Abstract
Acetyl coenzyme A (acteyl-CoA) carboxylase (ACC) is the first committed enzyme of the fatty acid synthesis pathway. Escherichia coli ACC is composed of four different proteins. The first enzymatic activity of the ACC complex, biotin carboxylase (BC), catalyzes the carboxylation of the protein-bound biotin moiety of another subunit with bicarbonate in an ATP-dependent reaction. Although BC is found as a dimer in cell extracts and the carboxylase activities of the two subunits of the dimer are interdependent, mutant BC proteins deficient in dimerization are reported to retain appreciable activity in vitro (Y. Shen, C. Y. Chou, G. G. Chang, and L. Tong, Mol. Cell 22:807–818, 2006). However, in vivo BC must interact with the other proteins of the complex, and thus studies of the isolated BC may not reflect the intracellular function of the enzyme. We have tested the abilities of three BC mutant proteins deficient in dimerization to support growth and report that the two BC proteins most deficient in dimerization fail to support growth unless expressed at high levels. In contrast, the wild-type protein supports growth at low expression levels. We conclude that BC must be dimeric to fulfill its physiological function.
INTRODUCTION
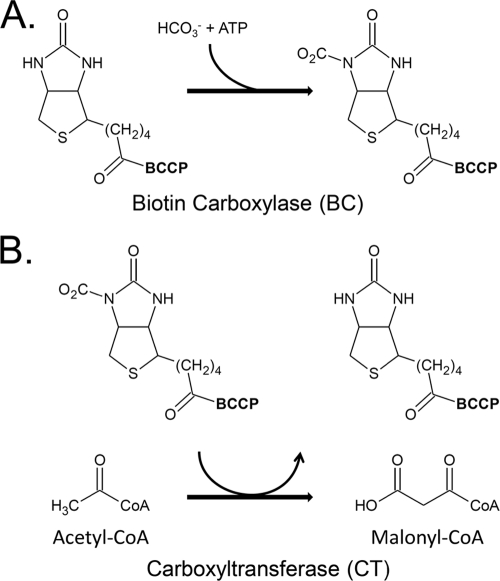
Fatty acid biosynthesis is essential for cell growth and viability. The initial step in fatty acid biosynthesis is the production of malonyl coenzyme A (malonyl-CoA) by acetyl-CoA carboxylase (ACC). Malonyl-CoA is produced from acetyl-CoA and bicarbonate in the first committed (and rate-limiting) step of fatty acid synthesis. In Escherichia coli, the sole destination for malonyl-CoA is fatty acid synthesis. Most bacteria have a multisubunit ACC composed of three functional entities: biotin carboxylase (BC; AccC), biotin carboxyl carrier protein (BCCP; AccB), and carboxyltransferase (CT; AccA plus AccD) (6). These components produce malonyl-CoA through two distinct partial reactions (6). The first partial reaction is the ATP-dependent carboxylation of the biotin moiety attached to BCCP using bicarbonate as the carboxy donor (Fig. 1A). The second partial reaction is the transfer of the carboxyl group from carboxybiotin to acetyl-CoA to form malonyl-CoA (Fig. 1B). The three functional components are encoded by four genes: accA, accB, accC, and accD (6). Biotin carboxylase is encoded by accC in a tightly controlled operon with accB, the gene encoding biotin carboxyl carrier protein (18, 20). The carboxyltransferase component consists of two proteins encoded by the accA (α subunit) and accD (β subunit) genes, which are located at sites distant from each other and from accBC (19). The functional ACC is thought to consist of a dimer of BC subunits in a complex with four molecules of BCCP and one α2β2 CT heterotetramer (3). Interactions among the components of the functional complex are weak, and upon cell lysis, they readily dissociate into stable CT and BC components plus a metastable complex of a BC dimer with four BCCP molecules. The subunits can readily be purified to study the partial reactions. Each of the acc genes is essential for growth of E. coli (2), and ACC is a validated antibiotic target (9, 21).
Fig 1.
The acetyl-CoA carboxylase (ACC) reaction. The synthesis of malonyl-CoA is carried out in two distinct partial reactions. The acetyl-CoA carboxylase (ACC) reaction is initiated by the carboxylation of biotin bound to the accB-encoded biotin carboxylase carrier protein (BCCP) by the accC-encoded biotin carboxylase (BC) (A). In the second partial reaction, the carbonyl group is transferred from the biotin moiety of the carrier protein to acetyl-CoA by the carboxyltransferase (CT) component (encoded by accA and accD), to give malonyl-CoA (B).
