Abstract
Background
Hydroxyurea is the only approved drug for treatment of sickle cell disease.
Objective
To synthesize the published literature on the efficacy, effectiveness, and toxicity of hydroxyurea when used in adults with sickle cell disease.
Data Sources
MEDLINE, EMBASE, TOXLine, and CINAHL were searched through 30 June 2007.
Study Selection
Randomized trials, observational studies, and case reports evaluating efficacy and toxicity of hydroxyurea in adults with sickle cell disease, and toxicity studies of hydroxyurea in other conditions that were published in English.
Data Extraction
Paired reviewers abstracted data on study design, patient characteristics, and outcomes sequentially and did quality assessments independently.
Data Synthesis
In the single randomized trial, the hemoglobin level was higher in hydroxyurea recipients than placebo recipients after 2 years (difference, 6 g/L), as was fetal hemoglobin (absolute difference, 3.2%). The median number of painful crises was 44% lower than in the placebo group. The 12 observational studies that enrolled adults reported a relative increase in fetal hemoglobin of 4% to 20% and a relative reduction in crisis rates by 68% to 84%. Hospital admissions declined by 18% to 32%. The evidence suggests that hydroxyurea may impair spermatogenesis. Limited evidence indicates that hydroxyurea treatment in adults with sickle cell disease is not associated with leukemia. Likewise, limited evidence suggests that hydroxyurea and leg ulcers are not associated in patients with sickle cell disease, and evidence is insufficient to estimate the risk for skin neoplasms, although these outcomes can be attributed to hydroxyurea in other conditions.
Limitation
Only English-language articles were included, and some studies were of lower quality.
Conclusion
Hydroxyurea has demonstrated efficacy in adults with sickle cell disease. The paucity of long-term studies limits conclusions about toxicity.
Sickle cell disease, which affects nearly 100 000 persons in the United States (1), decreases life expectancy by 25 to 30 years (2) and causes great morbidity (3). It is a group of disorders characterized by sickling of erythrocytes when they are deoxygenated due to a mutation in the B globin gene of hemoglobin. The sickled erythrocytes obstruct blood vessels and disrupt endothelial cell function, leading to tissue hypoxia and clinical complications. The erythrocytes have a markedly short life span, leading to anemia and release of free hemoglobin into the circulation, which is also injurious to the endothelium. Patients with sickle cell disease have both chronic and episodic pain and reduced quality of life (4). Painful crisis is the most common reason for emergency department use (5); life-threatening complications include the acute chest syndrome and stroke.
Until the U.S. Food and Drug Administration approved hydroxyurea for the treatment of adults with sickle cell disease, most therapies were supportive (episodic red blood cell transfusions, narcotics, antibiotics, and intravenous fluids); no approved therapies were available to modify the underlying pathophysiology of the disease. Hydroxyurea remains the only approved disease-modifying therapy, although repeated transfusions of red blood cells can greatly decrease disease severity and hematopoietic stem cell transplantation can cure it. The efficacy of hydroxyurea in the treatment of sickle cell disease is generally attributed to its ability to increase fetal hemoglobin (α2γ2). However, the mechanisms by which this occurs are unclear, and other mechanisms may account for the clinical benefit of this agent. The National Heart, Lung, and Blood Institute issued recommendations for the use of hydroxyurea in sickle cell disease in 2002 (6).
To clarify the role of hydroxyurea in the treatment of patients with sickle cell disease and to improve physician adherence to recommendations regarding its use, the National Institutes of Health Office of Medical Applications of Research commissioned a systematic review to summarize the available data on the efficacy, effectiveness, toxicity, and barriers to the use of hydroxyurea in people with sickle cell disease. We present the results of our systematic review. In addition, to evaluate the long-term adverse effects of hydroxyurea, we report on the toxicity of this drug in exposed individuals without sickle cell disease. The specific questions that the Office of Medical Applications of Research asked us to address were:
What is the efficacy of hydroxyurea treatment for patients with sickle cell disease?
What is the effectiveness of hydroxyurea treatment for patients with sickle cell disease?
What are the short- and long-term harms of hydroxyurea treatment?
What are barriers to the use of therapies that increase fetal hemoglobin, to the use of well-established therapies for disease management, and to the use of bone marrow transplantation for sickle cell disease?
The fourth question will be addressed in a separate report.
Methods
A detailed evidence report (7) describes fully the methods we used in our systematic review.
Data Sources
We searched MEDLINE, EMBASE, TOXLine, and CINAHL through 30 June 2007. We also reviewed reference lists and discussed search results with experts. All searches were limited to English-language publications describing treatment of humans. We excluded review articles from the searches (Appendix Table 1, available at www.annals.org). We defined efficacy trials as those showing a therapeutic effect of an intervention in an ideal setting, such as a clinical trial. We defined effectiveness studies as those showing a therapeutic effect of an intervention as demonstrated or observed in patients in their usual care setting.
Study Selection
For evidence of efficacy and effectiveness of hydroxyurea in adults with sickle cell disease, we included randomized, controlled trials (RCTs), cohort studies with a control population, and before-and-after studies. For evidence of toxicity, we included RCTs; cohort studies with a control population; before-and-after studies; and case reports, a weaker form of evidence. We included studies of children with sickle cell disease only if leukemia or lymphoma was described.
We knew that data on the long-term harms of hydroxyurea in individuals with sickle cell disease would be limited. Therefore, to describe all that is known about the long-term harms of hydroxyurea, we included indirect evidence from studies enrolling patients treated with hydroxyurea for other diseases (largely essential thrombocythemia, polycythemia vera, psoriasis, HIV, and chronic myelogenous leukemia). We included RCTs, cohort studies with a control population, before-and-after studies, case reports, and large case series (≥100 patients with diseases other than chronic myelogenous leukemia).
Two reviewers independently reviewed titles and abstracts for eligibility.
Data Extraction
A single reviewer abstracted data, and a co-investigator verified accuracy. Reviewers were not masked to the articles’ authors, institutions, or journal (8). Differences of opinion were resolved through discussion.
For all studies except case reports, reviewers extracted information on general study characteristics, participant characteristics, and efficacy and toxicity outcomes. Case reports were abstracted by using a separate form to record disease, participant age, reported adverse events, and causality according to the World Health Organization’s causality assessment instrument (9).
Quality Assessment
We assessed the quality of the included RCTs by using the scoring system developed by Jadad and colleagues (10). To assess quality of the observational studies, we developed a form to identify key elements that should be described in reports of observational research, as advocated by leaders in the field (11–13). For our quality assessment of surveys, we adapted information from the study by Ratanawongsa and associates (14). The quality assessments were done independently by paired reviewers. For the RCTs, a third reviewer reconciled the findings of the first 2 reviewers. For the other study designs, the results of the 2 reviewers were averaged.
Data Synthesis
We created detailed evidence tables with information extracted from eligible studies. We did not quantitatively pool the data for any of the outcomes because there were few RCTs. The substantial qualitative heterogeneity among the observational studies made pooling these studies problematic. We explored graphically the relationship across studies between potential predictors of response (age, mean corpuscular volume, sample size, change in leukocyte count) and the change in fetal hemoglobin. We considered the evidence about efficacy and effectiveness together because the observational studies were not easily categorized as efficacy or effectiveness studies.
Grading of Evidence
We graded the quantity, quality, and consistency of the evidence by adapting an evidence grading scheme recommended by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (15) and modified in the Evidence-based Practice Center manual (16). In this system, the grade of evidence depends on the required domains and not on the number of studies; consistency, directness, and precision are considered to be more informative than number of studies. If an outcome was evaluated in 1 or no RCTs, we also based our evidence grade on the best available nonrandomized trials or observational studies. We assessed the quality and consistency of the evidence by evaluating the risk for bias in the studies (as indicated by the study quality scores), the directness with which the data addressed the study question, and the precision and strength of the findings within individual studies. For each outcome of interest, 2 investigators graded the strength of the evidence for each question and all investigators then reached consensus.
The results from case reports were used as additional evidence of directness in our grading of toxicity. We graded the case reports according to the World Health Organization’s Collaborating Center for Drug Monitoring (9, 17). This method (Appendix Table 2, available at www.annals.org) uses 4 criteria to evaluate the case reports and determines from these criteria how strong the causal relationship is between the drug and the described toxicity. We determined from the body of case reports a level of causality based on the number of case reports and the strength of the causal relationships.
Role of the Funding Source
This topic was nominated by the National Institutes of Health Office of Medical Applications of Research and selected by the Agency for Healthcare Research and Quality for systematic review by an Evidence-based Practice Center. A representative from the Agency for Healthcare Research and Quality served as a Task Order Officer and provided technical assistance during the conduct of the full evidence report and provided comments on draft versions of the full evidence report. The Agency for Healthcare Research and Quality did not directly participate in the literature search; determination of study eligibility criteria; data analysis or interpretation; or preparation, review, or approval of the manuscript for publication.
