Abstract
Serum 1,3-β-d-glucan (BG) assay may be helpful as a marker for the diagnosis of Pneumocystis jiroveci pneumonia (PJP) and invasive fungal infection (IFI). We conducted a systematic review to assess the diagnostic accuracy of this assay. We searched MEDLINE, Web of Science, Cochrane Collaboration databases, Ichushi-Web, reference lists of retrieved studies, and review articles. Our search included studies of serum BG assay that used (i) positive cytological or direct microscopic examination of sputum or bronchoalveolar lavage fluid for PJP and (ii) European Organization for Research and Treatment of Cancer or similar criteria for IFI as a reference standard and provided data to calculate sensitivity and specificity. Only major fungal infections such as invasive candidiasis and invasive aspergillosis were included in the IFI group. Twelve studies for PJP and 31 studies for IFI were included from January 1966 to November 2010. The pooled sensitivity, specificity, diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUC-SROC) for PJP were 96% (95% confidence interval [95% CI], 92% to 98%), 84% (95% CI, 83% to 86%), 102.3 (95% CI, 59.2 to 176.6) and 0.96 (95% CI, 0.94 to 0.99), respectively. No heterogeneity was found. For IFI, the values were 80% (95% CI, 77% to 82%), 82% (95% CI, 81% to 83%), 25.7 (95% CI, 15.0 to 44.1), and 0.88 (95% CI, 0.82 to 0.93). Heterogeneity was significant. The diagnostic accuracy of the BG assay is high for PJP and moderate for IFI. Because the sensitivity for PJP is particularly high, the BG assay can be used as a screening tool for PJP.
INTRODUCTION
Pneumocystis jiroveci pneumonia (PJP) continues to be a serious problem among immunocompromised patients despite the decreased number of cases among human immunodeficiency virus (HIV)-infected patients over the past decade with the widespread use of prophylaxis. The high mortality of patients requiring mechanical ventilation has remained unchanged, ranging from 50 to 60% (35). Although the gold standard for diagnosis is microscopic visualization of the organism, the methods are not sensitive, particularly in HIV-negative patients (31).
The incidence of invasive fungal infection (IFI) has been increasing, especially among immunocompromised patients undergoing aggressive chemotherapy for cancer, bone marrow and organ transplantation, and advanced critical care. Despite advances in therapy, IFI is associated with considerable morbidity and a mortality rate of 30 to 70% for aspergillosis and 40 to 50% for candidiasis (15). Diagnosis of IFI is challenging because clinical and radiological signs and conventional microbiological and histological techniques are not sensitive enough (25). For these reasons, intensive research currently aims at the development of new diagnostic methods for PJP and IFI.
One of these new diagnostic techniques is the assay for the serum 1,3-β-d-glucan (BG) derived from major cell wall components of various medically important fungi. The Fungitell test (Associates of Cape Cod, Inc., East Falmouth, MA) is a chromogenic kinetic test that was approved in 2003 by the U.S. Food and Drug Administration for the presumptive diagnosis of IFI. The BG assay has been used frequently, and the results are included in the revised IFI diagnosis criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria (11). However, the results of test performances have varied, as Nakamura and colleagues (42) suggested that the detection rate of BG in HIV-negative patients was lower than that in HIV patients.
One systematic review (23) on the accuracy of BG assay only for diagnosing IFI has recently been published. The review did not assess the diagnostic accuracy for PJP. The article included 2 of 16 evaluated studies using inappropriate reference standards; such inappropriate reference standards, according to the EORTC/MSG, consisted of only mycological criteria (22, 39). The review also used language restrictions and did not investigate possible explanations for the observed heterogeneity. We report a new systematic review of the accuracy of the BG assay for diagnosing PJP and IFI. We also focus on study design, reference standard, and assay as explanations for between-study variability in diagnostic accuracy.
MATERIALS AND METHODS
Data sources and searches.
We developed a protocol for the review by following standard reporting guidelines (7, 21). The search was carried out using four different databases (MEDLINE, Web of Science, Cochrane Collaboration databases, and Ichushi-Web) from January 1966 until November 2010. Ichushi-Web, which is a major Japanese database, was included because the BG assay was first developed in Japan. The term “diagnosis” and the medical subject heading terms “fungi,” “mycoses,” and “beta-glucans” were used for searching MEDLINE, the terms “glucan” and “diagnosis” for searching the Web of Science and Cochrane Collaboration databases, and the terms “fungi,” “mycoses,” “Pneumocystis,” “carinii,” “jiroveci,” “aspergillosis,” “Aspergillus,” “Candida,” “candidiasis,” “glucan,” and “diagnosis” for searching Ichushi-Web. Reference lists of retrieved studies and review articles were also reviewed. Only papers published in full text were selected, while no language restrictions were applied to the search.
Study selection.
Studies relevant for determining the diagnostic validity of serum BG assay for PJP and IFI in humans were included if two sets of criteria were met. First, positive findings for cytological or direct microscopic examination of sputum or bronchoalveolar lavage fluid for PJP and the proven or probable presence of IFI according to the EORTC/MSG criteria (5) or similar criteria for IFI as a reference standard. If a study used both positive findings for cytological or microscopic examination and positive PCR as a reference standard for PJP, we excluded participants who had been diagnosed with positive PCR. Second, absolute numbers of true-positive, false-negative, true-negative, and false-positive observations were available or could be derived from the reported data. Only major fungi such as invasive candidiasis and invasive aspergillosis were included in the IFI group because the number of the other fungi was small in included studies and certain zygomycetes (Mucor and Rhizopus species) and because Cryptococcus species had no BG cell wall. Case reports and review articles were excluded. If a study appeared to meet selection criteria but had a patient population that appeared to be the same as or to overlap with the patient population of a similar study, we included the larger of the studies.
Data extraction and study quality assessment.
We wanted to extract the following variables: publication year; name(s) and institution(s) of the author(s); information on the original sample source; study design; patient demographics and comorbidities; type of invasive fungal infection; characteristics of control subjects; numbers of true-positive, false-negative, true-negative, and false-positive observations; type and manufacturer of the BG assay; reference standard; cutoff values for definition of a positive BG test result; and blinding of investigators to results.
Two investigators independently rated the quality of the findings by using a modified version of the Quality Assessment for Diagnostic Accuracy Studies (QUADAS) tool (58), which contains 11 items specifically developed to assess the quality of systematic reviews of primary studies of diagnostic tests, and is recommended by the Cochrane Diagnostic Reviewers' Handbook (52). As recommended by the designers of the QUADAS tool, we did not apply weights to the QUADAS item or use a summary score in the analysis. Instead, we used subgroup analyses to explore whether scores on the following quality items explained variation in diagnostic performance: representative spectrum, acceptable reference standard, differential verification avoided, and index test results blinded. These items have been shown to result in biased estimates of the diagnostic performance (30, 53). We resolved discrepancies about any item through discussion.
