Abstract
The purpose of this study was to evaluate the GenoType MTBDRsl assay (Hain Lifescience GmbH, Nehren, Germany) for its ability to detect resistance to fluoroquinolones (FLQ), injectable second-line antibiotics [kanamycin (KM) and capreomycin (CM)], and ethambutol (EMB) in Mycobacterium tuberculosis clinical strains and directly in clinical samples. A total of 34 clinical strains were characterized with the Bactec 460 TB system. Fifty-four clinical samples from 16 patients (5 were smear negative and 49 were smear positive) were also tested directly. The corresponding isolates of the clinical specimens were also analyzed with the Bactec 460TB. When there was a discrepancy between assays, pyrosequencing was performed. The overall rates of concordance of the MTBDRsl and the Bactec 460TB for the detection of FLQ, KM/CM, and EMB susceptibility in clinical strains were 72.4% (21/29), 88.8% (24/27), and 67.6% (23/34), whereas for clinical samples, rates were 86.5% (45/52), 92.3% (48/52), and 56% (28/50), respectively. In conclusion, the GenoType MTBDRsl assay may be a useful tool for making early decisions regarding KM/CM susceptibility and to a lesser extent regarding FLQ and EMB susceptibility. The test is able to detect mutations in both clinical strains and samples with a short turnaround time. However, for correct management of patients with extensively drug-resistant tuberculosis, results must be confirmed by a phenotypical method.
INTRODUCTION
Efficient tuberculosis (TB) control is based on an early diagnosis followed by the rapid identification of drug resistance, in order to treat patients adequately, break the chain of transmission, and avoid the spread of resistant strains (38). Multidrug-resistant (MDR) Mycobacterium tuberculosis strains resistant at least to isoniazid (INH) and rifampin (RIF), which are two of the main first-line anti-TB drugs, have emerged worldwide and seriously threaten TB control and prevention programs. At the same time, the emergence of extensively drug-resistant tuberculosis (XDR TB), defined as MDR TB with additional resistance to fluoroquinolones (FLQ) [moxifloxacin (MOX), ofloxacin (OFL), and levofloxacin] and at least one of the three injectable second-line drugs [amikacin (AM), kanamycin (KM), and capreomycin (CM)], has also become an important global health problem.
Conventional methods for detecting XDR strains are sequential, because they are applied once a strain has grown in solid or liquid medium and has been shown to be resistant to first-line drugs, mainly RIF and INH. As a consequence, the pattern of resistance to second-line drugs becomes available later. In addition, methods of detecting resistance to second-line drugs are not fully standardized (19, 39), so the comparison of resistance incidences between different geographical settings is difficult.
Regarding first-line drugs, mutations related to INH and RIF resistance have been extensively investigated and involve mainly the rpoB, katG, and inhA genes (30). Ethambutol (EMB) is another first-line drug, and its resistance has been related mainly to mutations in the arabinosyl transferase genes (embC, embA, and embB) (28). Mutations in embB and particularly in codon 306 confer resistance to EMB more frequently (27, 28, 33). However, the frequency can vary between 20 and 70% according to geographical area (28, 33). Mutations related to resistance against FLQ have been detected mainly in the quinolone resistance-determining region in gyrA, which encodes a DNA gyrase. Mutations mostly affect residues codons 90 and 94 and more rarely affect codons 88 and 91 (5, 24, 32, 36). Interestingly, cross-resistance to AM, KM, and CM has been reported (25). Mutations are most frequently found at positions 1401, 1402, and 1484 in the 16S rRNA (rrs) (2, 25).
The reference molecular method for the detection of mutations related to drug resistance has mainly been DNA sequencing. However, this technology is very laborious and not fully standardized for use in daily practice. An alternative is pyrosequencing, a semiautomated sequencing assay based on real-time monitoring of the DNA synthesis (9). Easy-to-use commercial assays have been also developed to address this issue, specifically to detect resistance in clinical practice, such as the Inno-Lipa and Hain line probe assays (10). This technology is based on a multiplex PCR combined with reverse hybridization on nitrocellulose strips. At the moment, there is one assay that can be performed directly on strains and clinical samples that targets common mutations related to RIF and INH resistance with good sensitivity and specificity (21, 22). In order to start a safe second-line treatment in cases of resistance to first-line drugs, it is also important to detect resistance to second-line drugs and EMB. The GenoType MTBDRsl test (Hain Lifescience, Nehren, Germany) enables the simultaneous molecular genetic identification of the M. tuberculosis complex and its resistance to FLQ, injectable antibiotics, and EMB by detecting common mutations in gyrA, rrs, and embB (7, 13, 18, 35).
The objective of this study was to assess the accuracy of the GenoType MTBDRsl for detecting resistance of M. tuberculosis to FLQ, KM/CM, and EMB in clinical strains and directly in clinical samples. GenoType MTBDRsl results were compared with those obtained with the Bactec 460TB system. In cases where there was a discrepancy between assays, pyrosequencing was performed.
