Abstract
Capnocytophaga species are known commensals of the oral cavity of humans and animals (mainly dogs and cats) and are a rare cause of respiratory tract infections. We report a case of cavitary lung abscess caused by a Capnocytophaga species in a patient with a metastatic neuroendocrine tumor.
CASE REPORT
A 39-year-old man with a metastatic, well-differentiated neuroendocrine tumor presented with fever and productive cough for 2 weeks. The patient's primary tumor was a large right infrahilar lung mass causing obstruction of the bronchus intermedius and extended to the right-middle-lobe (RML) and right-lower-lobe (RLL) bronchi. He also had multiple bilobar liver metastases. He had been treated with monthly octreotide injections for symptom control of carcinoid syndrome (hormone hypersecretion), which often occurs in metastatic neuroendocrine tumors. He had also recently been on chemotherapy with oral capecitabine and temozolomide.
Two weeks after planned right hepatic artery embolization for liver metastasis and symptom control, he experienced low-grade fevers with cough productive of foul-smelling melanoptysis, night sweats, malaise, and weight loss. The symptoms did not respond to a brief course of azithromycin. A computed tomograph (CT) of the chest revealed a new 8.7- by 6.4-cm cavitary abscess in the right lung. He was empirically treated with oral clindamycin for presumed aspiration pneumonia and anaerobic coverage but was admitted 2 days later for progressive symptoms.
His medical history included three episodes of pneumonia that predated the cancer diagnosis, heavy marijuana use for 20 years (he had quit 1 month prior to his current admission), and occasional alcohol use; he denied cigarette smoking. He had four cats at home and denied recent travel, sick contacts, exposure to tuberculosis, or prior incarceration; he worked as a salesman for a waste transfer facility.
On admission, he was febrile (38.4°C) and tachycardic without acute distress. Physical examination revealed an ill-appearing young man with normal dentition; a chest exam was remarkable for decreased breath sounds at the right base, egophony, and dullness to percussion on the right. Laboratory values showed an elevated white blood cell (WBC) count of 24,800/μl (neutrophils, 87%), thrombocytosis of 878,000/μl (normal, 160,000 to 400,000), mild hyponatremia (132 meq of sodium/liter; normal, 136 to 144), a low albumin level of 2.9 g/dl, and a high international normalized ratio (INR) of 1.83 (normal, 0.85 to 1.17). A chest radiograph obtained at admission and a repeat chest CT revealed an increase in the size of the right lung abscess to 10.2 by 8.3 cm (Fig. 1A and B). The patient was started on intravenous antibiotics, including piperacillin-tazobactam and vancomycin. A purified protein derivative (PPD) was placed and was negative; sputum samples were also negative for acid-fast bacilli (AFB) on smears, and mycobacterial culture remained negative after 42 days. Blood cultures were negative after 5 days of incubation. Serum Aspergillus galactomannan antigen, β-d glucan, and Legionella urinary antigen assays were all negative. A vasculitis panel, including p-ANCA (perinuclear antineutrophil cytoplasmic antibodies) and c-ANCA (cytoplasmic antineutrophil cytoplasmic antibodies), was negative. On hospital day 2, the patient underwent drainage of the lung abscess with CT-guided placement of a pigtail catheter, and 150 ml of a thick, tan-colored fluid was aspirated. The abscess fluid was negative for AFB on smear and culture, and the initial Gram stain showed 4+ (on a scale where 1+ represents the least and 4+ represents the most) polymorphonuclear cells, 3+ Gram-positive cocci in chains and 3+ Gram-negative rods. After 72 h, culture of the lung abscess fluid grew 4+ yellow-tan colonies with gliding motility on a sheep blood agar (SBA) plate. Gram stain of these colonies revealed thin, fusiform, Gram-negative bacilli (Fig. 2); no other organisms were recovered in culture. The Gram-negative bacillus was identified as Capnocytophaga species (99.99%) using the RapID ANA II system (Remel, Lenexa, KS) and further classified as human Capnocytophaga species based on negative oxidase and catalase tests (feline and canine species are oxidase and catalase positive). In an attempt to identify the organism to species level, additional biochemical reactions were set up, including nitrate reduction (negative), esculin hydrolysis (positive), ortho-nitrophenyl-β-d-galactopyranoside (ONPG) hydrolysis (positive), and gelatin hydrolysis (negative). Based on these reactions, three human Capnocytophaga species could be excluded—C. gingivalis (negative for all reactions), C. granulosa (esculin negative), and C. haemolytica (nitrate positive)—leaving two potential species, C. ochracea and C. sputigena. The organism tested positive for β-lactamase production by the cefinase disc test.
