Abstract
Object
The authors conducted a study to determine population-based estimates of survival following the diagnosis and treatment of nonmalignant intracranial meningioma in the US in the modern era.
Methods
Patients with nonmalignant intracranial meningioma were identified through the Surveillance, Epidemiology, and End Results (SEER) database for the years 2004–2007. Predictors of undergoing resection were identified and odds ratios calculated. Estimates of survival were calculated using Kaplan-Meier estimation method and Cox proportional hazards model.
Results
There were 12,284 patients with a diagnosis of nonmalignant intracranial meningioma included in the analysis. Only 55% had histological confirmation of the diagnosis of nonmalignant meningioma. Resection was used as an initial treatment in 43% of cases. Patients treated with surgery were more likely to be younger (OR 9.3, 95% CI 8.1–10.7, for resection in patients age 40–59 years compared with age > 80 years), male (OR 1.4, 95% CI 1.3–1.5, for males compared with females), white (OR 0.8, 95% CI 0.7–0.9, for black patients compared with white patients), and have larger tumors (OR 11.8, 95% CI 10.3–13.6, for tumors of the largest quartile compared with the smallest quartile). Patients treated with resection had a 3-year postdiagnosis survival estimate of 93.4% (95% CI 92.5%–94.3%) compared with 88.3% (95% CI 85.5%–90.6%) in patients not treated with resection (p < 0.01). Younger patient age, female sex, unilateral tumors, and resection were predictors of improved postdiagnosis survival after multivariate adjustment in patients with histologically confirmed meningiomas.
Conclusions
This analysis represents the first modern population-based analysis of treatment patterns and outcomes in US patients with nonmalignant intracranial meningioma. Over 85% of patients survive 3 years after diagnosis, and resection is associated with improved survival.
Keywords: meningioma, surgery, survival, epidemiology, outcomes, SEER, oncology
MeningioMas now represent the most frequently reported primary brain tumor in the US.4 As nonmalignant tumors have only recently begun to be formally registered, population-based information regarding the mortality rates following diagnosis and treatment of meningioma remains limited.3,6 To date, the largest outcome study on intracranial meningioma in the US included 8891 patients in the NCDB and reported 2- and 5-year survival rates of 82% and 70.1%, respectively, for benign meningioma.9 The role of treatment was examined in the NCDB project, which is based on data collected prior to 1992 from over 1000 US hospitals. The group reported that resection was associated with increased 5-year survival (75.1% with surgery compared with 49.9% without surgery).
Outside the US, population-based survival estimates for meningioma have been generated with data predating 1990s.14,16,17 Nonpopulation-based data from within the US exists with the majority predominantly drawn from single-institution case series.2,5,8,10 The passage of the Benign Brain Tumor Cancer Registries Amendment Act in 2002 (The Benign Brain Tumor Cancer Registries amendment Act, H.R. 5204) mandated the inclusion of benign intracranial tumors by the National Program of Cancer Registries.15 As a result, the SEER program formally added nonmalignant CNS tumors to case definitions as of January 1, 2004. The aim of the current analysis was to evaluate the survival following diagnosis of nonmalignant meningioma in the US population now that follow-up data are available for a relatively large population-based cohort of patients.
Methods
Patient Identification
Patients with a diagnosis of meningioma were identified from the SEER database.12 The SEER Program of the National Cancer Institute is a population-based tumor registry that contains data covering approximately 10%–26% of the US population depending on the year. The SEER Program added nonmalignant CNS tumors to case definitions as of January 1, 2004. Information concerning primary tumor type, patient demographics, initial cancer treatments, and survival are collected in the database.15 Data from the most recent SEER data set from 2004–2007, ASCII text data, were used for this analysis.
Patients with a diagnosis of meningioma in the brain histology grouping variable were eligible for inclusion. This included histology codes 9530–9534 and 9537–9539. Only patients with an ICD for Oncology (ICD-O-3) behavior code of “benign” were included in the analysis. Spinal meningiomas were excluded through elimination of patients with topography codes for spinal cord (C72.0), cauda equina (C72.1), or spinal meninges (C70.9). All other intracranial primary sites were eligible for inclusion in the analysis. Individuals with more than a single primary cancer were excluded from the analysis. Likewise, patients diagnosed at death (with a reporting source of autopsy only or death certificate only) were not included in this analysis.
Age at time of diagnosis of meningioma, sex, race, and marital status were obtained from the SEER data. Age was analyzed initially as a continuous variable and then as a categorical variable for ease of data presentation with the following categories: age in years less than 20, 20–39, 40–59, 60–79, and greater than 80. Due to the small number of minority patients in the data set, race was coded for the analysis as white, black, and other. Marital status at diagnosis was analyzed as the following 4 categories: single, married, divorced/separated/widowed, or unknown.
The size of the tumor was analyzed as categorical data based on quartiles for the largest reported dimension. The following size categories were used: 0–17, 18–27, 28–41, larger than 41 mm, and unknown size. The laterality of the tumor was analyzed as midline, left side, right side, bilateral sides, and unknown laterality. The information regarding histological confirmation of the diagnosis of meningioma and initial treatments were included.