MATERIALS AND METHODS
Clinical strains.
A total of 34 M. tuberculosis strains, isolated from 34 patients at the time of diagnosis, were tested by the radiometric Bactec 460TB method for first- and second-line-drug susceptibility.
Clinical samples.
A total of 54 clinical samples from 16 patients were retrospectively selected from the clinical routine visit at the time of diagnosis. All samples were collected directly from patients; they were not obtained by split, and therefore, there was no repeat testing of a single sample. Samples were processed as follows. First, they were digested and decontaminated using Kubica's N-acetyl-l-cysteine NaOH method (15, 17). After decontamination, the concentrated sediment was suspended in 2 ml sterile phosphate buffer (pH 7.0), and auramine-rhodamine acid-fast staining was performed. Specimens that were positive by fluorochrome staining were confirmed with Ziehl-Neelsen staining. Five samples were smear negative and 49 were smear positive. An aliquot of the decontaminated specimens was cultured on Lowenstein-Jensen solid and MB/BacT liquid media (bioMérieux, Marcy l'Etoile, France). After inoculation for growth detection, the remaining decontaminated specimen was stored at −20°C (34). Isolates of the M. tuberculosis complex were detected in all samples included in the evaluation. Identification of M. tuberculosis in cultures was confirmed by Inno-Lipa Mycobacteria v2 assay (Innogenetics, N.V., Ghent, Belgium).
Drug susceptibility.
Susceptibility testing for FLQ (specifically MOX), KM/CM, and EMB was performed with the Bactec 460TB radiometric method. Critical concentrations for MOX, KM, CM, and EMB were 0.5 μg/ml, 5 μg/ml, 1.25 μg/ml, and 7.5 μg/ml, respectively. For five strains, only an EMB pattern was obtained in the Bactec 460TB analysis. Resistance profiles for the clinical strains and samples included in the study are presented in Table 1. In this study, Bactec 460TB was considered the gold standard method.
Table 1.
Resistance patterns for FLQ, KM/CM, and EMB of clinical strains and samples included in the study
| Resistance pattern | No. with pattern |
|
|---|---|---|
| Clinical strains | Clinical samples | |
| FLQs KM/CMs EMBs | 7 | 8 |
| FLQs KM/CMs EMBr | 12 | 15 |
| FLQs KM/CMr EMBr | 3 | 23 |
| FLQr KM/CMs EMBs | 4 | 4 |
| FLQr KM/CMs EMBr | 1 | 4 |
| FLQr KM/CMr EMBr | 2 | 0 |
| EMBr | 5 | 0 |
| Total | 34 | 54 |
Genotypic characterization.
The pyrosequencing method consists of PCR amplification followed by the pyrosequencing reaction (1). PCRs were performed in a final volume of 25 μl under the following conditions: incubation at 95°C for 12 min; 45 cycles of amplification consisting of 94°C for 30s, 60°C for 1 min, and 72°C for 2 min; and 72°C for 7 min. PCR products were analyzed by electrophoresis in 1% agarose. PCR and pyrosequencing primers for gyrA, rrs, and embB were designed with pyrosequencing assay design software according to the position or codon analyzed. Primers sets are listed in Table 2. Mutations detected by pyrosequencing are located in codons 80 and 88 to 97 of gyrA, positions 1401, 1402, and 1484 of 16S rrs, and codon 306 of embB. Pyrosequencing analysis covers and analyzes the same gene codons and positions as GenoType MTBDRsl. Pyrosequencing reaction and data analysis were performed as recommended by the PSQ96MA and SQA software manufacturer (Biotage AB, Uppsala, Sweden).
Table 2.
PCR and sequencing primers used for pyrosequencing positions in gyrA, rrs, and embB genesa
| Gene and primer | Sequence (5′–3′) | Product size (bp) |
|---|---|---|
| gyrA | 111 | |
| Forward primer | CCGCAGCCACGCCAAGTC | |
| Reverse primer | Biotin-GACCAGGGCTGGGCCATG | |
| Pyrosequencing primer 1 | GCCCGGTCGGTTGCC | |
| Pyrosequencing primer 2 | AACTACCACCCGCAC | |
| rrs | 202 | |
| Forward primer | AGCAACGCTGCGGTGAAT | |
| Reverse primer | Biotin-TTTCGTGGTGCTCCTTAGAAAG | |
| Pyrosequencing primer 1 | TTGTACACACCGCCC | |
| Pyrosequencing primer 2 | ATCGGCGATTGGGAC | |
| embB | 76 | |
| Forward primer | ACGACGGCTACATCCTGG | |
| Reverse primer | Biotin-CCGAACCAGCGGAAATAG | |
| Pyrosequencing primer | CGACGGCTACATCCTG |
For gyrA, pyrosequencing primer 1 covers codon 80, and pyrosequencing primer 2 covers codons 88 to 95; for rrs, pyrosequencing primer 1 covers codons 1401 to 1402, and pyrosequencing primer 2 covers codon 1484; for embB, the pyrosequencing primer covers codon 306.
