were subcultured for 4 h, and then cell cultures were adjusted to an OD600 of 0.3 and a 10-μl aliquot was spotted onto an LBS plate containing 0.3% glycerol (vol/vol) and tetracycline. Plates were incubated at room temperature for 48 h before the spots were photographed. (B) Western blot of LPS and OMV extracts from vector control wild-type cells (KV1844) (lanes 1), RscS-overexpressing wild-type cells (KV1956) (lanes 2), vector control ΔsypK cells (KV5127) (lanes 3), and RscS-overexpressing ΔsypK cells (KV5128) (lanes 4). Samples were resolved on 16% SDS-PAGE and immunoblotted with biofilm-specific antibodies.
We next asked whether SypK was required for the production of the biofilm-associated molecules present on OMVs produced by RscS+ cells. Although we were able to collect OMVs from the sypK mutant (see below), we were unable to detect the 10- to 28-kDa bands in the OMV samples by the use of our biofilm-specific antibodies (Fig. 5B [OMV, lane 4]). These data suggest that both the syp locus generally and SypK specifically play a role in the production and/or localization of the biofilm-associated molecules found on OMVs in RscS+ cells.
Finally, we evaluated the role of SypK in generating the biofilm-associated molecules detected in our LPS preparations. Silver staining revealed that the profiles of the molecules isolated from RscS+ wild-type and RscS+ ΔsypK mutant cells following our LPS extraction protocol and gel electrophoresis were indistinguishable in appearance with respect to both the patterns and intensities of the bands (data not shown). However, Western immunoblotting using the biofilm-specific antibodies revealed that, as with the results seen with the OMV samples, the small molecules could not be detected in the LPS samples from the RscS+ ΔsypK cells (Fig. 5B [LPS, lane 4]). Complementation of the ΔsypK mutation with sypK expressed from plasmid pSS14 restored the production of those molecules under RscS-overexpressing conditions (data not shown).
Together, our data demonstrate that the SypK putative polysaccharide translocase is required for the RscS-induced production of the molecules that are recognized by biofilm-specific antibodies present on OMVs and extracted in LPS preparations.
OMV production correlates with biofilm formation.
We previously observed that OMV production was increased upon overexpression of RscS (Fig. 2). We hypothesized that the increase could be due to toxicity associated with protein overexpression or to the induction of biofilm formation. Overexpression of RscS did not impact the growth of the cells, suggesting that the levels of RscS did not reach toxicity (data not shown). If, instead, the latter hypothesis of the induction of biofilm formation were true, then a biofilm-defective mutant that overexpressed RscS should fail to exhibit the increase in OMV production. We therefore estimated OMV production by the RscS+ ΔsypK mutant, which does not form biofilms, by the use of the ammonium ferrothiocyanate assay. We found that the ΔsypK mutant indeed failed to induce the 2.5-fold increase in OMV production that we had observed previously for the RscS+ wild-type strain (Fig. 2). This defect could be restored by the co-overexpression of RscS and SypK from compatible plasmids but not by expression of only RscS or SypK in combination with the appropriate vector control or by the vectors alone (data not shown). We conclude that the increase in OMV production is not due to toxic levels of RscS but rather is dependent on the activity of the syp locus.
DegP plays a role in OMV production and biofilm formation.
It has been reported that the accumulation of misfolded outer membrane proteins in the periplasm is related to OMV production (37, 55). For example, a mutation in the gene encoding the periplasmic serine protease-chaperone DegP (MucD in P. aeruginosa), which is involved in degradation of misfolded proteins and in the biogenesis of outer membrane proteins (38), caused increased OMV production in both E. coli and P. aeruginosa (37, 55). To determine whether such a mutation would have the same effect on OMV production for V. fischeri, we searched for a degP homolog. We found that VF_2225 encodes a putative DegP protein with 55.8% amino acid identity to DegP of E. coli. We constructed a ΔdegP mutant and then evaluated its growth in the rich media (SWT and LBS) used for our experiments. The ΔdegP mutant and its parent ES114 strain grew at the same rate (data not shown), suggesting that the absence of DegP causes no significant defect in general bacterial metabolism.