Results
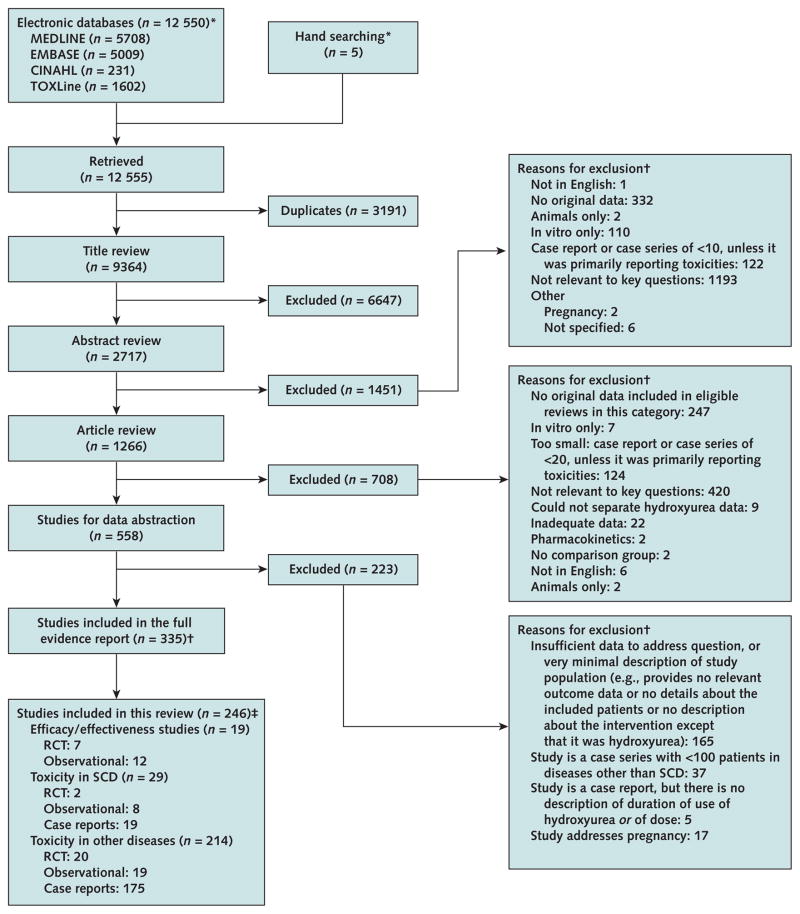
We identified 12 550 citations potentially relevant to these questions. An additional 5 articles were found by hand-searching, and we excluded 3191 duplicate citations. During the process of title, abstract, and article review, we eliminated studies that did not meet our criteria, leaving 246 articles for review (Figure).
Study flow diagram.
RCT = randomized, controlled trial; SCD = sickle cell disease. *The evidence report provides details of the search strategies (7). †The reasons for exclusion exceed the total number of studies because reviewers could enter multiple reasons for exclusion. ‡89 studies were not included in this review because they included children or infants only or examined barriers only. Toxicity and efficacy studies in SCD overlap.
Is Hydroxyurea Efficacious and Effective for Adults with Sickle Cell Disease?
A single RCT, the MSH (Multicenter Study of Hydroxyurea for Sickle Cell Anemia), tested the efficacy of hydroxyurea in adults with sickle cell disease (18). We identified 6 other articles associated with this trial (sub-studies or follow-up studies) (19 –24). The MSH was a high-quality, multicenter trial enrolling 299 adults with a mean age of 30.5 years. Almost all patients (n = 295) had sickle cell anemia; the remainder had hemoglobin Sβ0 thalassemia or hemoglobin Sβ+ thalassemia. Patients in the study received the maximum tolerated dose (that limited by toxicity) or a maximum dose of 35 mg/kg daily. The primary end point was a reduction in the frequency of painful crises. The investigators included several secondary end points for which they used a more stringent standard for determining significant differences between groups (P ≤ 0.01) (25) (Table 1).
Table 1.
Characteristics of RCTs and Observational Studies of HU for Treatment of SCD*
| Study, Year (Reference) |
Study Design and Aims | Inclusion Criteria | Exclusion Criteria | Study Group | Patients, n |
HU Dose | Follow-up (Range), mo |
Mean Age, y | Genotype, n | Haplotype, % | Quality† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Charache et al., 1995 (18) and 1996 (19)‡ | RCT of efficacy of HU in SCD | Age >18 y, SCA, Hb Sα+ thalassemia, ≥3 pain episodes/y | Hb Sβ+ or β0 thalassemia, sickle Hb C disease, transfusion-dependent, pregnant, using opioids, substance abuse, received antisickling agent, stroke in past 6 years, HIV positive, not using contraception, possible marrow suppression, Hb A level >15% if received transfusion, breastfeeding | HU | 152 | 15 mg/kg daily; increased by 5 mg/kg every 12 wk if ANC ≥2 × 109 cells/L, reticulocyte and platelet count ≥80 × 109 cells/L, and Hb level ≥45 g/L | 24 | 30 | SS: 151 Hb Sβo thalassemia: 1 |
Benin/Benin: 36 Benin/CAR: 21 Benin/Senegal: 3 Senegal/CAR: 3 Other: 23 |
No major deficits in reporting on source population, inclusion criteria, baseline characteristics, intervention, or adherence |
| Placebo | 147 | 31 | SS: 145 Hb Sβ0 thalassemia: 2 |
Benin/Benin: 43 Benin/CAR: 20 Benin/Senegal: 3 Senegal/CAR: 3 Other: 17 |
|||||||
|
| |||||||||||
| Steinberg et al., 1997 (20) | RCT of efficacy of HU in SCD | Same as those for Charache et al. (18, 19) | Same as those for Charache et al. (18, 19) | HU | 152 | Same as in Charache et al. (18, 19) | NR | 30 | NR | NR | |
| Placebo | 147 | 31 | NR | ||||||||
|
| |||||||||||
| Moore et al., 2000 (23) | RCT of efficacy of HU in SCD | Same as those for Charache et al. (18, 19) | Same as those for Charache et al. (18, 19) | HU | 152 | Same as in Charache et al. (18, 19) | NR | 30 | NR | NR | |
| Placebo | 147 | NR | 31 | NR | NR | ||||||
|
| |||||||||||
| Hackney et al., 1997 (21)§ | RCT of efficacy of HU in SCD | Same as those for Charache et al. (18, 19) | Same as those for Charache et al. (18, 19) | HU | 10 | Same as in Charache et al. (18, 19) | NR | 30.5 | SS: 100% | – | |
| Placebo | 14 | 29.8 | – | – | |||||||
|
| |||||||||||
| Ballas et al., 2006 (22) | RCT of efficacy of HU in SCD | Same as those for Charache et al. (18, 19) | Same as those for Charache et al. (18, 19) | HU | 141 | Same as in Charache et al. (18, 19) | NR | SS: 100% | |||
| Placebo | 136 | ||||||||||
|
| |||||||||||
| Steinberg et al., 2003 (24) | Prospective cohort study by MSH assignment | Same as those for Charache et al. (18, 19) | Same as those for Charache et al. (18, 19) | HU | 152 | Same as in Charache et al. (18, 19) | NR | NR | NR | ||
| Placebo | 147 | ||||||||||
|
| |||||||||||
| el-Hazmi et al., 1992 (26) | Prospective cohort study of effectiveness of HU for severe forms of SCD | NR | NR | HU | 21 | 20 mg/kg daily; no titration | 3 | Range, 17–32 | SS: 71% Sβ0 thalassemia: 28% |
NR | Did not adjust estimate of treatment effect for confounders or report losses to follow-up |
|
| |||||||||||
| Charache et al. 1992 (27) | Prospective cohort study on pharmacokinetics, toxicity, and increase in fetal Hb production in response to HU in patients with SCA | Age >18 y, SCA, Hb Sα+ thalassemia, >1 pain admissions in past year (including ED visits) | Hb Sβ+ or Sβ0 thalassemia; received transfusion; pregnant; renal failure; abnormal renal function; liver failure; abnormal hepatic function; HIV- positive; AST level >100 U/L; albumin level <30 g/L; use of theophylline- containing drugs, androgens, estrogens, or progesterones (other than birth control) | HU | 49 | 10–20 mg/kg daily depending on AUC at 6 h; increased by 5 mg/kg daily every 8 wk to MTD | 9 (0–25) | 27.6 | SS: 100% | Benin: 61 Senegal: 9.3 CAR: 25 |
Did not adjust estimate of treatment effect for confounders |
|
| |||||||||||
| Voskaridou et al., 1995 (28) | Prospective cohort study on response of white patients with SCD and complica- tions to high doses of HU | Age, adult, SCA, Hb Sα+ thalassemia, >2 crises in past year | Hb Sβ+ or Sβ0 thalassemia; received transfusion; pregnancy; renal failure; abnormal renal function; abnormal hepatic function; HIV-positive; use of theophylline- containing drugs, androgens, estrogens, or progesterones (other than birth control) | HU | 14 | 15 mg/kg daily, rounded up to the next 500 mg 4 days/wk; increased in 5-mg/kg increments, rounded up to the next 500 mg every 4 wk to MTD; maximum total dose, 2.5 g/d|| | (5–8.7) | 28.6 (range, 19–48) | Sβ+ thalassemia: 42% Sβ0 thalassemia: 58% |
NR | Did not adjust estimate of treatment effect for confounders or report losses to follow-up |
|
| |||||||||||
| Loukopoulos et al., 1998 (29) | Retrospective cohort study on effectiveness of HU in patients with thalassemia | Hb Sβ+ or Sβ0 thalassemia, frequent pain crises | NR | HU | 44 | 15–35 mg/kg daily 4–7 d wk; no titration | NR | NR | Sβ+ thalassemia: 34% Sβ0 thalassemia: 65% |
NR | Did not adequately describe the study design, adjust estimate of treatment effect for confounders, report losses to follow-up, or describe adherence |
|
| |||||||||||
| Loukopoulos et al., 2000 (30) | Prospective cohort study on HU in 55 Greek-origin patients with sickle β thalassemia or Hb SS disease receiving HU | Age, adults, Hb Sβ+ or Sβ0 thalassemia | NR | HU | 69 | 15 mg/kg daily for 4 d/wk, then titrated up to 25 mg/kg daily or to MTD; held for 6 mo and tapered to 1.0 g/d | (6–48) | Range, 17–50 | SS: 20% Sβ0 thalassemia: 79% |
All Hb S was Benin | Did not adjust estimate of treatment effect for confounders or describe adherence |
|
| |||||||||||
| Rigano et al., 2001 (31) | Prospective cohort study on efficacy of HU in Sicilian patients with sickle β thalassemia | Sicilian, ≥3 sickle crises (any type) in past year | HIV-positive, bone marrow hypoplasia | HU | 22 | 15 mg/kg daily; increased after 3 mo if no response and decreased for toxicity | >24 | Range, 29–53 | Sβ0 thalassemia: 76% Sβ+ thalassemia: 27% |
Benin: 100 | Did not adjust estimate of treatment effect for confounders |
|
| |||||||||||
| Ferguson et al., 2002 (32) | Retrospective cohort study on efficacy of HU in settings outside of a clinical trial with longer follow-up | Age, adults, SCA, treated at 2 hospitals | NR | HU ≥24 mo | 30 | 15 mg/kg daily; increased by 5 mg/kg every 8 wk to MTD | 21.6 (3–60)¶ | Range, 20–58 | SS: 100 | NR | Did not report losses to follow-up |
| HU <24 mo | 30 | 15 mg/kg daily; increased 5 mg/kg every 8 wk to MTD | NR | Range, 19–54 | NR | NR | |||||
|
| |||||||||||
| Cummins and Anie, 2003 (33) | Prospective cohort study with comparison; patients with SCD treated with CBT vs. HU in terms of quality of life, pain experience, health service utilization, and pain coping strategies | Adults with SCD treated at 1 hospital | NR | HU | 15 | Weight-based | 23 (12–39) | 33 | SS: 93% | NR | Did not adjust estimate of treatment effect for confounders, report losses to follow-up, or describe adherence |
| CBT | 12 | NR | NR | 30.9 | SS: 57% SC: 29% |