GenoType MTBDRsl.
The GenoType MTBDRsl test was carried out according to the manufacturer's instructions. The GenoType MTBDRsl strip contains 20 reaction zones; 14 of them are mutation probes, and 6 are control probes to verify the test procedures [conjugate control (CC), amplification control (AC), M. tuberculosis complex-specific control (TUB), gyrA amplification control, rrs amplification control, and embB amplification control]. For the detection of FLQ resistance, probes cover gyrA; for the detection of aminoglycosides and cyclic peptides resistance probes cover positions in the 16S rRNA; and finally, for the detection of EMB resistance, probes cover embB (Fig. 1). The absence of at least one of the wild-type bands or the presence of mutation bands for each drug resistance-related gene implies that the sample tested is resistant to the respective antibiotic. When all the wild-type probes for a gene stain positive, and there is no detectable mutation within the examined region, the sample tested is susceptible to the respective antibiotic. In order to consider a result valid, all six control bands should appear as expected; otherwise, the result is considered invalid. According to the manufacturer's instructions, when one of the mutation probes as well as the corresponding wild-type probe stains positive on the respective strip, the possibility of a heteroresistant strain should be considered. Persons who read and recorded the GenoType MTBDRsl bands results were blind to the Bactec 460TB and pyrosequencing results.
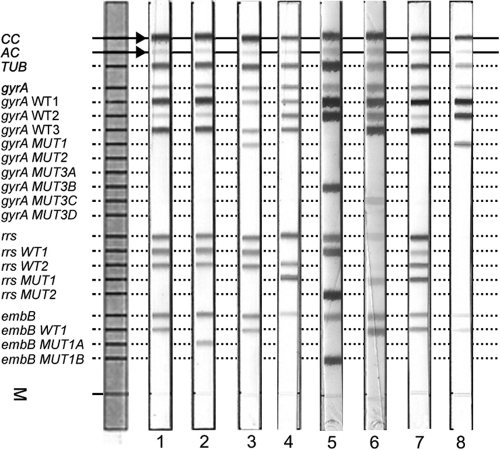
Fig 1.
Representative DNA patterns obtained with GenoType MTBDRsl. The positions of the oligonucleotides and control probes are given on the left. The targeted genes and specific probes lines are shown from top to bottom as follows: conjugate control (CC); amplification control (AC); M. tuberculosis complex-specific control (TUB); gyrA amplification control; gyrA wild-type probes WT1 to WT3 (85–90, 89–93 and 92–97); gyrA mutant probes MUT1, MUT2, MUT3A, MUT3B, MUT3C, and MUT3D for codons A90V, S91P, D94A, D94N, D94Y, D94G, and D94H, respectively; rrs amplification control; rrs wild-type probes WT1 (codons 1401 and 1402) and WT2 (codon 1484); rrs mutant probes MUT1 and MUT2, with A1401G and G1484T changes, respectively; embB amplification control; embB wild-type probe WT1, covering codon 306; and embB probes MUT1A and MUT1B for the mutations M306I and M306V, respectively. Lane 1, example of an FLQs KM/CMs EMBs pattern; lane 2, FLQs KM/CMs EMBr; lane 3, FLQr KM/CMs EMBs; lane 4, FLQs KM/CMr EMBr; lane 5, FLQr KM/CMr EMBr; lane 6, FLQr KM/CMr EMBs; lane 7, FLQs KM/CMr (mixed WT1 and MUT1 bands) EMBs; lane 8, FLQr, invalid result for rrs, and EMBs.
RESULTS
GenoType MTBDRsl assay results for the clinical strains.
Thirty-four M. tuberculosis clinical strains were processed by the GenoType MTBDRsl assay. All results for FLQ and EMB were valid, whereas for KM/CM, no valid results were obtained in two cases. Invalid results corresponded to two strips in which the locus control was absent, as well as the wild-type and mutation bands for the rrs gene. Susceptibility pattern results obtained by the GenoType MTBDRsl were compared with those obtained by the Bactec 460TB and are shown in Table 3.
Table 3.
Distribution of GenoType MTBDRsl results according to Bactec 460TB result for the 34 clinical strains
| Drug and Bactec result (no. of isolates) | No. of isolates (%) with the following GenoType MTBDRsl result |
||
|---|---|---|---|
| Resistant | Sensitive | No valid result | |
| FLQ | |||
| Resistant (7) | 4 (57.1) | 3 (42.9) | |
| Sensitive (22) | 5 (22.7) | 17 (77.3) | |
| Total (29) | 9 (31) | 20 (69) | |
| KM/CM | |||
| Resistant (5) | 5 (100) | 0 | |
| Sensitive (24) | 3 (13.6) | 19 (86.4) | 2 |
| Total (29) | 8 (29.6) | 19 (70.4) | |
| EMB | |||
| Resistant (23) | 14 (60.9) | 9 (39.1) | |
| Sensitive (11) | 2 (18.1) | 9 (81.9) | |
| Total (34) | 16 (47) | 18 (53) | |
For 17 strains, there was complete agreement between Bactec 460TB and GenoType MTBDRsl results, while for 15, there was disagreement for some of the drugs. These 15 strains presented a valid result for the three genes analyzed. Eleven strains had a disagreement result for one drug, three strains had disagreements for two drugs, and for one strain, none of the drug results matched. In addition, we obtained invalid results for one drug test (KM/CM) in two strains. For one of these strains, the results for the two remaining drugs (FLQ and EMB) matched, and for the other strain, the two drug results did not match.
