Abstract
Mycobacterium tuberculosis is a global public health concern, particularly with the emergence of drug-resistant strains. Immediate identification of drug-resistant strains is crucial to administering appropriate treatment before the bacteria are allowed to spread. However, developing countries, which are most affected by drug resistance, are struggling to combat the disease without the facilities or funds for expensive diagnostics. Recent studies have emphasized the suitability of isothermal microcalorimetry (IMC) for the rapid detection of mycobacteria. In this study, we investigate its suitability for rapid and reliable M. tuberculosis drug susceptibility testing. Specifically, IMC was used to determine the MICs of three drugs, namely, isoniazid, ethambutol, and moxifloxacin, against three mycobacteria, namely, Mycobacterium smegmatis, Mycobacterium avium, and Mycobacterium tuberculosis. The Richards growth model was used to calculate growth parameters, namely, the maximum bacterial growth rate and the lag phase duration from integrated heat flow-versus-time results. For example, MICs of isoniazid, ethambutol, and moxifloxacin were determined to be 1.00, 8.00, and 0.25 μg/ml, respectively. IMC, as described here, could be used not just in industrialized countries but also in developing countries because inexpensive and sensitive microcalorimeters are now available.
INTRODUCTION
Drug-resistant strains of Mycobacterium tuberculosis are becoming increasingly widespread, creating a global health concern and making the treatment of tuberculosis even more difficult. Most of the cases reported in 2009 were in European countries and South Africa. However, it is likely that the occurrence of multidrug-resistant M. tuberculosis (MDR-TB), which is defined as a strain of M. tuberculosis that is resistant to both isoniazid and rifampin, is greatly underestimated in developing countries because the diagnosis is difficult and expensive (28). Direct identification of MDR-TB requires exposing an M. tuberculosis specimen to antibiotics to which it might be resistant at a series of concentrations and assessing the effects on growth—i.e., determining whether the MIC is abnormally high. In addition to M. tuberculosis, the incidence of infections caused by mycobacteria other than M. tuberculosis (MOTT) is continuously increasing, with Mycobacterium abscessus being the most pathogenic fast-growing mycobacterium (17, 18, 26). However, most MOTT infections have been overlooked, drug susceptibility test results are inconsistent, and some MOTT still need study to determine effective treatment (27). The determination of MICs for M. tuberculosis and MOTT by standard means is labor-intensive and/or expensive and can produce ambiguous results (11, 24). Using the proportion method to determine drug susceptibility of an M. tuberculosis specimen (potentially MDR-TB) entails inoculating plates with various concentrations of each antibiotic and waiting up to 6 weeks for visible colonies to be formed. Results of tetrazolium salt assays can fluctuate depending on the cells' ability to reduce these salts (15). In addition, such metabolic assays generally run a high risk of cross-contamination and pose a significant biosafety hazard as a result of repeated manipulations of open microplate wells containing live liquid cultures. They are also read at the first sign of growth in the control (no-antibiotic) sample (2, 5, 13). Unfortunately, this is not effective, since many drug-resistant mycobacterium strains show delayed growth in the presence of antibiotic, and so signs of growth may not show up until days or weeks after growth is observed in controls. Automated methods for assessing M. tuberculosis, such as Bactec, are fast but require expensive equipment and, therefore, present a financial problem in developing countries. Genetic tests for MDR-TB detection (for example, based on the PCR method) are much faster, but the exact relationship between a genetic signature and drug resistance is not fully understood (11). This makes gene-based quantitative estimation of the MIC impossible or, at best, highly speculative. Furthermore, genetic mutations resulting in new forms of drug resistance continue to occur, making the use of gene-based methods to screen for new forms of drug resistance challenging (24). Thus, there is room for direct and quantitative methods for determining resistance levels for a given drug against a given mycobacterium. Such a method should be sensitive, reliable (minimal false positives and false negatives), faster than manual methods, and simple to use. For use in developing countries, these methods should also be inexpensive, in terms of costs for both equipment and consumables.