NR | |||||
|
| |||||||||||
| Vicari et al., 2005 (34) | Prospective cohort study of the influence of genetic determinants on response to and toxicity of HU | Age, >18 y, SCA, ≥3 pain episodes/y, ACS | Hb Sβ+ or Sβ0 thalassemia, Hb SSα+ thalassemia, sickle Hb C disease, received transfusion, pregnancy, stroke in past 6 years, HIV-positive, bone marrow depression | HU | 22 | NR | 30.45 (12–60) | 25.6 (range, 18–46) | SS: 100% | Homozygous Bantu: 41 Homozygous Benin: 18 Heterozygous Bantu–Benin: 31 |
Did not describe adequately the study design or intervention, adjust estimate of treatment effect for confounders, report losses to follow- up, or describe adherence |
|
| |||||||||||
| Ataga et al., 2006 (35) | Prospective cohort study on the development of pulmonary HTN and the association of pulmonary HTN in patients with SCD | Age, adult, SCA, Hb Sβ+ or Sβ0 thalassemia, Hb SSα + thalassemia, sickle Hb C disease | ACS in past 4 wk, current crisis or acute illness | With pulmonary HTN, HU | 9 | NR | NR | 42.3 (SD, 11) | Both groups combined: SS: 74% Sickle Hb C disease: 12% Sβ0 thalassemia: 5% Sβ+ thalassemia: 9% |
NR | Did not describe adequately the study design or intervention, adjust estimate of treatment effect for confounders, report losses to follow-up, or describe adherence |
| No pulmonary HTN, HU | 32 | NR | NR | 38.4 (SD, 12) | |||||||
|
| |||||||||||
| Chaine et al., 2001 (36) | Retrospective cohort study on risk for cutaneous adverse reactions in patients with SCD treated with HU | SCA, Hb Sβ+ or Sβ0 thalassemia, Hb SSα+ thalassemia, sickle Hb C disease receiving HU | NR | 17 | NR | 12 (MTD plus time to escalate) | 27.1 (19–51) | SS: 94% Sβo thalassemia: 6% |
Benin: 12.5 Senegal: 2 CAR: 3 |
Did not report losses to follow-up or describe adherence | |
|
| |||||||||||
| Schultz and Ware, 2003 (37) | Survey to report on cancer in patients with SCD | NR | NR | With cancer, no HU | 49 | NR | 22 | 34 (median, 1.2–62) | SS: 63% Sickle Hb C disease: 22% Sβ thalassemia: 14% |
NR | Did not describe study participants or use of a validated instrument or discuss validity or reliability of instrument |
| With cancer, HU | 3 | NR | NR | NR | NR | NR | |||||
ACS = acute chest syndrome; ANC = absolute neutrophil count; AST = aspartate aminotransferase; AUC = area under the curve; CAR = Central African Republic; CBT = cognitive behavioral therapy; ED = emergency department; Hb = hemoglobin; HTN = hypertension; HU = hydroxyurea; MSH = Multicenter Study of Hydroxyurea for Sickle Cell Anemia; MTD = maximum tolerated dose; NR = not reported; RCT = randomized, controlled trial; SCA = sickle cell anemia; SCD = sickle cell disease.
All criteria for assessing RCT quality are shown in the table. For observational studies, the following criteria were used to assess quality: study description, inclusion and exclusion criteria, description of key characteristics of study participants, description of intervention, description of adherence, adjusted estimate of treatment effect, report of ≥1 objective outcome, report of number of participants lost to follow-up. Only criteria with the worst scores are reported in the table.
Results presented from final analysis (19).
Subgroup of the MSH.
Maintenance phase (after the first 24 wk) was 1000 mg/d for 4 days/wk; patients were then assigned to 1 of 3 groups that differed.
9.7 months in patients receiving HU for <24 mo.
The significant hematologic effects of hydroxyurea after 2 years included a small mean increase in total hemoglobin level of 6 g/L relative to placebo recipients and a moderate absolute increase in fetal hemoglobin of 3.2% (Table 2). The median number of painful crises was 44% lower among persons receiving hydroxyurea (2.5 vs. 4.5 per year; P < 0.001), and the time to the first painful crisis was 3 months compared with 1.5 months (P < 0.01). The hydroxyurea group had fewer episodes of the acute chest syndrome (25 vs. 51; P < 0.001) and fewer patients who required transfusions (55 vs. 79; P = 0.002) compared with the placebo group, but no significant differences in deaths, strokes, or hepatic sequestration were observed (19). In the long-term follow-up study, which followed patients for a median of 7 years, mortality rates were 40% lower (P = 0.04) while patients were receiving hydroxyurea (1.5 deaths per 3-month period) compared with no hydroxyurea (2.6 deaths per 3-month period) (24). However, long-term mortality, when analyzed by initial treatment assignment, was similar for patients randomly assigned to hydroxyurea (3.1 per 100 person-years) and those assigned to placebo (3.6 per 100 person-years). Rates of stroke, sepsis, and renal and hepatic failure were also similar between the 2 groups.
Table 2.
Outcomes of RCTs and Observational Studies of HU for Treatment for Sickle Cell Disease*
| Study, Year (Reference) | Group | Outcome
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths, n (%) |
Fetal Hb Level (SD), % |
Fetal Hb Cells (SD), % |
Hb Level (SD), g/L |
MCV (SD), fL |
Reticulocyte Count (SD), cells × 109/L |
Pain Episodes, n | Events, n | Received Transfusion, n |
Admitted to Hospital, n |
Other | ||
| Charache et al., 1995 (18) and 1996 (19)† | HU | 2 | 8.6 (6.8)‡ | 0.48 (0.23)‡ | 91 (15)‡ | 103 (14)‡ | 2.31 (1.00)‡ | 2.5/y§ | ACS: 23|| Stroke: 2 |
55¶ | – | – |
| Placebo | 6 | 4.7 (3.3)‡ | 0.35 (0.18)‡ | 85 (13)‡ | 93 (9)‡ | 3.00 (0.99)‡ | 4.5/y | ACS: 51 Stroke: 3 |
79** | – | – | |
|
| ||||||||||||
| Steinberg et al., 1997 (20) | HU | – | 3.6 (5.4)†† | 0.152 (0.173)†† | – | 9.7 (11.2)†† | 0.97 (1.07)†† | – | – | – | – | – |
| Placebo | – | 0.4 (2.0)†† | 0.023 (0.071)†† | – | −0.4 (4.8)‡ | 0.21 (0.72)†† | – | – | – | – | – | |
|
| ||||||||||||
| Hackney et al., 1997 (21)‡‡ | HU | – | – | – | – | – | – | – | – | – | Weight gain (±SE), 3.2 ± 0.8 kg with HU vs. 1.8 ± 0.8 kg with placebo; change in peak power during exercise (±SE), 104.9 ± 31 W with HU vs. 58 ± 20 W with placebo | |
| Placebo | – | – | – | – | ||||||||
|
| ||||||||||||
| Steinberg et al., 2003 (24)§§ | HU | 36 (23.7) | – | – | – | – | – | – | – | – | – | – |
| Placebo | 39 (26.5) | – | – | – | – | – | – | Stroke: 8 | – | – | – | |
| – | – | – | Stroke: 6 | – | – | – | ||||||
|
| ||||||||||||
| el-Hazmi et al., 1992 (26) | HU | – | 19.8 (4.0)† | – | NR | – | – | – | – | – | – | – |
| Pre-HU | – | 11.8 (3.5) | – | – | – | – | – | – | – | – | – | |
| – | – | 1.3/6 mo (range, 0–9)‡ | – | – | – | – | ||||||
|
| ||||||||||||
| Charache et al. 1992 (27) | HU at MTD | – | 15.0 (6.0)† | – | 97 (18)† | – | – | 4/6 mo (range, 0–20) | – | – | – | – |
| Pre-HU | – | 4.0 (2.0) | – | 84 (14) | ||||||||
|
| ||||||||||||
| Voskaridou et al., 1995 (28) | HU | – | 22.9 (7.7) | – | 9 (13) | – | – | – | – | – | – | – |
| Pre-HU | – | 3.6 (2.1) | – | 9 (12) | – | – | – | – | – | – | ||
|
| ||||||||||||
| Loukopoulos et al., 1998 (29) | HU | – | 23.1 (9.2) | – | 93 | – | – | – | – | – | – | – |
| Pre-HU | – | 6.7 (4.7) | – | 89 | – | – | – | – | – | – | – | |
|
| ||||||||||||
| Loukopoulos et al., 2000 (30) | HU | – | – | – | – | – | – | – | – | – | Hematologic results reported stratified by sex and genotype; mean clinical severity score, 81.7 over 12 018 patient-weeks (baseline score, 1182) (arbitrary scale) | |
|
| ||||||||||||
| Rigano et al., 2001 (31) | HU | – | 25.2 (5.2)† | – | 10 (15) | – | – | 1.1 (SD, 1.8)/y; median, 0.5† | – | – | Hospital-days, 1.2 (SD, 2.3)‡ | |
| Pre-HU | – | 7.5 (5.3) | – | 6 (13) | – | – | 9.2 (SD, 6.9); median, 7.5 (all crises, not just pain crises) | – | – | Hospital-days: 22.4 (SD, 21.9) | – | |
|
| ||||||||||||
| Ferguson et al., 2002 (32) | HU ≥24 mo | – | – | – | – | – | – | – | – | – | 2.1/y; P = 0.04 relative to baseline | – |
| Pre-HU | – | – | – | – | – | – | – | – | – | 3.1/y | – | |
| HU <24 mo | – | – | – | – | – | – | – | – | – | 4.8/y; P = 0.49 relative to baseline | – | |
| Pre-HU | – | – | – | – | – | – | – | – | – | 5.7/y | – | |
|
| ||||||||||||
| Cummins and Anie, 2003 (33) | HU | – | – | – | – | – | – | 1.4/y (SD, 2.1) || || | – | – | 1.1/y (SD, 2.4) | – |
| CBT | – | – | – | – | – | – | 4.3/y (SD, 4.3) | – | – | 0.9/y (SD, 1.2) | – | |
|
| ||||||||||||
| Vicari et al., 2005 (34) | HU | – | 10.2 (5.0) | – | 86 (11)‡ | – | – | – | – | – | – | – |
| Pre-HU | – | 5.0 (3.0) | – | 79 (9) | – | – | – | – | – | – | – | |
The studies by Moore et al. (23), Ballas et al. (22), Ataga et al. (35), Chaine et al. (36), and Schultz and Ware (37) are not included in the table because they did not report the specific outcomes described in the table. ACS = acute chest syndrome; CBT = cognitive behavioral therapy; Hb = hemoglobin; HTN = hypertension; HU = hydroxyurea; MCV = mean corpuscular volume; MTD = maximum tolerated dose; NR = not reported; RCT = randomized, controlled trial.