Structural studies have demonstrated that each BC monomer contains a complete active site, and the two active sites of a dimer are separated by 25 Å (4, 6, 27). Thus, the BC active site is not comprised of residues from both subunits (i.e., a shared active site), as seen in many dimeric enzymes. Kinetic analyses have shown no cooperative behavior for any of the substrates (6), suggesting that there is no communication between the subunits. So why does BC exist as a dimer? To answer this question, hybrid BC molecules were made in which one subunit was wild type and the other contained an active site mutation that caused at least a 100-fold decrease in activity (14). If the subunits act independently, then the activity of the hybrid should be about one-half of the wild-type activity. However, if communication between the subunits is required for activity, then the activity would be less than half of the wild-type level. The activity of the hybrid enzymes ranged from 0.35% to 3.6% of the wild-type activity, indicating intersubunit communication (14). A plausible explanation for the dominant-negative effect of the mutations is that the two BC subunits cannot catalyze the reaction simultaneously. Instead, the two subunits might alternate catalytic reactions, such that while one subunit is binding substrate and undergoing catalysis, the other subunit is releasing product (8, 14). If one of the subunits contains a mutation that results in a significant decrease in catalytic rate, it might also lead to a decrease in the catalytic rate of the wild-type subunit, indicating that the catalytic sequences of the subunits are inextricably linked. This scenario has received support from a BC crystal structure in which an ATP analogue is found bound to only one of the two subunits of a dimer (25).
More recently, Shen and coworkers (27) investigated the catalytic activity of mutant biotin carboxylase proteins deficient in their ability to form dimers. They made amino acid substitutions in the BC dimer interface that hindered its ability to form dimers to various degrees. They showed that the activities of the monomeric BC proteins were only slightly diminished (about 3-fold) compared to the dramatic increases in the Kd (dissociation constant) values for dimer formation (5,000- to 8,000-fold, depending on incubation conditions). They concluded that dimer formation may not be required for the catalytic activity of BC and that although the protein likely exists as a dimer in the ACC complex, monomers might also interact with the other subunits. However, these experiments have the caveat that none of the other ACC subunits were present, and only the first partial reaction was assayed. This raised the possibility that dimerization might be a prerequisite for the interaction of BC with the other subunits to form the complex required for the overall ACC reaction. Therefore, we tested the importance of BC dimerization in vivo and report that it is essential for growth.
MATERIALS AND METHODS
Media and culture condition.
All strains were grown in LB liquid or solid agar medium or M9 liquid medium. The M9 medium contained 0.4% glycerol as the carbon source. Antibiotics were utilized at the following concentrations: kanamycin sulfate, 20 μg/ml; sodium ampicillin, 100 μg/ml; spectinomycin sulfate, 100 μg/ml; and tetracycline hydrochloride, 20 μg/ml. LB medium was used for all strain constructions, whereas M9 minimal salts medium was used for growth curve analysis. Arabinose was added at concentrations between 1.3 μM and 13 mM (0.2%) for induction of plasmid-based genes. Glucose was added to 0.8% for repression of the arabinose promoter.
Plasmid constructions.
All strains used in this study were derived from Escherichia coli K-12 strain MG1655. The characteristics of the strains and plasmids used are shown in Table 1. Plasmid pACS221 was constructed by inserting the coding sequences of the E. coli accBC operon (aacBCEc) with the native accB ribosome binding site into EcoRI- and XbaI-digested pBAD322K. The insert was generated by PCR amplification using primer 5′ EcoRI accBCEc-341 plus primer 3′ Xba accBCEc and MG1655 genomic DNA as a template. Plasmid pACS199 was constructed by inserting a fragment containing the Salmonella enterica LT2 accBC operon (called accBCSe) together with its native promoter into BamHI- and XbaI-digested vector pAH144. The insert was generated using primers 5′ BamHI accBCSe-553 and 3′ XbaI accBCSe and with S. enterica LT2 genomic DNA as the template. The ligation products were transformed into strain WM95 to allow the Pi protein-requiring plasmid to replicate.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| MG1655 | Wild type | Lab collection |
| AS59 | MG1655 attHK022::accBCSeaadA | This work |
| AS61 | AS59 ΔaccBC::cat | This work |
| AS105 | MG1655 ΔlacZYΔaraBAD | This work |
| AS109 | AS105 ΔaccBC::catattHK022::accBCSeaadA | This work |
| AS110 | AS109 zcc-282::Tn10/pACS221 | This work |
| CAG18466 | MG1655 zcc-282::Tn10 | 28 |
| WM95 | pir+ cloning strain | 24 |
| Plasmids | ||
| pBAD322K | Expression vector; Kanr | 5 |
| pACS199 | pAH144 containing S. entericaaccBC | This work |
| pACS221 | pBAD322K encoding wild-type E. coli AccB and AccC | This work |
| pACS222 | pACS221 encoding AccB and AccC(R19E) | This work |
| pACS223 | pACS221 encoding AccB and AccC(E23R) | This work |
| pACS224 | pACS221 encoding AccB and AccC(F363A) | This work |
| pAH69 | IntHK022 integrase expression plasmid | 11 |
| pAH144 | pir-dependent attP HK022 integration plasmid; Spcr Strr | 11 |
| pKD3 | Chloramphenicol cassette plasmid | 7 |
| pKD46 | λred recombinase expression plasmid | 7 |
CRIM plasmid integration.