Data synthesis and analysis.
For main outcomes, we evaluated the diagnostic accuracy of the serum BG assay for PJP and for IFI. Subgroup analyses were performed only when each subgroup included data of at least three diagnostic studies. If several cutoffs were reported in one study, we used the cutoff that offered the best test performance. A random-effects model was used to combine estimates of sensitivity, specificity, diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUC-SROC) with 95% confidence interval (95% CI) (10, 37). We also assessed heterogeneity by means of the Cochran Q method and the test of inconsistency (I2)(9, 18). Sensitivity analysis was based on control participants, exclusion of possible IFI in control patients, language, and prophylactic antifungal therapy. We conducted a stratified analysis for study design, the brand-name assay, the reference standard, the kind of mycosis, and the QUADAS item and used metaregression to identify the possible sources of heterogeneity among studies (41). We explored the possibility of publication bias by means of funnel plots and the Egger test for diagnostic odds ratios (13, 55). For all analyses, we used MetaDiSc, version 1.4 (Hospital Universitario Ramón y Cajal, Madrid, Spain) and Comprehensive Meta Analysis version 2 (Biostat Inc., Englewood, CA).
RESULTS
Search results and characteristics of studies.
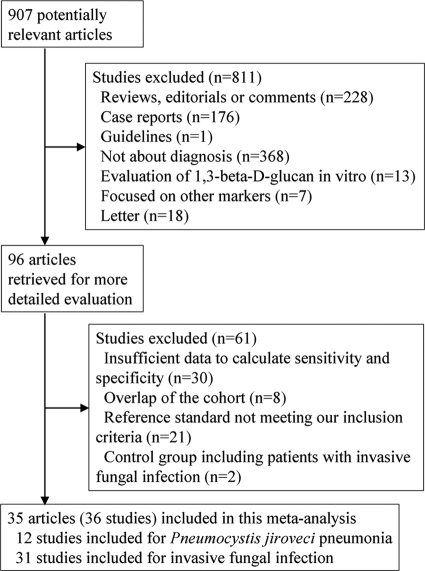
We identified 907 possibly relevant articles from four different databases (MEDLINE, Web of Science, Cochrane Collaboration databases, and Ichushi-Web), and 96 full-length articles were selected for detailed analysis on the basis of title or abstract. Retrieval and inclusion flow is shown in Fig. 1. Eventually, 35 articles met the inclusion criteria (1–4, 12, 14, 16, 17, 19, 20, 24, 26, 27, 29, 32–34, 38, 40, 43–51, 54, 56, 57, 59–62), but since the article by Odabasi et al. (45) included two studies, a case-control study and a retrospective cohort study, 36 studies in all were included. Twelve studies were included for PJP and 31 studies for IFI. The characteristics of these studies are outlined in Tables 1, 2, and 3. A total of 5,453 participants were covered by the studies, with women accounting for a median of 43.3%, and the median of the mean or median age was 55.1 years (interquartile range, 47.0 to 58.3 years). Hematological disorders constituted the underlying diseases of most patients. Characteristics of control groups varied, with most studies using patients with hematological disorders. The two studies published by Obayashi and colleagues included different patient cohorts, one study analyzing 50 patients who were enrolled in 1992 and 1993 at nine hospitals in Japan (43) and the other study 58 patients who had been enrolled from 2000 to 2005 and who had undergone autopsy after death (44).
Fig 1.
Flow diagram for the selection of studies.
Table 1.
Characteristics of studies included in the meta-analysisb
| Author (reference) | Year | Region | Language | Assay | Cutoff (pg/ml) | Mean or median age (yr) | Sex (female) | Patient population | Study design | Prophylactic antifungal therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Obayashi et al. (43) | 1995 | Japan | English | Fungitec G | 20 | NA | NA | Patients with various underlying diseases | Case-control study | Not reported |
| Yasuoka et al. (59) | 1996 | Japan | English | Fungitec G | 20 | NA | NA | Patients with HIV | Case-control study | Not reported |
| Mitsutake et al. (38) | 1996 | Japan | English | Fungal index | 60 | NA | NA | Patients with various underlying diseases | Case-control study | Not reported |
| Moro et al. (40) | 2003 | Japan | Japanese | Wako | 11 | 58.5 | 0.412 | Patients with various underlying diseases | Case-control study | Not reported |
| Odabasi-1 et al.a (45) | 2004 | USA | English | Fungitell | 60 | NA | NA | NA | Case-control study | Not reported |
| Odabasi-2 et al.a (45) | 2004 | USA | English | Fungitell | 60 | NA | NA | Patients with HM | Retrospective cohort | Yes |
| Kondori et al. (26) | 2004 | Sweden | English | Fungitec G | 20 | 52.9 | 0.636 | Patients who are immunocompromised | Case-control study | Yes |
| Kawazu et al. (24) | 2004 | Japan | English | Wako | 11 | 45.1 | 0.338 | Patients with HM | Prospective cohort | Yes |
| Horiguchi (20) | 2004 | Japan | Japanese | Fungitec G | 20 | 61.5 | 0.397 | Patients with HM | Prospective cohort | Not reported |
| Pickering et al. (49) | 2005 | USA | English | Fungitell | 60 | NA | NA | NA | Case-control study | Not reported |
| Pazos et al. (47) | 2005 | Spain | English | Fungitell | 120 | 44.0 | 0.432 | Patients with HM | Prospective cohort | Yes |
| Ostrosky-Zeichner et al. (46) | 2005 | USA | English | Fungitell | 60 | 46.0 | 0.583 | Patients with various underlying diseases | Case-control study | Yes |
| Yoshida et al. (60) | 2006 | Japan | Japanese | Fungitec G | 20 | 62.8 | 0.347 | NA | Case-control study | Not reported |
| Fujita et al. (14) | 2006 | Japan | English | Wako | 11 | 52.8 | 0.648 | Patients with various underlying diseases | Case-control study | Yes |
| Tasaka et al. (56) | 2007 | Japan | English | Wako | 31.1 | NA | 0.452 | Patients with various underlying diseases | Case-control study | Not reported |
| Akamatsu et al. (2) | 2007 | Japan | English | Fungitec G | 40 | 51.0 | 0.461 | Solid-organ transplant recipients | Prospective cohort | Not reported |
| Alam et al. (3) | 2007 | Kuwait | English | Fungitell | 80 | NA | NA | Patients with various underlying diseases | Case-control study | Not reported |
| Lu et al. (33) | 2007 | China | Chinese | Fungitec G | 20 | 43.8 | 0.376 | NA | Case-control study | Not reported |
| Persat et al. (48) | 2008 | France | English | Fungitell | 80 | NA | NA | Patients with various underlying diseases | Case-control study | Not reported |
| Senn et al. (54) | 2008 | Switzerland | English | Wako | 7 | 57.0 | 0.389 | Patients with HM | Prospective cohort | Yes |
| Obayashi et al. (44) | 2008 | Japan | English | Fungitec G | 30 | NA | NA | Patients with various underlying diseases | Case-control study | Not reported |