Results presented from final analysis (19).
P < 0.001.
P = 0.001.
P < 0.001); median time to first crisis was 3 mo (P < 0.01); 2.5 pain crises per year (odds ratio, 0.6–7.0).
423 red blood cell units were transfused (P = 0.002).
670 red blood cell units were transfused.
Change from baseline.
Cohort study grouped by MSH (Multicenter Study of Hydroxyurea for Sickle Cell Anemia) assignment.
Subgroup of MSH.
P < 0.005.
The MSH also evaluated costs and quality of life. Annualized total costs were similar in the hydroxyurea group and the placebo group (P = 0.21), but costs for hospitalization for pain were statistically significantly lower in the hydroxyurea group (mean, $12 160 vs. $17 290; P < 0.05) (23). The hydroxyurea and placebo groups were similar in all quality-of-life measures (22).
Twelve observational studies enrolling only adults were available (26–37). Two studies were retrospective (29, 32), and 10 were prospective (26–28, 30, 31, 33–37) (Table 1); 2 of the prospective studies (36, 37) reported only on toxicities and not on efficacy outcomes. These observational studies included patients with various sickle genotypes. Only 1 study could be considered high quality (27). Most reports lacked descriptions of adherence to the intervention and careful accounting for losses to follow-up. Table 1 shows the omissions that may compromise the validity of these observational studies.
The mean or median age of participants in observational studies ranged from 21 to 33 years (35). Three studies from Europe included white patients exclusively (28, 30, 31). The duration of observation varied markedly across studies and within studies; the longest median follow-up was 36 to 45 months. Some individuals in each study were followed for much longer, although their exposure to hydroxyurea did not span the total duration of observation. In half of the studies, patients received the maximum tolerated dose of the drug (27, 38 – 43).
These observational studies generally support the findings from the RCT (Table 2). In the 6 studies that reported hematologic outcomes (26, 27, 30, 31, 34, 44), fetal hemoglobin was significantly higher during treatment. Mean fetal hemoglobin ranged from 4% to 12% at baseline and 10% to 23% during hydroxyurea treatment. The greatest increase occurred in white patients with hemoglobin Sβ+ thalassemia or hemoglobin Sβ0 thalassemia who received high doses of hydroxyurea (up to 2.5 g/d) (28). These 14 patients had an increase in mean fetal hemoglobin level from 3.6% (SD, 2.1%) to 23% (SD, 7.7%).
Three studies (27, 31, 33) assessed the frequency of pain crises. For the 32 patients who completed the study by Charache and colleagues (27), the frequency of crises decreased from 4 in 6 months (range, 0 to 20) to 1.3 in 6 months (range, 0 to 9), which was not statistically significant (27). In contrast, in a study of Sicilians with hemoglobin Sβ+ thalassemia or hemoglobin Sβ0 thalassemia, the annual frequency of crises decreased significantly from a mean of 9 events to 1.8 events (P < 0.001) (31). In a nonrandomized study comparing patients receiving hydroxyurea with those receiving cognitive behavioral therapy, hydroxyurea recipients had fewer pain crises (1.4 per year vs. 4.3 per year; P < 0.05); however, we suspect that the patients choosing each of these therapies differed substantially, which could affect the reported outcomes (33).
Hospitalization rates generally decreased for adults treated with hydroxyurea. In the study of Sicilians (31), the annual number of days hospitalized declined from 22.4 to 1.2 (SD, 2.3) (P < 0.001). In another study, rates of hospitalization decreased from baseline in the group treated for longer than 24 months (from 3.1 to 2.1 hospitalizations per year; P = 0.04) (32). In the study comparing hydroxyurea with cognitive behavioral therapy (33), hospitalization rates were similar among patients receiving hydroxyurea and those receiving therapy.
We did not identify any relationship across studies between the mean age of the participants or the number of enrolled participants and outcomes (pain reduction or increase in fetal hemoglobin). In the 4 studies with relevant data, the greater the change in fetal hemoglobin, the greater the decrease in the number of painful crises (19, 27, 31, 45).
On the basis of 1 high-quality RCT in adults and many lower-quality observational studies, we conclude that high-grade evidence supports that hydroxyurea increases fetal hemoglobin in adults with sickle cell disease. High-grade evidence also indicates that hydroxyurea reduces the frequency of pain crises, transfusions, and frequency or duration of hospitalization in adults. Only low-grade evidence indicates that hydroxyurea reduces mortality. The evidence base is insufficient to comment on neurologic events in adults.
Are There Predictors of Response to Hydroxyurea?
Several studies described patient characteristics that predicted response to hydroxyurea. Predictors of fetal hemoglobin response to hydroxyurea were most frequently reported. In the MSH, fetal hemoglobin increased most in participants with a history of fewer painful events and, at baseline, a reticulocyte count greater than 300 × 109 cells/L, F reticulocyte level greater than 12%, absolute neutrophil count greater than 7.5 × 109 cells/L, and fetal hemoglobin greater than 7.5%. Fetal hemoglobin increased less in men, those with the Central African Republic haplotype, those with less than 80% adherence to therapy, and those with fewer than 2 episodes of hematologic toxicity during treatment (18). In a phase II study from the same group (27), fetal hemoglobin was directly associated with the most recent plasma level of hydroxyurea and baseline fetal hemoglobin percentage and leukocyte count.
Clinical responses to hydroxyurea (a decreased rate of painful episodes during treatment) were associated with fewer baseline painful episodes, decreases in the absolute neutrophil count and absolute reticulocyte count, and increases in mean corpuscular volume (19). Hospital admissions were significantly decreased in patients with at least 2 years of hydroxyurea treatment with no interruptions longer than 2 weeks, compared with those with a shorter duration of therapy or interruptions (32).
Most patients were homozygous for hemoglobin S, but most studies also included some patients with other genotypes. No studies meeting our inclusion criteria specifically focused on outcomes in adults with hemoglobin SC disease. In general, patients with hemoglobin Sβ+ thalassemia or hemoglobin Sβ0 thalassemia responded to treatment similarly to patients with hemoglobin SS. Sex and age had little influence on outcomes.
What Toxicities Are Associated with Use of Hydroxyurea in Adults with Sickle Cell Disease?
The National Toxicology Program and the National Institute of Environmental Health Sciences has a Center for the Evaluation of Risks to Human Reproduction (CERHR). In 2007, the CERHR reported on the effect of hydroxyurea on growth and development, after a literature review and expert panel discussion (46, 47). On the basis of 1 study, the panel concluded that nursing infants of women taking hydroxyurea may be exposed to 1 to 6 mg of hydroxyurea daily depending on the mother’s dose, but they did not comment on the effect of this exposure. The expert panel found no data on the effects of hydroxyurea on female human or animal reproductive processes. Likewise, no data were available on the effect of germ cell exposure to hydroxyurea. The expert panel found sufficient data to conclude that developmental toxicity occurs in rat and mice fetuses exposed in utero to hydroxyurea. In addition, they concluded that hydroxyurea has reproductive toxicity in male mice, causing decreased testis weight and sperm count. The expert panel believed that this experimental animal data were relevant to humans. On this basis, the expert panel had concerns about the adverse effect of hydroxyurea on spermatogenesis in men receiving hydroxyurea at therapeutic doses.