Of the seven strains identified as FLQr by Bactec 460TB, four were correctly identified as resistant by GenoType MTBDRsl. The three strains identified as FLQs had the following pattern: presence of WT1, WT2, and WT3 and no MUT bands. According to pyrosequencing, the gyrA codons analyzed were wild type in all three strains. Of the 22 strains identified as FLQs by Bactec 460TB, 17 were correctly identified as sensitive by GenoType MTBDRsl and 5 were identified as resistant. For one strain, GenoType MTBDRsl reported the band pattern WT1, WT2, and MUT1, which corresponds to an example of a heteroresistant strain. In addition, the absence of WT3 also indicates that the strain has a mutation between codons 92 and 97. In fact, pyrosequencing detected a D94Y mutation that should theoretically have been detected by GenoType MTBDRsl (MUT3B). Another strain had the following pattern: WT1, WT3, and MUT1, with the absence of WT2. By pyrosequencing, we confirmed the presence of the mutation A90V. Another strain had the pattern WT1, WT2, and MUT3B (D94Y or D94N), and pyrosequencing did not identify mutations in the codons analyzed. For the two remaining strains, GenoType MTBDRsl and pyrosequencing results agreed: one strain had D94G and the other had S91P.
All five strains identified as KM/CMr by Bactec 460TB were correctly identified as resistant by GenoType MTBDRsl. Of the 24 strains identified as sensitive by Bactec 460TB, GenoType MTBDRsl identified 19 as sensitive and 3 as resistant, and 2 did not yield a valid result. Among strains that were resistant according to GenoType MTBDRsl, one had the profile WT1, WT2, and MUT1. This combination of WT and MUT bands indicates that the tested strain is heteroresistant for this drug. According to pyrosequencing, positions 1401, 1402, and 1484 were wild type. For the remaining two strains, the profile was WT2 and MUT1, which corresponds to A1401G; both cases were confirmed by pyrosequencing. For the two strains with no valid result in the GenoType assay, no bands were obtained in the strip section corresponding to the rrs gene.
Of the 23 strains identified as EMBr by Bactec 460TB, 14 were correctly identified as resistant and 9 were identified as sensitive by GenoType MTBDRsl (presence of WT1 and absence of MUT1A and MUT1B); for these, pyrosequencing detected wild-type sequence at codon 306 (ATG). Among the 11 strains identified as EMBs by Bactec 460TB, nine were correctly identified as sensitive and two as resistant by GenoType MTBDRsl. The latter had the absence of the WT and presence of the MUT1B band, which corresponds to the M306V mutation. By pyrosequencing, the codon sequence was GTG, which also codes for valine, confirming the GenoType MTBDRsl result.
GenoType MTBDRsl assay results for the clinical specimens.
Fifty-four clinical samples were processed by GenoType MTBDRsl. Five samples were smear negative, and the remaining 49 were smear positive. For four clinical samples (two smear positive and two smear negative), we obtained invalid GenoType MTBDRsl results. Two samples had invalid results only for the embB gene/position. The remaining two samples (one smear positive and one smear negative) had invalid results for three genes tested. Susceptibility patterns obtained with the GenoType MTBDRsl were compared with those obtained with the Bactec 460TB and are shown in Table 4. Overall, in 25 cases there was complete agreement between Bactec 460TB and GenoType MTBDRsl results. In 27 cases, there was no agreement: in 22 of these, the disagreement was for 1 drug; in 4, the disagreement was for 2 drugs; and in 1, the disagreement was for the three drugs. For two samples, two drug patterns matched, and for the last drug, no valid result was obtained. For the remaining two samples, no valid results were obtained for any of the drugs.
Table 4.