| Watanabe et al. (57) | 2009 | Japan | English | Fungitec G | 23.2 | 39.4 | NA | Patients with HIV | Case-control study | Not reported |
| Desmet et al. (12) | 2009 | Belgium | English | Fungitell | 100 | 42.4 | 0.267 | Patients with HIV and HM | Case-control study | Not reported |
| Presterl et al. (50) | 2009 | Austria | English | Fungitell | 40 | NA | 0.317 | Patients who were admitted to ICU | Retrospective cohort | Not reported |
| Koo et al. (27) | 2009 | USA | English | Fungitell | 80 | 54.0 | 0.436 | Patients who were admitted to hospital | Case-control study | Yes |
| Hachem et al. (16) | 2009 | USA | English | Fungitell | 80 | NA | NA | Patients with HM and solid tumor | Prospective cohort | Not reported |
| Lunel et al. (34) | 2009 | The Netherlands | English | Fungitell | 60 | NA | 0.373 | Patients with various underlying diseases | Retrospective cohort | No |
| Zhao et al. (62) | 2009 | China | Chinese | GKT-25 M | 10 | 6.2 | 0.315 | Patients with various underlying diseases | Prospective cohort | Not reported |
| Racil et al. (51) | 2009 | Czech Republic | Czech | Fungitell | 80 | NA | 0.374 | Patients with HM | Retrospective cohort | Yes |
| Leon et al. (29) | 2009 | Spain, Argentina, and France | English | Fungitell | 75 | 60.0 | 0.327 | Patients with various underlying diseases | Prospective cohort | No |
| Liu et al. (32) | 2009 | China | Chinese | GKT-25 M | 20 | 26.0 | 0.457 | Patients with HM | Retrospective cohort | Not reported |
| Yu et al. (61) | 2010 | China | Chinese | GKT-25 M | 20 | 54.0 | 0.365 | Patients who were admitted to hospital | Retrospective cohort | Not reported |
| Hirata et al. (19) | 2010 | Japan | English | Wako | 8.9 | 57.3 | 0.433 | Patients with HM | Retrospective cohort | Yes |
| Held et al. (17) | 2010 | Germany | English | Fungitell | 85 | 53.8 | 0.420 | Patients with various underlying diseases | Case-control study | Not reported |
| Acosta et al. (1) | 2011 | Spain | English | Fungitell | 80 | 57.5 | 0.667 | Patients with various underlying diseases | Prospective cohort | Yes |
| Alexander et al. (4) | 2010 | USA | English | Fungitell | 60 | 52.0 | 0.452 | Lung transplant patients | Prospective cohort | Yes |
Since the article by Odabasi et al. included two studies, Odabasi-1 indicates a case-control study and Odabasi-2 indicates a retrospective cohort study.
NA, not available; HIV, human immunodeficiency virus; HM, hematological malignancy; ICU, intensive care unit.
Table 2.
Diagnostic performance for Pneumocystis jiroveci pneumonia according to data extracted from different studies
| Author (reference) | HIV status of patient population (no.)a | True positive | False negative | False positive | True negative |
|---|---|---|---|---|---|
| Yasuoka et al. (59) | HIV positive (7) | 6 | 1 | 0 | 23 |
| Moro et al. (40) | HIV negative (4) | 4 | 0 | 13 | 82 |
| Tasaka et al. (56) | Not available | 53 | 4 | 14 | 87 |
| Akamatsu et al. (2) | HIV negative (2) | 2 | 0 | 26 | 130 |
| Persat et al. (48) | HIV positive (16), HIV negative (4) | 20 | 0 | 39 | 123 |
| Obayashi et al. (44) | Not available | 6 | 0 | 9 | 98 |
| Watanabe et al. (57) | HIV positive (111) | 105 | 6 | 51 | 371 |
| Desmet et al. (12) | HIV positive (8), HIV negative (6) | 14 | 0 | 3 | 25 |
| Koo et al. (27) | HIV negative (14) | 13 | 1 | 124 | 635 |
| Yu et al. (61) | Not available | 2 | 0 | 32 | 72 |
| Held et al. (17) | Not available | 45 | 1 | 3 | 47 |
| Acosta et al. (1) | HIV positive (3) | 3 | 0 | 7 | 31 |
HIV, human immunodeficiency virus.
Table 3.
Diagnostic performance for invasive fungal infection according to data extracted from different studies
| Author (reference) | Reference standardb | True positive | False negative | False positive | True negative |
|---|---|---|---|---|---|
| Obayashi et al. (43) | Autopsy, microbiologically documented | 37 | 4 | 0 | 153 |
| Mitsutake et al. (38) | Microbiological culture from blood or sterile material or autopsy | 32 | 5 | 0 | 30 |
| Moro et al. (40) | Similar criteria (Japanese guideline) | 7 | 0 | 13 | 82 |
| Odabasi-1 et al.a (45) | Microbiological culture from blood | 29 | 1 | 2 | 28 |
| Odabasi-2 et al.a (45) | EORTC/MSG | 15 | 0 | 10 | 220 |
| Kondori et al. (26) | Microbiological culture from blood or sterile material | 14 | 0 | 0 | 19 |
| Kawazu et al. (24) | EORTC/MSG | 6 | 5 | 2 | 123 |
| Horiguchi (20) | EORTC/MSG | 7 | 1 | 9 | 52 |
| Pickering et al. (49) | Histopathologic examination or blood culture | 15 | 1 | 16 | 44 |
| Pazos et al. (47) | EORTC/MSG | 7 | 1 | 3 | 26 |
| Ostrosky-Zeichner et al. (46) | EORTC/MSG | 95 | 22 | 22 | 148 |
| Yoshida et al. (60) | Similar criteria (Japanese guideline) | 11 | 1 | 14 | 81 |
| Fujita et al. (14) | Microbiological culture from blood | 72 | 4 | 28 | 147 |
| Akamatsu et al. (2) | EORTC/MSG | 12 | 7 | 26 | 130 |
| Alam et al. (3) | Microbiological culture from blood | 14 | 13 | 0 | 26 |
| Lu et al. (33) | Histopathologic examination or blood culture | 25 | 3 | 5 | 50 |
| Persat et al. (48) | EORTC/MSG | 70 | 26 | 39 | 123 |
| Senn et al. (54) | EORTC/MSG | 20 | 10 | 28 | 85 |
| Obayashi et al. (44) | Autopsy | 39 | 2 | 9 | 98 |
| Presterl et al. (50) | Microbiological culture from blood or sterile material | 12 | 11 | 14 | 44 |
| Koo et al. (27) | EORTC/MSG | 50 | 23 | 124 | 635 |
| Hachem et al. (16) | EORTC/MSG | 29 | 16 | 2 | 18 |
| Lunel et al. (34) | Microbiological culture from blood or sterile material | 16 | 5 | 12 | 18 |
| Zhao et al. (62) | EORTC/MSG | 18 | 4 | 19 | 89 |
| Racil et al. (51) | EORTC/MSG | 8 | 1 | 51 | 35 |
| Leon et al. (29) | Histopathologic examination or microbiological culture from blood or sterile material | 14 | 4 | 105 | 117 |
| Liu et al. (32) | EORTC/MSG | 15 | 5 | 6 | 61 |
| Yu et al. (61) | EORTC/MSG | 5 | 3 | 32 | 72 |
| Hirata et al. (19) | EORTC/MSG | 8 | 2 | 2 | 196 |
| Acosta et al. (1) | EORTC/MSG | 7 | 2 | 7 | 31 |
| Alexander et al. (4) | EORTC/MSG | 8 | 3 | 54 | 5 |
Since the article by Odabasi included two studies, Odabasi-1 indicates a case-control study and Odabasi-2 indicates a retrospective cohort study.