The CERHR panel identified 21 papers relevant to use of hydroxyurea in pregnancy. The report reviewed studies examining pregnancy outcomes in women with sickle cell disease and essential thrombocythemia who were receiving hydroxyurea. No RCTs on the use of hydroxyurea during pregnancy were available. The largest case series (48) described outcomes of 32 pregnancies in 31 patients treated with hydroxyurea for diverse conditions. The panel concluded that the 2 cases of intrauterine fetal growth restriction and the 9 patients with preterm delivery demonstrated an increase over that expected for this population, but causality could not be attributed in these cases. The additional 20 articles were case reviews or small case series with no clear evidence for causality for any of the 10 abnormal pregnancy outcomes. The CERHR report concluded that the use of hydroxyurea in pregnancy does not seem to be commonly associated with adverse perinatal outcomes and that no data are available on long-term outcomes in children who were exposed in utero. The CERHR is concerned that hydroxyurea may increase the risk for congenital anomalies or abnormalities of fetal growth after exposure of pregnant women, but this concern is based on few data from experimental studies.
The MSH described few significant toxicities in adults (Table 3) (18 –20, 24). The investigators found lower absolute neutrophil counts among hydroxyurea recipients than placebo recipients (4.9 vs. 6.4 × 109/L cells), but the number of patients with thrombocytopenia, thrombocytosis, cancer, aplastic crisis, aseptic necrosis, lymphadenopathy, and bleeding was similar between groups. The proportion of patients reporting hair loss, fever, rash or nail changes, or gastrointestinal disturbance at 3 or more follow-up visits was similar for the hydroxyurea and placebo groups (18). The 1 article that described long-term follow-up of the MSH participants reported 3 cases of cancer, 2 of which occurred in the hydroxyurea group (24).
Table 3.
Toxicity Results in Studies of HU for Sickle Cell Disease*
| Study, Year (Reference) | Group | Adverse Effect, n |
|||
|---|---|---|---|---|---|
| Bone Marrow Depression | Rash or Nail Changes | Leg Ulcer | Other | ||
| Charache et al., 1996 (19) | HU | Bone marrow depression: 120 Neutropenia: 100 |
38 | 23 | Hair loss: 18 Fever: 91 Aseptic necrosis: 14 Lymphadenopathy: 68 Bleeding tendency: 11 Hb level >128 g/L: 11† |
| 54 | 37 | 25 | Hair loss: 28 Fever: 95 Aseptic necrosis: 13 Lymphadenopathy: 82 Bleeding tendency: 4 Hb level >12.8 g/L: 0† |
||
|
| |||||
| Steinberg et al., 2003 (24)‡ | HU Placebo |
NR | NR | NR | Cancer: 2 Sepsis/infection: 18 Hepatic failure: 3 Renal failure: 14 Stroke: 8 |
| NR | NR | NR | Cancer: 1 Sepsis/infection 20 Hepatic failure: 10 Renal failure: 14 Stroke: 6 |
||
|
| |||||
| el-Hazmi et al., 1992 (26) | HU | Leukocyte count <45 × 109 cells/L: 6 | NR | NR | |
|
| |||||
| Charache et al., 1992 (27) | HU | Thrombocytopenia: 1 Reticulocytopenia: 5 Neutropenia: 17 |
NR | NR | No unusual infections; karyotypic analysis showed no difference in proportion of abnormal chromosomes before and after treatment |
|
| |||||
| Voskaridou et al., 1995 (28) | HU | Leukopenia or thrombocytopenia: 6 | NR | NR | Bone marrow suppression rapidly corrected with holding therapy; 2 patients could not tolerate dose increase because of marrow suppression |
|
| |||||
| Loukopoulos et al., 2000 (30) | HU | Severe anemia: 2 Required splenectomy for thrombocytopenia and leukopenia: 2 |
0 | 3 | 0/40 with mutations in p53: N-ras: K-ras 0/10 with cytogenetic abnormalities |
|
| |||||
| Ferguson et al., 2002 (32) | HU ≥24 mo | 0 | NR | NR | No adverse events, including hematologic toxicity |
| HU <24 mo | 0 | NR | NR | ||
|
| |||||
| Vicari et al., 2005 (34) | HU | Neutropenia: 3 | NR | NR | 2 patients with labyrinthitis |
|
| |||||
| Chaine et al., 2001 (36) | HU | NR | 13 | 5 | Previous leg ulcer was associated with ulcer during treatment (P < 0.005); patients with ulcer were older than those without (P < 0.001); 3 of 5 cases resolved after withholding HU |
|
| |||||
| Schultz and Ware, 2003 (37) | Patients with SCD and cancer | NR | NR | NR | Survey of providers: 49 cases of cancer in patients with SCD (not all patients were receiving HU) |
| Patients with cancer and SCD receiving HU | NR | NR | NR | Unknown number of patients receiving HU, but among 49 patients with cancer (including 1 with leukemia), 3 were receiving HU | |
Hb = hemoglobin; HU = hydroxyurea; NA = not applicable; NR = not reported; SCD = sickle cell disease.
Data reported in reference 18.
According to group assigned in trial.
Although observational studies are not optimal for attributing causality for an event because they lack comparison groups, they are useful for characterizing people exposed to a drug outside of a randomized trial (Table 3). In the studies we reviewed (37, 49 –51), 3 cases of leukemia (2 children and 1 adult) were reported. A 10-year-old girl in France who received hydroxyurea for 18 months had Philadelphia chromosome–positive acute lymphoblastic leukemia on bone marrow examination (49, 50). This 1 case of leukemia was detected among 225 children observed in a well-characterized cohort of treated patients. Gulbis and associates (51) reported on a 21-year-old woman who developed acute promyelocytic leukemia after 8 years of hydroxyurea therapy. Data on cancer development were collected in 16 613 patients with sickle cell disease (37). Cancer, including 7 cases of leukemia, was diagnosed in 49 patients. Three of the 49 patients had been using hydroxyurea; 1 patient was a 14-year-old who developed acute lymphoblastic leukemia 3 months after initiating hydroxyurea therapy. No data are available on the prevalence of hydroxyurea use among the 16 613 persons with sickle cell disease.
We identified 2 additional case reports about leukemia. One case occurred in a 25-year-old Saudi Arabian woman who received hydroxyurea for 2 years, with good response. Acute myelocytic leukemia was subsequently diagnosed; cytogenetic studies revealed no abnormal clone (45). Acute myelocytic leukemia also occurred in a 42-year-old woman with hemoglobin SS who received hydroxyurea for 6 years; no cytogenetic analysis was performed (52).
We are aware of a case of acute nonlymphocytic leukemia that occurred in a 27-year-old woman with sickle cell anemia after 8 years of hydroxyurea therapy. This case was reported in abstract form (53). Bone marrow aspiration suggested that leukemia developed in the setting of myelodysplasia (53).
We reviewed 19 case reports in 15 publications (45, 52– 65) about adverse effects of hydroxyurea use in patients with sickle cell disease (Appendix Table 3, available at www.annals.org). Two of these reports described a Greek child who developed Hodgkin lymphoma (54, 55). The 18 unique case reports included 4 reports of low sperm count or decreased sperm motility; 2 cases of avascular necrosis; 2 cases of skin hyperpigmentation; 1 case each of leg ulcer, cytopenia, splenomegaly, cryptosporidium infection, intracerebral hemorrhage, and acute myocardial infarction; and the 3 cases of leukemia described earlier. Evidence for causality in each of these toxicities is low (level 3), with the exception of cytopenia, for which evidence is moderate (level 2). The reports of leukemia are difficult to score with the World Health Organization’s causality scale, because there is no possibility of regression of disease with removal of the putative causal agent.
We also reviewed studies of patients with diseases other than sickle cell disease treated with hydroxyurea to determine long-term harms. We identified 39 studies (20 randomized trials and 19 observational studies) and 235 case reports in 175 publications (Appendix Table 4, available at www.annals.org). Among the RCTs (66 – 85), none showed more cases of leukemia in the hydroxyurea group. This harm could not be assessed in trials that enrolled patients with chronic myeloid leukemia because acute leukemia was evaluated as an outcome rather than as a medication-related toxicity. Our review of case reports in diseases other than sickle cell disease found level 1 evidence to support the causal role of hydroxyurea in leg ulcers, interstitial pneumonitis, hepatitis, azoospermia or decreased sperm motility, limbal stem cell deficiency (a corneal condition), pruritus, and skin neoplasms.
On the basis of our review of toxicities in patients with sickle cell disease and patients with other diseases, we conclude that limited evidence suggests that hydroxyurea treatment in adults with sickle cell disease does not increase the risk for leukemia. High-grade evidence supports that hydroxyurea is not associated with leg ulcer development in patients with sickle cell disease, although high-grade evidence supports that hydroxyurea is associated with leg ulcers in patients with other conditions. It has not been determined whether the increase in hemoglobin, the effects of hydroxyurea on nitric oxide bioavailability, or another effect of hydroxyurea might explain this discrepancy. The evidence is insufficient in sickle cell disease to know whether hydroxyurea contributes to skin neoplasms, although high-grade evidence in other conditions supports that it does. Similarly, evidence is insufficient to know whether hydroxyurea is associated with secondary cancer in adults with sickle cell disease, and the evidence in other diseases is only low grade.
Are There Predictors of Toxicity from Hydroxyurea?
