Abstract
Disseminated aspergillosis in dogs has been associated with Aspergillus terreus or A. deflectus infection. We report a case of disseminated A. versicolor infection presenting as diskospondylitis, osteomyelitis, and pyelonephritis. The diagnosis was made based on clinical, radiographic, and pathological findings. The etiologic agent was identified by fungal culture and internal transcribed spacer (ITS) ribosomal DNA (rDNA) sequencing. This is the first description of canine aspergillosis caused by A. versicolor.
CASE REPORT
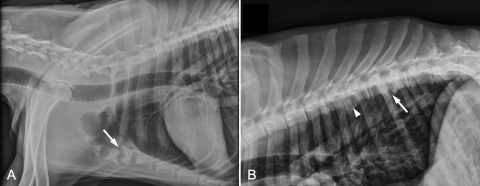
A 31-kg, 2.5-year-old male castrated German shepherd dog was examined at the Texas A&M University Veterinary Medicine Teaching Hospital because of nonambulatory paraparesis, weight loss, and hyporexia for 3 months. At admission, the dog had a body temperature of 104.6°F, heart rate of 160 beats/min, respiratory rate of 80 breaths/min, and blood pressure of 173/99 mm Hg. Neurological examination revealed a hyperreflexive patellar reflex bilaterally. Motor function was absent in the right pelvic limb and questionable in the left. The patient had marked generalized muscle atrophy. A lateral radiograph of the cranial thorax revealed lysis and shortening of the first four sternebrae (Fig. 1A). The second and third sternebrae were most severely affected and had irregular margins with loss of their end plates. Lateral radiographs of the thoracic vertebral column showed lysis and shortening of the 9th (T9) and 10th (T10) thoracic vertebrae with loss of the end plates and spondylosis deformans ventrally (Fig. 1B). Narrowing of the intervertebral space, end plate sclerosis, and ventral spondylosis derformans were also found between the seventh and eighth thoracic vertebrae. The clinical diagnoses were diskospondylitis involving T9-T10, osteomyelitis of the sternum and left humerus, and T3-L3 myelopathy resulting in nonambulatory paraparesis. Suspected causes included disseminated aspergillosis, blastomycosis, coccidioidomycosis, bacterial infection, and neoplasia. Due to the poor prognosis, the dog was subsequently euthanized and a complete necropsy was performed.
Fig 1.
(A) Lateral radiograph of the thorax. There is lysis of the first four sternebrae and marked shortening of the second and third sternebrae, which have irregular margins and loss of the end plates (arrow). (B) Lateral radiograph of the thoracic vertebral column. There is end plate lysis of the 9th (T9) and 10th (T10) thoracic vertebrae that is centered on the intervertebral space (arrow), with spondylosis deformans ventrally. There is also narrowing, end plate sclerosis, and spondylosis deformans between the seventh (T7) and eighth (T8) vertebrae (arrowhead).
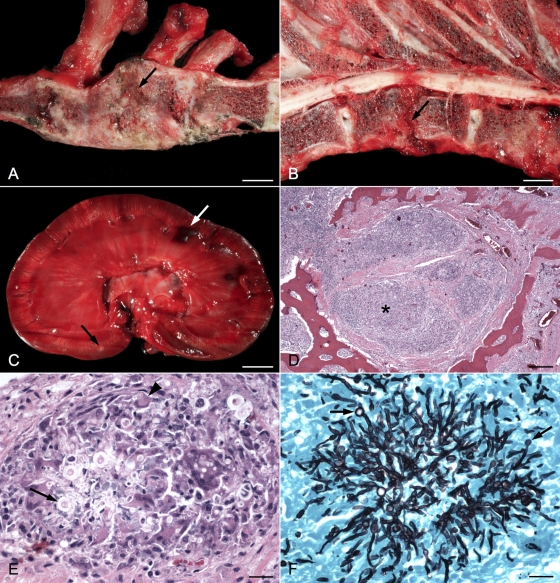
The major skeletal changes found at postmortem examination included marked bony proliferation of the cranial end of the sternum, extending from the first to the fourth sternebrae, with loss of the joint space between the second and third sternebrae (Fig. 2A). A soft gray area of necrotizing osteomyelitis was in the center of the collapsed and fused sternebrae. In the thoracic vertebral column, there was loss of the intervertebral disk at T9-T10 with lysis of the associated vertebral end plates (Fig. 2B). The latter changes resulted in joint instability, overriding of the vertebral bodies, and spinal cord compression that was exacerbated with ventroflexion of the vertebral column. In the kidneys, there was dark red to purple mottling of the cortical region with dozens of white to tan areas throughout the cortex and medulla (Fig. 2C). The renal crests were ulcerated, and the pelvises were dilated and contained a small amount of cloudy fluid with clumps of fibrin.