EORTC/MSG, the European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria.
The 36 studies included six assays: 17 used Fungitell (1, 3, 4, 12, 16, 17, 27, 29, 34, 45–51), 9 used the Fungitec G test (2, 20, 26, 33, 43, 44, 57, 59, 60), 6 used the Wako β-glucan test (14, 19, 24, 40, 54, 56), 3 used the GKT-25 M set (32, 61, 62), and 1 used the fungal index (38). All studies but one were based in 1 of 13 countries, with the majority of assays in Japan (n = 13), as well as in the United States (n = 7), China (n = 4), Spain (n = 2), Austria (n = 1), Belgium (n = 1), Czech Republic (n = 1), France (n = 1), Germany (n = 1), Kuwait (n = 1), The Netherlands (n = 1), Sweden (n = 1), and Switzerland (n = 1); one study that performed assays in multiple countries (Spain, Argentina, and France) was also included. Prophylactic antifungal therapy was used in 12 studies.
Assessment of study quality.
Table 4 presents the results of the quality assessment. All studies used acceptable reference standard and avoided differential verification bias. Incorporation bias was avoided in 97% (35 of 36) of the studies. Most studies did not report whether the interpreters of BG results were blinded to the final diagnosis and vice versa. We therefore conducted stratification based only on the representative spectrum item among preplanned subgroup analyses. A cohort design rather than a case-control design was used by 47% (17 of 36) of the studies (1, 2, 4, 16, 19, 20, 24, 29, 32, 34, 45, 47, 50, 51, 54, 61, 62). Characteristics of enrolled patients were fully described in 64% (23 of 36) of the studies (1, 2, 4, 12, 14, 16, 17, 19, 20, 24, 26, 27, 29, 32–34, 40, 46, 47, 54, 56, 61, 62). While 55% (17 of 31) of the studies used the EORTC/MSG criteria as the reference standard for IFI, 12 studies used microbiological culture from blood or sterile material, autopsy, or histopathological examination which consisted of the proven presence of IFI according to the EORTC/MSG criteria. Enrollment was prospective in 28% (10 of 36) of the studies (1, 2, 4, 16, 20, 24, 29, 47, 54, 62).
Table 4.
Results of the risk of bias assessment per studya
| Author (reference) | Representative spectrum | Acceptable reference standard | Acceptable delay between tests | Partial verification avoided | Differential verification avoided | Incorporation avoided | Index test results blinded | Reference standard results blinded | Relevant clinical information | Uninterpretable results reported | Withdrawals reported |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obayashi et al. (43) | No | Yes | Unclear | Unclear | Yes | Yes | Unclear | Unclear | No | Unclear | Unclear |
| Yasuoka et al. (59) | No | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | No | Yes | Yes |
| Mitsutake et al. (38) | No | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | No | Yes | Yes |
| Moro et al. (40) | No | Yes | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear |
| Odabasi-1 et al. (45) | No | Yes | Unclear | Unclear | Yes | Yes | Yes | Unclear | No | Yes | Yes |
| Odabasi-2 et al. (45) | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Unclear | No | Unclear | Yes |
| Kondori et al. (26) | No | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Kawazu et al. (24) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear |
| Horiguchi (20) | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Pickering et al. (49) | No | Yes | Unclear | No | Yes | Yes | Unclear | Unclear | No | Yes | No |
| Pazos et al. (47) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Ostrosky-Zeichner et al. (46) | No | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes |
| Yoshida et al. (60) | No | Yes | Unclear | No | Yes | Yes | Yes | Unclear | No | Yes | Yes |
| Fujita et al. (14) | No | Yes | Unclear | No | Yes | Yes | Unclear | Unclear | Yes | Unclear | No |
| Tasaka et al. (56) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | No | No |
| Akamatsu et al. (2) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear |
| Alam et al. (3) | No | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | No | Yes | Unclear |
| Lu et al. (33) | No | Yes | Unclear | No | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear |
| Persat et al. (48) | No | Yes | Unclear | Unclear | Yes | Yes | Unclear | Unclear | No | Yes | Yes |
| Senn et al. (54) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Obayashi et al. (44) | No | Yes | Yes | No | Yes | Yes | Unclear | Unclear | No | Yes | Yes |
| Watanabe et al. (57) | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | No | Yes | Yes |
| Desmet et al. (12) | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Presterl et al. (50) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | No | No | No |
| Koo et al. (27) | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Hachem et al. (16) | Yes | Yes | Yes | UNclear | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear |
| Lunel et al. (34) | Yes | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Zhao et al. (62) | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Racil et al. (51) | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear | Unclear | No | Unclear | Unclear |
| Leon et al. (29) | Yes | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes |
| Liu et al. (32) | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Yu et al. (61) | Yes | Yes | Unclear | Unclear | Yes | No | Unclear | Unclear | Yes | Unclear | Unclear |
| Hirata et al. (19) | Yes | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes |
| Held et al. (17) | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Unclear | Yes |
| Acosta et al. (1) | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes |
| Alexander et al. (4) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Yes |
Yes indicates no bias; No indicates potential bias; Unclear indicates bias unclear.
Diagnostic accuracy for PJP.
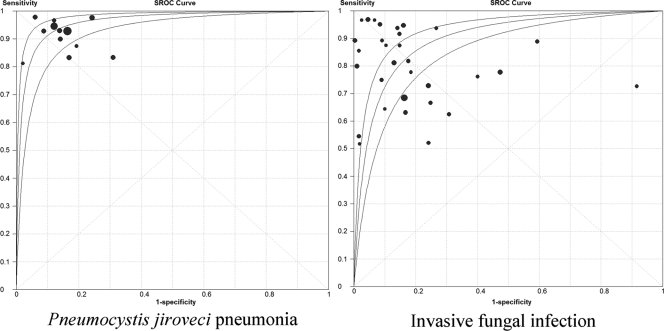
Table 5 shows the pooled analysis findings for sensitivity, specificity, DOR, and AUC-SROC of the BG assay for PJP and IFI. The pooled findings for PJP showed sensitivity of 96% (95% CI, 92% to 98%), specificity of 84% (95% CI, 83% to 86%), DOR of 102.3 (95% CI, 59.2 to 176.6), and AUC-SROC of 0.96 (95% CI, 0.94 to 0.99). The DOR was not heterogeneous (Q = 7.24; P = 0.77; I2 = 0%). The SROC curve is shown in Fig. 2.