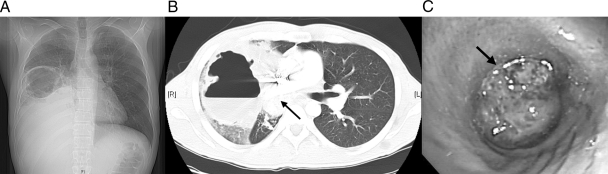
Fig 1.
Chest X-ray (A) and CT scan (B) done at admission, showing a large, irregular air and fluid intraparenchymal collection up to 10.2 by 8.3 cm and obliteration of the bronchus intermedius by a calcified mass (arrow) with increased necrosis and collapse of the RML and RLL. (C) Initial bronchoscopy showing complete obstruction of bronchus intermedius by the tumor (arrow).
Fig 2.
Gram-negative rods identified as human Capnocytophaga species (oil immersion). Magnification, ×1,000.
The patient defervesced with prompt resolution of leukocytosis and clinically improved after a CT-guided drainage catheter was placed into the abscess. The antibiotic regimen was changed to ampicillin/sulbactam to allow him to complete six more weeks as an outpatient. He was then switched to piperacillin-tazobactam via a pump for easier home dosing for two more weeks (total IV antibiotic therapy of 9 weeks). The pigtail catheter was removed 4 weeks after placement. Rigid bronchoscopy at the end of his antibiotic course confirmed complete obstruction of the bronchus intermedius (Fig. 1C), which was palliated via mechanical debulking and laser ablation, to achieve partial patency and regain both RML and RLL ventilation. Therapeutic aspiration of purulent secretions during this procedure grew pharyngeal flora with no evidence of Capnocytophaga species, and bronchoscopic biopsy confirmed typical carcinoid.
The patient continued to improve with marked resolution of the abscess on follow-up chest CT as early as 6 weeks after presentation. His metastatic carcinoid showed clinical and radiologic response after a left hepatic artery embolization, systemic chemotherapy, and maintenance monthly octreotide. He required two additional palliative rigid bronchoscopies to gain maximal patency of the bronchus intermedius, RML, and RLL with concurrent negative bronchial cultures and continued improvement on chest CT (Fig. 3). He has returned to work, is asymptomatic, and is doing well.
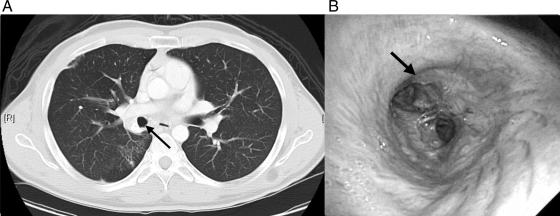
Fig 3.
(A) Chest CT 10 months after presentation. Interval clearing of dense RLL consolidation and the decreased size of the partially calcified mass in the right hilum can be seen. The patent bronchus intermedius (arrow) was viewed by CT (A) and follow-up bronchoscopy (B) after laser ablation and mechanical debulking.
The genus Capnocytophaga is a group of long, thin, fusiform, slowly growing, facultatively anaerobic, Gram-negative rods with gliding motility whose growth is optimal in a CO2-enriched atmosphere; hence the name Capnocytophaga (consumption of CO2) (10). Capnocytophaga species are part of the normal oral flora in dogs, cats, and humans and were once associated with periodontal disease but are now considered commensals in dental plaque of humans (10). In immunocompetent patients, respiratory tract infections occur due to secretions from the oral cavity and are usually polymicrobial (5, 17). In immunocompromised hosts with neutropenia or oral ulcerations, Capnocytophaga species can cause severe systemic infections and even death (10, 17).
Capnocytophaga infections can have varied clinical presentations, such as periodontal disease (9, 22), respiratory tract infections (3), ophthalmic lesions (1, 6, 18), traumatic pericarditis, mediastinal abscess (16), brain abscess (21), meningitis (12, 20), and peritonitis (10, 15). The species colonizing the human oral tract are C. ochracea, C. sputigena, C. gingivalis, C. haemolytica, and C. granulosa (10). Patients with compromised oral mucosa, including those undergoing intensive chemotherapy for the treatment of cancer, can develop septicemia, which has sometimes led to multiorgan failure and death (2, 3, 8), endocarditis (4), pyonephrosis (23), osteomyelitis, and septic arthritis (10, 24). Significant risk factors include splenectomy and alcoholism (10). Isolation of this organism from lung abscesses is rare; we found only one other case report in the English-language literature (17). The patient was a 66-year-old man with lung cancer who developed the infection after at least 3 days of hospitalization, was initially treated with cefamandole, and was then treated with drainage, pneumonectomy, cephalothin, and tobramycin and recovered; no further details are provided. Like our patient, he had underlying lung cancer, was not immunosuppressed, and recovered after drainage and antibiotic treatment.
