Abstract
Streptococcus gallolyticus subsp. pasteurianus, previously known as Streptococcus bovis biotype II.2, is known to cause multiple infectious complications, including bacterial meningitis, in adults. Only sporadic individual case reports have identified this pathogen as a cause of meningitis in infants. This study is the first to longitudinally document S. gallolyticus subsp. pasteurianus as a cause of meningitis in four epidemiologically unrelated infants less than 2 weeks of age. The 16S rRNA gene sequences of all 4 isolates were identical, and further were identical to 3 central nervous system (CNS) strains (two adults and one child) reported in existing literature. S. gallolyticus subsp. pasteurianus is an increasingly recognized cause of meningitis and bacteremia in the newborn period, and it merits further study with respect to etiology of infection.
INTRODUCTION
Streptococcus gallolyticus subsp. pasteurianus is a Lancefield group D streptococcus, formerly known as Streptococcus bovis biotype II.2. Although S. gallolyticus subsp. pasteurianus can be unambiguously differentiated by 16S rRNA gene sequence analysis from other Streptococcus gallolyticus subspecies, namely, Streptococcus gallolyticus subsp. gallolyticus and Streptococcus gallolyticus subsp. macedonicus, as well as the closely related subspecies Streptococcus infantarius subsp. coli and Streptococcus infantarius subsp infantarius (2, 11), biochemical differentiation using only a few biochemicals is not always as clear-cut (2). A combination of tests for β-glucuronidase, β-mannosidase, β-galactosidase, and the ability to acidify trehalose, mannitol, starch, inulin, and glycogen allows identification of all but the rare isolates (2, 11). In the past, however, all of these groups have been referred to as Streptococcus bovis.
S. bovis in this wider sense has been primarily associated with bacteremia and endocarditis and less commonly with meningitis and bone and joint infections in adults (4, 9). S. bovis has not been studied as extensively in children, and studies in which the organism has been identified to the subspecies level in children are rare. Sporadic case reports, including those by Cheung et al. in 2000, Gavin et al. in 2003, and Onoyama et al. in 2009, have identified S. bovis biotype II.2, now known as S. gallolyticus subsp. pasteurianus, as a cause of neonatal meningitis (3, 8, 10). Recently, Floret et al. described a cluster of five infections due to S. gallolyticus subsp. pasteurianus in a 16-bed neonatal intensive care unit (NICU) in France. All five infections occurred within 11 days of one another, and the authors could not rule out health care worker transmission from one child to another (7).
No previous studies or reports to date have longitudinally documented evidence of S. gallolyticus subsp. pasteurianus as a cause of neonatal meningitis and/or bacteremia. We describe the first case series of four unrelated patients, whose time frames of reference differ, with neonatal or infantile meningitis with or without bacteremia due to confirmed S. gallolyticus subsp. pasteurianus infection.
CASE REPORTS
Patient 1.
A full-term male infant was born via spontaneous vaginal delivery after a pregnancy complicated by a maternal urinary tract infection with an unknown pathogen during the month prior to delivery, as well as a maternal stray-cat bite 1 week prior to delivery. He presented at 13 days of age with congestion, increased work of breathing, and increased sleepiness for 1 day (Table 1). He did not exhibit fever at presentation. Initial laboratory values included a complete blood count (CBC), with a white blood cell (WBC) count of 6,570/mm3, with 37% neutrophils, 33% lymphocytes, 23% monocytes, and 3% eosinophils, a hemoglobin level of 17 g/dl, and a platelet count of 382,000/mm3. Cerebrospinal fluid (CSF) revealed a glucose level of 35 mg/dl, a protein level of 60 mg/dl, a red blood cell (RBC) count of 23/mm3, and a WBC count of 13/mm3. CSF Gram staining exhibited no organisms and rare WBCs. He received intravenous (IV) ampicillin and IV cefotaxime empirically. On hospital day 2, the CSF culture grew Gram-negative rods and gamma-hemolytic streptococcus, which were identified on hospital day 4 as Klebsiella oxytoca and S. gallolyticus subsp. pasteurianus. The patient underwent a repeat lumbar puncture on hospital day 4 due to development of emesis and diarrhea, and as the treating physician desired to assess the CSF WBC trend, since the original sample of 13/mm3 was within the normal range for age. Repeat CSF cultures on hospital day 4 showed no bacterial growth, though the CSF exhibited pleocytosis suggestive of infection, with a WBC count of 33/mm3. Herpes simplex virus (HSV) and enterovirus PCR testing (PCR) of the first CSF specimen were negative. After discontinuation of the ampicillin on hospital day 4, the patient was successfully treated with 16 total days of cefotaxime at 300 mg/kg/day, divided and administered every 6 h. Bilateral auditory brainstem response (ABR) evaluation of hearing at discharge revealed no evidence of hearing loss.