Table 5.
Results of meta-analyses for diagnostic accuracy of PJP and IFIa with serum 1,3-β-d-glucan
| Test | No. of studies | % sensitivity (95% CI) | % specificity (95% CI) % | Diagnostic odds ratio (95% CI) | AUC-SROC (95% CI) |
|---|---|---|---|---|---|
| PJP | |||||
| All data | 12 | 96 (92–98) | 84 (83–86) | 102.3 (59.2–176.6) | 0.96 (0.94–0.99) |
| Healthy control excluded | 12 | 96 (92–98) | 84 (82–86) | 99.8 (57.8–172.4) | 0.96 (0.94–0.99) |
| Effect of HIV status | |||||
| HIV-positive patients | 5 | 95 (90–98) | 85 (82–88) | 117.3 (55.0–250.4) | 0.97 (0.95–0.99) |
| HIV-negative patients | 5 | 97 (83–100) | 83 (81–85) | 50.3 (15.0–169.4) | 0.93 (0.80–1.00) |
| Effect of study design | |||||
| Cohort study | 3 | 100 (59–100) | 78 (73–83) | 20.1 (3.4–117.8) | 0.91 (0.74–1.00) |
| Case-control study | 9 | 95 (92–98) | 85 (84–87) | 121.4 (68.4–215.6) | 0.97 (0.95–0.99) |
| Effect of assay type | |||||
| Fungitell | 5 | 98 (93–100) | 83 (81–85) | 139.2 (44.5–435.5) | 0.96 (0.88–1.00) |
| Fungitec G test | 4 | 94 (89–98) | 88 (85–90) | 117.8 (53.8–258.3) | 0.97 (0.94–0.99) |
| Only paper in English included | 10 | 95 (92–98) | 85 (83–87) | 112.8 (64.1–198.4) | 0.96 (0.94–0.99) |
| Effect of methodological quality (representative spectrum) | |||||
| Yes (no bias) | 8 | 95 (92–98) | 84 (83–86) | 100.5 (55.9–180.6) | 0.86 (0.61–1.00) |
| No (potential bias) | 4 | 97 (86–100) | 84 (80–88) | 115.2 (25.6–517.0) | 0.97 (0.93–1.00) |
| IFI | |||||
| All data | 31 | 80 (77–82) | 82 (81–83) | 25.7 (15.0–44.1) | 0.88 (0.82–0.93) |
| Healthy control excluded | 28 | 78 (75–81) | 80 (79–82) | 19.2 (11.0–33.7) | 0.86 (0.81–0.92) |
| Per event excluded | 26 | 80 (77–83) | 82 (80–83) | 25.3 (14.0–45.8) | 0.90 (0.86–0.95) |
| Possible IFI in control excluded | 28 | 81 (78–83) | 82 (81–84) | 30.7 (16.4–57.5) | 0.89 (0.83–0.94) |
| Effect of study design | |||||
| Cohort study | 17 | 72 (67–77) | 78 (76–80) | 12.3 (6.0–25.1) | 0.79 (0.73–0.85) |
| Case-control study | 14 | 83 (80–86) | 86 (84–88) | 68.3 (30.7–151.8) | 0.95 (0.92–0.98) |
| Effect of reference standard | |||||
| EORTC/MSG | 17 | 77 (70–78) | 83 (81–84) | 15.2 (8.5–27.3) | 0.80 (0.73–0.86) |
| Similar criteria | 14 | 86 (82–90) | 81 (79–83) | 61.4 (20.3–185.5) | 0.95 (0.90–0.99) |
| Effect of assay type | |||||
| Fungitell | 15 | 75 (71–79) | 77 (75–79) | 12.0 (6.1–23.7) | 0.86 (0.79–0.93) |
| Fungitec G test | 7 | 89 (83–93) | 90 (88–92) | 100.7 (23.2–437.6) | 0.96 (0.96–1.00) |
| Wako | 5 | 84 (77–90) | 90 (87–92) | 60.1 (11.2–321.3) | 0.94 (0.86–1.00) |
| Effect of kind of mycosis | |||||
| Candidiasis | 19 | 81 (77–85) | 81 (80–83) | 25.7 (12.9–51.2) | 0.90 (0.85–0.95) |
| Aspergillosis | 17 | 77 (71–82) | 83 (82–85) | 23.2 (9.9–54.4) | 0.86 (0.77–0.94) |
| Effect of antifungal therapy | 12 | 81 (77–85) | 84 (82–85) | 25.9 (10.0–66.8) | 0.87 (0.78–0.96) |
| Only papers in English included | 23 | 79 (76–82) | 83 (82–84) | 26.8 (13.9–51.5) | 0.87 (0.80–0.95) |
| Effect of methodological quality (representative spectrum) | |||||
| Yes (no bias) | 18 | 71 (66–76) | 80 (78–81) | 11.8 (6.4–21.9) | 0.78 (0.71–0.85) |
| No (potential bias) | 13 | 85 (82–88) | 87 (85–89) | 89.3 (36.4–219.1) | 0.96 (0.93–0.98) |
PJP, Pneumocystis jiroveci pneumonia; HIV, human immunodeficiency virus; IFI, invasive fungal infection; EORTC/MSG, the European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria.
Fig 2.
Summary receiver operating characteristic (SROC) curves for Pneumocystis jiroveci pneumonia and invasive fungal infection. Individual study estimates of sensitivity and 1 − specificity are represented by the circles. Circle sizes are proportional to study weights; however, sizes are not to scale. The lateral lines represent 95% confidence intervals.
Of the 12 included studies for PJP, 5 studies for patients with HIV (1, 12, 48, 57, 59) and 5 studies for HIV-negative patients (2, 12, 27, 40, 48) provided specific data for the diagnostic accuracy of the BG assay. The diagnostic accuracy was not significantly different between HIV patients and HIV-negative patients (Table 5).
Stratification based on findings of the brand-name assay, study design, or QUADAS item (representative spectrum) did not produce statistically significant differences in the accuracy of the BG assay, either. When we excluded studies including healthy controls or blood donors, no statistically significant differences in the accuracy of the BG assay were noted. Even after the exclusion of studies written in languages other than English, the results did not change significantly. In the result of multivariable metaregression, DOR was not significantly influenced by language, the brand-name assay, study design, age, or sex.
Diagnostic accuracy for IFI.