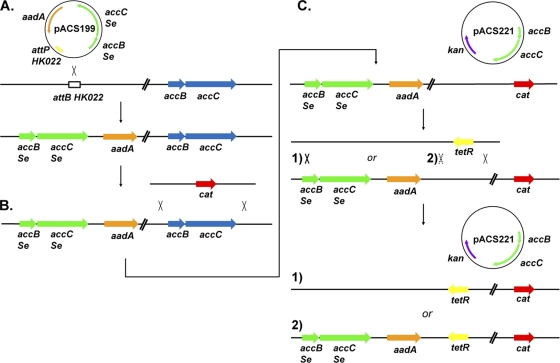
MG1655 was first transformed with CRIM helper plasmid pAH69, which is temperature sensitive for replication and encodes intHK022 phage integrase (11). The cells were then grown at 30°C, made electrocompetent, transformed with plasmid pACS199, and incubated at 42°C for 1 h to induce expression of intHK022. Plating on LB agar containing spectinomycin at 37°C selected for integration of pACS199, which is unable to replicate in wild-type E. coli strains. Spectinomycin-resistant transformants were then streaked for single colonies and tested for the presence of single integrants by colony PCR using the following pool of primers: CRIM HK022 P1, CRIM P2, CRIM P3, and CRIM HK022 P4 (11). A successful single integrant that gave PCR products of 289 and 824 bp was called strain AS59. This strain contains two copies of the accBC operon—the endogenous operon plus the S. enterica LT2 copy integrated into the phage attachment site (Fig. 2A).
Fig 2.
Construction of the plasmid-complemented accBC deletion strain and the test for BC function. (A) Plasmid pACS199 was transformed into a wild-type strain carrying the integrase expression plasmid pAH69. Plasmid pACS199 contains the entire S. enterica LT2 accBC operon with its native promoter plus the attP HK022 integration site. This allows for the integration of the plasmid into the attB HK022 site of the E. coli chromosome. (B) Using λ red-catalyzed site-specific recombination, a PCR product containing a chloramphenicol resistance cassette (cat) flanked by sequences homologous to the endogenous accBC operon was inserted into the chromosome, replacing the native sequence. After selection for chloramphenicol resistance, the resulting strain, AS61, contained only the ectopic S. enterica accBC operon. (C) Plasmid pACS221, which contains the E. coli accBC operon under arabinose promoter control, was then introduced into the strain. The transformed strain was then transduced with a phage P1 lysate grown on strain CAG18466, which contains a tetracycline-resistant Tn10 linked to the attB HK022 site. In this depiction of the genetic cross, a crossover to the right of the Tn10 is required. The second crossover needed for tetracycline resistance can occur either within the interval between the Tn10 and the ectopic accBC operon or to the left of the aadA gene. Tetracycline-resistant transductants were selected and screened for streptomycin sensitivity. If crossover path 1 is taken, then the transductants would be spectinomycin sensitive, indicating that the plasmid copy allows growth and that the resulting strain is dependent on plasmid-based accBC expression for viablity. If crossover path 2 occurs, all transductants would retain spectinomycin resistance and the ectopic S. enterica copy linked to aadA, indicating that the plasmid-borne accBC construct was unable to provided sufficient expression for growth.
Construction of the ΔaccBC strain.
A linear DNA fragment was amplified from the template plasmid pKD3 using the knockout (KO) primers 5′ accBC KO −362 and 3′ accBC KO. The resulting linear PCR product contained a chloramphenicol resistance cassette flanked by FLP recognition target sites along with sequences in the primers homologous to the 5′ and 3′ ends of the accBC operon. Strain AS59 was transformed with the λ red recombinase expressing plasmid pKD46. The transformed strain was grown at the temperature permissive for plasmid replication (30°C) with arabinose and then made electrocompetent. These cells were transformed with the linear DNA fragment with selection for chloramphenicol resistance to replace accBC with the resistance cassette (Fig. 2B). The deletion was verified by colony PCR using external primers 5′ accBCEc check for and 3′ accBCEc check rev. In the resulting construct, strain AS61, the native accBC operon had been replaced with the chloramphenicol resistance cassette, and cell growth was supported by the ectopic S. enterica accBC operon.
accBC complementation.