Few studies specified what predicted toxicity from hydroxyurea. In the MSH, patients with the greatest response in fetal hemoglobin were more likely to have 2 or more episodes of hematologic toxicity (20). In addition, leg ulcers at baseline were associated with leg ulcers during therapy, and 36 viral-like infections were associated with neutropenia during therapy (86). Patients with hemoglobin SC disease did not tolerate dose increases as well as patients with other genotypes (38). Charache and colleagues (27) found no association between hydroxyurea clearance and toxicity.
Discussion
Since its approval for the treatment of sickle cell disease in 1998, hydroxyurea has been under intense study. On the basis of 1 high-quality randomized trial in adults and many observational studies, we conclude that hydroxyurea increases fetal hemoglobin in adults with sickle cell disease, reduces the frequency of pain crises, reduces the frequency or duration of hospitalization, and reduces transfusions (Table 4). Although the data convincingly demonstrate an increase in fetal hemoglobin percentage with use of this drug, far less evidence is available regarding the clinically relevant outcomes of hospitalization, stroke, pain crises, the acute chest syndrome, and death.
Table 4.
Summary of the Evidence in Sickle Cell Disease*
| Criterion | Outcome | Evidence Grade | Basis for Grade |
|---|---|---|---|
| Efficacy/effectiveness | Increase in fetal hemoglobin level | High | 1 good RCT, plus consistent observational studies |
| Reduction in pain crises | High | 1 good RCT, plus consistent observational studies | |
| Reduction in hospitalizations | High | 1 good RCT, plus consistent observational studies | |
| Reduction in neurologic events | Insufficient | No studies | |
| Reduction in transfusion frequency | High | 1 good RCT, plus consistent observational studies | |
| Mortality | Low | Inconsistent observational studies | |
| Toxicity | Leukemia (MDS, AML, or cytogenetic abnormalities) | Low (supports absence of increased risk) | Indirect evidence and inconsistent results |
| Leg ulcer | High (supports absence of increased risk) | 1 good RCT, plus consistent observational studies | |
| Skin neoplasms | Insufficient | No studies in sickle cell, high-grade evidence in other populations | |
| Secondary cancer | Insufficient | No studies in sickle cell, low-grade evidence in other populations | |
| Adverse pregnancy outcome | Insufficient | CEHER report | |
| Spermatogenesis defects | Low | Case reports with evidence of causality |
AML = acute myelogenous leukemia; CEHER = Center for the Evaluation of Risks to Human Reproduction; MDS = myelodysplastic syndrome; RCT = randomized, controlled trial.
Although the evidence is sparse, it suggests that hydroxyurea treatment in adults is not associated with leukemia. In addition, hydroxyurea is not associated with leg ulcers in patients with sickle cell disease, although it is in patients with other conditions. We hypothesize that the improvement in rheology offsets any increase in leg ulcer risk associated with the drug. We cannot draw conclusions from the evidence about whether hydroxyurea contributes to skin neoplasms in sickle cell disease, although it convincingly does in other conditions. The other populations studied were largely light-skinned, and we were not surprised that the skin cancer risk differed across populations.
This evidence base has important limitations. Most notably, only 1 randomized trial studied efficacy (18, 87). Although the MSH was a high-quality study, concerns have been raised (88) about its overreliance on secondary analyses. The MSH investigators used a more stringent threshold for statistical significance in the secondary analysis of the results of the randomized trial, and these analyses were planned before study initiation. Similar adjustments, however, were not made in the secondary analysis of overall mortality published in the long-term observational follow-up study of MSH.
We are also concerned that the observational data may be confounded by regression to the mean. For example, if physicians started hydroxyurea therapy in patients after a period of increased disease activity, it is likely that in time, the patient may have returned to his or her usual level of disease activity even without a change in therapy. This effect would overstate the benefits of the therapy (89). No trial data are available with which to comment on effectiveness of this drug in a population that may take the medication for many years with less intense supervision and encouragement than is received in a typical efficacy trial. Thus, the evidence base is limited by the lack of effectiveness trials and the paucity of trials of efficacy, even though the MSH may be considered a definitive efficacy trial of this drug in adults (18). These results cannot be generalized to all patients with sickle cell disease because the MSH included almost entirely patients with hemoglobin SS; clinical response and toxicities seem to differ to some extent by genotype.
These relatively short studies cannot provide strong evidence for toxicities that may require many years of exposure. The follow-up studies from these trials are important contributors to the literature, but they become observational studies after the randomization period ends and thus are subject to the limitations of any observational study. The losses to follow-up were substantial in most of the observational studies. The studies of toxicity were also weakened by the absence of control groups.
Many subgroups require further study. Patients with hemoglobin SC, the second most common genotype of sickle cell disease, were particularly understudied. Additional studies of hydroxyurea at doses other than the maximum tolerated dose are appropriate, particularly as the use of a maximum tolerated dose in resource-poor populations may be less practical or safe. Other subgroups of interest are patients with comorbid illnesses, specifically HIV, AIDS, or hepatitis C. The interactions between hydroxyurea and these underlying diseases, and between hydroxyurea and therapies for these diseases, need to be clarified. Additional longer-term studies are needed to further assess toxicities of hydroxyurea.
Further research on the role of hydroxyurea in the lifelong treatment of sickle cell disease is indicated; current studies do not tell us the optimal timing of initiation of hydroxyurea therapy and the indicators of failed hydroxyurea therapy. Several other questions also remain: Is there a role for rechallenge with the drug if there was no previous efficacy? Is there a role for hydroxyurea as an adjunctive therapy with other drugs? What are the best intermediate outcomes that will predict clinical response to the drug? Additional trials with other clinical outcomes are appropriate, including randomized trials to prevent other complications of sickle cell disease, such as kidney disease, pulmonary hypertension, and neurologic events in adults. Effectiveness trials are needed to test hydroxyurea in a regular care setting. These could be clustered randomized trials in which some providers are randomly assigned to prescribe hydroxyurea in all patients and others are randomly assigned to provide usual care, including the use of hydroxyurea when clinically indicated, or effectiveness studies in which a group of providers is actively encouraged to consider hydroxyurea when appropriate and another group is not targeted for education.
Hydroxyurea is the only U.S. Food and Drug Administration–approved medication for the treatment of sickle cell disease. It is the only available drug that alters the disease process, and its toxicities or potential toxicities should be interpreted in this light, particularly when it is used to treat a disease that causes great morbidity and predictably shortens the life span.
Acknowledgments
Grant Support: This project was funded under contract no. 290-02-0018 from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services.
Footnotes
Disclaimer: The authors of this report are responsible for its content. Statements in the report should not be construed as endorsements by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Potential Financial Conflicts of Interest: Grants received: C. Haywood (National Institutes of Health [grant no. 5F31HL082037-03]).
Requests for Single Reprints: Sophie Lanzkron, MD, Johns Hopkins University, 1830 East Monument Street, Suite 7300, Baltimore, MD 21205.
Current author addresses are available at www.annals.org.
References
- 1.Hassell K. Sickle cell disease population estimation: application of available contemporary data to traditional methods [Abstract]. Presented at the 35th Anniversary Convention of the National Sickle Cell Disease Program; 17–22 September 2007; Washington, DC. Baltimore: Sickle Cell Disease Assoc of America; 2007. p. Abstract no. 275. [Google Scholar]
- 2.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2. Philadelphia: Williams & Wilkins; 1996. Screening for hemoglobinopathies. [Google Scholar]
- 4.McClish DK, Penberthy LT, Bovbjerg VE, Roberts JD, Aisiku IP, Levenson JL, et al. Health related quality of life in sickle cell patients: the PiSCES project. Health Qual Life Outcomes. 2005;3:50. doi: 10.1186/1477-7525-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro BS, Benjamin LJ, Payne R, Heidrich G. Sickle cell-related pain: perceptions of medical practitioners. J Pain Symptom Manage. 1997;14:168–74. doi: 10.1016/S0885-3924(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. The Management of Sickle Cell Disease. 4. Washington, DC: National Institutes of Health; Jun, 2002. National Heart, Lung, and Blood Institute. NIH publication no. 02-2117 2002. [Google Scholar]
- 7.Segal JB, Strouse JJ, Beach MC, Haywood C, Witkop C, Park HS, et al. Evidence Report/Technology Assessment No. 165. (Prepared by Johns Hopkins University Evidence-based Practice Center under contract No. 290-02-0018) Rockville, MD: Agency for Healthcare Research and Quality; Feb, 2008. Hydroxyurea for the Treatment of Sickle Cell Disease. AHRQ publication no. 08-E007. [Google Scholar]
- 8.Berlin JA. Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group [Letter] Lancet. 1997;350:185–6. doi: 10.1016/s0140-6736(05)62352-5. [DOI] [PubMed] [Google Scholar]
- 9.WHO Collaborating Center for International Drug Monitoring. The Uppsala Monitoring Centre. Accessed at www.who-umc.org/dynpage.aspx?id=22682 on 10 April 2008.
