Abstract
The agar dilution method has been standardized by the CLSI for the susceptibility testing of Campylobacter species, and according to these standards, the disk diffusion method should be used only in screening for macrolide and ciprofloxacin resistance. Nevertheless, the disk diffusion test is currently widely used, since it is easy to perform in clinical microbiology laboratories. In this study, the disk diffusion method was compared to the agar dilution method by analyzing the in vitro activities of seven antimicrobial agents against 174 Campylobacter strains collected in Finland between 2003 and 2008. Recommendations of the CLSI were followed using Mueller-Hinton agar plates with 5% of sheep blood. For each strain, the disk diffusion tests were performed two to four times. Of the 33 erythromycin-resistant strains (MIC, ≥16 μg/ml), 24 (73%) constantly showed a 6-mm erythromycin inhibition zone (i.e., no inhibition), while for seven strains the inhibition zone varied from 6 to 44 mm in repeated measurements. Among the 141 erythromycin-susceptible strains (MIC, <16 μg/ml), erythromycin inhibition zones varied between 6 and 61 mm. Of the 87 ciprofloxacin-resistant strains, 47 (54%) showed 6-mm inhibition zones, while 40 strains showed inhibition zones between 6 and 60 mm. Significant differences between the repetitions were observed in the disk diffusion for all antimicrobial agents and all strains except for the macrolide-resistant strains regarding the macrolides. For 17 (10%) strains, the variation in repeated measurements was substantial. These results show that the disk diffusion method may not be a reliable tool for the susceptibility testing of Campylobacter spp. Further studies are needed to assess whether the disk diffusion test could be improved or whether all susceptibilities of campylobacters should be tested using an MIC-based method.
INTRODUCTION
Campylobacters are one of the most common causes of bacterial gastroenteritis in humans (2). Though bacterial gastroenteritis is usually a mild and self-limiting disease, antimicrobial therapy is needed in severe and prolonged cases, in immunocompromised patients, and in pregnant women as well as in very young and very old patients (2). Currently, macrolides and fluoroquinolones are considered to be the first- and second-choice alternatives for the antimicrobial treatment of campylobacteriosis. In recent years, however, resistance against both of these antimicrobial groups has complicated the empirical treatment of bacterial gastroenteritis (1). Therefore, reliable routine susceptibility testing is required to provide an adequate antimicrobial treatment for severely ill patients with campylobacteriosis. Moreover, correct measures are needed to monitor the Campylobacter resistance situation worldwide.
Several laboratory methods have been applied for the susceptibility testing of Campylobacter species. The agar dilution and broth dilution methods have been standardized by the Clinical and Laboratory Standards Institute (CLSI) (3, 4, 5, 6). The Etest is also an MIC-based method which has been widely used although not standardized. The disk diffusion method has been standardized by the CLSI, but according to those standards, it should be used only as a screening method for resistance to erythromycin and ciprofloxacin: a disk diffusion zone of 6 mm (growth up to the edge of a 6-mm disk) indicates resistance, while any inhibition zone would require an MIC determination of susceptibility (4).
A number of previous studies have compared the disk diffusion method with the other susceptibility testing methods for Campylobacter spp. In some of those studies, the results have been in line, though in some other studies, inconsistencies between the methods have been observed (8, 9, 15, 17–20). Nevertheless, the disk diffusion test is currently widely used, since it is easy to perform in clinical microbiology laboratories.
The aim of the present study was to compare the performance of the disk diffusion method with the agar dilution method in susceptibility testing of campylobacters to seven different antimicrobial agents. In addition, the repeatability of the disk diffusion method was evaluated in determining the in vitro efficacy of these same antimicrobial agents toward Campylobacter spp. The main interest was focused on erythromycin and ciprofloxacin susceptibilities, since they are clinically the two most important antimicrobial agents in the treatment of campylobacteriosis. Five additional antimicrobial agents were chosen based on possible clinical relevance and in order to find out whether there are differences within antimicrobial groups.
(This work was presented in part at the 20th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Vienna, Austria, 2010.)
MATERIALS AND METHODS
Campylobacter isolates.