Plasmid pACS221 was tested for its ability to complement an ΔaccBC null mutant. Strain AS61 was transformed with plasmid pACS221 and grown in LB medium containing 0.2% arabinose to induce expression of AccB and AccC. This strain was then transduced with a phage P1 lysate grown on strain CAG18466 which contains a Tn10 element closely linked to attHK022, the site at which accBCSe is inserted (Fig. 2C). After selection for tetracycline resistance, the resulting colonies were screened for sensitivity to spectinomycin, indicating that the ectopic accBCSe copy had been replaced. These transductants required arabinose-induced accBC expression from plasmid pACS221 for robust growth and failed to grow in the presence of glucose, which represses basal expression from the arabinose promoter. Strain AS109 was constructed by first inserting the attHK022::accBCSe ectopic copy and then the ΔaccBC::cat locus from strain AS61 into strain AS105 by P1 transduction. Since plasmid pACS221 provided complementation, it was transformed into strain AS109, and in the presence of arabinose, the transformed strain was transduced with P1 phage grown on strain CAG18466. Tetracycline-resistant transductants were selected and then screened for spectinomycin sensitivity. The resulting strain, AS110, lacks a chromosomal copy of accBC and requires plasmid pACS221 plus arabinose for rapid growth.
Site-directed mutagenesis.
Complementary primers (Table 2) were designed to anneal to plasmid pACS221 at the site of the desired mutations to introduce the R19E, E23R, or F363A mutation into AccC. These primer pairs were then used to amplify the entire pACS221 plasmid, using Pfu polymerase. The resulting products were then digested with DpnI to minimize template background and transformed. The resulting plasmid inserts were then sequenced to verify the expected mutational changes.
Table 2.
PCR primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| 5′ BamHI accBCSe −553 | GGATCCTGGCAAACACTGCATAACGTCGTC |
| 3′ XbaI accBCSe | TCTAGAAAGCGGGAATTGTACATG |
| 5′ EcoRI accBCEc −341 | CGGGGAATTCGCTTTACAGACGG |
| 3′ Xba accBCEc | TCTAGAAAGCGGGGATTGTACCTTA |
| 5′ accBC KO −362 | CGGTGTTGAAGGTTATTTACATGTTAGCTGTTGATTATCTTCCCTTGTGTAGGCTGGAGCTGCTTCGA |
| 3′ accBC KO | CGGCCTTTTGACGCTTTAGCAGTCTTATTTTTCCTGAAGACCGAGCATATGAATATCCTCCTTAG |
| 5′ accBCEc check for | TCTATCTTGTCGCGATCCTGGCAT |
| 3′ accBCEc check rev | TAAGCGGCTACTAACCAAACTGCC |
| CRIM HK022 P1 | GGAATCAATGCCTGAGTG |
| CRIM HK022 P4 | GGCATCAACAGCACATTC |
| CRIM P2 | ACTTAACGGCTGACATGG |
| CRIM P3 | ACGAGTATCGAGATGGCA |
| accC(R19E) SDM | GATTGCATTGCGTATTCTTGAAGCCTGTAAAGAACTGGG |
| accC(R19E) SDM RC | CCCAGTTCTTTACAGGCTTCAAGAATACGCAATGCAATC |
| accC(E23R) SDM | CGTGCCTGTAAACGACTGGGCATCAAGAC |
| accC(E23R) SDM RC | GTCTTGATGCCCAGTCGTTTACAGGCACG |
| accC(F363A) SDM | CACCTGGCGGTGCTGGCGTACGTT |
| accC(F363A) SDM RC | CAACGTACGCCAGCACCGCCAGGTG |
Growth measurements.
The desired plasmid and strain combinations were inoculated into LB containing the appropriate antibiotics and arabinose and grown overnight. The strains were then subcultured into M9 minimal medium with 0.4% glycerol as the primary carbon source and the appropriate concentrations of kanamycin and arabinose. These overnight cultures were then diluted to an approximate optical density at 600 nm (OD600) of 0.001, and 300 μl of each sample was pipetted into 5 wells of a Bioscreen C plate. The medium was supplemented with 1.3 μM arabinose, 0.8% glucose, or 13 mM (0.2%) arabinose or left unsupplemented. The plates were placed into the Bioscreen C analyzer (Growth Curves USA, Piscataway, NJ) and allowed to grow for 48 h with continuous high-intensity shaking and monitoring every 15 min. The resulting growth data were averaged and plotted.
Biotin carboxylase partial purification and assay.
The method of Guchhait et al. (10) was used for the partial purification and assay of wild-type and mutant BC proteins. Strains requiring plasmid-based BC expression were grown in 500 ml of LB medium with kanamycin and 0.2% arabinose until an OD600 of 1.0 was reached. The cultures were then pelleted and stored at −80°C. The samples were suspended in 20 ml of 100 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 5 mM 2-mercaptoethanol. All procedures were performed at 4°C. The cells were then lysed by multiple passages through a French pressure cell. The lysates were centrifuged to remove cell debris, and the supernatant was fractionated with ammonium sulfate at 4°C. The supernatants were stirred while sitting in ice as 144 g per liter (25% of saturation) of crushed ammonium sulfate was slowly added. The mixture was allowed to stir for 15 min on ice and then centrifuged. The recovered supernatant was then brought to 45% of saturation by the addition of 125 g per liter of ammonium sulfate. The precipitated protein was recovered by centrifugation and stored at −80°C. Prior to use, the protein precipitates were suspended in a minimal volume of buffer and then dialyzed for 3 h at 4°C against the above phosphate buffer to remove ammonium sulfate, and the protein concentrations were then measured by the Bio-Rad protein assay.