- 10.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln TM, Rief W. How much do sample characteristics affect the effect size? An investigation of studies testing the treatment effects for social phobia. J Anxiety Disord. 2004;18:515–29. doi: 10.1016/S0887-6185(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 12.Tobin GA, Begley CM. Methodological rigour within a qualitative framework. J Adv Nurs. 2004;48:388–96. doi: 10.1111/j.1365-2648.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- 13.Horsburgh D. Evaluation of qualitative research. J Clin Nurs. 2003;12:307–12. doi: 10.1046/j.1365-2702.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Ratanawongsa N, Thomas PA, Marinopoulos SS, Dorman T, Wilson LM, Ashar BH, et al. The reported validity and reliability of methods for evaluating continuing medical education: a systematic review. Acad Med. 2008;83:274–283. doi: 10.1097/ACM.0b013e3181637925. [DOI] [PubMed] [Google Scholar]
- 15.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality. Methods Reference Manual for Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2007. pp. 1–127. [Google Scholar]
- 17.Jones JK. Determining causation from case reports. In: Strom B, editor. Pharmacoepidemiology. 4. Chichester, UK: Wiley; 2005. [Google Scholar]
- 18.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 19.Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26. doi: 10.1097/00005792-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood. 1997;89:1078–88. [PubMed] [Google Scholar]
- 21.Hackney AC, Hezier W, Gulledge TP, Jones S, Strayhorn D, Busby M, et al. Effects of hydroxyurea administration on the body weight, body composition and exercise performance of patients with sickle-cell anaemia. Clin Sci (Lond) 1997;92:481–6. doi: 10.1042/cs0920481. [DOI] [PubMed] [Google Scholar]
- 22.Ballas SK, Barton FB, Waclawiw MA, Swerdlow P, Eckman JR, Pegelow CH, et al. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual Life Outcomes. 2006;4:59. doi: 10.1186/1477-7525-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore RD, Charache S, Terrin ML, Barton FB, Ballas SK. Cost-effectiveness of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Am J Hematol. 2000;64:26–31. doi: 10.1002/(sici)1096-8652(200005)64:1<26::aid-ajh5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–51. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 25.Charache S, Terrin ML, Moore RD, Dover GJ, McMahon RP, Barton FB, et al. Design of the multicenter study of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea. Control Clin Trials. 1995;16:432–46. doi: 10.1016/s0197-2456(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 26.el-Hazmi MA, Warsy AS, al-Momen A, Harakati M. Hydroxyurea for the treatment of sickle cell disease. Acta Haematol. 1992;88:170–4. doi: 10.1159/000204681. [DOI] [PubMed] [Google Scholar]
- 27.Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–65. [PubMed] [Google Scholar]
- 28.Voskaridou E, Kalotychou V, Loukopoulos D. Clinical and laboratory effects of long-term administration of hydroxyurea to patients with sickle-cell/beta-thalassaemia. Br J Haematol. 1995;89:479–84. doi: 10.1111/j.1365-2141.1995.tb08352.x. [DOI] [PubMed] [Google Scholar]
- 29.Loukopoulos D, Voskaridou E, Stamoulakatou A, Papassotiriou Y, Kalotychou V, Loutradi A, et al. Hydroxyurea therapy in thalassemia. Ann N Y Acad Sci. 1998;850:120–8. doi: 10.1111/j.1749-6632.1998.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 30.Loukopoulos D, Voskaridou E, Kalotychou V, Schina M, Loutradi A, Theodoropoulos I. Reduction of the clinical severity of sickle cell/beta-thalassemia with hydroxyurea: the experience of a single center in Greece. Blood Cells Mol Dis. 2000;26:453–66. doi: 10.1006/bcmd.2000.0328. [DOI] [PubMed] [Google Scholar]
- 31.Rigano P, Rodgers GP, Renda D, Renda MC, Aquino A, Maggio A. Clinical and hematological responses to hydroxyurea in Sicilian patients with Hb S/beta-thalassemia. Hemoglobin. 2001;25:9–17. doi: 10.1081/hem-100103065. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson RP, Arun A, Carter C, Walker SD, Castro O. Hydroxyurea treatment of sickle cell anemia in hospital-based practices. Am J Hematol. 2002;70:326–8. doi: 10.1002/ajh.10184. [DOI] [PubMed] [Google Scholar]
- 33.Cummins O, Anie KA. A comparison of the outcome of cognitive behaviour therapy and hydroxyurea in sickle cell disease. Psychol Health Med. 2003;8:199–204. [Google Scholar]
- 34.Vicari P, Barretto de Mello A, Figueiredo MS. Effects of hydroxyurea in a population of Brazilian patients with sickle cell anemia. Am J Hematol. 2005;78:243–4. doi: 10.1002/ajh.20293. [DOI] [PubMed] [Google Scholar]
- 35.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 36.Chaine B, Neonato MG, Girot R, Aractingi S. Cutaneous adverse reactions to hydroxyurea in patients with sickle cell disease. Arch Dermatol. 2001;137:467–70. [PubMed] [Google Scholar]
- 37.Schultz WH, Ware RE. Malignancy in patients with sickle cell disease. Am J Hematol. 2003;74:249–53. doi: 10.1002/ajh.10427. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–45. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 39.Ware RE, Eggleston B, Redding-Lallinger R, Wang WC, Smith-Whitley K, Daeschner C, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99:10–4. doi: 10.1182/blood.v99.1.10. [DOI] [PubMed] [Google Scholar]
- 40.Kinney TR, Helms RW, O’Branski EE, Ohene-Frempong K, Wang W, Daeschner C, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94:1550–4. [PubMed] [Google Scholar]
- 41.Olivieri NF, Vichinsky EP. Hydroxyurea in children with sickle cell disease: impact on splenic function and compliance with therapy. J Pediatr Hematol Oncol. 1998;20:26–31. doi: 10.1097/00043426-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 42.de Montalembert M, Belloy M, Bernaudin F, Gouraud F, Capdeville R, Mardini R, et al. Three-year follow-up of hydroxyurea treatment in severely ill children with sickle cell disease. The French Study Group on Sickle Cell Disease. J Pediatr Hematol Oncol. 1997;19:313–8. doi: 10.1097/00043426-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Scott JP, Hillery CA, Brown ER, Misiewicz V, Labotka RJ. Hydroxyurea therapy in children severely affected with sickle cell disease. J Pediatr. 1996;128:820–8. doi: 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- 44.Aslanidis S, Pyrpasopoulou A, Perifanis V, Kartali N, Zamboulis C. Acute arthritis in sickle-cell anaemia: rare presentation of a peripheral bone infarct. Eur J Haematol. 2006;77:89. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2643.x. [DOI] [PubMed] [Google Scholar]
- 45.Al-Jam’a AH, Al-Dabbous IA, Al-Khatti AA, Esan FG. Are we underestimating the leukemogenic risk of hydroxyurea. Saudi Med J. 2002;23:1411–3. [PubMed] [Google Scholar]
- 46.Shelby MD. Center for the Evaluation of Risks to Human Reproduction Expert Panel. National Toxicology Program Center for the Evaluation of Risks to Human Reproduction: guidelines for CERHR expert panel members. Birth Defects Res B Dev Reprod Toxicol. 2005;74:9–16. doi: 10.1002/bdrb.20029. [DOI] [PubMed] [Google Scholar]
- 47.Shelby MD Center for the Evaluation of Risks to Human Reproduction. NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Hydroxyurea. 2007 January; Accessed at http://cerhr.niehs.nih.gov/chemicals/hydroxyurea/Hydroxyurea_final.pdf on 28 December 2007.
- 48.Thauvin-Robinet C, Maingueneau C, Robert E, Elefant E, Guy H, Caillot D, et al. Exposure to hydroxyurea during pregnancy: a case series [Letter] Leukemia. 2001;15:1309–11. doi: 10.1038/sj.leu.2402168. [DOI] [PubMed] [Google Scholar]
- 49.de Montalembert M, Bégué P, Bernaudin F, Thuret I, Bachir D, Micheau M. Preliminary report of a toxicity study of hydroxyurea in sickle cell disease. French Study Group on Sickle Cell Disease. Arch Dis Child. 1999;81:437–9. doi: 10.1136/adc.81.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Montalembert M, Brousse V, Elie C, Bernaudin F, Shi J, Landais P. French Study Group on Sickle Cell Disease. Long-term hydroxyurea treatment in children with sickle cell disease: tolerance and clinical outcomes. Haematologica. 2006;91:125–8. [PubMed] [Google Scholar]
- 51.Gulbis B, Haberman D, Dufour D, Christophe C, Vermylen C, Kagambega F, et al. Hydroxyurea for sickle cell disease in children and for prevention of cerebrovascular events: the Belgian experience. Blood. 2005;105:2685–90. doi: 10.1182/blood-2004-07-2704. [DOI] [PubMed] [Google Scholar]
- 52.Wilson S. Acute leukemia in a patient with sickle-cell anemia treated with hydroxyurea [Letter] Ann Intern Med. 2000;133:925–6. doi: 10.7326/0003-4819-133-11-200012050-00029. [DOI] [PubMed] [Google Scholar]
- 53.Rauch A, Borromeo M, Ghafoor A, Khoyratty B, Maheshwari J. Leukemogenesis of hydroxyurea in the treatment of sickle cell anemia [Abstract] Blood. 1999;94(Suppl 1):415a. [Google Scholar]
- 54.Moschovi M, Psychou F, Menegas D, Tsangaris GT, Tzortzatou-Stathopoulou F, Nicolaidou P, et al. Hodgkin’s disease in a child with sickle cell disease treated with hydroxyurea. Pediatr Hematol Oncol. 2001;18:371–6. doi: 10.1080/088800101316921985. [DOI] [PubMed] [Google Scholar]