Table 1.
Neonatal meningitis due to S. gallolyticus subsp. pasteurianusa
| Case | Age (days) | Gestational age | Delivery method | Sex | Presenting symptoms | Site(s) of isolation | CSF WBCs/mm3 | Serum WBCs/mm3 (% PMNs) | Final antimicrobial therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Term | SVD | M | Congestion, increased WOB, increased sleepiness | CSF | 13 (33 on repeat exam) | 6,570 (37) | Cefotaxime |
| 2 | 5 | Term | IVD | F | Fever, seizure | CSF, blood | 1,340 | 3,490 (67) | Ampicillin |
| 3 | 2 | Term | SVD | M | Lethargy, poor feeding, seizure | CSF, blood | 4,470 | 7,000 (60) | Ampicillin |
| 4 | 5 | Term | SVD | M | Fever, congestion, fussiness | CSF, blood | 2,000 | 25,740 (23) | Cefotaxime |
WOB, work of breathing; SVD, spontaneous vaginal delivery; IVD, induced vaginal delivery; M, male; F, female.
Patient 2.
A full-term female infant was born via induced vaginal delivery after a pregnancy complicated by late prenatal care and maternal tobacco abuse. She presented at 5 days of age with fever and seizure-like activity for 1 day, which consisted of bilateral eye fluttering movements and upper extremity tremors (Table 1). She received an initial loading dose of phenobarbital. Blood, urine, and CSF cultures were obtained. Initial CBC values included a WBC count of 3,490/mm3, with 67% neutrophils, 20% lymphocytes, and 12% monocytes, a hemoglobin level of 16.1 mg/dl, and a platelet count of 224,000/mm3. The initial C-reactive protein level was 4.1 mg/dl. CSF studies included a glucose level of less than 20 mg/dl, an RBC count of 29,250/mm3, and a WBC count of 1,340/mm3. Ampicillin, cefotaxime, and acyclovir were initiated, and cefotaxime was switched to gentamicin while the patient was in the emergency department. HSV PCR did not detect the virus, and acyclovir was discontinued. Cefotaxime was restarted on hospital day 2 when the CSF Gram stain revealed Gram-positive cocci in chains and pairs. Magnetic resonance imaging of the head to evaluate seizure activity on hospital day 2 was significant only for a small focus of hemorrhage in the posterior fossa, thought to be due to birth trauma. Blood culture and CSF culture isolates were both identified as S. gallolyticus subsp. pasteurianus on hospital day 3, with the isolate showing susceptibility to both ampicillin and cefotaxime. Cefotaxime and gentamicin were both discontinued on hospital day 3, and the patient received ampicillin at a dosage of 300 mg/kg/day divided and administered every 8 h, for 14 total days of therapy. Bilateral ABR at discharge revealed no evidence of hearing loss.
Patient 3.
A full-term male infant was born via spontaneous vaginal delivery at home after a pregnancy complicated by preeclampsia; additionally, the mother had not had prenatal testing performed. The maternal history also included a subjective report of a urinary tract infection during pregnancy which was successfully treated with over-the-counter remedies. Rupture of membranes occurred approximately 3 h prior to delivery. Maternal exposure history was significant for multiple animals on the family farm, including chickens, horses, dogs, cats, and dairy cattle, with maternal consumption of unpasteurized milk. Within 1 week prior to delivery, 70 baby chicks on the parents' farm died due to an unknown illness.