Isothermal microcalorimetry (IMC) is, potentially, such a method. It is a general analytical technique that allows measurements of heat production rates in the microwatt range from small (e.g., ∼1 ml or ∼1 g) specimens of any type. Since all chemical and physical processes either consume or produce heat, IMC provides a means for continuously monitoring the rate and extent of all reactions. IMC microbiology, using the methods that we describe, entails real-time, continuous measurement of microbial metabolic heat produced in sealed ampoules containing growth medium, bacteria, and antibiotic (3, 21). The use of IMC ampoules sealed in a microbiology lab prior to IMC measurements substantially augments microbiologic safety. In addition, since IMC measures growth-related heat rather than, for example, change in an optical quantity (for example, absorbance or fluorescence), it allows use of either liquid or solid media (21, 25). This is of special interest since (i) measuring growth on solid media by means other than IMC is a difficult task and (ii) there are strains of M. tuberculosis and MOTT that grow faster, or even only, on solid media (16). IMC-determined cumulative heat production over time has been shown to be correlated with the cumulative increase in bacterial biomass. Therefore, IMC heat production data also quantitatively record any changes in growth due to the presence of an antimicrobial substance (3). IMC microbiologic measurements are minimally labor-intensive since no repetitive assessments over time are needed to determine a growth curve under a given set of conditions. The cumulative heat flow signal over time is a representation of the growth curve (4).
The purpose of this study was to establish proof of concept for use of IMC in mycobacterial drug susceptibility testing. The study thus employed a standard multichannel microcalorimetry instrument and simple (but mycobacterium suited) media for determining the phenotypic drug susceptibilities of three different mycobacteria to three drugs, namely, isoniazid, ethambutol, and moxifloxacin.
MATERIALS AND METHODS
IMC was employed to detect the heat production, and therefore related growth, of Mycobacterium smegmatis (DSM 43756), Mycobacterium avium (clinical isolates kindly provided by K. Jaton from the University Hospital of Lausanne), and Mycobacterium tuberculosis (ATCC 25177; an attenuated strain). Each mycobacterium was tested against both ethambutol and isoniazid at the concentrations specified below. In addition, M. tuberculosis was also tested against moxifloxacin, due to new interest in the drug as a treatment for tuberculosis.
Four-milliliter IMC ampoules containing Middlebrook 7H9 medium (Sigma-Aldrich Co., St. Louis, MO) with added OADC (oleic acid-albumin-dextrose-catalase; 10%) and agar were prepared as 2-ml slants with various concentrations of either isoniazid, ethambutol, or moxifloxacin. Stock solutions of isoniazid, ethambutol (Sigma-Aldrich Co., St. Louis, MO), and moxifloxacin (Alcon Switzerland SA, Hünenberg, Switzerland) were prepared with concentrations of 2,500 μg/ml, 3,200 μg/ml, and 500 μg/ml, respectively. To determine MICs, dilutions were made to yield a range of drug concentrations commonly found in the literature. The concentrations were as follows: isoniazid, 0, 0.01, 0.1, 1.0, 5.0, 12.5, and 25 μg/ml; ethambutol, 0, 2.0, 4.0, 8.0, 16.0, and 32.0 μg/ml; and moxifloxacin, 0, 0.1, 0.25, 0.5, 1.0, and 2.0 μg/ml (1, 6, 7, 8, 9, 12, 19, 20, 22, 23, 27, 29). Ampoules were inoculated from liquid medium cultures using sterile, disposable inoculating loops. Measurements were performed in triplicate.
A TAM48 microcalorimetry instrument (Waters/TA) was previously equilibrated at 37°C for at least 2 days. The instrument has 48 independent microcalorimeters available at the set temperature. Sealed ampoules were then introduced into the microcalorimeters, and the individual heat production rates (i.e., W = J/s = heat flow or thermal power) of each ampoule were measured continuously in real time as previously described (3).
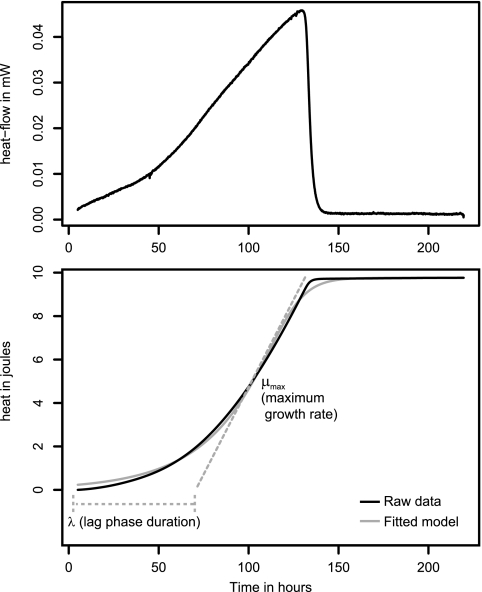
The grofit statistical package (10, 30) was used to analyze the heat flow-versus-time results to determine the maximum bacterial growth rate (μmax) and the lag phase duration (λ) (Fig. 1).
Fig 1.