Fig 2.
(A) Right sagittal section of sternum; the first sternebra is on the left. The second and third sternebrae are collapsed, and areas of bony proliferation obscure the joint space. An area of necrotizing osteomyelitis partially separates the two sternebrae (arrow). Bar, 1 cm. (B) Left sagittal section of thoracic vertebrae; the cranial end is to the right. The intervertebral disk at T9-T10 is missing (arrow). The end plates are eroded, and a wedge-shaped piece of tissue compresses the spinal cord dorsally. Bar, 1 cm. (C) Sagittal section of left kidney. Small white areas are scattered throughout the cortex and medulla (black arrow). The pelvis is dilated, and the renal crest is ulcerated. Areas of hemorrhage (white arrow) are visible in the cortex. Bar, 1 cm. (D) Photomicrograph of second sternebra showing areas of inflammation (*) and surrounding fibrous tissue invading and replacing the marrow cavity. Bar, 250 μm. (E) Higher magnification of sternebra showing marked granulomatous inflammation with giant cell formation (arrowhead) surrounding septate fungal hyphae and bulbous spore-like structures (arrow). Bar, 25 μm. (F) Grocott's methenamine silver-stained section of sternebra taken from same area as previous image, demonstrating prominent fungal hyphae and terminal conidiophores (arrows). Bar, 25 μm.
Samples of tissue from all major organs were placed in 10% formalin and processed in a standard manner, and 4-μm sections were stained with hematoxylin and eosin or Grocott's methenamine silver. Histologically, the affected sternebrae and vertebrae contained large, locally extensive areas of chronic granulomatous inflammation that replaced the marrow cavity and resulted in extensive areas of bone loss (Fig. 2D). Affected areas contained large numbers of epithelioid macrophages and many Langhans-type multinucleated giant cells that often surrounded clusters of poorly discernible septate fungal hyphae and bulbous spore-like structures (Fig. 2E). Grocott's methenamine silver-stained sections revealed large numbers of regularly septate fungal hyphae with 5-μm-wide parallel walls; dichotomous branching; bulbous, 10-μm-diameter terminal conidiophores; and occasional laterally branching spherical aleuriospores, whose morphology was consistent with Aspergillus spp. (Fig. 2F). Similar areas of granulomatous inflammation with intralesional fungal hyphae and bulbous structures were found in the kidney, spleen, and liver and sternal, vertebral, and axillary lymph nodes. Additionally, areas of granulomatous and necrotizing vasculitis were found in the kidneys and peristernal soft tissues.
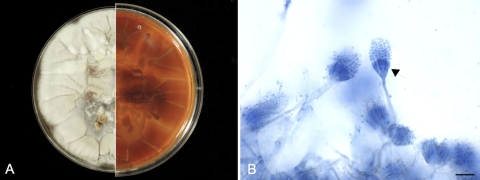
Sternebral and vertebral swabs taken from affected areas of bone and a sample of kidney were cultured on Trypticase soy agar with 5% sheep blood, Sabouraud's dextrose agar, and potato dextrose agars (Remel, Lenexa, KS). After 1 week of incubation at room temperature, pure fungal growth was obtained. The surface of the fungal colonies, which had a downy texture, was initially white but turned to a light tan color after 7 days (Fig. 3A). The reverse side was yellow to brown. Microscopically, the colonies had brush-like and radiate conidial heads, ellipsoidal to round vesicles, biseriate phialides, and spherical conidia in short chains (Fig. 3B). The morphological characteristics of the fungus were suggestive of A. versicolor. To confirm the identification, PCR amplification was performed on a sample of DNA extracted from the fungus to amplify the internal transcribed spacer (ITS) region of rRNA genes, using primers ITS-1 and ITS-4 (27). A BLAST search against the GenBank nr database showed 99.8% and 98.7% identities between the amplicon and the rRNA ITS regions of A. versicolor and A. sydowii, respectively. The amplicon sequence was then compared with the ITS sequences of Aspergillus species commonly associated with canine aspergillosis using the San Diego Supercomputer Center (SDSC) Biology Workbench nucleic acid tools. The results indicated that the isolate shared 97.3%, 88.8%, 87.2%, 85.9%, and 85.7% identities with A. nidulans, A. deflectus, A. fumigatus, A. flavus, and A. terreus, respectively. According to CLSI interpretive criteria for identification of bacteria and fungi by DNA target sequencing, the fungal isolate was identified as A. versicolor (34).