The patient presented at 2 days of age with poor feeding, lethargy, and hypoglycemia, with a blood glucose of 16 mg/dl (Table 1). He did not have fever before or during his hospitalization. According to the maternal report, he also had seizure activity on the day of presentation, consisting of full-body stiffening and upward deviation of gaze. Initial CBC showed a WBC count of 7,000/mm3, with 60% neutrophils, 21% banded neutrophils, 16% lymphocytes, and 2% monocytes, along with a hemoglobin level of 20 mg/dl and a platelet count of 83,000/mm3. Initial CSF results included a glucose level of 65 mg/dl, an RBC count of 34,650/mm3, and a WBC count of 4470/mm3. HSV PCR was negative. The patient was initially given two glucose boluses, and ampicillin and gentamicin were administered 2.5 h and 1.5 h before the lumbar puncture, respectively. Vancomycin was added on hospital day 1 when Gram stain of the CSF showed Gram-positive cocci in chains and pairs. Gentamicin was discontinued on hospital day 2. Cefotaxime was added on hospital day 3 when the organism, isolated in the blood and CSF, was identified as presumptive streptococcal species. The vancomycin was discontinued on hospital day 4, when the isolate was identified as S. gallolyticus subsp. pasteurianus. Cefotaxime was continued along with ampicillin until hospital day 6, when the cefotaxime was discontinued at the request of the Infectious Diseases team. Ampicillin was continued for a total of 16 days, or 14 days from the first negative blood culture, with the dose of ampicillin at 300 mg/kg/day IV divided every 8 h. Bilateral ABR at discharge and at a follow-up evaluation 1 month after discharge revealed no deficiencies.
Patient 4.
A postterm male was born via spontaneous vaginal delivery after a pregnancy complicated only by maternal hyperemesis gravidarum. Delivery history was significant for prolonged maternal rupture of membranes prior to delivery, lasting approximately 13 h. Meconium staining was observed at birth, but the patient did not suffer any adverse effects.
The patient presented at 5 days of life with fever, rhinorrhea, and fussiness for 1 day (Table 1). Upon presentation to the emergency department, blood, urine, and CSF cultures were obtained. Initial CBC showed a WBC count of 25,740/mm3, with 23% neutrophils, 22% banded neutrophils, 31% lymphocytes, 14% monocytes, and 7% eosinophils, along with a hemoglobin level of 16.2 mg/dl; a platelet count was not performed. Initial CSF studies showed a glucose level of less than 20 mg/dl, a protein level of 234 mg/dl, an RBC count of 150/mm3, and a WBC count of 2,000/mm3. Enterovirus PCR of the CSF was negative. He was placed on ampicillin and cefotaxime. A Gram stain of the CSF exhibited Gram-positive cocci in pairs, and ampicillin was changed to vancomycin therapy. On hospital day 2, the CSF culture and blood culture both revealed evidence of gamma-hemolytic streptococci, not pneumococci or enterococci. On hospital day 3, the organism isolated from the blood and CSF was identified as S. gallolyticus subsp. pasteurianus. Vancomycin was discontinued on hospital day 3, when susceptibilities of the isolate were determined. Of note, this S. gallolyticus subsp. pasteurianus isolate was intermediately resistant to penicillin, with a MIC of 0.19 μg/ml. The patient received 14 total days of IV cefotaxime, at 300 mg/kg/day, divided and administered every 6 h. Bilateral ABR at discharge and at a follow-up 1 month after hospital discharge showed no deficiencies.
MATERIALS AND METHODS
Patient data, including patient histories, physical examination findings, daily inpatient progress notes, laboratory findings, imaging findings, medication reconciliation information, discharge information, and follow-up outpatient appointment data, were collected retrospectively using the computerized medical record system at Children's Mercy Hospitals and Clinics (Kansas City, MO). Study accession numbers were used to pull frozen S. gallolyticus subsp. pasteurianus isolates originally isolated from cerebrospinal fluid or blood. All specimens were obtained from four subjects who were less than 2 weeks old at presentation. Dates of patient visits ranged from 9 November 2008 through 14 July 2010. Meningitis was defined as the presence of cerebrospinal fluid pleocytosis (>30 white blood cells/mm3) in addition to the growth of S. gallolyticus subsp. pasteurianus from cerebrospinal fluid culture. Three of the four patients also presented with bacteremia, defined as isolation of S. gallolyticus subsp. pasteurianus from blood culture.
Microbiologic data for each isolate, including identification and antimicrobial susceptibility patterns for penicillin, vancomycin, and cefotaxime against S. gallolyticus subsp. pasteurianus, were collected from computerized records. The organisms were identified with a Vitek 2 (bioMérieux, Inc.) system, and the MICs were obtained by Etest susceptibility testing.