The species that colonize the saliva of dogs and cats are C. canimorsus and C. cynodegmi, and they have been found to cause disease after pet contact (mostly cellulitis) and severe sepsis after dog bites, especially in splenectomized or alcoholic patients (10).
Capnocytophaga species may be difficult to isolate. Most isolates require more than 2 days to show growth under anaerobic conditions; in our case, the culture revealed growth after 3 days of incubation. Drugs of choice for treatment of severe infections include a penicillin–β-lactamase inhibitor combination, imipenem, or broad-spectrum cephalosporin, such as ceftriaxone, ceftazidime, or cefepime. Milder infections may be treated with oral clindamycin, doxycycline, or a fluoroquinolone. Beta-lactamase-producing strains may be more resistant to cephalosporins and should ideally be treated with a penicillin–β-lactamase inhibitor combination or imipenem (19). The frequency of β-lactamase production has varied depending on the study but appears to be increasing (10). A large survey of the oral flora of hospitalized pediatric oncology patients in France showed that 70% of 440 Capnocytophaga isolates produced β-lactamase (11). Smaller series have shown a frequency of β-lactamase production of 75% (18 of 24 bloodstream isolates from febrile neutropenic patients in France), 30% (of periodontal isolates from periodontitis patients in Spain), or 32% (6 of 19 mostly clinical isolates in Canada) (14, 13, 19). Our Capnocytophaga isolate was β-lactamase positive, and our patient was treated with ampicillin-sulbactam, followed by piperacillin-tazobactam. Vancomycin and metronidazole have no activity against Capnocytophaga species. A case of infection with fluoroquinolone-resistant C. gingivalis has been reported (7).
Our patient was not neutropenic and likely developed the cavitary lung abscess after aspiration of oral flora via the RUL bronchus during moderate sedation for his right hepatic artery embolization procedure. He was unlikely to clear the aspirate given the high-grade obstruction of the bronchus intermedius (Fig. 1C). Exposure to his cats at home was likely unrelated, since his particular Capnocytophaga species is not present in feline saliva.
Capnocytophaga species are unusual opportunistic pathogens that are a rare cause of lung abscess; we report the second case in the English-language literature (17). Our case is remarkable for the sheer size of the lung abscess, which occurred in the setting of airway obstruction, and the rapidity of resolution after drainage and IV antibiotics with minimal residual parenchymal damage. Clinicians treating patients with cancer should be aware that β-lactamase-producing organisms are becoming increasingly common and empirical coverage with vancomycin, cephalosporins, or fluoroquinolones may not be adequate in this setting (10, 19). Prompt diagnosis and appropriate treatment are imperative in the management of these infections, as the organism is capable of causing sepsis, multiorgan failure, and death in certain patient populations, such as immunosuppressed patients undergoing treatment for cancer.
(This case was presented in part as a poster at the New York State Thoracic Society Annual Scientific Assembly, West Point, NY, 2010.)