The relative activities of the wild-type and mutant biotin carboxylases were determined by ATP-dependent carboxylation of free biotin with [14C]bicarbonate (American Radiolabeled Chemicals) (10, 26). The reaction mixtures contained 100 mM Tris-HCl buffer (pH 8.0), 3 mM ATP, 8 mM MgCl2, 8 mM NaH14CO3 (1.28 mCi/mmol), 100 mM potassium d-biotin, 3 mM glutathione, 10% ethanol, and 250 μg of partially purified biotin carboxylase protein in a volume of 500 μl. All of the reaction components were mixed, and the reaction was initiated by addition of enzyme. After incubation of the reaction mixtures at 30°C for 15 min, 400 μl of the reaction mixture was removed to a test tube containing 1 ml of ice-cold water containing a few drops of octanol (to prevent foaming). These tubes were placed in ice, and CO2 was bubbled through the solution for 30 min to remove unincorporated [14C]bicarbonate by exchange with excess CO2. After thorough gassing, 100 μl of 0.1 N NaOH was added to each reaction mixture. The contents were transferred to a vial of scintillation fluid, and the 14C incorporation was measured. Note that due to exchange of [14C]bicarbonate with CO2 present in the buffers and atmosphere, the results are given as counts incorporated rather than molar quantities.
RESULTS
Construction of an E. coli strain dependent on plasmid-based BC expression.
In order to test the effects of the dimer interface mutations in vivo, it was necessary to construct a strain in which the sole sources of BC were plasmids encoding either the wild-type or mutant BCs under a controllable promoter. BC is an essential protein, and since it was unclear if the mutant BCs would be able to support growth, we used the approach developed for another essential gene, fabH (17). In this approach, a second highly homologous copy of the accBC operon, that of Salmonella enterica LT2, was integrated into the HK022 phage attachment site of the E. coli chromosome, using the phage integrase system developed by Haldiman et al. (11), to produce strain AS59 (Fig. 2A). We then used recombineering to delete the normal chromosomal copy of accBC to obtain a strain having only the S. enterica accBC operon, which was marked with a spectinomycin resistance cassette to obtain strain AS61 (Fig. 2B). This strain was transformed with plasmid pACS221, which expresses the E. coli accBC operon using the native accB ribosome binding site but with the araBAD promoter, which replaced the native promoter. The resulting transformed strain contained two functional (but nonidentical) copies of the accBC operon. We then introduced plasmids encoding either the mutant or wild-type BC proteins and tested whether or not the S. enterica accBC operon could be removed by transduction using a phage lysate grown on strain CAG18466, which carries a Tn10 transposon insertion closely linked to the HK022 phage attachment site (Fig. 2C). The resulting tetracycline-resistant transductants were then scored for spectinomycin sensitivity. If the plasmid-encoded BC mutant protein could functionally replace the S. enterica protein, then spectinomycin-sensitive colonies would result (Fig. 2C, path 1), whereas the opposite result (only spectinomycin-resistant transductants) would indicate that the S. enterica gene could not be removed (Fig. 2C, path 2), demonstrating that the mutant gene was unable to provide sufficient BC function for growth. The resulting spectinomycin-sensitive strains contained a single plasmid-borne accBC operon under arabinose regulation. Note that although we were testing only mutant BC proteins, all constructs also encoded a wild-type accB gene. This is because sole overproduction of either of the accBC operon proteins is growth inhibitory, presumably due to disruption of the stoichiometry of ACC assembly (or of a subcomplex) (1, 13, 15, 20). The S. enterica accBC operon was used because the gene organization and encoded proteins are essentially identical to those of E. coli, but the genes differ sufficiently at the nucleotide level (mainly due to differing bases in codon wobble positions) that homologous recombination is blocked. Hence, the mutations of plasmid-borne accC genes could not be repaired by recombination with the chromosomal copy. The accBC expression plasmid was pBAD322K, a medium-copy-number plasmid with inducible expression from the araBAD promoter (5). Strain AS110, an ΔaraBAD strain blocked in arabinose catabolism, was then constructed to allow induction by low arabinose concentrations.