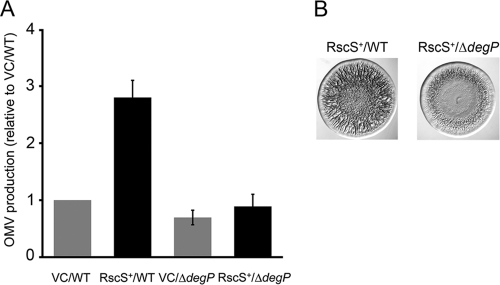
Next, we introduced the RscS overexpression plasmid or the vector control into the ΔdegP mutant and evaluated OMV production. In contrast to the increase observed upon mutation of degP (mucD) in E. coli and P. aeruginosa, OMV production of the V. fischeri ΔdegP mutant carrying empty vector was slightly decreased (to approximately 70%) compared to the level of production seen with the wild-type vector control strain. Importantly, OMV production of the ΔdegP mutant was not induced under RscS-overexpressing conditions (Fig. 6A). Complementation of the ΔdegP mutant with degP on plasmid pSS13 restored OMV production to wild-type levels under RscS-overexpressing conditions (data not shown). These results suggested that DegP is involved in OMV production in V. fischeri.
Fig 6.
Impact of degP mutation on biofilm formation and OMV production. (A) OMV production of vector control wild-type cells (VC/WT) (KV1844), RscS-overexpressing wild-type cells (RscS+/WT) (KV1956), vector control ΔdegP cells (VC/ΔdegP) (KV5135) and RscS-overexpressing ΔdegP cells (RscS+/ΔdegP) (KV5134) grown at 23°C for 18 h. The levels of OMV production were identified by a phospholipid-based assay and standardized to the optical density of cell culture. Relative OMV production levels were determined by comparison with the vector control wild-type cell results. Data are expressed as means ± standard deviations (error bars) of the results of three independent experiments. (B) RscS-induced wrinkled colony morphology of wild-type cells (KV1956) and ΔdegP cells (KV5134). Cells were prepared and assayed as described in the Fig. 5A legend.
Because our results with respect to sypK suggested a correlation between OMV induction and biofilm formation, we wondered whether the degP mutant would exhibit a decrease in biofilm formation corresponding to its decrease in OMV induction. Indeed, the RscS+ ΔdegP mutant exhibited a subtle but reproducible defect in biofilm formation, as measured by wrinkled colony development: 24 h after the cultures were spotted, the wild-type control exhibited extensive wrinkling throughout the whole colony, while the ΔdegP mutant still exhibited a smooth morphology in the central part of the colony (Fig. 6B). To eliminate the concern that the defect in biofilm formation could be attributed to a decrease in the production of syp-dependent molecules, we applied our LPS extraction protocol to the RscS+ ΔdegP mutant and RscS+ wild-type cells and analyzed the extracted molecules by Western immunoblotting. Similar amounts of syp-dependent, biofilm-associated molecules were detected in the two samples (data not shown). Thus, our data demonstrate a relationship between OMV production and biofilm formation in V. fischeri.
DISCUSSION
We report here that V. fischeri, like other Gram-negative bacteria, is capable of releasing OMVs. Production of OMVs by Gram-negative bacteria appears to be a versatile way for bacteria to carry out a number of different functions, such as delivering proteins and other molecules to eukaryotic cells, combating stress induced by environmental conditions, and promoting horizontal gene transfer (11, 28). For V. fischeri, OMVs are likely used for multiple function(s), as we find that non-biofilm-producing wild-type cells produce OMVs, and yet OMV production clearly correlates with biofilm formation. For example, the increase in the number of OMVs produced by V. fischeri coincided with the induction of genes (syp genes) involved in biofilm formation. Furthermore, the loss of the ability to produce a biofilm (via sypK disruption) eliminated the observed increase in OMV production, as estimated from the amount of vesicle-associated membrane phospholipid detected. Finally, mutation of the gene degP, a disruption that in E. coli increases OMV production (36), decreased both the amount of OMVs and the amount of biofilm formation by V. fischeri. Together, these data indicate that one important function of OMV production in V. fischeri may be modulation of biofilm formation.
We found that OMVs produced by V. fischeri under RscS-overexpressing conditions contain a set of molecules that are recognized by biofilm-specific antibodies. The biochemical identity of these molecules is as yet unknown, although they are resistant to protease treatment, suggesting that they are not proteins. One possibility is that they are LPS molecules, as OMVs, which are derived from the cell surface, generally contain LPS molecules (11, 27). Indeed, it has been reported that OMV production can be impacted by the LPS structure. For example, P. aeruginosa PAO1 expresses two types (A and B) of O-antigen side chains of LPS and produces OMVs enriched in the highly charged and longer “B-band” form of LPS (4, 22, 42). The enhanced formation of B-band LPS was related to increased formation of OMVs under conditions of oxygen stress (47). Furthermore, mutants expressing LPS that lacked the O-antigen side chain showed increased OMV production in both Salmonella enterica serovar Typhimurium and P. aeruginosa (48, 50).