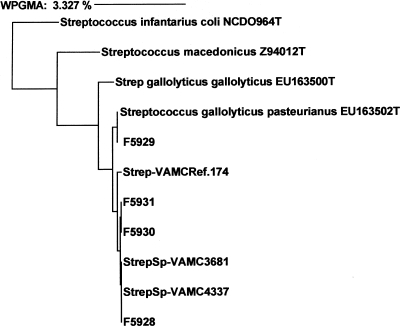
Approximately the first 500 bases (positions 20 to 510) of the 16S rRNA gene sequence of each strain were obtained by standard methods. Electrophoretograms were analyzed by the MicroSeq program (Applied Biosystems, Foster City, CA). The resulting 16S rRNA sequences were compared with sequences in several databases, including a database consisting of in-house strains, the MicroSeq database, and the public National Center for Biotechnology Information (NCBI) database. An analysis of our four strains, as well as type strains and S. gallolyticus subsp. pasteurianus central nervous system (CNS) isolates previously reported, showed that all had a T in the 182 position and a C in the 238 position as the only and defining differences from S. gallolyticus subsp. gallolyticus (2, 4, 5). A dendrogram was generated by the weighted-pair algorithm for constructing phylogenetic trees (Fig. 1).
Fig 1.
Dendrogram of phylogenetic relationships between four patient Streptococcus gallolyticus subsp. pasteurianus isolates, labeled F5928 to F5931. Sequences of the four isolates are compared with those of type strains (designated with a “T” and the NCBI number) and Streptococcus bovis biotype II.2 CNS isolates sequenced in a previous study (Ref 174, VAMC 3681 and VAMC 4337) (4). The bar at the top indicates a 3% difference measured horizontally. All Streptococcus gallolyticus subsp. pasteurianus isolates were identical and differed from Streptococcus gallolyticus subsp. gallolyticus by two base pairs, a T at position 182 and a C at position at position 238, as described by Beck et al. (2). WPGMA, weighted-pair group method using arithmetic averages.
RESULTS
Of the four patients with S. gallolyticus subsp. pasteurianus meningitis, three had concurrent bacteremia with this pathogen. Patient 1 was coinfected with Klebsiella oxytoca in the CSF but did not exhibit bacteremia with either organism. CSF pleocytosis was initially observed in three of four infants (average, 1,956 WBCs/mm3; range, 13 to 4,470 WBCs/mm3), while the serum WBC count was low to low-normal in three of the four patients. The average age of the patients at presentation was 6.25 days (range, 2 to 13 days). Three patients were male, and one was female.
Two of the four infants presented with fever, while two presented with seizure or seizure-like activity. Two of four subjects also presented with congestion and rhinorrhea.
MIC values and susceptibilities to penicillin, cefotaxime, and vancomycin were reported for each isolate. For penicillin, three of the four isolates were reported as susceptible, each with a MIC of 0.125 μg/ml. The fourth isolate was reported as intermediately resistant to penicillin, with a MIC of 0.19 μg/ml. Each isolate was reported as susceptible to cefotaxime, with all four having MICs of 0.25 μg/ml. All isolates were reported as susceptible to vancomycin, with MICs of 0.75 μg/ml, 0.75 μg/ml, 0.5 μg/ml, and 0.38 μg/ml.
DISCUSSION
S. gallolyticus, subsp. pasteurianus, formerly known as S. bovis biotype II.2, was previously described in three individual case reports as a cause of neonatal meningitis and/or bacteremia. In 2006, a cluster of five preterm infants in a neonatal intensive care unit in France were diagnosed as having bacteremia secondary to S. gallolyticus subsp. pasteurianus. Four of five isolates were genetically identical to each other, with the fifth isolate closely resembling the other four (7). The etiology of this outbreak was postulated to be environmental contamination followed by transient hand carriage by a staff member; therefore, these infections were likely the result of one index case, followed by a breakdown in infection control measures.
In contrast, our series longitudinally evaluated cases of meningitis with or without associated bacteremia due to S. gallolyticus subsp. pasteurianus. The cases were unrelated with respect to epidemiology and time frame, having occurred between 2008 and 2010. The 16S rRNA gene sequences of all four isolates were identical, and furthermore, they were identical to the sequences of three CNS strains (from two adults and one child) reported previously, as well as the type strain of S. gallolyticus subsp. pasteurianus (4, 5). One of the isolates exhibited intermediate resistance to penicillin, with a MIC of 0.19 μg/ml. The prognostic outlook for this patient was not different from that of the other three patients. The isolate that was intermediately resistant to penicillin was the most recently reported of the four listed above. Whether this intermediate resistance is an anomaly or whether this is the beginning of a trend for S. gallolyticus subsp. pasteurianus remains to be seen. All four of our patients were successfully treated with a minimum of 14 days of IV ampicillin or IV cefotaxime.