Distribution of GenoType MTBDRsl results according to Bactec 460TB result for the 54 clinical samples
| Drug and Bactec result (no. of clinical samples) | No. of samples (%) with the following GenoType MTBDRsl result |
||
|---|---|---|---|
| Resistant | Sensitive | No valid result | |
| FLQ | |||
| Resistant (8) | 3 (37.5) | 5 (62.5) | |
| Sensitive (46) | 2 (4.5) | 42 (95.5) | 2 |
| Total (54) | 5 (9.6) | 47 (90.4) | |
| KM/CM | |||
| Resistant (23) | 23 (100) | 0 | |
| Sensitive (31) | 4 (13.8) | 25 (86.2) | 2 |
| Total (54) | 27 (52) | 25 (48) | |
| EMB | |||
| Resistant (42) | 22 (55) | 18 (45) | 2 |
| Sensitive (12) | 4 (40) | 6 (60) | 2 |
| Total (54) | 26 (52) | 24 (48) | |
Of the eight FLQr samples, three were identified as resistant and five as sensitive. For the latter, pyrosequencing identified all sequenced codons as wild type. Of the 46 FLQs strains, 42 were identified as sensitive and 2 as resistant, and for the remaining two, no result was obtained. According to GenoType MTBDRsl, one strain had WT1, WT2, WT3, and MUT3B bands, and the other had WT1, WT2, WT3, and MUT3C bands, indicating heteroresistance. For both cases, a mutation was identified at codon 94. Interestingly, by pyrosequencing, all codons were wild type.
All 23 KM/CMr samples were identified as resistant. GenoType MTBDRsl patterns were as follows: 22 strains had WT2 and MUT1 bands, and 1 had WT1, WT2, and MUT1 bands. Of the 31 samples identified as KM/CMs by Bactec 460TB, GenoType MTBDRsl identified 25 as sensitive and 4 as resistant, and for the last 2 samples, no valid results were obtained. For the four strains identified as resistant by GenoType MTBDRsl, there were discrepancies between the GenoType MTBDRsl and pyrosequencing results. One sample showed the absence of both WT bands and the presence of MUT1, which theoretically corresponds only to A1401G. However, by pyrosequencing we detected two mutations, C1402A and G1484T. Although the GenoType MTBDRsl and pyrosequencing results are discordant, both methods identified the sample as resistant. One sample showed only the WT1 band, which correspond to a G1484T mutation (absence of WT2), but pyrosequencing identified all positions as wild type. The two remaining samples showed only the WT2 band, which corresponds to C1402T (absence of WT1), but pyrosequencing identified all positions as wild type.
Of the 42 samples identified as EMBr by Bactec 460TB, 22 were identified as resistant and 18 as sensitive, and the last 2 samples did not yield valid results. The 18 samples with the sensitive pattern showed the presence of only the WT1 band. By pyrosequencing, 17 of them showed the wild-type sequence for codon 306 (ATG). The remaining discordant sample had a mutation at codon 306 (ATC) identified by pyrosequencing that should had been identified by GenoType MTBDRsl as the absence of the WT band. Of the 12 EMBs samples, 6 were identified as sensitive and 4 as resistant, and the last 2 samples did not yield valid results. Three samples identified as resistant by GenoType MTBDRsl showed the same mutation profile in GenoType MTBDRsl and pyrosequencing: absence of WT and presence of MUT1A (M306I), which corresponds to ATA for codon 306. The last sample's pattern showed the presence of both WT and MUT1A bands, and by pyrosequencing, we found ATA for codon 306.
Agreement values between Bactec 460TB and GenoType MTBDRsl results according to the drug considered are shown in Table 5. The highest agreement value was found for KM/CM, while the lowest value corresponded to EMB. Specificity and sensitivity values of GenoType MTBDRsl for detecting FLQ, KM/CM, and EMB resistance in clinical strains and clinical samples (considering only one sample per patient) are shown in Table 6.
Table 5.
Agreement between Bactec 460TB and GenoType MTBDRsl results according to the drug considered
| Drug | Clinical strains |
Clinical samples |
||||
|---|---|---|---|---|---|---|
| Agreement (%) | Kappa | SE | Agreement (%) | Kappa | SE | |
| FLQ | 21/29 (72.4) | 0.314 | 0.190 | 45/52 (86.5) | 0.389 | 0.184 |
| KM/CM | 24/27 (88.8) | 0.701 | 0.155 | 48/52 (92.3) | 0.847 | 0.073 |
| EMB | 23/34 (67.6) | 0.366 | 0.144 | 28/50 (56) | 0.098 | 0.116 |
Table 6.
Sensitivity and specificity of GenoType MTBDRsl for detecting resistance to FLQ, KM/CM, and EMB in clinical strains and clinical samplesa
| Drug | Clinical strains |
Clinical samples |
||
|---|---|---|---|---|
| Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | |
| FLQ | 4/7 (57.1) (20.2–88.1) | 17/22 (77.3) (54.1–91.3) | 1/3 (33.3) (1.7–87.4) | 11/13 (84.6) (53.6–97.2) |
| KM/CM | 5/5 (100) (46.2–100) | 19/22 (86.4) (64–96.4) | 4/4 (100) (39.5–100) | 7/12 (58.3) (28.5–83.5) |
| EMB | 14/23 (60.9) (38.7–79.5) | 9/11 (81.9) (47.7–96.7) | 3/9 (33.3) (9–69) | 5/7 (71.4) (30.2–94.8) |
A total of 16 patients were included (one sample per patient). Sensitivity values are expressed as numbers of true detections of resistance by GenoType MTBDRsl/total number of resistant cases by Bactec 460TB. Specificity values are expressed as numbers of true detections of susceptibility by GenoType MTBDRsl/total number of susceptible cases by Bactec 460TB. CI, confidence interval.