In keeping with the idea that the biofilm-associated molecules could be LPS molecules, our LPS preparations extracted a set of small molecules that were similar in size to those isolated from OMVs and that were detected by biofilm-specific antibodies. Furthermore, TEM experiments indicated that the molecules were localized to the surface of OMVs. Finally, our preliminary results indicate that a mutation in sypL, which encodes a putative lipid A core O-antigen ligase (63), causes a defect in the production of the antigenically distinct molecules (our unpublished results). It remains possible, however, that the antigenically distinct molecules merely copurify with LPS and migrate similarly during SDS-PAGE. For example, the molecules could be derivatives of a distinct lipooligosaccharide. In either case, the production of these antigenically distinct molecules depends upon both induction of the syp genes by RscS overexpression and the function of the Syp proteins, including SypK (Fig. 5) and SypL (data not shown). One possible explanation for these results is that a syp-dependent polysaccharide is incorporated into the LPS molecules (or the distinct lipooligosaccharide) and ultimately localizes to the surfaces of OMVs. Additional work is needed to determine the biochemical identity of these antigenically distinct, biofilm-associated molecules and the impact of syp on their structure.
If the biofilm-associated molecules are, in fact, modified LPS molecules, then this modification could have important implications for the association of V. fischeri with its symbiotic host, as LPS is an integral part of that interaction (14, 15, 26, 56). In particular, LPS induces programmed cell death (apoptosis) (14) and, together with the peptidoglycan monomer tracheal cytotoxin TCT, controls tissue remodeling (26), apparently by attenuating nitric oxide production (1). syp is already known to be required for symbiotic biofilm formation and thus colonization (62, 63). However, it is possible that OMVs with syp-dependent molecules could play additional key roles during symbiosis. For example, OMVs can serve as vehicles to deliver molecules to the host. We have not yet determined whether there are differences in the types or amounts of proteins or other molecules present inside the OMVs. These molecules could permit additional signaling between V. fischeri and its host, such as the apparent education of hemocytes, the primitive immune system of the squid, in recognizing V. fischeri (43, 44). Our future directions include determining whether OMVs produced under biofilm conditions contain additional different molecules and whether OMVs can induce host development.
Response to stress may be another role of OMV production in V. fischeri, as DegP appears to be involved in the production of OMVs. However, in contrast to the role of DegP (MucD in P. aeruginosa) in repressing OMV production in E. coli and P. aeruginosa (37, 55), DegP promotes OMV production in V. fischeri (Fig. 6B). Thus, DegP may play different roles in OMV production in different bacteria. In keeping with this idea, DegP exhibits not only chaperone activity but also periplasmic serine protease activity, and these functions switch depending on temperature: the chaperone function is activated at low temperatures, while the protease activity is activated at elevated temperatures (51). V. fischeri strains are grown at 23°C, a temperature at which the chaperone function could dominate. Additional work would be necessary to determine whether DegP functions as a chaperone in V. fischeri and, if so, what molecule(s) it acts on to control OMV production.
Because deletion of degP caused decreases in both biofilm formation and OMV production (Fig. 6), we wondered whether degP contributed to symbiotic colonization. Our preliminary data indicated that ΔdegP mutant cells are capable of colonization but that they are slightly defective compared to wild-type cells (the average numbers of CFU per squid at an early time point were 1.4 × 104 for the wild type and 2.5 × 103 for the ΔdegP strain). These results suggest that DegP may be required for efficient colonization of squid by V. fischeri. However, whether OMVs themselves play a role or whether the defect can be attributed to a decrease in biofilm formation, a failure to respond to stress, or a symbiosis-specific role of DegP itself (independent of OMV production) are issues that remain to be investigated.
Another protein known to mediate control over OMV production is the lipoprotein YfgL: deletion of yfgL causes a severe decrease in OMV production in E. coli (46). To determine the role of yfgL in OMV production by V. fischeri, we constructed an insertional mutant of VF_0632, a yfgL homolog, and examined the effect of this mutation on OMV production. However, the mutation did not affect the quantity of OMVs compared to the quantity produced by the wild-type cells under RscS-overexpressing conditions (our unpublished data). Taken together with the degP mutant data, these results suggest that the mechanisms for biogenesis of OMVs in V. fischeri are different from those in other bacteria.