The study collection consisted of 174 Campylobacter strains isolated from stool specimens from Finnish patients between 2003 and 2008. Of these strains, 23 were collected locally in the area of the Turku University Hospital. The rest of the strains were collected from 10 different hospital districts around Finland; their susceptibilities have been reported in our earlier paper (14). The cultivation of the stool samples and the preliminary identification of the samples were carried out by standard microbiological methods. The hippurate hydrolysis test was used for determination of the C. jejuni strains. All hippurate-positive isolates were determined as Campylobacter jejuni, and all hippurate-negative isolates were confirmed by PCR as either C. jejuni or C. coli (16). The final study collection was composed of 151 C. jejuni strains and 23 C. coli strains. Campylobacter jejuni type strain DSM 4688 (same as ATCC 33560 and NCTC 11351), C. coli type strain DSM 4689 (ATCC 33559 and NCTC 11366), Staphylococcus aureus ATCC 29213, and Escherichia coli ATCC 25922 were used as controls in susceptibility testing.
Agar dilution and the Etest method.
The MICs of the Campylobacter strains were determined by the standard agar plate dilution method for six antimicrobials (3, 12). The 90-mm plates were incubated in a microaerobic atmosphere (CampyPak; BBL) at 35 ± 1°C for 48 h. The MIC determinations were done twice for each strain. The antimicrobial agents evaluated were clindamycin, erythromycin, nalidixic acid, and tetracycline (Sigma, Steinheim, Germany) and azithromycin and ciprofloxacin (Fluka, Buchs, Switzerland). The MICs of the isolates to tigecycline were determined by the Etest (Biodisk AB, Solna, Sweden) as described previously (14).
Disk diffusion method.
The following antimicrobial disks were used: clindamycin 2 μg, erythromycin 15 μg, nalidixic acid 30 μg, tetracycline 30 μg, azithromycin 15 μg, ciprofloxacin 5 μg, and tigecycline 15 μg (Oxoid, United Kingdom). The disk diffusion tests were made according to the following instructions. Plates were dried at 35°C for 1.5 to 2 h. Inocula prepared in sterile NaCl at a density adjusted to a 0.5 McFarland turbidity standard were delivered onto Mueller-Hinton agar plates supplemented with 5% defibrinated sheep blood filled with an amount of agar giving a uniform depth of 4 ± 0.5 mm. The disks were added and incubated in a microaerobic atmosphere at 35 ± 1°C for 48 h. Sterile blotting paper was used between the lid and plate to inhibit excess moisture during the incubation. Three plates were made for each strain for the testing of susceptibility toward the different antimicrobials from the same inoculum at one measurement time. A maximum of three disks at the same time were applied onto one agar plate to make sure of a proper reading of even large inhibition zones. Tigecycline was always placed alone onto one agar plate. All disk diffusion tests were done according to the same instructions for each strain, three to four times for erythromycin and two to four times for the other antimicrobials. No inhibition zone indicated a disk diffusion zone of 6 mm, i.e., growth up to the edge of a 6-mm disk.
Data analysis.
The susceptibility data were analyzed using the WHONET5.4 computer program (http://www.whonet.org). Statistical analyses were made using SPSS 17.0 and SAS for Windows 9.1 programs. To describe the variability within the two or four disk diffusion test results, coefficients of variation (%) and maximum differences (mm) between the tests were determined for all strains. For the susceptible strains, repeated-measures analysis of variance was used to compare the repetitions. Due to the skewed distributions for the resistant strains, pairwise differences between the repetitions were tested by nonparametric Wilcoxon rank sum test. The Bonferroni method was used to adjust for the multiple comparisons. A P value of <0.05 was considered statistically significant.
RESULTS
As determined by the agar dilution method, a total of 33 Campylobacter strains were erythromycin resistant (MIC ≥ 16 μg/ml) and 141 strains were susceptible (MIC < 16 μg/ml) (Table 1). A total of 87 strains were ciprofloxacin resistant (MIC ≥ 4 μg/ml) and 87 strains were susceptible (MIC < 4 μg/ml). According to the Etest, all 174 strains were susceptible to tigecycline. The MIC determinations were made twice for each strain obtaining identical susceptibility results.