The pooled findings for IFI showed sensitivity of 80% (95% CI, 77% to 82%), specificity of 82% (95% CI, 81% to 83%), DOR of 25.7 (95% CI, 15.0 to 44.1), and AUC-SROC of 0.88 (95% CI, 0.82 to 0.93) (Table 5). The DOR was significantly heterogeneous (Q = 144.33; P < 0.001; I2 = 79%). The SROC curve is shown in Fig. 2.
In 17 cohort studies (1, 2, 4, 16, 19, 20, 24, 29, 30, 34, 45, 47, 50, 51, 54, 61, 62), the pooled sensitivity, specificity, DOR, and AUC-SROC were 72% (95% CI, 67% to 77%), 78% (95% CI, 76% to 80%), 12.3 (95% CI, 6.0 to 25.1), and 0.79 (95% CI, 0.73 to 0.85), respectively. In 14 case-control studies (3, 14, 26, 27, 33, 38, 40, 43–46, 48, 49, 60), the pooled sensitivity, specificity, DOR, and AUC-SROC for IFI were 83% (95% CI, 80% to 86%), 86% (95% CI, 84% to 88%), 68.3 (95% CI, 30.7 to 151.8), and 0.95 (95% CI, 0.92 to 0.98), respectively. AUC-SROC was significantly lower in cohort studies than in case-control studies (P < 0.001) (Table 5). This difference was attributable to both significantly low sensitivity and significantly low specificity in the cohort study. When we conducted subgroup analysis for a reference standard, AUC-SROC of EORTC/MSG criteria and similar criteria were 0.80 (95% CI, 0.73 to 0.86) and 0.95 (95% CI, 0.90 to 0.99). The diagnostic accuracy was significantly lower in EORTC/MSG criteria than in similar criteria. Because the pooled specificity was similar, the lower diagnostic accuracy of EORTC/MSG criteria was mainly attributable to lower sensitivity. When stratified analysis was conducted based on findings of the brand-name assay, AUC-SROC of Fungitell, Fungitec G test, and Wako were 0.86 (95% CI, 0.79 to 0.93), 0.96 (95% CI, 9.96 to 1.00), and 0.94 (95% CI, 0.86 to 1.00). Fungitell had an accuracy statistically lower than that of the Fungitec G test or Wako. Fungitell was lower in both sensitivity and specificity. Subgroup analysis within the QUADAS item (representative spectrum) showed lower accuracy in the no-bias group than in the potential-bias group.
Of the 31 included studies for IFI, 19 studies for invasive candidiasis (2–4, 14, 16, 19, 26, 27, 29, 34, 38, 40, 44–46, 48, 49, 61) and 17 studies for invasive aspergillosis (1, 2, 4, 16, 19, 20, 24, 27, 38, 40, 44–48, 51, 61) provided specific data for the diagnostic accuracy of the BG assay. When we conducted subgroup analysis for underlying disease, the pooled findings for invasive candidiasis showed sensitivity of 81% (95% CI, 77% to 85%), specificity of 81% (95% CI, 80% to 83%), DOR of 25.7 (95% CI, 12.9 to 51.2), and AUC-SROC of 0.90 (95% CI, 0.85 to 0.95). The pooled findings for invasive aspergillosis showed sensitivity of 77% (95% CI, 71% to 82%), specificity of 83% (95% CI, 82% to 85%), DOR of 23.2 (95% CI, 9.9 to 54.4), and AUC-SROC of 0.86 (95% CI, 0.77 to 0.94) (Table 5). The diagnostic accuracies for invasive candidiasis and invasive aspergillosis were not significantly different.
When we excluded studies including healthy controls, studies in which diagnostic performance was analyzed per episode and not per patient, or studies including possible IFI in control population, no statistically significant differences in the accuracy of the BG assay were noted. Even after the exclusion of studies written in languages other than English and studies in which prophylactic antifungal therapy was not reported or used, the results did not change significantly. In the multivariable metaregression results, DOR was not significantly influenced by language, reference standard, age, or sex. Although we performed sensitivity analysis, stratified analysis, and metaregression based on various factors to determine the source of this majority of heterogeneity among the studies, heterogeneity was still significant.
Publication bias.
The appearance of funnel plots was asymmetrical, and Egger's test results were significant (P = 0.01). This suggested that publication bias may be present. After exclusion of the four studies that included 50 or fewer participants, differences in the accuracy of the BG assay findings were not statistically significant.
DISCUSSION
Our meta-analysis to examine the diagnostic accuracy of the serum BG assay for PJP and IFI has resulted in several significant findings.
First, the BG assay showed the high AUC-SROC for PJP with no heterogeneity. Moreover, the BG assay is not an invasive test. The gold standard for diagnosis is microscopic visualization of the organism, and bronchoalveolar lavage fluid, sputum, or tissue is necessary for diagnosis. Because patients with PJP tend to present with nonproductive or minimally productive cough, sputum is often insufficient for diagnosis and an invasive procedure such as bronchoscopy is needed. Noninvasive BG assay is useful as a screen to avoid unnecessary invasive procedures.
Second, we showed that the BG assays have high sensitivity for PJP. The BG assay can therefore be used as a screening tool. If any of the BG assays are negative, PJP can be ruled out and unnecessary procedures or treatments for PJP can be avoided.
Third, the pooled specificity was moderate for PJP because the BG assay could be positive for various fungal infections and the presence of factors such as use of intravenous amoxicillin-clavulanic acid, treatment of patients with immunological preparations (albumins or globulins), use of cellulose membranes and filters made from cellulose in hemodialysis, and use of cotton gauze swabs/packs/pads and sponges during surgery (8). If the BG assay is positive, it is important to consider the factor associated with false-positive results and exclude IFI by other modalities or invasive procedures such as computed tomography scan and bronchoscopy.
Fourth, the diagnostic accuracies for HIV patients and HIV-negative patients were not significantly different. Nakamura and colleagues (42) suggested that the detection rate of BG in HIV-negative patients was lower than that in HIV patients. HIV-negative patients usually have significantly fewer organisms in bronchoalveolar lavage fluid than do HIV patients. This can lead to false-negative results, particularly in HIV-negative patients (8). However, our results showed that the BG assay was useful not only in HIV patients but also in HIV-negative patients.
Fifth, the diagnostic accuracy for IFI of the BG assay was moderate, with high statistical heterogeneity. While there are several molecular and serological assays for the diagnosis of specific types of IFI, which can be detected by means of galactomannan, mannan, and DNA sequences (36), only the BG assay can be used for various fungal infections. For instance, the diagnostic accuracy of the galactomannan assay for invasive aspergillosis is similar to that of BG for IFI. Leeflang and colleagues (28) used a meta-analysis to demonstrate that the galactomannan assay had an overall sensitivity of 78% (95% CI, 61% to 89%) and an overall specificity of 81% (95% CI, 72% to 88%) for proven or probable cases of invasive aspergillosis. However, patients at risk for one type of IFI are often at risk for one or more other types of IFI. Because both the sensitivity and the specificity of the BG assay are moderate for IFI, combination with other modalities and procedures is necessary to either rule out or rule in IFI. Therefore, it is reasonable that the BG assay positivity is used only as a mycological criterion in the revised EORTC/MSG criteria.