- 55.Kattamis A, Lagona E, Orfanou I, Psichou F, Ladis V, Kanavakis E, et al. Clinical response and adverse events in young patients with sickle cell disease treated with hydroxyurea. Pediatr Hematol Oncol. 2004;21:335–42. doi: 10.1080/08880010490440473. [DOI] [PubMed] [Google Scholar]
- 56.Fattori A, de Souza RA, Saad ST, Costa FF. Acute myocardial infarction in sickle cell disease: a possible complication of hydroxyurea treatment. Hematol J. 2005;5:589–90. doi: 10.1038/sj.thj.6200572. [DOI] [PubMed] [Google Scholar]
- 57.Huang Y, Ananthakrishnan T, Eid JE. Hydroxyurea-induced splenic re-growth in an adult patient with severe hemoglobin SC disease. Am J Hematol. 2003;74:125–6. doi: 10.1002/ajh.10388. [DOI] [PubMed] [Google Scholar]
- 58.Venigalla P, Motwani B, Allen S, Agarwal M, Alva M, Westerman M, et al. A patient on hydroxyurea for sickle cell disease who developed an opportunistic infection [Letter] Blood. 2002;100:363. doi: 10.1182/blood-2002-03-0754. [DOI] [PubMed] [Google Scholar]
- 59.O’Branski EE, Ware RE, Prose NS, Kinney TR. Skin and nail changes in children with sickle cell anemia receiving hydroxyurea therapy. J Am Acad Dermatol. 2001;44:859–61. doi: 10.1067/mjd.2001.113471. [DOI] [PubMed] [Google Scholar]
- 60.Ferster A, Sariban E, Meuleman N. Belgian Registry of Sickle Cell Disease patients treated with Hydroxyurea. Malignancies in sickle cell disease patients treated with hydroxyurea [Letter] Br J Haematol. 2003;123:368–9. doi: 10.1046/j.1365-2141.2003.04614.x. [DOI] [PubMed] [Google Scholar]
- 61.Kersgard C, Osswald MB. Hydroxyurea and sickle cell leg ulcers [Letter] Am J Hematol. 2001;68:215–6. doi: 10.1002/ajh.1183. [DOI] [PubMed] [Google Scholar]
- 62.Grigg A. Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern Med J. 2007;37:190–2. doi: 10.1111/j.1445-5994.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 63.Garozzo G, Disca S, Fidone C, Bonomo P. Azoospermia in a patient with sickle cell disease treated with hydroxyurea [Letter] Haematologica. 2000;85:1216–8. [PubMed] [Google Scholar]
- 64.Vichinsky EP, Lubin BH. A cautionary note regarding hydroxyurea in sickle cell disease. Blood. 1994;83:1124–8. [PubMed] [Google Scholar]
- 65.Callot-Mellot C, Bodemer C, Chosidow O, Frances C, Azgui Z, Varet B, et al. Cutaneous carcinoma during long-term hydroxyurea therapy: a report of 5 cases [Letter] Arch Dermatol. 1996;132:1395–7. doi: 10.1001/archderm.132.11.1395. [DOI] [PubMed] [Google Scholar]
- 66.Frank I, Bosch RJ, Fiscus S, Valentine F, Flexner C, Segal Y, et al. ACTG 307 Protocol Team. Activity, safety, and immunological effects of hydroxyurea added to didanosine in antiretroviral-naive and experienced HIV type 1-infected subjects: a randomized, placebo-controlled trial, ACTG 307. AIDS Res Hum Retroviruses. 2004;20:916–26. doi: 10.1089/aid.2004.20.916. [DOI] [PubMed] [Google Scholar]
- 67.Havlir DV, Gilbert PB, Bennett K, Collier AC, Hirsch MS, Tebas P, et al. ACTG 5025 Study Group. Effects of treatment intensification with hydroxyurea in HIV-infected patients with virologic suppression. AIDS. 2001;15:1379–88. doi: 10.1097/00002030-200107270-00007. [DOI] [PubMed] [Google Scholar]
- 68.Swindells S, Cohen CJ, Berger DS, Tashima KT, Liao Q, Pobiner BF, et al. NZTA4008 Study Team. Abacavir, efavirenz, didanosine, with or without hydroxyurea, in HIV-infected adults failing initial nucleoside/protease inhibitor-containing regimens. BMC Infect Dis. 2005;5:23. doi: 10.1186/1471-2334-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seminari E, Lisziewicz J, Tinelli C, Foli A, Lori F, Maserati R. Hydroxyurea toxicity combined with didanosine (ddl) in HIV-1-seropositive asymptomatic individuals. Int J Clin Pharmacol Ther. 1999;37:514–8. [PubMed] [Google Scholar]
- 70.Bloch MT, Smith DE, Quan D, Kaldor JM, Zaunders JJ, Petoumenos K, et al. The role of hydroxyurea in enhancing the virologic control achieved through structured treatment interruption in primary HIV infection: final results from a randomized clinical trial (Pulse) J Acquir Immune Defic Syndr. 2006;42:192–202. doi: 10.1097/01.qai.0000219779.50668.e6. [DOI] [PubMed] [Google Scholar]
- 71.Rutschmann OT, Opravil M, Iten A, Malinverni R, Vernazza PL, Bucher HC, et al. A placebo-controlled trial of didanosine plus stavudine, with and without hydroxyurea, for HIV infection. The Swiss HIV Cohort Study. AIDS. 1998;12:F71–7. doi: 10.1097/00002030-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Rutschmann OT, Opravil M, Iten A, Malinverni R, Vernazza PL, Bucher H, et al. Didanosine plus stavudine with or without hydroxyurea in HIV-1-infected patients: 1 year follow-up. Swiss HIV Cohort Study. Antivir Ther. 1998;3 (Suppl 4):65–7. [PubMed] [Google Scholar]
- 73.Rutschmann OT, Vernazza PL, Bucher HC, Opravil M, Ledergerber B, Telenti A, et al. Long-term hydroxyurea in combination with didanosine and stavudine for the treatment of HIV-1 infection. Swiss HIV Cohort Study. AIDS. 2000;14:2145–51. doi: 10.1097/00002030-200009290-00011. [DOI] [PubMed] [Google Scholar]
- 74.Hehlmann R, Berger U, Pfirrmann M, Hochhaus A, Metzgeroth G, Maywald O, et al. German CML-Study Group. Randomized comparison of interferon alpha and hydroxyurea with hydroxyurea monotherapy in chronic myeloid leukemia (CML-study II): prolongation of survival by the combination of interferon alpha and hydroxyurea. Leukemia. 2003;17:1529–37. doi: 10.1038/sj.leu.2403006. [DOI] [PubMed] [Google Scholar]
- 75.Randomized study on hydroxyurea alone versus hydroxyurea combined with low-dose interferon-alpha 2b for chronic myeloid leukemia. The Benelux CML Study Group. Blood. 1998;91:2713–21. [PubMed] [Google Scholar]
- 76.Hehlmann R, Heimpel H, Hasford J, Kolb HJ, Pralle H, Hossfeld DK, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064–77. [PubMed] [Google Scholar]
- 77.Broustet A, Reiffers J, Marit G, Fiere D, Jaubert J, Reynaud J, et al. Hydroxyurea versus interferon alfa-2b in chronic myelogenous leukaemia: preliminary results of an open French multicentre randomized study. Eur J Cancer. 1991;27 (Suppl 4):S18–21. doi: 10.1016/0277-5379(91)90558-u. [DOI] [PubMed] [Google Scholar]
- 78.Hehlmann R, Heimpel H, Hasford J, Kolb HJ, Pralle H, Hossfeld DK, et al. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: prolongation of survival by hydroxyurea. The German CML Study Group. Blood. 1993;82:398–407. [PubMed] [Google Scholar]
- 79.Stephens RL, Vaughn C, Lane M, Costanzi J, O’Bryan R, Balcerzak SP, et al. Adriamycin and cyclophosphamide versus hydroxyurea in advanced prostatic cancer. A randomized Southwest Oncology Group study. Cancer. 1984;53:406–10. doi: 10.1002/1097-0142(19840201)53:3<406::aid-cncr2820530307>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 80.Loening SA, Scott WW, deKernion J, Gibbons RP, Johnson DE, Pontes JE, et al. A comparison of hydroxyurea, methyl-chloroethyl-cyclohexy-nitrosourea and cyclophosphamide in patients with advanced carcinoma of the prostate. J Urol. 1981;125:812–6. doi: 10.1016/s0022-5347(17)55216-7. [DOI] [PubMed] [Google Scholar]
- 81.Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997;90:3370–7. [PubMed] [Google Scholar]
- 82.Kiladjian JJ, Rain JD, Bernard JF, Briere J, Chomienne C, Fenaux P. Long-term incidence of hematological evolution in three French prospective studies of hydroxyurea and pipobroman in polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:417–21. doi: 10.1055/s-2006-942762. [DOI] [PubMed] [Google Scholar]
- 83.Finazzi G, Ruggeri M, Rodeghiero F, Barbui T. Second malignancies in patients with essential thrombocythaemia treated with busulphan and hydroxyurea: long-term follow-up of a randomized clinical trial. Br J Haematol. 2000;110:577–83. doi: 10.1046/j.1365-2141.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- 84.Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332:1132–6. doi: 10.1056/NEJM199504273321704. [DOI] [PubMed] [Google Scholar]
- 85.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. United Kingdom Medical Research Council Primary Thrombocythemia 1 Study. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 86.Wang WC, Wynn LW, Rogers ZR, Scott JP, Lane PA, Ware RE. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139:790–6. doi: 10.1067/mpd.2001.119590. [DOI] [PubMed] [Google Scholar]
- 87.Ferster A, Tahriri P, Vermylen C, Sturbois G, Corazza F, Fondu P, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97:3628–32. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 88.Debaun MR, Field JJ. Limitations of clinical trials in sickle cell disease: a case study of the Multi-center Study of Hydroxyurea (MSH) Trial and the Stroke Prevention (STOP) Trial. Hematology Am Soc Hematol Educ Program. 2007;2007:482–8. doi: 10.1182/asheducation-2007.1.482. [DOI] [PubMed] [Google Scholar]
- 89.Wright CC, Abbess CR, Jarrett DF. Estimating the regression-to-mean effect associated with road accident black spot treatment: towards a more realistic approach. Accid Anal Prev. 1988;20:199–214. doi: 10.1016/0001-4575(88)90004-8. [DOI] [PubMed] [Google Scholar]