Table 1.
Correlation between MICs and inhibition zone valuesa
| Antimicrobial agent | Agar plate dilution |
Disk diffusionb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of strains | Resistance breakpoint (μg/ml) | Susceptibility according to MIC determinations | MIC range (μg/ml) | No. of total measurements | Inhibition zone variation (mm) | No. of measurements with no inhibition zone | Mean value for max difference (mm) | Mean value for coefficient of variation (%) | Pc | |
| Erythromycin | 33 | ≥16 | Resistant | 16–>128 | 129 | 6–44 | 115 (89%) | 2.79 | 10.04 | No significant pairwise differences |
| 141 | Susceptible | 0.5–8 | 477 | 6–61 | 2 (0.42%) | 8.91 | 11.5 | <0.001 | ||
| Azithromycin | 31 | ≥4 | Resistant | 64–>128 | 121 | 6–57 | 110 (91%) | 3.55 | 10.68 | No significant pairwise differences |
| 143 | Susceptible | ≤0.062–1 | 480 | 6–64 | 4 (0.83%) | 10.66 | 12.28 | <0.001 | ||
| Clindamycin | 33 | ≥8 | Resistant | 8–128 | 127 | 6–58 | 103 (81%) | 6.21 | 18.32 | <0.05 for 3 of 6 pairwise differences |
| 141 | Susceptible | 0.125–4 | 479 | 6–60 | 7 (1.5%) | 10.54 | 14.91 | 0.026 | ||
| Ciprofloxacin | 87 | ≥4 | Resistant | 4–>32 | 312 | 6–60 | 231 (74%) | 5.49 | 19.24 | <0.01 for all pairwise differences |
| 87 | Susceptible | 0.06–1 | 267 | 6–66 | 9 (3.4%) | 10.38 | 11.07 | 0.013 | ||
| Nalidixic acid | 86 | ≥32 | Resistant | 32–256 | 310 | 6–44 | 239 (77%) | 4.42 | 16.86 | <0.01 for all pairwise differences |
| 87 | Susceptible | 2–16 | 234 | 6–56 | 9 (3.8%) | 8.72 | 13.19 | <0.001 | ||
| Tetracycline | 65 | ≥16 | Resistant | 16–>128 | 230 | 6–62 | 101 (44%) | 6.91 | 22.2 | <0.01 for all pairwise differences |
| 107 | Susceptible | 0.1–4 | 332 | 6–70 | 2 (0.60%) | 10.05 | 10.08 | 0.014 | ||
| Tigecycline | ≥0.5 | Resistant | ||||||||
| 174 | Susceptible | ≤0.008–0.064 | 609 | 6–80 | 1 (0.16%) | 10.8 | 9.93 | 0.81 | ||
The maximum difference between different measurement times and the mean value for coefficient of variation were determined for five antimicrobial agents. Also, P values were determined for different measurement times.
Disk diffusion tests were repeated for each strain 2 to 4 times.
Significance of the difference between repetitions. Due to very skewed distributions in the resistant strains, differences between the repetitions were tested by nonparametric Wilcoxon rank sum test. In the susceptible strains, repeated measures were tested by analysis of variance.
For the erythromycin-resistant strains, the total number of the measurements for the erythromycin disk was 129. Of the 33 erythromycin-resistant strains, 24 (73%) showed an erythromycin inhibition zone of 6 mm (i.e., no inhibition zone) in all repeated measurements (total number, 115). Seven (21%) erythromycin-resistant strains with MICs of ≥128 μg/ml showed inhibition zones between 6 and 44 mm; two (6.1%) of the erythromycin-resistant strains, with MICs of 16 and 64 μg/ml, respectively, showed inhibition zones between 22 and 42 mm in all repeated measurements. For the 141 erythromycin-susceptible isolates, a total of 477 measurements were performed. For these susceptible strains, the inhibition zones for erythromycin varied from 6 to 61 mm. Ten (2.1%) measurements were equal to or less than 20 mm, and two (0.42%) were 6 mm.