Growth parameters of M. tuberculosis analyzed using R (a control curve without antibiotic was used here as an example). (Top) Heat flow data. (Bottom) Heat over time curve (i.e., integrated heat flow data; solid black line), fitted growth model (solid gray line), and calculated growth parameters (dashed lines). μmax is the maximum growth rate corresponding to the maximum slope of the heat over time curve. λ is the duration of the lag phase measured as the time from the start of the experiment to the interception of a line tangent to the maximum growth rate point and the baseline.
RESULTS
For each combination of mycobacterium and drug, the results are presented as variations of μmax and λ with drug concentration (Fig. 2 to 4).
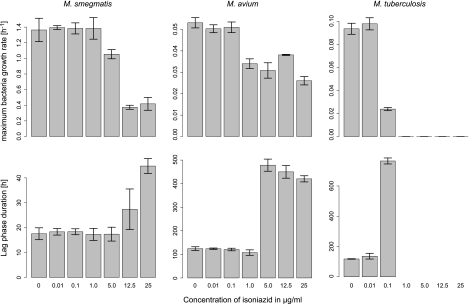
Fig 2.
Mean and standard deviation (n = 3) of the growth rate and lag phase duration with increasing concentrations of isoniazid for M. smegmatis, M. avium, and M. tuberculosis. No growth results in a maximum growth rate equal to 0 and an infinite lag phase (not represented).
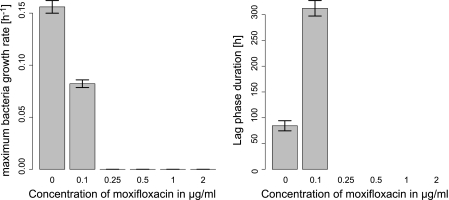
Fig 4.
Mean and standard deviation (n = 3) of the growth rate and lag phase duration with increasing concentrations of moxifloxacin for M. tuberculosis. No growth results in a maximum growth rate equal to 0 and an infinite lag phase (not represented).
Isoniazid had a limited effect on the growth of both M. smegmatis and M. avium. Total inhibition was not reached at the drug concentrations used for either of these two species. Only a minimal effect on μmax was visible with isoniazid; however, a strong increase in λ was observed for M. smegmatis and M. avium above 12.5 μg/ml and 1 μg/ml, respectively. This increase of the lag phase is consistent with the bactericidal effect of the drug. Much higher antimicrobial activity was observed for M. tuberculosis, for which MIC was reached at 1.0 μg/ml (Fig. 2).
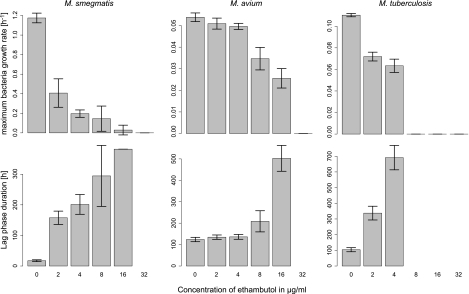
Similarly, ethambutol had also a limited effect on the growth of both M. smegmatis and M. avium, with a MIC value of 32 μg/ml for both strains. Clear relationships could be seen between increasing ethambutol concentrations, decreasing μmax, and increasing λ. A stronger antimicrobial effect on M. tuberculosis was seen, with a MIC being 8.0 μg/ml (Fig. 3).
Fig 3.
Mean and standard deviation (n = 3) of the growth rate and lag phase duration with increasing concentrations of ethambutol for M. smegmatis, M. avium, and M. tuberculosis. No growth results in a maximum growth rate equal to 0 and an infinite lag phase (not represented).
Moxifloxacin was evaluated only against M. tuberculosis, against which it showed pronounced inhibition. The MIC value was found to be 0.25 μg/ml (Fig. 4).