Fig 3.
(A) Macroscopic colonial morphology of the A. versicolor isolate. The culture was incubated at room temperature for 10 days. The surface of the fungal colonies is white to light tan (left), and the reverse side is yellow to brown (right). (B) Lactophenol blue staining reveals brush-like and radiate conidial heads with round vesicles, biseriate phialides (arrow), and spherical conidia in short chains. Bar, 15 μm.
The genus Aspergillus has recently been classified into 8 distinct subgenera, including Aspergillus, Fumigati, Circumdati, Terrei, Nidulantes, Ornati, Warcupi, and Candidi (33). These subgenera are further divided into 22 sections, each of which encompasses a number of related species (33). Although there are more than 200 known species in the genus, only a small percentage are associated with infections. Among them, A. fumigatus (subgenus Fumigati, section Fumigati), A. flavus (subgenus Circumdati, section Flavi), and A. niger (subgenus Circumdati, section Nigri) are the most frequently encountered species (13, 17, 33). Others, such as A. terreus (subgenus Terrei, section Terrei) and A. versicolor (subgenus Nidulantes, section Nidulantes), are occasionally isolated from clinical specimens (3, 29). The disease conditions, ranging from localized skin infection, nail infection, ocular infection, and pulmonary disorder to invasive systemic Aspergillus infection causing diskospondylitis, osteomyelitis, and pyelonephritis, are collectively referred to as “aspergillosis,” an umbrella term coined by Hinson, Moon, and Plummer in 1952 (7, 30, 31, 38, 42).
In dogs, the three major forms of aspergillosis are nasal, bronchopulmonary, and disseminated infections. The nasal form, frequently accompanied by invasive sinusitis, occurs most commonly in medium to large, dolichocephalic or mesaticephalic breeds (32). The primary etiologic agent is A. fumigatus, followed by A. flavus and A. niger (39). The clinical signs include sneezing, unilateral or bilateral nasal discharge, rhinalgia, epistaxis, frontal sinus osteomyelitis, anorexia, and lethargy (28, 32). In advanced cases, ulceration of the nares, facial deformity due to paranasal extension, and ocular involvement may be evident. Radiographs may show turbinate tissue destruction with large radiolucent spaces. Fungal plaques in the nasal cavity may be observed by rhinoscopy (28, 32). Bronchopulmonary aspergillosis is a rare disease in dogs (1, 40, 44). The causative agents and the breeds being affected are similar to those seen in the nasal form of aspergillosis (1, 5, 8, 25). The clinical signs are nonspecific, including depression, fever, and cough (1, 5, 25). Cytologic evaluation of the bronchoalveolar lavage fluid often reveals a mixed inflammatory response dominated by neutrophils and macrophages but rarely reveals the presence of fungal elements (5). Chest radiographs can demonstrate diffuse nodular lesions in the lung. Disseminated aspergillosis is a relatively infrequent but potentially fatal disease in dogs. The two most common etiologic agents are A. terreus and A. deflectus, followed by A. fumigatus, A. niger, and A. flavipes in order of decreasing frequency (37). Disseminated A. terreus infection in a dog was first reported in 1978 (45). This was followed by a description of disseminated A. deflectus infection in 4 dogs in 1986 (20). Additional case reports and case series have provided useful information regarding the etiology, clinical course, pathological changes, and prognosis pertaining to this disease (12, 14, 22, 24). In some of these cases, aleuriospores were observed in infected tissues, which led to the hypothesis that the ability of A. terreus and A. deflectus to produce aleuriospores enhances their ability to effectively disseminate via hematogenous routes (12, 20).