For the ciprofloxacin-resistant strains, the total number of the measurements for the ciprofloxacin disk was 312. Of the 87 ciprofloxacin-resistant strains, 47 (54%) showed a ciprofloxacin inhibition zone of 6 mm in all repeated measurements (total number, 231), while for 40 (46%) strains, the inhibition zone varied from 6 to 60 mm. In 11 (12%) measurements, the inhibition zone was over 10 mm. For six ciprofloxacin-resistant strains with MICs between 4 and 32 μg/ml, the inhibition zones were equal to or over 20 mm in all repeated measurements. For the 87 ciprofloxacin-susceptible isolates, a total of 267 measurements were performed. For these susceptible strains, the inhibition zones for ciprofloxacin varied between 6 and 66 mm. A 6-mm inhibition zone was observed in nine (3.4%) measurements. The results for azithromycin, clindamycin, nalidixic acid, tetracycline, and tigecycline are shown in Table 1.
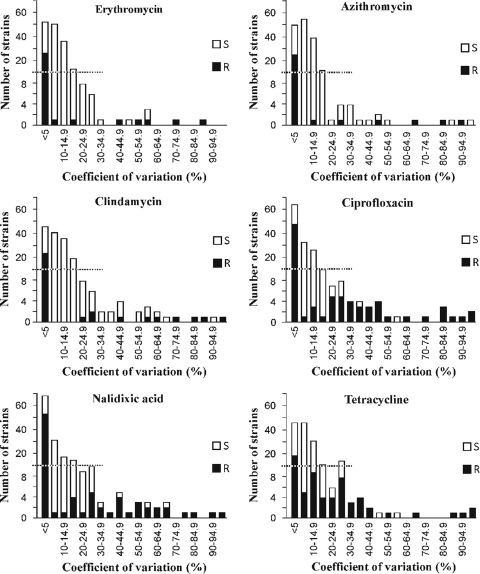
For all measurements of azithromycin, ciprofloxacin, clindamycin, erythromycin, nalidixic acid, and tetracycline, the coefficients of variation (CV) were determined for the resistant and susceptible strains (Fig. 1; Table 1). For all compounds, strains were found to have a substantial variation between the repetitions of the disk diffusion test. For the erythromycin disk diffusion tests, the CV variation was less than 5% for 53 strains and greater than or equal to 15% for 36 strains. For ciprofloxacin, the variation was small for 64 strains and substantial for 52 strains. Seventeen strains showed substantial variations for both erythromycin and ciprofloxacin. For all of the strains susceptible to these antimicrobial agents, the mean of maximum difference between two different measurements was over 8 mm (Table 1). Even for the resistant strains, the mean values of maximum difference over 4 mm were found for ciprofloxacin, nalidixic acid, and tetracycline. The mean values of coefficient of variation for all antimicrobial agents were over 10%. When the different repetition times were evaluated, significant differences were observed for all antimicrobial agents and for all strains except for the macrolide-resistant strains regarding erythromycin and azithromycin (Table 1).
Fig 1.
Coefficients of variation for all repeated measurements of six antimicrobial agents studied were determined for 174 Campylobacter jejuni and Campylobacter coli strains. Measurements were repeated two to four times for each strain. R means a resistant strain and S a susceptible strain.
DISCUSSION
The aim of the present study was to evaluate the adequacy of the disk diffusion method in comparison with the agar dilution method for determining the efficacy of important antimicrobial compounds toward Campylobacter spp. In so doing, significant differences were found for the majority of the antimicrobial agents analyzed when the disk diffusion tests were repeated and the results obtained at different measurement times compared. As many as 17 (10%) of the 174 strains showed a substantial variation in repeated measurements for erythromycin and ciprofloxacin. However, no significant differences in repeatability were observed in the group of macrolide-resistant strains regarding erythromycin and azithromycin. The reasons for the better performance regarding the macrolide-resistant strains are still unclear. One explanation for the result could be that the number of those strains was rather small. Thus, further studies are needed to evaluate this finding with a greater number of macrolide-resistant Campylobacter strains.