In summary, our experiments have provided insights into OMV production by V. fischeri. The correlation between OMV production and biofilm formation is strong, and we have identified genes that contribute to these processes. This report thus makes an important contribution to the developing OMV field and lays a foundation for future studies addressing the role(s) of OMVs in colonization and in colonization-relevant biofilm formation.
ACKNOWLEDGMENTS
We are grateful to Linda Fox for her help with TEM experiments, Andrew Morris, Kevin Quirke, and Valerie Ray for constructing plasmids and strains, Laurie Comstock for her suggestions on antibody production, Christine Bassis for preparing samples for antibody production, and Marie-Eve Val for providing plasmid pSW7848 prior to publication. We also thank Alan Wolfe for comments on the manuscript.
This work was supported by NIH R01 grant GM59690 to K.L.V.
Footnotes
Published ahead of print 21 October 2011
REFERENCES
- 1. Altura MA, Stabb E, Goldman W, Apicella M, McFall-Ngai MJ. 2011. Attenuation of host NO production by MAMPs potentiates development of the host in the squid-Vibrio symbiosis. Cell Microbiol. 13:527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauman SJ, Kuehn MJ. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8:2400–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beveridge TJ. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20:291–303 [DOI] [PubMed] [Google Scholar]
- 5. Boettcher KJ, Ruby EG. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branda SS, Vik S, Friedman L, Kolter R. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20–26 [DOI] [PubMed] [Google Scholar]
- 7. DeLoney CR, Bartley TM, Visick KL. 2002. Role for phosphoglucomutase in Vibrio fischeri-Euprymna scolopes symbiosis. J. Bacteriol. 184:5121–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devoe IW, Gilchrist JE. 1973. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 138:1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorward DW, Garon CF, Judd RC. 1989. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171:2499–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV. 2006. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 72:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellis TN, Kuehn MJ. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fiocca R, et al. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220–226 [DOI] [PubMed] [Google Scholar]
- 13. Flemming HC, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633 [DOI] [PubMed] [Google Scholar]
- 14. Foster JS, Apicella MA, McFall-Ngai MJ. 2000. Vibrio fischeri lipopolysaccharide induces developmental apoptosis, but not complete morphogenesis, of the Euprymna scolopes symbiotic light organ. Dev. Biol. 226:242–254 [DOI] [PubMed] [Google Scholar]
- 15. Foster JS, McFall-Ngai MJ. 1998. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Dev. Genes Evol. 208:295–303 [DOI] [PubMed] [Google Scholar]
- 16. Graf J, Dunlap PV, Ruby EG. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176:6986–6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrero M, de Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoekstra D, van der Laan JW, de Leij L, Witholt B. 1976. Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta 455:889–899 [DOI] [PubMed] [Google Scholar]
- 19. Horstman AL, Kuehn MJ. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275:12489–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hussa EA, Darnell CL, Visick KL. 2008. RscS functions upstream of SypG to control the syp locus and biofilm formation in Vibrio fischeri. J. Bacteriol. 190:4576–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kadurugamuwa JL, Beveridge TJ. 1999. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology 145(Pt. 8):2051–2060 [DOI] [PubMed] [Google Scholar]
- 22. Kadurugamuwa JL, Beveridge TJ. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karatan E, Watnick P. 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73:310–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khandelwal P, Banerjee-Bhatnagar N. 2003. Insecticidal activity associated with the outer membrane vesicles of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 69:2032–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kondo K, Takade A, Amako K. 1993. Release of the outer membrane vesicles from Vibrio cholerae and Vibrio parahaemolyticus. Microbiol. Immunol. 37:149–152 [DOI] [PubMed] [Google Scholar]
- 26. Koropatnick TA, et al. 2004. Microbial factor-mediated development in a host-bacterial mutualism. Science 306:1186–1188 [DOI] [PubMed] [Google Scholar]
- 27. Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 [DOI] [PubMed] [Google Scholar]
- 28. Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee EY, et al. 2007. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7:3143–3153 [DOI] [PubMed] [Google Scholar]
- 30. Le Roux F, Binesse J, Saulnier D, Mazel D. 2007. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl. Environ. Microbiol. 73:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu D, Cole RA, Reeves PR. 1996. An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flippase. J. Bacteriol. 178:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425 [DOI] [PubMed] [Google Scholar]