The majority of the reported cases of disseminated aspergillosis in dogs involve young to middle-aged females. A recent study of systemic aspergillosis in 30 dogs reported a mean age of 4.5 years, with a range of 2 to 8 years and a female-to-male ratio of approximately 3.1:1 (37). The German shepherd dog is the most commonly affected breed; however, other breeds, including the Dalmatian, English setter, pug, Rhodesian ridgeback, springer spaniel, and whippet, have occasionally been affected (21, 22, 37). Comparative studies of serum immunoglobulin concentrations in healthy dogs indicate that the IgA level in the German shepherd dog is significantly lower than that in other breeds (11, 18, 43). This IgA deficiency has been suggested as a possible predisposing factor for disseminated aspergillosis (11). German shepherd dogs with disseminated aspergillosis usually have normal complement activity and increased serum IgG levels. However, Aspergillus-specific serum antibody can be detected in only 44% to 69% of infected dogs, depending on the serological test used (10). Some infected German shepherd dogs have depressed IgM responses or impaired mitogen-induced lymphocyte transformation (9). These findings highlight the importance of humoral mucosal immunity and cell-mediated immunity in the prevention and clearance of Aspergillus infection (9). In the present case, tests were not performed to determine the immune status of the dog. A review of medical records showed no history of specific immunosuppression; however, this could not rule out any undiagnosed immunodeficiency.
Clinical signs of disseminated aspergillosis may develop suddenly or slowly over a few months. Clinical presentations of disseminated aspergillosis may include diskospondylitis, osteomyelitis, spinal hyperpathia, vestibular abnormalities, ataxia, paraparesis, weight loss, anorexia, uveitis, lameness, renal failure, and respiratory distress (4, 6, 35, 37). Leukocytosis, hyperglobulinemia, azotemia, and hypercalcemia are common clinicopathologic features (37). Granulomatous inflammation in multiple organs, including bone, kidney, and spleen, is frequently observed (4, 6, 21, 35), as it was in the current case. The disease can generally be diagnosed based on clinical, radiographic, and pathological findings. Since disseminated mycosis caused by other fungal species, including Penicillium spp., may mimic disseminated aspergillosis (37), fungal culture is necessary to confirm the clinical diagnosis and identify the specific organism involved. Treatment of disseminated aspergillosis is difficult, and the prognosis is usually unfavorable. Retrospective studies indicate that the disease is refractory to amphotericin B treatment (37, 45). Long-term treatment of up to 3 years with itraconazole may clear the infection or prolong the survival time (24). Although the causes of therapeutic failure are multifactorial, delayed initiation of treatment is certainly a major reason, as the dogs are usually presented in advanced stages of the infection. In addition, fungal resistance to chemotherapeutic agents is another key contributing factor. Analyses of in vitro antifungal susceptibility patterns of various Aspergillus isolates suggest that non-A. fumigatus Aspergillus species are intrinsically resistant to amphotericin B (41).
In the present case, the dog was presented with advanced disease and a portal of entry for A. versicolor was not identified by clinical or postmortem examinations. We hypothesized that fungal spores gained entry through inhalation or an open wound that had healed at the time of examination. Subsequent hematogenous dissemination was possible because the fungal species did produce terminal and lateral spores in the infected tissue (Fig. 2F). The clinical and pathological changes of the present case resembled those caused by A. terreus and A. deflectus. Due to the severity of the disease and the poor prognosis, antifungal therapy was not initiated. Like other species of Aspergillus, A. versicolor is ubiquitous in nature and can be isolated from soil, water, organic matter, bathrooms, carpets, and mattresses (2, 15, 16). It is commonly found on water-damaged building material such as wallpaper or fiberboard insulation. A. versicolor infections in people have been associated with onychomycosis and pulmonary aspergillosis (19, 29, 30). Mycotoxins produced by A. versicolor such as sterigmatocystin have been shown to cause DNA damage in vitro and are potentially carcinogenic (36). A. versicolor has been implicated in a case of equine subcutaneous mycetoma and two cases of equine guttural pouch mycosis (23, 26). The findings from the present case indicate that A. versicolor should be considered one of the causative agents of canine disseminated aspergillosis.
ACKNOWLEDGMENTS
We thank Jay Griffin for assistance in interpretation of the radiographs and Amanda Engram for technical assistance.