Of the 129 measurements made for the strains classified as erythromycin resistant by the MICs, 89% showed no inhibition zones (i.e., an inhibition zone of 6 mm), while in 11% of them, the inhibition zones exceeded 6 mm, indicating a need for a more accurate susceptibility determination to demonstrate erythromycin resistance. For ciprofloxacin, the situation was even more worrisome: for the ciprofloxacin-resistant strains, only 74% of the results showed no inhibition zones in 312 measurements. It is noteworthy that even 66% of the measurements for tetracycline would have required MIC-based determinations of susceptibility, if the rule of the 6-mm inhibition zone as an indication of resistance would have been followed also for this antimicrobial. On the other hand, 0.42% and 3.4% of all measurements for the erythromycin-susceptible and ciprofloxacin-susceptible strains, respectively, did not show any inhibition zones. Thus, following the CLSI guidelines, a small number of susceptible Campylobacter strains would have been falsely classified as resistant. Apart from these false-resistant strains, our findings support the CLSI standardization that the disk diffusion method should be used as a screening method only for resistance to erythromycin and ciprofloxacin in Campylobacter spp. and that any inhibition zone around the disk demands an MIC-based determination of susceptibility (3, 4, 5, 6, 13).
These results indicate that there is a need for a standardized protocol for susceptibility testing in clinical microbiology laboratories, as well as determining clear resistance breakpoints and interpretive criteria for Campylobacter spp. The falsely diagnosed resistant strains may lead to an excessive use of more toxic and possibly even less effective antimicrobial treatment for patients with campylobacteriosis (19). The most serious threat is that resistant strains falsely diagnosed as susceptible may lead to ineffective antimicrobial treatment even in invasive and life-threatening infections. Infections with resistant strains have been reported in association with a 5-fold increase of the risk of invasive illness or death (7). Especially for that reason, it is of importance to be able to correctly distinguish the resistant strains. Moreover, adequate worldwide monitoring of Campylobacter resistance is impossible if the resistance rates are falsely reported due to unreliable susceptibility testing.
Several previous papers have focused on the efficacy and accuracy of the disk diffusion method and the Etest method compared to the agar plate dilution or broth microdilution methods, with somewhat contradictory results (8, 9, 15, 17, 19, 20). In these studies, the disk diffusion tests were performed only once for each strain. Gaudreau et al. (8, 9) have found the disk diffusion method to be a reliable, easy and inexpensive method for the testing of the susceptibility of C. jejuni to erythromycin, ciprofloxacin, and tetracycline. Corroborating the present study, the results of, e.g., van der Beek et al. are different (19). They reinvestigated 48 erythromycin-resistant C. jejuni and C. coli strains retrospectively to reevaluate erythromycin resistance, and only 11 to 14% of the C. jejuni strains and 67% of the C. coli strains were erythromycin resistant in the second analysis. In that study, the initial susceptibility testing was performed in most cases by the disk diffusion method and the reinvestigation was carried out using broth microdilution. The authors conclude that routine determination of the erythromycin resistance in C. jejuni and C. coli shows unacceptable interlaboratory variation. Nonstandardized susceptibility testing methods may be involved in the differences of the susceptibility results obtained by the disk diffusion method, including (i) various protocols of the methods used, (ii) long incubation time for campylobacters, (iii) inaccuracy of the measurements between different times or between different persons measuring the inhibition zone, and (iv) different methods for achieving microaerobic conditions during the incubation. In their paper, van der Beek et al. (19) speculated on the possibility that differences could also be caused by instability of the erythromycin resistance. In the present study, no rising trend during the repetitions was observed in the inhibition zone variation between the different measurement times. Therefore, the instability of the erythromycin resistance does not seem to be the factor underlying the variation in our strains.
In conclusion, our results show that the disk diffusion method may not be a reliable tool for susceptibility testing of Campylobacter spp. This is a major concern due to the wide use of the disk diffusion method in routine clinical laboratories as well as in some research laboratories. Accurate determination of Campylobacter susceptibility and resistance is of vital importance to ensure an adequate antimicrobial therapy for patients with severe forms of the disease and, also, to efficiently monitor the antimicrobial resistance situation of Campylobacter spp. worldwide. Further studies are needed to assess whether the disk diffusion test method could be improved or whether all susceptibility testing of campylobacters should be done using an MIC-based method.