DISCUSSION
At the moment, there are different molecular tests that identify MDR and XDR M. tuberculosis, and these are mainly DNA sequencing and line probe assays (11, 21, 29, 37). Interestingly, in recent years pyrosequencing has also appeared as a molecular test for the detection of M. tuberculosis strains resistant to INH and RIF (4, 12, 23). Resistance to EMB and FLQ has also been detected by means of pyrosequencing (6, 16). In order to increase the ability to detect resistance to second-line drugs and EMB, the GenoType MTBDRsl assay was developed. This test has been widely evaluated with clinical strains, but there is limited experience of its performance with clinical samples (7, 13, 18).
The sensitivities of the MTBDRsl test in confirming FLQ resistance in clinical strains and samples were 57.1% and 33.3%, respectively. Surprisingly, these values are lower than the ones reported in previous studies, which ranged from 70 to 90% (7, 8, 13, 14, 18, 20). One possible explanation is that the total number of FLQ-resistant specimens was 15, which is low in comparison to the number of specimens tested in other published studies. A total of eight cases (three strains and five clinical samples) had a gyrA wild-type pattern according to GenoType MTBDRsl and pyrosequencing, while Bactec 460TB indicated that these cases were resistant. Given that phenotypical methods are not standardized for second-line drugs, we cannot exclude the possibility that Bactec 460TB might have misclassified some results. Different mutations at gyrB have also been related to FLQ resistance (5, 32, 36). Given that mutation probes for gyrB are not included in the assay, it is possible that some isolates might have had mutations in these positions, which the assay could not detect. In seven cases (five strains and two clinical samples), we detected a mixture of WT and MUT bands for the same position, indicating the presence of heteroresistance. For two strains, the GenoType MTBDRsl profile was WT1, WT2, WT3, and MUT2; for another two strains, the profile was WT1, WT3, and MUT 1; and for the last strain, the profile was WT1, WT2, and MUT 1. Regarding clinical samples (from two different patients), profiles detected were WT1, WT2, WT3, and MUT3B and WT1, WT2, WT3, and MUT3C. The presence of a mixed wild-type and resistant population with regard to this drug has also been reported in previous studies (7, 13). Specificity values were around 80% and similar to values in the literature (7, 8, 13, 14, 18, 20). A natural polymorphism at gyrA codon 95 (ACC) unrelated to FLQ resistance has been described (31), and by pyrosequencing, we found it in 9 clinical samples (1 per patient) and 28 clinical strains.
The sensitivity of confirmation of KM/CM resistance by targeting rrs in clinical strains and samples was 100%, indicating that the test performs well in detecting the presence of mutations at rrs positions 1401, 1402, and 1482 (25, 26). Values from other studies are also in an acceptable range, from 75% to 80% (7, 13, 18). The specificity of detecting KM/CM resistance in clinical strains was 86.4%, which is slightly lower than values reported in the literature (7, 13, 14, 18). For two clinical strains, we reported the presence of WT1, WT2, and MUT1 bands, indicating a mixture of resistant and susceptible bacilli. The other example found corresponded to a clinical sample which also had the pattern WT1, WT2, and MUT1.
The low detection rate of EMB resistance is consistent with previous reports (7, 18, 40). It has been reported that 30 to 70% of EMB-resistant strains have a mutation in embB (3, 16, 40). Indeed, mutations at codon 306 have shown a strong correlation with EMB resistance. However, the low sensitivity implies that there is a need to identify other mutations conferring resistance to this drug. Huang et al. identified several mutations in embB other than that at codon 306 (14). Interestingly, we found that two EMB-susceptible strains harbored a mutation at codon 306. Brossier et al. also reported this fact, highlighting the need for a better understanding of the molecular basis of EMB resistance (7). Two strains resistant to EMB according to Bactec 460TB had an MTBDRsl profile indicating the absence of WT1, MUT1A, and MUT1B bands. By pyrosequencing, these strains showed an ATG → ATC mutation. One clinical sample also harbored a mixture of WT1 and MUT1A bands.
The agreement between the phenotypic reference method (Bactec 460TB) and GenoType MTBDRsl varies according to the drug considered. In our experience, the concordance for clinical strains was 88.8% and 72.4% for KM/CM and FLQ, respectively, whereas for EMB, the value was 67.6%. For clinical samples, agreement values were higher for FLQ and KM/CM and slightly lower for EMB. Values reported in the literature, obtained only for clinical strains, are around 90% for FLQ and KM/CM and 70% for EMB (14, 18).