To test if expression of accBC from plasmid pBAD322K was sufficient for growth of E. coli, a strain lacking a chromosomal copy was constructed in three steps. Integration of S. enterica accBC into a wild-type E. coli strain gave strain AS59, which contained two functional copies of the accBC operon (Fig. 2A). The native operon was then replaced with a chloramphenicol resistance cassette (7) to give strain AS61 which contained only the S. enterica accBC operon under its native promoter (Fig. 2B). This strain was transformed with plasmid pACS221, which expressed the E. coli accBC operon with the native accB ribosome binding site but in which the araBAD promoter replaced the native promoter. The resulting transformed strain contained two functional (but nonidentical) copies of the accBC operon. The S. enterica copy, located at the HK022 phage integration site, was then eliminated by P1 transduction of phage grown on strain CAG18466, which contains Tn10 linked to the HK022 phage attachment site (17, 28). Tetracycline-resistant transductants were selected and screened for spectinomycin sensitivity to score for elimination of the integrated S. enterica accBC operon (Fig. 2C). The resulting spectinomycin-sensitive strains contained a single plasmid-borne accBC operon under arabinose regulation. The strains were viable with arabinose induction but grew poorly when glucose repressed basal expression of the ara promoter. Strain AS110, a ΔaraBAD strain blocked in arabinose catabolism, was then constructed to allow induction by low arabinose concentrations.
Site-directed accC alleles were constructed in plasmid pACS221 to introduce the R19E, E23R, and F363A residue substitutions implicated in dimerization (27). Relative to the wild-type BC, the R19E, E23R, and F363A mutations were reported to increase the dimer dissociation constants by approximately 7,000- to 8,000-fold, 5,500- to 6,000-fold, and 4- to 28-fold, respectively, with only modest effects on in vitro BC activity (27). These plasmids were introduced into strains having the integrated S. enterica accBC operon as the sole source of BC activity. The ability to remove the ectopic S. enterica accBC operon was tested by transduction as described above, and the transductants were plated on LB agar plates containing 13 mM (0.2%) arabinose. All three strains carrying plasmids that encoded a mutant BC protein grew on this medium, indicating that the ectopic S. enterica accBC had been functionally replaced, although the colonies were smaller than those formed by the strain expressing wild-type BC. Moreover, unlike the strain expressing wild-type BC, the strains encoding the R19E and E23R proteins failed to grow in the absence of arabinose (data not shown).
Complementation of the ΔaccC strain by plasmid-borne accC alleles.
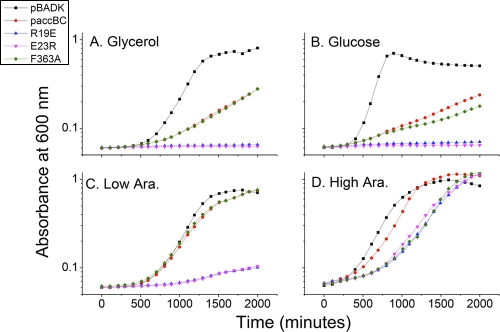
The growth rates in liquid media of all four strains were analyzed in detail using the Bioscreen C growth curve analysis system. The strains encoding the mutant BCs all grew under maximal induction conditions, as previously seen by colony formation, but the growth curves lagged behind those of the strain carrying the wild-type plasmid and the wild-type strain carrying an empty vector (Fig. 3D). Under low-arabinose conditions (1.3 μM), the mutant least defective in dimerization (F363A) grew similarly to the wild type, whereas the other two strains that encoded BC mutant proteins with much higher dissociation constants grew poorly (Fig. 3C). In medium devoid of arabinose or supplemented with glucose (to repress promoter function), the two severely defective dimerization mutants (R19E and E23R) failed to grow, while expression of the minimally impaired mutant F363A or wild-type enzymes allowed slow growth under these low-expression conditions (Fig. 3A and B).
Fig 3.
Growth of strains dependent on plasmid-encoded mutant BC proteins. Derivatives of the accBC deletion strain (AS109), cured of the S. enterica LT2 accBC operon by transduction, that contained plasmids encoding accB plus one of the accC alleles were grown in M9 minimal medium in a Bioscreen C analyzer with 0.4% glycerol as the primary carbon source and supplemented with either arabinose (Ara.) or glucose, as shown. The plasmids encoding the dimerization-deficient BC R19E and E23R mutant proteins were unable to allow growth (complement) at low levels of expression. Panel A shows growth of the strains in the absence of supplementation with arabinose or glucose. Panel B shows the strains grown with the addition of 0.8% glucose to repress expression from the araBAD promoter. Panel C shows the strains grown with 1.3 μM arabinose in addition to glycerol to give a low level of induction. Panel D shows the strains grown with the addition of 13 mM (0.2%) arabinose for maximum induction. The measurements were averages of at least 3 independent repetitions with 5 duplicates per repetition.
BC activities of cell extracts.