The summary results from our overall analysis for IFI are similar to those of the earlier review of Karageorgopoulos and colleagues (sensitivity, 80% vs. 77%; specificity, 82% vs. 85%; AUC-SROC, 0.88 vs. 0.89) (23). However, Karageorgopoulos and colleagues included only 23 studies because of language restrictions and did not stratify their final analysis by study design or reference standard. Our meta-analysis showed that the substantial between-study heterogeneity for IFI resulted from differences in study design, reference standard, brand-name assay, and QUADAS item. We observed that case-control studies seemed to overestimate both sensitivity and specificity (3, 14, 26, 27, 33, 38, 40, 43–46, 48, 49, 60). We also observed that the sensitivity of the EORTC/MSG criteria was lower than that of similar criteria (1, 2, 4, 16, 19, 20, 24, 27, 32, 45–48, 51, 54, 61, 62). Fungitell was statistically lower in accuracy than Fungitec G test or Wako. Subgroup analysis within the QUADAS item (representative spectrum) showed lower accuracy in the no-bias group than in the potential-bias group. Well-designed, high-quality prospective cohort studies on the BG assay for IFI are needed.
Only one study, that by Alexander and colleagues (4), reported low diagnostic accuracy for IFI in lung transplants. These authors suggested that the BG assay might have limited utility as a screening tool for lung transplants. However, further studies are needed to confirm these findings of this study, because the sample size was small.
Our review has several limitations. First, there was evidence of publication bias, so it is possible that our results constitute an overestimation of the performance of the test. However, when we excluded small studies that have a greater tendency to overestimate diagnostic performance, differences in the accuracy of the BG assay still were not statistically significant. It is thus reasonable to conclude that the effect of publication bias was only minor (6).
Second, the quality of our included studies was moderate. The bias in which low-quality studies overestimate test performance has been seen previously in studies of diagnostic tests (30, 53). Although this relationship was found for diagnostic accuracy for IFI, stratification based on findings of the QUADAS item (representative spectrum) for PJP did not produce statistically significant differences in the accuracy of the BG assay.
In conclusion, the diagnostic accuracy of the BG assay is high for PJP and moderate for IFI. Because the sensitivity for PJP is particularly high, the BG assay can be used as a screening tool for PJP.
ACKNOWLEDGMENT
All authors declare no conflict of interest.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Acosta J, et al. 2011. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1→3)-beta-d-glucan chromogenic assay in serum samples. Clin. Microbiol. Infect. 17:1053–1060 [DOI] [PubMed] [Google Scholar]
- 2. Akamatsu N, Sugawara Y, Kaneko J, Tamura S, Makuuchi M. 2007. Preemptive treatment of fungal infection based on plasma (1→3)beta-d-glucan levels after liver transplantation. Infection 35:346–351 [DOI] [PubMed] [Google Scholar]
- 3. Alam FF, Mustafa AS, Khan ZU. 2007. Comparative evaluation of (1, 3)-beta-d-glucan, mannan and anti-mannan antibodies, and candida species-specific snPCR in patients with candidemia. BMC Infect. Dis. 7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB. 2010. The (1,3){beta}-d-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J. Clin. Microbiol. 48:4083–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ascioglu S, et al. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7–14 [DOI] [PubMed] [Google Scholar]
- 6. Avina-Zubieta JA, et al. 2008. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 59:1690–1697 [DOI] [PubMed] [Google Scholar]
- 7. Bossuyt PM, et al. 2003. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 326:41–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carmona EM, Limper AH. 2011. Update on the diagnosis and treatment of pneumocystis pneumonia. Ther. Adv. Respir. Dis. 5:41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cochran WG. 1954. The combination of estimates from different experiments. Biometrics 10:101–129 [Google Scholar]
- 10. Deeks J. 2001. Systematic reviews of evaluations of diagnostic and screening tests, p 248–282 In Egger M, Davey Smith G, Altman D. (ed), Systematic reviews in health care: meta-analysis in context, 2nd ed. BMJ, London, United Kingdom [Google Scholar]
- 11. De Pauw B, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desmet S, et al. 2009. Serum (1–3)-beta-d-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J. Clin. Microbiol. 47:3871–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egger M, Davey Smith G, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujita S, Takamura T, Nagahara M, Hashimoto T. 2006. Evaluation of a newly developed down-flow immunoassay for detection of serum mannan antigens in patients with candidaemia. J. Med. Microbiol. 55:537–543 [DOI] [PubMed] [Google Scholar]
- 15. Fukuda T, et al. 2003. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 102:827–833 [DOI] [PubMed] [Google Scholar]
- 16. Hachem RY, et al. 2009. Utility of galactomannan enzyme immunoassay and (1,3) beta-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J. Clin. Microbiol. 47:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Held J, Koch M, Reischl U, Danner T, Serr A. 2011. Serum (1→3)-beta-d-glucan measurement as early indicator for Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin. Microbiol. Infect. 17:595–602 [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirata Y, et al. 2010. Antifungal prophylaxis with micafungin in neutropenic patients with hematological malignancies. Leuk. Lymphoma 51:853–859 [DOI] [PubMed] [Google Scholar]
- 20. Horiguchi Y. 2004. The performance of (1, 3)-beta-d-glucan and aspergillus galactomannan measurement for early diagnosis of invasive aspergillosis in patients with hematological diseases. Kansenshogaku Zasshi 78:566–573 [DOI] [PubMed] [Google Scholar]
- 21. Irwig L, et al. 1994. Guidelines for meta-analyses evaluating diagnostic tests. Ann. Intern. Med. 120:667–676 [DOI] [PubMed] [Google Scholar]
- 22. Kami M, et al. 2000. Computed tomographic scan of the chest, latex agglutination test and plasma (1-3)-beta-d-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745–752 [PubMed] [Google Scholar]
- 23. Karageorgopoulos DE, et al. 2011. Beta-d-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin. Infect. Dis. 52:750–770 [DOI] [PubMed] [Google Scholar]
- 24. Kawazu M, et al. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-beta-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J. Clin. Microbiol. 42:2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kedzierska A, Kochan P, Pietrzyk A, Kedzierska J. 2007. Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1→3)-beta-d-glucan antigens. Eur. J. Clin. Microbiol. Infect. Dis. 26:755–766 [DOI] [PubMed] [Google Scholar]