It is important to remember that the main limitation of the molecular tests is that they are unable to target all possible mutations involved in resistance, and as a consequence, some resistant strains will not be detected. This is one of the explanations for our sensitivity values. Since only the more frequent mutations related to resistance are detected by the assay, results must be confirmed by phenotypic methods. Although common mutations predictive of resistance are well known for some drugs, in some cases the mutations identified are silent and are not always related to the acquisition of resistance. It is important to bear in mind that mutation prevalences differ by geographical region and setting. In addition, the exact ratio of resistant to susceptible bacilli that results in phenotypic resistance is unclear. In general, if the proportion of resistant cells in an isolate is less than 10% of mutant DNA, it will be difficult to detect by molecular methods, while the phenotypical methods might give a more sensitive test result in these cases (29). This means that in practice, as we have noticed, the GenoType MTBDRsl result can differ from that obtained by a proportion-based method, such as the Bactec 460TB system. Overall, we found 55 discordant drug results between these two assays (22 for strains and 33 for clinical samples). Although no systematic confirmation of Bactec 460TB was performed, one of these discordant strains was studied with the Bactec MGIT 960 system (Becton Dickinson Microbiology System, Sparks, MD), confirming the FQL, KM/CM, and EMB resistance result obtained with the Bactec 460TB. For clinical strains, pyrosequencing confirmed GenoType MTBDRsl results in 90% of the cases, while for clinical specimens, pyrosequencing confirmed 81% of the cases. These results confirm that GenoType MTBDRsl is an accurate method for the detection of mutations involved in M. tuberculosis resistance. However, the presence of discordant results, especially when GenoType MTBDRsl detected mutations but the Bactec 460TB obtained a susceptible pattern, raises some concerns about its utility in low-prevalence areas.
On the other hand, it is important to emphasize that phenotypical methods for detecting second-line drug susceptibility are not totally standardized, and therefore, strains which are identified as drug susceptible by Bactec 460TB but drug resistant (showing mutations) by molecular methods may be falsely drug susceptible, and Bactec 460TB results indicating that strains are drug resistant could be false if the molecular tests do not show any mutational changes. In addition, it continues to be unclear whether all mutations observed using the molecular methods are associated with drug resistance or could be just polymorphisms (29).
In conclusion, the GenoType MTBDRsl assay may be a useful tool for making early decisions regarding KM/CM susceptibility and, to a lesser extent, FLQ and EMB susceptibility. The test is able to detect mutations in both clinical strains and samples with a short turnaround time. However, for correct management of XDR TB patients, results have to be confirmed by a phenotypical method.
ACKNOWLEDGMENTS
This work was supported by grants from Fondo de Investigaciones Sanitarias (FIS07/0551) (Ministerio de Sanidad y Consumo, Madrid, Spain); and Sociedad Española de Neumología y Cirugía Torácica (SEPAR). J. Domínguez is funded by the “Miguel Servet” program of the Instituto de Salud Carlos III (Spain). N. García-Sierra is a FI predoctoral student and is a recipient of a grant from AGAUR (Agència de Gestió d'Ajuts Universitaris i de Recerca), Generalitat de Catalunya, Spain.
We especially thank Oriol Martos for his kind technical assistance.
None of the investigators has any financial interest or financial conflict with the subject matter or materials discussed in this report. Hain Lifescience did not play a role in the study design, conduct, collection, management, analysis, or interpretation of the data or the preparation, review, or approval of the manuscript.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Ahmadian A, Ehn M, Hober S. 2006. Pyrosequencing: history, biochemistry and future. Clin. Chim Acta 363:83–94 [DOI] [PubMed] [Google Scholar]
- 2. Alangaden GJ, et al. 1998. Mechanism of resistance to amikacin and kanamycin in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:1295–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alcaide F, Pfyffer GE, Telenti A. 1997. Role of embB in natural and acquired resistance to ethambutol in mycobacteria. Antimicrob. Agents Chemother. 41:2270–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arnold C, et al. 2005. Single-nucleotide polymorphism-based differentiation and drug resistance detection in Mycobacterium tuberculosis from isolates or directly from sputum. Clin. Microbiol. Infect. 11:122–130 [DOI] [PubMed] [Google Scholar]
- 5. Aubry A, et al. 2006. Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob. Agents Chemother. 50:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bravo LT, et al. 2009. Pyrosequencing for rapid detection of Mycobacterium tuberculosis resistance to rifampin, isoniazid, and fluoroquinolones. J. Clin. Microbiol. 47:3985–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. 2010. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 48:1683–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang KC, Yew WW, Chan RC. 2010. Rapid assays for fluoroquinolone resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J. Antimicrob. Chemother. 65:1551–1561 [DOI] [PubMed] [Google Scholar]
- 9. Diggle MA, Clarke SC. 2004. Pyrosequencing: sequence typing at the speed of light. Mol. Biotechnol. 28:129–137 [DOI] [PubMed] [Google Scholar]