ACKNOWLEDGMENT
We thank Paul Albert, Digital Services Librarian of Weill Cornell Medical College, for assistance with image formatting.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Alexandrakis G, Palma LA, Miller D, Alfonso EC. 2000. Capnocytophaga keratitis. Ophthalmology 107:1503–1506 [DOI] [PubMed] [Google Scholar]
- 2. Bilgrami S, et al. 1992. Capnocytophaga bacteremia in a patient with Hodgkin's disease following bone marrow transplantation: case report and review. Clin. Infect. Dis. 14:1045–1049 [DOI] [PubMed] [Google Scholar]
- 3. Bonatti H, et al. 2003. A series of infections due to Capnocytophaga spp in immunosuppressed and immunocompetent patients. Clin. Microbiol. Infect. 9:380–387 [DOI] [PubMed] [Google Scholar]
- 4. Buu-Hoi AY, Joundy S, Acar JF. 1988. Endocarditis caused by Capnocytophaga ochracea. J. Clin. Microbiol. 26:1061–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell JR, Edwards MS. 1991. Capnocytophaga species infections in children. Pediatr. Infect. Dis. J. 10:944–948 [DOI] [PubMed] [Google Scholar]
- 6. Chodosh J. 2001. Cat's tooth keratitis: human corneal infection with Capnocytophaga canimorsus. Cornea 20:661–663 [DOI] [PubMed] [Google Scholar]
- 7. Geisler WM, Malhotra U, Stamm WE. 2001. Pneumonia and sepsis due to fluoroquinolone-resistant Capnocytophaga gingivalis after autologous stem cell transplantation. Bone Marrow Transplant. 28:1171–1173 [DOI] [PubMed] [Google Scholar]
- 8. Gomez-Garces JL, Alos JI, Sanchez J, Cogollos R. 1994. Bacteremia by multidrug-resistant Capnocytophaga sputigena. J. Clin. Microbiol. 32:1067–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Handal T, et al. 2005. Chromosome- and plasmid-encoded beta-lactamases in Capnocytophaga spp. Antimicrob. Agents Chemother. 49:3940–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z, Bonnaure-Mallet M. 2007. Antimicrobial treatment of Capnocytophaga infections. Int. J. Antimicrob. Agents 29:367–373 [DOI] [PubMed] [Google Scholar]
- 11. Jolivet-Gougeon A, et al. 2005. Prevalence of oropharyngeal beta-lactamase-producing Capnocytophaga spp. in pediatric oncology patients over a ten-year period. BMC Infect. Dis. 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. 2003. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin. Infect. Dis. 36:e42–e46 [DOI] [PubMed] [Google Scholar]
- 13. Maestre JR, et al. 2007. Odontogenic bacteria in periodontal disease and resistance patterns to common antibiotics used as treatment and prophylaxis in odontology in Spain. Rev. Esp. Quimioter. 20:61–67 [PubMed] [Google Scholar]
- 14. Maury S, et al. 1999. Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta-lactamase-producing strains. Clin. Infect. Dis. 28:1172–1174 [DOI] [PubMed] [Google Scholar]
- 15. Mortensen JE, LeMaistre A, Moore DG, Robinson A. 1985. Peritonitis involving Capnocytophaga ochracea. Diagn. Microbiol. Infect. Dis. 3:359–362 [DOI] [PubMed] [Google Scholar]
- 16. Mosher CB, Corp R. 1986. Mediastinal abscess with Capnocytophaga spp. in a competent host. J. Clin. Microbiol. 24:161–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parenti DM, Snydman DR. 1985. Capnocytophaga species: Infections in nonimmunocompromised and immunocompromised hosts. J. Infect. Dis. 151:140–147 [DOI] [PubMed] [Google Scholar]
- 18. Phipps SE, Tamblyn DM, Badenoch PR. 2002. Capnocytophaga canimorsus endophthalmitis following cataract surgery. Clin. Experiment. Ophthalmol. 30:375–377 [DOI] [PubMed] [Google Scholar]
- 19. Roscoe DL, Zemcov SJ, Thornber D, Wise R, Clarke AM. 1992. Antimicrobial susceptibilities and β-lactamase characterization of Capnocytophaga species. Antimicrob. Agents Chemother. 36:2197–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenman JR, Reynolds JK, Kleiman MB. 2003. Capnocytophaga canimorsus meningitis in a newborn: an avoidable infection. Pediatr. Infect. Dis. J. 22:204–205 [PubMed] [Google Scholar]
- 21. Sabbatani S, Manfredi R, Frank G, Chiodo F. 2004. Capnocytophaga spp. brain abscess in an immunocompetent host: problems in antimicrobial chemotherapy and literature review. J. Chemother. 16:497–501 [DOI] [PubMed] [Google Scholar]
- 22. Sixou JL, de Medeiros-Batista O, Bonnaure-Mallet M. 1996. Modifications of the microflora of the oral cavity arising during immunosuppressive chemotherapy. Eur. J. Cancer B. Oral Oncol. 32B:306–310 [DOI] [PubMed] [Google Scholar]
- 23. Tay JS, Chusid MJ, Dunne WM., Jr 1985. Capnocytophaga ochracea pyonephrosis in an infant with obstructive nephropathy. Pediatr. Infect. Dis. 4:555–556 [DOI] [PubMed] [Google Scholar]
- 24. Winn RE, Chase WF, Lauderdale PW, McCleskey FK. 1984. Septic arthritis involving Capnocytophaga ochracea. J. Clin. Microbiol. 19:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]