- 33. Mashburn-Warren L, et al. 2008. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mashburn-Warren L, McLean RJ, Whiteley M. 2008. Gram-negative outer membrane vesicles: beyond the cell surface. Geobiology 6:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mayrand D, Grenier D. 1989. Biological activities of outer membrane vesicles. Can. J. Microbiol. 35:607–613 [DOI] [PubMed] [Google Scholar]
- 36. McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188:5385–5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McBroom AJ, Kuehn MJ. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meltzer M, et al. 2009. Structure, function and regulation of the conserved serine proteases DegP and DegS of Escherichia coli. Res. Microbiol. 160:660–666 [DOI] [PubMed] [Google Scholar]
- 39. Morris AR, Darnell CL, Visick KL. 2011. Inactivation of a novel response regulator is necessary for biofilm formation and host colonization by Vibrio fischeri. Mol. Microbiol. 82:114–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mug-Opstelten D, Witholt B. 1978. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim. Biophys. Acta 508:287–295 [DOI] [PubMed] [Google Scholar]
- 41. Nevot M, Deroncele V, Messner P, Guinea J, Mercade E. 2006. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ. Microbiol. 8:1523–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nguyen TT, Saxena A, Beveridge TJ. 2003. Effect of surface lipopolysaccharide on the nature of membrane vesicles liberated from the Gram-negative bacterium Pseudomonas aeruginosa. J. Electron Microsc. (Tokyo) 52:465–469 [DOI] [PubMed] [Google Scholar]
- 43. Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol. 2:632–642 [DOI] [PubMed] [Google Scholar]
- 44. Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. 2009. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ. Microbiol. 11:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. 2006. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 4:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rolhion N, Barnich N, Claret L, Darfeuille-Michaud A. 2005. Strong decrease in invasive ability and outer membrane vesicle release in Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J. Bacteriol. 187:2286–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sabra W, Lunsdorf H, Zeng AP. 2003. Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology 149:2789–2795 [DOI] [PubMed] [Google Scholar]
- 48. Salkinoja-Salonen M, Nurmiaho EL. 1978. The effect of lipopolysaccharide composition on the ultrastructure of Pseudomonas aeruginosa. J. Gen. Microbiol. 105:23–28 [DOI] [PubMed] [Google Scholar]
- 49. Schooling SR, Beveridge TJ. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smit J, Kamio Y, Nikaido H. 1975. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J. Bacteriol. 124:942–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spiess C, Beil A, Ehrmann M. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339–347 [DOI] [PubMed] [Google Scholar]
- 52. Stabb EV, Reich KA, Ruby EG. 2001. Vibrio fischeri genes hvnA and hvnB encode secreted NAD(+)-glycohydrolases. J. Bacteriol. 183:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stabb EV, Ruby EG. 2002. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 358:413–426 [DOI] [PubMed] [Google Scholar]
- 54. Stewart JC. 1980. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 104:10–14 [DOI] [PubMed] [Google Scholar]
- 55. Tashiro Y, et al. 2009. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J. Bacteriol. 191:7509–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Troll JV, et al. 2009. Peptidoglycan induces loss of a nuclear peptidoglycan recognition protein during host tissue development in a beneficial animal-bacterial symbiosis. Cell Microbiol. 11:1114–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Visick KL. 2009. An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri. Mol. Microbiol. 74:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Visick KL, Ruby EG. 2006. Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 9:632–638 [DOI] [PubMed] [Google Scholar]
- 59. Visick KL, Skoufos LM. 2001. Two-component sensor required for normal symbiotic colonization of Euprymna scolopes by Vibrio fischeri. J. Bacteriol. 183:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yaganza ES, Rioux D, Simard M, Arul J, Tweddell RJ. 2004. Ultrastructural alterations of Erwinia carotovora subsp. atroseptica caused by treatment with aluminum chloride and sodium metabisulfite. Appl. Environ. Microbiol. 70:6800–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yaron S, Kolling GL, Simon L, Matthews KR. 2000. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 66:4414–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yip ES, Geszvain K, DeLoney-Marino CR, Visick KL. 2006. The symbiosis regulator rscS controls the syp gene locus, biofilm formation and symbiotic aggregation by Vibrio fischeri. Mol. Microbiol. 62:1586–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yip ES, Grublesky BT, Hussa EA, Visick KL. 2005. A novel, conserved cluster of genes promotes symbiotic colonization and sigma-dependent biofilm formation by Vibrio fischeri. Mol. Microbiol. 57:1485–1498 [DOI] [PubMed] [Google Scholar]
- 64. Yonezawa H, et al. 2009. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 9:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou L, Srisatjaluk R, Justus DE, Doyle RJ. 1998. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol. Lett. 163:223–228 [DOI] [PubMed] [Google Scholar]