DISCUSSION
Our results using IMC clearly demonstrate that rapid, safe, and clear MIC determinations can be achieved for representative mycobacteria. The MIC values determined for the different combinations of mycobacteria and antibiotics fit in the ranges previously observed in the literature for closely related experiments (1, 6, 7, 8, 9, 12, 19, 20, 22, 23, 27, 29). The main advantage of IMC over other approaches is the fact that it provides quantitative measurements of the effect (i.e., the dose response) of an antimicrobial on the growth rate and lag phase of the mycobacteria. In contrast, most methods only determine the MIC values without providing further knowledge on the nature of the dose response (11, 24). Further, the results suggest that IMC can provide additional valuable information during the testing of new drugs, for example, the extent to which the effects are bactericidal or bacteriostatic. As a bactericidal agent kills a portion of the inoculum, the time before exponential growth can be observed (i.e., the lag phase) in the microcalorimeter increases. However, with a bactericidal agent, the growth rate is either not affected or only minimally affected, resulting in a decoupling of the lag phase duration and the maximum growth rate. Such a bactericidal effect is best seen here with M. avium and isoniazid, for which a sudden increase in lag phase does not correspond to a significant change in the maximum growth rate. On the other hand, a bacteriostatic agent affects the cellular process and decreases the growth rate. In this case, the increase in the lag phase duration is directly linked to the decrease in growth rate. In our study, this is best seen with M. smegmatis and ethambutol. Other studies focusing on fast-growing microorganisms have shown that microcalorimetry could be very effective in deciphering whether an antibiotic is either bactericidal or bacteriostatic or has a combined effect (25).
IMC can, potentially, be readily adopted for use in developing countries as a safe and reliable method of determining mycobacterial MICs against antitubercular drugs. Studies using a less expensive instrument have shown promising results in the detection of M. tuberculosis and mycobacteria other than M. tuberculosis (MOTT) (21). The IMC procedure is easy and requires little training; the curve fitting using the grofit package can be automated and does not constitute a limitation. In addition, due to the use of sealed ampoules, the risk of cross-contamination is low, as are biosafety risks. These characteristics make IMC an attractive method for MIC determination in countries lacking the funds and trained specialists required for the use of methods such as Bactec. IMC could be a valuable diagnostic tool in the field to directly determine drug susceptibilities of M. tuberculosis strains, thus facilitating monitoring the levels of resistance of MDR-TB and helping to implement effective treatment plans in areas most affected by MDR-TB. Although the cost of an IMC instrument such as the TAM48 used in this study may be too high for users in developing countries, several other instrument designs are available at a much lower cost (3, 21). With IMC, the cost of consumables is low since only simple medium formulations are needed and no special indicators (e.g., fluorescent markers) have to be added (3, 16). We have estimated the cost per sample of our microcalorimetric approach using such a cheaper instrument. In industrialized countries, complete processing of a sample would cost ca. $15.00, most of that being the salary cost of ca. $10.50, with remaining costs being ca. $2.50 for consumables and ca. $2.00 for microcalorimeter use and maintenance. In developing countries, microcalorimeter usage time and maintenance as well as consumable costs would remain the same. However, salary costs are generally much lower (e.g., ca. $1.00), resulting in an overall estimated cost of ca. $5.50 per sample. Looking further ahead, “microcalorimeter-on-a-chip” designs (3, 14) or multiwell plate calorimeters (reportedly under development) will likely allow higher throughput at lower cost. Currently, the throughput constitutes the main limitation of IMC since only low to medium throughput can be achieved with today's instruments. An added attraction for using IMC in developing countries is that the method can be used for determining MICs for other drugs and microbes besides mycobacteria, e.g., methicillin-resistant Staphylococcus aureus (MRSA) (25).
A final point is that using IMC in developed countries for M. tuberculosis detection and MDR-TB identification has advantages over existing methods. This is because there are strains of M. tuberculosis that grow much more readily and rapidly—or only—on solid media (16). Only IMC offers a simple means (heat flow measurement) to semiautomate detection and continuous monitoring of growth on solid media.
Conclusions.
We have provided proof of concept for the use of IMC for mycobacterium drug susceptibility testing. Not only are MIC values determined accurately, but also the dose response is clearly visible and can be quantified. This includes an indication as to whether drug action is bactericidal or bacteriostatic. Finally, the ability of IMC to accomplish such studies with inexpensive solid growth media sets it apart as a special tool for mycobacterial microbiology. The method presented here may be suitable for determining the MICs of other drugs for other microorganisms, including resistant strains.
ACKNOWLEDGMENTS
We are greatly indebted to the three anonymous reviewers who contributed to increase the original quality of the manuscript.