The mother of patient 3 had confirmed contact with multiple chickens that had died within 1 week prior to the birth of the patient. S. bovis species are a known cause of septicemia and sudden death in fowl, including pigeons, turkeys, chickens, and goslings (1, 12). This exposure history could be a potential clue to the etiology of this patient's S. gallolyticus subsp. pasteurianus infection; however, no postmortem testing was performed on the affected chickens.
One of the limitations of this case series is its retrospective nature; namely, mothers were not asked for an in-depth history of illness during pregnancy. Two of the four mothers had regular prenatal care throughout their entire pregnancy, while one had regular follow-up with a midwife. The mother of patient 2 did not receive prenatal care until her third trimester.
S. gallolyticus subsp. pasteurianus has been associated with colonic neoplasia, endocarditis, peritonitis, and chorioamnionitis in adults (8). A recent case report by Sturt et al. describes S. gallolyticus subsp. pasteurianus as the causative agent of meningitis in a 75-year-old male (13). S. bovis is part of normal colonic flora in 10% of healthy subjects. In one study, S. bovis was isolated from 3.4% of normal vaginal secretions (6); however, that study did not identify the S. bovis isolates to the subspecies level. Future studies should focus on rates of maternal colonization and infection with S. gallolyticus subsp. pasteurianus.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Barnett J, Ainsworth H, Boon JD, Twomey DF. 2008. Streptococcus gallolyticus subsp. pasteurianus septicaemia in goslings. Vet. J. 176:251–253 [DOI] [PubMed] [Google Scholar]
- 2. Beck Frodl MR, Funke G. 2008. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J. Clin. Microbiol. 46:2966–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung M, Pelot M, Nadarajah R, Kohl S. 2000. Neonate with late onset Streptococcus bovis meningitis: case report and review of the literature. Pediatr. Infect. Dis. J. 19:891–892 [DOI] [PubMed] [Google Scholar]
- 4. Clarridge JE, III, Attori SM, Zhang Q, Bartell J. 2001. 16S Ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Streptococcus bovis biotype II/2 is a separate genospecies and the predominant clinical isolate in adult males. J. Clin. Microbiol. 39:1549–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen LF, Dunbar SA, Sirbasku DM, Clarridge JE., III 1997. Streptococcus bovis infection of the central nervous system: report of two cases and review. Clin. Infect. Dis. 25:819–823 [DOI] [PubMed] [Google Scholar]
- 6. Egido JM, Maestre JR, Pena Izquierdo MY. 1995. The isolation of Streptococcus morbillorum from vaginal exudates. Rev. Soc. Bras. Med. Trop. 28:117–122 [DOI] [PubMed] [Google Scholar]
- 7. Floret N, et al. 2010. A cluster of bloodstream infections caused by Streptococcus gallolyticus subspecies pasteurianus that involved 5 preterm neonates in a university hospital during a 2-month period. Infect. Control Hosp. Epidemiol. 31:194–196 [DOI] [PubMed] [Google Scholar]
- 8. Gavin P, Thomson R, Jr, Horng S-J, Yogev R. 2003. Neonatal sepsis caused by Streptococcus bovis variant (biotype II/2): report of a case and review. J. Clin. Microbiol. 41:3433–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerber J, Glas M, Frank G, Shah S. 2006. Streptococcus bovis infection in young infants. Pediatr. Infect. Dis. J. 25:1069–1073 [DOI] [PubMed] [Google Scholar]
- 10. Onoyama S, et al. 2009. Neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J. Med. Microbiol. 58:1252–1254 [DOI] [PubMed] [Google Scholar]
- 11. Schlegel L, Grimont F, Ageron E, Grimont PAD, Bouvet A. 2003. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int. J. Syst. Evol. Microbiol. 53:631–645 [DOI] [PubMed] [Google Scholar]
- 12. Sekizaki T, et al. 2008. Endocarditis in chickens caused by subclinical infection of Streptococcus gallolyticus subsp. gallolyticus. Avian Dis. 52:183–186 [DOI] [PubMed] [Google Scholar]
- 13. Sturt A, et al. 2010. Streptococcus gallolyticus subspecies pasteurianus (biotype II/2), a newly reported cause of adult meningitis. J. Clin. Microbiol. 48:2247–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]