Footnotes
Published ahead of print 26 October 2011
REFERENCES
- 1. Adamama-Moraitou KK, et al. 2011. Aspergillus fumigatus bronchopneumonia in a Hellenic shepherd dog. J. Am. Anim. Hosp. Assoc. 47:e13–e18 [DOI] [PubMed] [Google Scholar]
- 2. Andersen B, Frisvad JC, Søndergaard I, Rasmussen IS, Larsen LS. 2011. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 77:4180–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balajee SA. 2009. Aspergillus terreus complex. Med. Mycol. 47(Suppl. 1):S42–S46 [DOI] [PubMed] [Google Scholar]
- 4. Berry WL, Leisewitz AL. 1996. Multifocal Aspergillus terreus discospondylitis in two German shepherd dogs. J. S. Afr. Vet. Assoc. 67:222–228 [PubMed] [Google Scholar]
- 5. Billen F, Clercx C, Le Garérrès A. 2009. Effect of sampling method and incubation temperature on fungal culture in canine sinonasal aspergillosis. J. Small Anim. Pract. 50:67–72 [DOI] [PubMed] [Google Scholar]
- 6. Bruchim Y, Elad D, Klainbart S. 2006. Disseminated aspergillosis in two dogs in Israel. Mycoses 49:130–133 [DOI] [PubMed] [Google Scholar]
- 7. Camus M, et al. 2010. Primary cutaneous aspergillosis in an immunocompetent farmworker. Ann. Dermatol. Venereol. 137:373–376 [DOI] [PubMed] [Google Scholar]
- 8. Clercx C, McEntee K, Snaps F, Jacquinet E, Coignoul F. 1996. Bronchopulmonary and disseminated granulomatous disease associated with Aspergillus fumigatus and Candida species infection in a golden retriever. J. Am. Anim. Hosp. Assoc. 32:139–145 [DOI] [PubMed] [Google Scholar]
- 9. Day MJ, Eger CE, Shaw SE, Penhale WJ. 1985. Immunologic study of systemic aspergillosis in German shepherd dogs. Vet. Immunol. Immunopathol. 9:335–347 [DOI] [PubMed] [Google Scholar]
- 10. Day MJ, Penhale WJ. 1988. Humoral immunity in disseminated Aspergillus terreus infection in the dog. Vet. Microbiol. 16:283–294 [DOI] [PubMed] [Google Scholar]
- 11. Day MJ, Penhale WJ. 1988. Serum immunoglobulin A concentrations in normal and diseased dogs. Res. Vet. Sci. 45:360–363 [PubMed] [Google Scholar]
- 12. Day MJ, et al. 1986. Disseminated aspergillosis in dogs. Aust. Vet. J. 63:55–59 [DOI] [PubMed] [Google Scholar]
- 13. de Hoog GS, Guarro J, Gene J, Figueras MJ. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili, Utrecht, The Netherlands [Google Scholar]
- 14. Elad D, Lahav DD, Blum S. 2008. Transuterine transmission of Aspergillus terreus in a case of disseminated canine aspergillosis. Med. Mycol. 46:175–178 [DOI] [PubMed] [Google Scholar]
- 15. Engelhart S, et al. 2002. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl. Environ. Microbiol. 68:3886–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flanning B, Samson RA, Miller DJ. (ed). 2001. Microorganisms in home and indoor work environments: diversity, health impacts, investigation and control. Taylor and Francis, New York, NY [Google Scholar]
- 17. Greub G, Bille J. 1998. Aspergillus species isolated from clinical specimens: suggested clinical and microbiological criteria to determine significance. Clin. Microbiol. Infect. 4:710–716 [DOI] [PubMed] [Google Scholar]
- 18. Griot-Wenk ME, et al. 1999. Total serum IgE and IgA antibody levels in healthy dogs of different breeds and exposed to different environments. Res. Vet. Sci. 67:239–243 [DOI] [PubMed] [Google Scholar]
- 19. Hinson KF, Moon AJ, Plummer NS. 1952. Bronchopulmonary aspergillosis; a review and a report of eight new cases. Thorax 7:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jang SS, Dorr TE, Biberstein EL, Wong A. 1986. Aspergillus deflectus infection in four dogs. J. Med. Vet. Mycol. 24:95–104 [PubMed] [Google Scholar]
- 21. Kabay MJ, Robinson WF, Huxtable CR, McAleer R. 1985. The pathology of disseminated Aspergillus terreus infection in dogs. Vet. Pathol. 22:540–547 [DOI] [PubMed] [Google Scholar]
- 22. Kahler JS, Leach MW, Jang S, Wong A. 1990. Disseminated aspergillosis attributable to Aspergillus deflectus in a springer spaniel. J. Am. Vet. Med. Assoc. 197:871–874 [PubMed] [Google Scholar]