Footnotes
Published ahead of print 16 November 2011
REFERENCES
- 1. Angeby K, Kristian A, Klintz L, Hoffner SE. 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J. Clin. Microbiol. 40:553–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bastian I, Rigouts L, Palomino JC, Portaels F. 2001. Kanamycin susceptibility testing of Mycobacterium tuberculosis using Mycobacterium growth indicator tube and a colorimetric method. Antimicrob. Agents Chemother. 45:1934–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braissant O, Wirz D, Göpfert B, Daniels AU. 2010. Review: biomedical use of isothermal microcalorimeters. Sensors 10:9369–9383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braissant O, Wirz D, Goepfert B, Daniels AU. 2010. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 303:1–8 [DOI] [PubMed] [Google Scholar]
- 5. Caviedes L, Delgado J, Gilman RH. 2002. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1873–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danilchanka O, Pavlenok M, Niederweiss M. 2008. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob. Agents Chemother. 52:3127–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Logu A, et al. 2005. In vitro antimcobacterial activity of newly synthesised S-alylisothiosemicarbazone derivatives and synergistic interactions in combination with rifamycins against Mycobacterium avium. Int. J. Antimicrob. Agents. 26:28–32 [DOI] [PubMed] [Google Scholar]
- 8. Heym B, Cole ST. 1992. Isolation and characterization of isoniazid-resistant mutants of Mycobacterium smegmatis and Mycobacterium aurum. Res. Microbiol. 143:721–730 [DOI] [PubMed] [Google Scholar]
- 9. Ji B, et al. 1998. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:2066–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahm M, Hasenbrink G, Lichtenberg-Fraté H, Ludwig J, Kschischo M. 2010. Grofit: fitting biological growth curves with R. J. Stat. Softw. 33(7):1–2120808728 [Google Scholar]
- 11. Kim SJ. 2005. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J. 25:564–569 [DOI] [PubMed] [Google Scholar]
- 12. Kumar M, Khan IA, Verma V, Kalyan N, Qazi GN. 2005. Rapid, inexpensive MIC determination of Mycobacterium tuberculosis isolates by using microplate nitrate reductase assay. Diagn. Microbiol. Infect. Dis. 53:121–124 [DOI] [PubMed] [Google Scholar]
- 13. Martin A, Camacho M, Portaels F, Palomino JC. 2003. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob. Agents Chemother. 47:3616–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maskow T, Lerchner J, Peitzsch M, Harms H, Wolf G. 2006. Chip calorimetry for the monitoring of whole cell biotransformation. J. Biotechnol. 122:431–442 [DOI] [PubMed] [Google Scholar]
- 15. Mshana R, Tadasse G, Abate G, Miörner H. 1998. Use of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1214–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parsons LM, et al. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin. Microbiol. Rev. 24:314–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrini B. 2006. Non-tuberculous mycobacterial infections. Scand. J. Infect. Dis. 38:246–255 [DOI] [PubMed] [Google Scholar]
- 18. Petrini B. 2006. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 114:319–328 [DOI] [PubMed] [Google Scholar]
- 19. Piersimoni C, et al. 2007. Validation of the agar proportion and 2 liquid systems for testing the susceptibility of Mycobacterium tuberculosis to moxifloxacin. Diagn. Microbiol. Infect. Dis. 57:283–287 [DOI] [PubMed] [Google Scholar]
- 20. Ren H, Liu J. 2006. AsnB is involved in natural resistance of Mycobacterium smegmatis to multiple drugs. Antimicrob. Agents Chemother. 50:250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez D, Daniels AU, Urrusti JL, Wirz D, Braissant O. 2011. Evaluation of a low-cost calorimetreic approach for rapid detection of tuberculosis and other mycobacteria in culture. J. Appl. Microbiol. 111:1016–1024 [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez JC, Ruiz M, Lopez M, Royo G. 2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin, and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464–467 [DOI] [PubMed] [Google Scholar]
- 23. Stephan J, Mailaender C, Etienne G, Daffé M, Niederweiss M. 2004. Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48:4163–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Deun A, Martin A, Palomino JC. 2010. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int. J. Tuberc. Lung Dis. 14:131–140 [PubMed] [Google Scholar]
- 25. von Ah U, Wirz D, Daniels AU. 2008. Rapid MSSA-MRSA differentiation and MIC determinations by isothermal microcalorimetry. J. Clin. Microbiol. 46:2083–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wayne LG, Sramek HA. 1992. Agent of newly recognized or infrequently encountered mycobacterial diseases. Clin. Microbiol. Rev. 5:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woods GL, Washington JA. 1987. Mycobacteria other than Mycobacterium tuberculosis: review of microbiologic and clinical aspects. Rev. Infect. Dis. 9:275–294 [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization 2010. Global tuberculosis control: WHO report 2010. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf [Google Scholar]
- 29. Zimmer BL, DeYoung DR, Roberts GD. 1982. In vitro synergistic activity of ethambutol, isoniazid, kanamycin, rifampin, and streptomycin against Mycobacterium avium-intracellulare complex. Antimicrob. Agents Chemother. 22:148–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zwietering MH, Jongenburger I, Rombouts FM, van't Riet K. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56:1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]