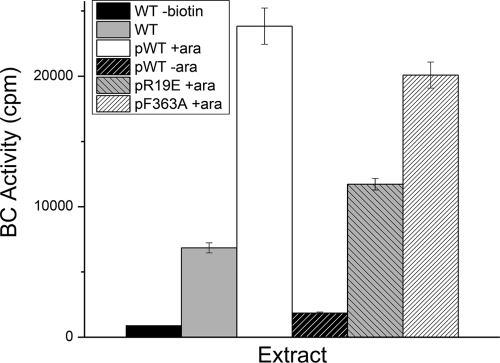
All of the dimer interface mutant proteins allowed growth when highly expressed, whereas the R19E and E23R mutants failed to grow at lower expression levels at which the growth of strains expressing the wild-type and F363A proteins proceeded. Shen et al. (27) reported that the catalytic activities of the dimer interface mutant proteins were only modestly diminished relative to the loss of their ability to form dimers. In their assays, the R19E and E23R mutant proteins had kcat values about 30% that of the wild-type enzyme compared to a roughly 5,500- to 8,000-fold increases in Kd, while the F363A mutant retained around 90% of the kcat, with a much smaller (4- to 28-fold) increase in Kd. Our analyses assumed that the mutant proteins were expressed as efficiently as the wild-type protein. If so, we would expect that the ratios of the BC activities in partially purified cell extracts would reflect those of the purified proteins studied by Shen et al. (27). We used a more direct assay than that used by the prior researchers—the incorporation of [14C]bicarbonate into carboxybiotin (10)—and found that the R19E mutant extract had about half the activity of the wild-type protein extract, whereas the F363A mutant extract had approximately 84% of the wild-type activity, values in good agreement with those reported by Shen et al. (27). However, for unknown reasons, multiple attempts to measure activity of the E23R mutant extracts showed only background activity (data not shown). We had chosen the pBAD322K vector because its range of protein expression levels seemed likely to overlap with the level of BC expression in wild-type cells, and this was the case. The extract of a wild-type strain had 29% of the activity of a strain expressing the fully induced plasmid-borne wild-type BC (Fig. 4), indicating only modest overexpression of BC. We also assayed the basal (uninduced) level of expression and found it to be 12-fold lower than the fully induced level.
Fig 4.
Relative BC activities of partially purified cell extracts. Biotin carboxylase preparations of the various strains shown were partially purified from cell extracts as described in Materials and Methods. Partially purified protein (250 μg) was incubated with 100 mM biotin, 3 mM ATP, and 8 mM [14C]bicarbonate. The reaction mixtures were incubated at 30°C for 15 min, and the reactions were terminated by the addition of ice-cold water. CO2 was then bubbled through the samples for 30 min on ice to eliminate residual [14C]bicarbonate, and the gassed samples were then transferred to scintillation counter vials for counting. The control experiments were performed in exactly the same manner, except that biotin was omitted. All background values were essentially identical to that of the protein extract of the wild-type (WT) strain, and for simplicity, only that control is given. The activity of the wild-type strain MG1655 protein extract was included to allow comparison with the activities given by plasmid-based expression. ara, arabinose.
DISCUSSION
Our data demonstrate that ACC activity in vivo depends on the ability of BC to efficiently form dimers. It should be noted that proteomic data obtained by three mass spectral analyses based on the yield of BC (AccC) peptides (12, 16, 23) or on quantitative two-dimensional (2D) gel separations (16, 22) give a consistent value of 1,477 ± 150 BC molecules per E. coli cell. This translates to an intracellular BC concentration of about 2.4 μM, a concentration well above the reported Kd (∼0.1 μM) for dimer dissociation of the wild-type BC (27) and consistent with its isolation as a stable dimer. In our experiments, basal expression of accBC from the plasmid constructs (Fig. 4) gives BC levels about one-fourth that of wild-type cells, and thus the intracellular BC concentration would be roughly 0.6 μM, whereas the Kd values of the R19E and E23R mutant proteins (703 to 843 and 540 to 600 μM, respectively) are hundreds of fold higher (27). Thus, if the Kd values obtained in vitro reflect the in vivo situation, it would be expected that strains producing R19E and E23R proteins would be unable to grow at basal levels of expression, whereas strains expressing the wild-type protein or the more modestly defective F363A mutant protein (Kd values of 4 to 28 μM) might grow slowly in the absence of induction. Indeed, these expectations were fulfilled (Fig. 3). The increased intracellular BC concentration resulting from induction of accBC expression allowed growth of strains expressing the R19E and E23R proteins, and increased inducer concentration resulted in more rapid growth. These are the results expected if higher intracellular BC concentrations can partially overcome the dimerization defects of the mutant proteins. Indeed, the E23R protein is not completely defective in dimer formation because it formed dimers at 300 μM, the concentration used for its crystallization (the R19E protein failed to crystallize) (27). Finally the subtle dimerization defect of the F363A protein was seen when glucose was used to repress basal expression of the ara pBAD promoter (Fig. 3B).