- 23. Keegan KG, Dillavou CL, Turnquist SE, Fales WH. 1995. Subcutaneous mycetoma-like granuloma in a horse caused by Aspergillus versicolor. J. Vet. Diagn. Invest. 7:564–567 [DOI] [PubMed] [Google Scholar]
- 24. Kelly SE, Shaw SE, Clark WT. 1995. Long-term survival of four dogs with disseminated Aspergillus terreus infection treated with itraconazole. Aust. Vet. J. 72:311–313 [DOI] [PubMed] [Google Scholar]
- 25. Kim SH, et al. 2003. Aspergillus niger pulmonary infection in a dog. J. Vet. Med. Sci. 65:1139–1140 [DOI] [PubMed] [Google Scholar]
- 26. Ludwig A, Gatineau S, Reynaud MC, Cadoré JL, Bourdoiseau G. 2005. Fungal isolation and identification in 21 cases of guttural pouch mycosis in horses (1998-2002). Vet. J. 169:457–461 [DOI] [PubMed] [Google Scholar]
- 27. Manter DK, Vivanco JM. 2007. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 71:7–14 [DOI] [PubMed] [Google Scholar]
- 28. Meler E, Dunn M, Lecuyer M. 2008. A retrospective study of canine persistent nasal disease: 80 cases (1998-2003). Can. Vet. J. 49:71–76 [PMC free article] [PubMed] [Google Scholar]
- 29. Moreno G, Arenas R. 2010. Other fungi causing onychomycosis. Clin. Dermatol. 28:160–163 [DOI] [PubMed] [Google Scholar]
- 30. Morgan J, et al. 2005. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med. Mycol. 43(Suppl. 1):S49–S58 [DOI] [PubMed] [Google Scholar]
- 31. Ozer B, Kalaci A, Duran N, Dogramaci Y, Yanat AN. 2009. Cutaneous infection caused by Aspergillus terreus. J. Med. Microbiol. 58:968–970 [DOI] [PubMed] [Google Scholar]
- 32. Peeters D, Clercx C. 2007. Update on canine sinonasal aspergillosis. Vet. Clin. North Am. Small Anim. Pract. 37:901–916 [DOI] [PubMed] [Google Scholar]
- 33. Peterson SW, Varga J, Frisvad JC, Samson RA. 2008. Phylogeny and subgeneric taxonomy of Aspergillus, p 33–56 In Varga J, Samson RA. (ed), Aspergillus in the genomics era. Wageningen Academic Publishers, Wageningen, The Netherlands [Google Scholar]
- 34. Petti AC, et al. 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI document MM18-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 35. Robinson WF, Connole MD, King TJ, Pitt JI, Moss SM. 2000. Systemic mycosis due to Aspergillus deflectus in a dog. Aust. Vet. J. 78:600–602 [DOI] [PubMed] [Google Scholar]
- 36. Sakai M, et al. 1992. Genotoxicity of fungi evaluated by SOS microplate assay. Nat. Toxins 1:27–34 [DOI] [PubMed] [Google Scholar]
- 37. Schultz RM, et al. 2008. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J. Vet. Intern. Med. 22:851–859 [DOI] [PubMed] [Google Scholar]
- 38. Shah A. 2010. Concurrent allergic bronchopulmonary aspergillosis and aspergilloma: is it a more severe form of the disease? Eur. Respir. Rev. 19:261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharp NJH, Mathews KG. 2006. Canine nasal aspergillosis-penicilliosis, p 613–627 In Greene CE. (ed), Infectious diseases of the dog and cat, 3rd ed. Elsevier Saunders, Edinburgh, United Kingdom [Google Scholar]
- 40. Southard C. 1987. Bronchopulmonary aspergillosis in a dog. J. Am. Vet. Med. Assoc. 190:875–877 [PubMed] [Google Scholar]
- 41. Van Der Linden JW, Warris A, Verweij PE. 2011. Aspergillus species intrinsically resistant to antifungal agents. Med. Mycol. 49(Suppl. 1):S82–S89 [DOI] [PubMed] [Google Scholar]
- 42. Veraldi S, Chiaratti A, Harak H. 2010. Onychomycosis caused by Aspergillus versicolor. Mycoses 53:363–365 [DOI] [PubMed] [Google Scholar]
- 43. Whitbread TJ, Batt RM, Garthwaite G. 1984. Relative deficiency of serum IgA in the German shepherd dog: a breed abnormality. Res. Vet. Sci. 37:350–352 [PubMed] [Google Scholar]
- 44. Whitley NT, et al. 2010. Long term survival in two German shepherd dogs with Aspergillus-associated cavitary pulmonary lesions. J. Small Anim. Pract. 51:561. [DOI] [PubMed] [Google Scholar]
- 45. Wood GL, Hirsh DC, Selcer RR, Rinaldi MG, Boorman GA. 1978. Disseminated aspergillosis in a dog. J. Am. Vet. Med. Assoc. 172:704–707 [PubMed] [Google Scholar]