ACKNOWLEDGMENTS
We thank Erkki Nieminen for technical assistance and Tarja Boman, Katri Kylä-Mattila, Minna Lamppu, Tarja Laustola, and Tuula Randell for laboratory assistance.
This study was financially supported by grants from the Maud Kuistila Memorial Foundation, Finnish Cultural Foundation, and the Turku University Central Hospital Research Fund.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Aarestrup FM, Engberg J. 2001. Antimicrobial resistance of thermophilic Campylobacter. Vet. Res. 32:311–321 [DOI] [PubMed] [Google Scholar]
- 2. Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201–1206 [DOI] [PubMed] [Google Scholar]
- 3. CLSI 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement M100–S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. CLSI 2006. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline M45-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. CLSI 2008. Performance standards for disk and dilution susceptibility testing from bacteria isolated from animals 31-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. CLSI 2009. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard—8th edition. CLSI document M07–A8 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Coker AO, et al. 2002. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaudreau C, et al. 2008. Comparison of disk diffusion and agar dilution methods for erythromycin, ciprofloxacin, and tetracycline susceptibility testing of Campylobacter coli and for tetracycline susceptibility testing of Campylobacter jejuni subsp. jejuni. Antimicrob. Agents Chemother. 52:4475–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudreau C, et al. 2007. Comparison of disk diffusion and agar dilution methods for erythromycin and ciprofloxacin susceptibility testing of Campylobacter jejuni subsp. jejuni. Antimicrob. Agents Chemother. 51:1524–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reference deleted.
- 11. Reference deleted.
- 12. Hakanen AJ, et al. 2003. Multidrug resistance in Campylobacter jejuni strains collected from Finnish patients during 1995-2000. J. Antimicrob. Chemother. 52:1035–1039 [DOI] [PubMed] [Google Scholar]
- 13. King A. 2001. Recommendations for susceptibility tests on fastidious organisms and those requiring special handling. J. Antimicrob. Chemother. 48(Suppl. 1):77–80 [DOI] [PubMed] [Google Scholar]
- 14. Lehtopolku M, et al. 2010. Antimicrobial susceptibilities of multidrug-resistant Campylobacter jejuni and C. coli strains: in vitro activities of 20 antimicrobial agents. Antimicrob. Agents Chemother. 54:1232–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGill K, et al. 2009. Comparison of disc diffusion and epsilometer (E-test) testing techniques to determine antimicrobial susceptibility of Campylobacter isolates of food and human clinical origin. J. Microbiol. Methods 79:238–241 [DOI] [PubMed] [Google Scholar]
- 16. Nakari UM, et al. 2008. Correct identification and discrimination between Campylobacter jejuni and C. coli by a standardized hippurate test and species-specific polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 27:513–518 [DOI] [PubMed] [Google Scholar]
- 17. Schönberg-Norio D, et al. 2006. Activities of telithromycin, erythromycin, fluoroquinolones, and doxycycline against Campylobacter strains isolated from Finnish subjects. Antimicrob. Agents Chemother. 50:1086–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valdivieso-Garcia A, et al. 2009. Cost analysis and antimicrobial susceptibility testing comparing the E test and the agar dilution method in Campylobacter jejuni and Campylobacter coli. Diagn. Microbiol. Infect. Dis. 65:168–174 [DOI] [PubMed] [Google Scholar]
- 19. van der Beek MT, et al. 2010. Inaccuracy of routine susceptibility tests for detection of erythromycin resistance of Campylobacter jejuni and Campylobacter coli. Clin. Microbiol. Infect. 16:51–56 [DOI] [PubMed] [Google Scholar]
- 20. Varela NP, et al. 2008. Comparison of agar dilution and E-test for antimicrobial susceptibility testing of Campylobacter coli isolates recovered from 80 Ontario swine farms. Can. J. Vet. Res. 72:168–174 [PMC free article] [PubMed] [Google Scholar]