Although the in vivo results are consistent with the in vitro data, it may be too much to expect strict quantitative agreement. This is because it is unclear what fraction of BC subunits must be present as dimers to allow growth and whether or not the presence of the other ACC subunits aids BC dimerization. Moreover, the presence or absence of substrates gave large changes (up to 6-fold) in the Kd values obtained in vitro for the mutant proteins (27), which introduces additional uncertainties.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health (NIH) grant AI15650 from the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Published ahead of print 28 October 2011
REFERENCES
- 1. Abdel-Hamid AM, Cronan JE. 2007. Coordinate expression of the acetyl coenzyme A carboxylase genes, accB and accC, is necessary for normal regulation of biotin synthesis in Escherichia coli J. Bacteriol. 189:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi-Rhee E, Cronan JE. 2003. The biotin carboxylase-biotin carboxyl carrier protein complex of Escherichia coli acetyl-CoA carboxylase. J. Biol. Chem. 278:30806–30812 [DOI] [PubMed] [Google Scholar]
- 4. Chou CY, Yu LP, Tong L. 2009. Crystal structure of biotin carboxylase in complex with substrates and implications for its catalytic mechanism. J. Biol. Chem. 284:11690–11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cronan JE. 2006. A family of arabinose-inducible Escherichia coli expression vectors having pBR322 copy control. Plasmid 55:152–157 [DOI] [PubMed] [Google Scholar]
- 6. Cronan JE, Jr, Waldrop GL. 2002. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 41:407–435 [DOI] [PubMed] [Google Scholar]
- 7. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Queiroz MS, Waldrop GL. 2007. Modeling and numerical simulation of biotin carboxylase kinetics: implications for half-sites reactivity. J. Theor. Biol. 246:167–175 [DOI] [PubMed] [Google Scholar]
- 9. Freiberg C, et al. 2004. Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity. J. Biol. Chem. 279:26066. [DOI] [PubMed] [Google Scholar]
- 10. Guchhait RB, et al. 1974. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J. Biol. Chem. 249:6633–6645 [PubMed] [Google Scholar]
- 11. Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183:6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishihama Y, et al. 2008. Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. James ES, Cronan JE. 2004. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J. Biol. Chem. 279:2520. [DOI] [PubMed] [Google Scholar]
- 14. Janiyani K, Bordelon T, Waldrop GL, Cronan JE., Jr 2001. Function of Escherichia coli biotin carboxylase requires catalytic activity of both subunits of the homodimer. J. Biol. Chem. 276:29864–29870 [DOI] [PubMed] [Google Scholar]
- 15. Karow M, Fayet O, Georgopoulos C. 1992. The lethal phenotype caused by null mutations in the Escherichia coli htrB gene is suppressed by mutations in the accBC operon, encoding two subunits of acetyl coenzyme A carboxylase. J. Bacteriol. 174:7407–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuntumalla S, et al. 2009. Comparison of two label-free global quantitation methods, APEX and 2D gel electrophoresis, applied to the Shigella dysenteriae proteome. Proteome Sci. 7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai CY, Cronan JE. 2003. 3-Ketoacyl-acyl carrier protein synthase III (FabH) is essential for bacterial fatty acid synthesis. J. Biol. Chem. 278:51494. [DOI] [PubMed] [Google Scholar]
- 18. Li SJ, Cronan J. 1992. The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J. Biol. Chem. 267:855. [PubMed] [Google Scholar]
- 19. Li SJ, Cronan JE., Jr 1992. The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J. Biol. Chem. 267:16841–16847 [PubMed] [Google Scholar]
- 20. Li SJ, Cronan JE., Jr 1993. Growth rate regulation of Escherichia coli acetyl coenzyme A carboxylase, which catalyzes the first committed step of lipid biosynthesis. J. Bacteriol. 175:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu X, Fortin PD, Walsh CT. 2008. Andrimid producers encode an acetyl-CoA carboxyltransferase subunit resistant to the action of the antibiotic. Proc. Natl. Acad. Sci. U. S. A. 105:13321–13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez-Campistrous A, et al. 2005. Localization, annotation, and comparison of the Escherichia coli K-12 proteome under two states of growth. Mol. Cell Proteomics 4:1205–1209 [DOI] [PubMed] [Google Scholar]
- 23. Lu P, Vogel C, Wang R, Yao X, Marcotte EM. 2007. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 25:117–124 [DOI] [PubMed] [Google Scholar]
- 24. Metcalf WW, Jiang W, Wanner BL. 1994. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6K gamma origin plasmids at different copy numbers. Gene 138:1–7 [DOI] [PubMed] [Google Scholar]
- 25. Mochalkin I, et al. 2008. Structural evidence for substrate-induced synergism and half-sites reactivity in biotin carboxylase. Protein Sci. 17:1706–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polakis SE, Guchhait RB, Zwergel EE, Lane MD, Cooper TG. 1974. Acetyl coenzyme A carboxylase system of Escherichia coli. J. Biol. Chem. 249:6657. [PubMed] [Google Scholar]
- 27. Shen Y, Chou CY, Chang GG, Tong L. 2006. Is dimerization required for the catalytic activity of bacterial biotin carboxylase? Mol. Cell 22:807–818 [DOI] [PubMed] [Google Scholar]
- 28. Singer M, et al. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]