- 10. Dominguez J, et al. 2008. Utility of molecular biology in the microbiological diagnosis of mycobacterial infections. Enferm. Infecc. Microbiol. Clin. 26(Suppl. 9):33–41 [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Sierra N, et al. 2011. Pyrosequencing for rapid molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 49:3683–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garza-Gonzalez E, et al. 2010. A pyrosequencing method for molecular monitoring of regions in the inhA, ahpC and rpoB genes of Mycobacterium tuberculosis. Clin. Microbiol. Infect. 16:607–612 [DOI] [PubMed] [Google Scholar]
- 13. Hillemann D, Rusch-Gerdes S, Richter E. 2009. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 47:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang WL, Chi TL, Wu MH, Jou R. 2011. Performance assessment of the GenoType MTBDRsl test and DNA sequencing for detection of second-line and ethambutol drug resistance among patients infected with multidrug-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 49:2502–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isenberg HD. (ed.). 2007. Clinical microbiology procedures handbook, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 16. Isola D, et al. 2005. A pyrosequencing assay for rapid recognition of SNPs in Mycobacterium tuberculosis embB306 region. J. Microbiol. Methods 62:113–120 [DOI] [PubMed] [Google Scholar]
- 17. Kent PT, Kubica GP. 1985. Public health mycobacteriology—a guide for the level III laboratory. U.S. Department of Health and Human Services, U.S. Government Printing Office, Washington, DC [Google Scholar]
- 18. Kiet VS, et al. 2010. Evaluation of the MTBDRsl test for detection of second-line-drug resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 48:2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim SJ, et al. 2004. Is second-line anti-tuberculosis drug susceptibility testing reliable? Int. J. Tuberc. Lung Dis. 8:1157–1158 [PubMed] [Google Scholar]
- 20. Kontsevaya I, et al. 2011. Evaluation of two molecular assays for the rapid detection of Mycobacterium tuberculosis resistance to fluoroquinolones in high TB and multidrug resistance settings. J. Clin. Microbiol. 49:2832–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacoma A, et al. 2008. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J. Clin. Microbiol. 46:3660–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ling DI, Zwerling AA, Pai M. 2008. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur. Respir. J. 32:1165–1174 [DOI] [PubMed] [Google Scholar]
- 23. Marttila HJ, Makinen J, Marjamaki M, Soini H. 2009. Prospective evaluation of pyrosequencing for the rapid detection of isoniazid and rifampin resistance in clinical Mycobacterium tuberculosis isolates. Eur. J. Clin. Microbiol. Infect. Dis. 28:33–38 [DOI] [PubMed] [Google Scholar]
- 24. Matrat S, et al. 2006. Functional analysis of DNA gyrase mutant enzymes carrying mutations at position 88 in the A subunit found in clinical strains of Mycobacterium tuberculosis resistant to fluoroquinolones. Antimicrob. Agents Chemother. 50:4170–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maus CE, Plikaytis BB, Shinnick TM. 2005. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 49:3192–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maus CE, Plikaytis BB, Shinnick TM. 2005. Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 49:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plinke C, Rusch-Gerdes S, Niemann S. 2006. Significance of mutations in embB codon 306 for prediction of ethambutol resistance in clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 50:1900–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramaswamy SV, et al. 2000. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richter E, Rusch-Gerdes S, Hillemann D. 2009. Drug-susceptibility testing in TB: current status and future prospects. Expert Rev. Respir. Med. 3:497–510 [DOI] [PubMed] [Google Scholar]
- 30. Somoskovi A, Parsons LM, Salfinger M. 2001. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir. Res. 2:164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sreevatsan S, et al. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. U. S. A. 94:9869–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takiff HE, et al. 1994. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Telenti A, et al. 1997. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 3:567–570 [DOI] [PubMed] [Google Scholar]
- 34. Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. 2011. Rate of recovery of Mycobacterium tuberculosis from frozen acid-fast-bacillus smear-positive sputum samples subjected to long-term storage in northwest Ethiopia. J. Clin. Microbiol. 49:2557–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Ingen J, et al. 2010. Comparative study on genotypic and phenotypic second-line drug resistance testing of Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 48:2749–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang JY, et al. 2007. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates: associated genetic mutations and relationship to antimicrobial exposure. J. Antimicrob. Chemother. 59:860–865 [DOI] [PubMed] [Google Scholar]
- 37. Wilson ML. 2011. Recent advances in the laboratory detection of Mycobacterium tuberculosis complex and drug resistance. Clin. Infect. Dis. 52:1350–1355 [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization 2010. Global tuberculosis control. (WHO/HTM/TB/2010.7) World Health Organization, Geneva, Switzerland [Google Scholar]
- 39. World Health Organization 2001. Guidelines for drug susceptibil-ity testing for second-line anti-tuberculosis drugs for DOT-plus. WHO/CDS/TB/2001.288 World Health Organization, Geneva, Switzerland [Google Scholar]
- 40. Zhang Y, Yew WW. 2009. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 13:1320–1330 [PubMed] [Google Scholar]