- 26. Kondori N, Edebo L, Mattsby-Baltzer I. 2004. Circulating beta (1-3) glucan and immunoglobulin G subclass antibodies to Candida albicans cell wall antigens in patients with systemic candidiasis. Clin. Diagn. Lab. Immunol. 11:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koo S, Bryar JM, Page JH, Baden LR, Marty FM. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650–1659 [DOI] [PubMed] [Google Scholar]
- 28. Leeflang MM, et al. 2008. Galactomannan detection for invasive aspergillosis in immunocompromized patients. Cochrane Database Syst. Rev. 2008:CD007394. [DOI] [PubMed] [Google Scholar]
- 29. Leon C, et al. 2009. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit. Care Med. 37:1624–1633 [DOI] [PubMed] [Google Scholar]
- 30. Lijmer JG, et al. 1999. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 282:1061–1066 [DOI] [PubMed] [Google Scholar]
- 31. Limper AH, Offord KP, Smith TF, Martin WJ., II 1989. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204–1209 [DOI] [PubMed] [Google Scholar]
- 32. Liu F, et al. 2009. Diagnostic value of plasma (1, 3)-beta-d glucan assay for invasive fungal infections in patients with hematological disorders. Zhongguo Shi Yan Xue Ye Xue Za Zhi 17:1043–1046 [PubMed] [Google Scholar]
- 33. Lu PH, Zhao BL, Shi Y, Wen YT. 2007. The diagnostic value of detecting plasma 1, 3-beta-d-glucan for invasive fungal infections. Zhonghua Jie He He Hu Xi Za Zhi 30:31–34 [PubMed] [Google Scholar]
- 34. Lunel FM, et al. 2009. Value of Candida serum markers in patients with invasive candidiasis after myeloablative chemotherapy. Diagn. Microbiol. Infect. Dis. 64:408–415 [DOI] [PubMed] [Google Scholar]
- 35. Mansharamani NG, Garland R, Delaney D, Koziel H. 2000. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 118:704–711 [DOI] [PubMed] [Google Scholar]
- 36. McLintock LA, Jones BL. 2004. Advances in the molecular and serological diagnosis of invasive fungal infection in haemato-oncology patients. Br. J. Haematol. 126:289–297 [DOI] [PubMed] [Google Scholar]
- 37. Midgette AS, Stukel TA, Littenberg B. 1993. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med. Decis. Making 13:253–257 [DOI] [PubMed] [Google Scholar]
- 38. Mitsutake K, et al. 1996. Enolase antigen, mannan antigen, Cand-Tec antigen, and beta-glucan in patients with candidemia. J. Clin. Microbiol. 34:1918–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mori T, et al. 1997. Evaluation of plasma (1→3)-beta-d-glucan measurement by the kinetic turbidimetric limulus test, for the clinical diagnosis of mycotic infections. Eur. J. Clin. Chem. Clin. Biochem. 35:553–560 [PubMed] [Google Scholar]
- 40. Moro H, et al. 2003. Comparison of four diagnostic methods using clinical blood by measuring (1→3)-beta-d-glucan. Kansenshogaku Zasshi 77:227–234 [DOI] [PubMed] [Google Scholar]
- 41. Moses LE, Shapiro D, Littenberg B. 1993. Combining independent studies of a diagnostic test into a summary roc curve: data-analytic approaches and some additional considerations. Stat. Med. 12:1293–1316 [DOI] [PubMed] [Google Scholar]
- 42. Nakamura H, et al. 2009. Clinical utility of serum beta-d-glucan and kl-6 levels in Pneumocystis jirovecii pneumonia. Intern. Med. 48:195–202 [DOI] [PubMed] [Google Scholar]
- 43. Obayashi T, et al. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17–20 [DOI] [PubMed] [Google Scholar]
- 44. Obayashi T, Negishi K, Suzuki T, Funata N. 2008. Reappraisal of the serum (1→3)-beta-d-glucan assay for the diagnosis of invasive fungal infections–a study based on autopsy cases from 6 years. Clin. Infect. Dis. 46:1864–1870 [DOI] [PubMed] [Google Scholar]
- 45. Odabasi Z, et al. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199–205 [DOI] [PubMed] [Google Scholar]
- 46. Ostrosky-Zeichner L, et al. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654–659 [DOI] [PubMed] [Google Scholar]
- 47. Pazos C, Ponton J, Del Palacio A. 2005. Contribution of (1→3)-beta-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 43:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Persat F, et al. 2008. Contribution of the (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 46:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. 2005. Evaluation of a (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Presterl E, et al. 2009. Invasive fungal infections and (1,3)-beta-d-glucan serum concentrations in long-term intensive care patients. Int. J. Infect. Dis. 13:707–712 [DOI] [PubMed] [Google Scholar]
- 51. Racil Z, et al. 2009. Detection of 1,3-beta-d glucan for diagnosis of invasive fungal infections in hematooncological patients: usefulness for screening of invasive mycosis and for confirmation of galactomannan positive results. Klin. Mikrobiol. Infekc. Lek. 15:48–57 [PubMed] [Google Scholar]
- 52. Reitsma JB, et al. 2009. Chapter 9: assessing methodological quality. In Deeks JJ, Bossuyt PM, Gatsonis C. (ed). Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0.0. The Cochrane Collaboration. http://srdta.cochrane.org/
- 53. Rutjes AW, et al. 2006. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Senn L, et al. 2008. 1,3-Beta-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878–885 [DOI] [PubMed] [Google Scholar]
- 55. Sutton A, Jones D, Sheldon T, Song F. 2000. Methods for meta-analysis in medical research. Wiley, London, United Kingdom [Google Scholar]
- 56. Tasaka S, et al. 2007. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest 131:1173–1180 [DOI] [PubMed] [Google Scholar]
- 57. Watanabe T, et al. 2009. Serum (1→3) beta-d-glucan as a noninvasive adjunct marker for the diagnosis of pneumocystis pneumonia in patients with aids. Clin. Infect. Dis. 49:1128–1131 [DOI] [PubMed] [Google Scholar]
- 58. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. 2003. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yasuoka A, Tachikawa N, Shimada K, Kimura S, Oka S. 1996. (1→3) Beta-d-glucan as a quantitative serological marker for Pneumocystis carinii pneumonia. Clin. Diagn. Lab Immunol. 3:197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoshida K, et al. 2006. Clinical usefulness of the (1→3)-beta-d-glucan measurement kit using the improved alkaline pretreatment method—comparison with conventional method. Kansenshogaku Zasshi 80:701–705 [DOI] [PubMed] [Google Scholar]
- 61. Yu J, Li RY, Gao LJ, Lu QY, Wang XH. 2010. Utility of galactomannan enzyme immunoassay and (1,3)beta-d-glucan assay in invasive fungal infection. Zhonghua Yi Xue Za Zhi 90:371–374 [PubMed] [Google Scholar]
- 62. Zhao L, et al. 2009. Value of plasma beta-glucan in early diagnosis of invasive fungal infection in children. Zhongguo Dang Dai Er Ke Za Zhi 11:905–908 [PubMed] [Google Scholar]