Outcomes
The primary outcome for this study was postdiagnosis survival time. The date of death or censoring was obtained from the SEER data set. The initial analysis included descriptive statistics of clinical and demographic information using means, proportions, and standard deviations. Predictors of undergoing surgical treatment com pared with observation were identified and odds ratios calculated. One- and 3-year estimates of survival and 95% CIs were calculated using the Kaplan-Meier method. The effect of clinical and demographic covariates on the timing of the outcome was estimated using a Cox proportional hazards model. Separate analyses were performed for patients with and without histological confirmation of the diagnosis of meningioma. Hazard ratios were estimated with 95% CIs. All variables found to be significant at p = 0.10 in unadjusted analyses were included in multivariate analyses. Statistical significance was defined as a Type 1 error < 5%. All analyses were 2 sided and performed using SAS version 9.2 (SAS Institute).
Results
There were 15,259 patients with a diagnosis of benign, nonspinal meningioma identified in the SEER data set. There were 2819 patients excluded with more than a single primary cancer type and 156 patients excluded with a diagnosis of meningioma after death. The final study cohort included 12,284 patients. Of this cohort, histological confirmation of the diagnosis of meningioma was established in 55% (6737) of the patients. The remaining 45% (5547) of the patients had clinical and radiographic diagnoses only. Table 1 displays the descriptive clinical and demographic variables for the entire study cohort. The mean age at diagnosis of meningioma was 62 years ± 16 years (SD). The majority of patients were female (75%) and white (79%), with 53% of the patients married at the time of diagnosis. The interquartile range for maximal size of the tumor was 18–41 mm, although in 28% of patients tumor size data were not recorded. The majority of tumors were unilateral—43% left sided and 44% right sided. The location of the lesion was classified as “cerebral meninges” in 98% of cases, “brain” in 2%, and “cranial nerves” or “endocrine glands” in less than 1% of cases. Initial treatments included gross-total resection in 34%, subtotal resection in 9%, biopsy in 7%, and neither resection nor biopsy in 47% of patients. Radiation therapy was administered in 9% of patients.
TABLE 1.
Demographic and clinical profile of 12,284 patients with a diagnosis of nonmalignant intracranial meningioma in the SEER database from 2004–2007 included in the study cohort
| Variable | Percentage/Value (no. of patients) |
|---|---|
| age category (yrs) | |
| <20 | 0.5% (56) |
| 20–39 | 8% (930) |
| 40–59 | 37% (4505) |
| 60–79 | 38% (4661) |
| ≥80 | 17% (2132) |
| mean age at diagnosis (yrs) | 62 ± 16 |
| sex | |
| male | 25.0% (3077) |
| female | 75.0% (9207) |
| race | |
| white | 79% (9728) |
| black | 11% (1346) |
| other | 10% (1210) |
| marital status | |
| single | 16% (1913) |
| married | 53% (6538) |
| divorced/widowed/separated | 26% (3218) |
| unknown | 5% (615) |
| year of diagnosis | |
| 2004 | 23% (2861) |
| 2005 | 25% (3108) |
| 2006 | 26% (3181) |
| 2007 | 26% (3134) |
| histology | |
| tissue diagnosis | 55% (6737) |
| radiographic diagnosis | 45% (5547) |
| size of tumor (quartile) | |
| <17 mm | 19% (2356) |
| 18–27 mm | 17% (2129) |
| 28–41 mm | 17% (2147) |
| ≥42 mm | 18% (2152) |
| unknown | 28% (3500) |
| side of tumor | |
| midline | 10% (1184) |
| left | 43% (5300) |
| right | 44% (5401) |
| bilateral | 2% (278) |
| unknown | 1% (121) |
| surgical treatment | |
| total resection | 34% (4222) |
| partial resection | 9% (1151) |
| biopsy | 7% (868) |
| no surgery or biopsy | 47% (5812) |
| unknown | 2% (231) |
| radiation treatment | |
| any radiation | 8.9% (1088) |
| no radiation | 90.2% (11082) |
| unknown | 1.0% (114) |
There were significant differences in the clinical and demographic data in the patients who underwent resection (partial or complete) compared with those who underwent conservative management (biopsy or no surgical intervention). The predictors associated with undergoing resection are listed in Table 2. Increased odds of undergoing resection was associated with younger age, male sex, increasing tumor size, and lateral compared with midline tumors. Compared with patients older than age 80 years, those in the 40–59 age group had a 9-fold increase in the odds of undergoing resection (OR 9.3, 95% CI 8.1–10.7), whereas patients in the 20–39 age group had a 12-fold increase in the odds of undergoing resection (OR 12.5, 95% CI 10.4–14.9). Male patients were more likely to undergo resection than female patients (OR 1.4, 95% CI 1.3–1.5, for male vs female). The patient’s race was also associated with the likelihood of undergoing resection, with black patients less likely to undergo resection than white patients (OR 0.8, 95% CI 0.7–0.9), although there was no difference between white patients and patients classified as other races. Marital status at the time of diagnosis was also associated with the treatment received. Divorced, widowed, or separated patients had lower chance of undergoing resection than married patients (OR 0.4, 95% CI 0.4–0.5). The odds of undergoing resection also increased with each quartile of tumor size. Tumors with a diameter equal to or greater than 42 mm were associated with an OR of 11.8 (95% CI 10.3–13.6) for resection compared with tumors less than 17 mm in diameter. Finally, patients who underwent radiation therapy had approximately half the odds of undergoing resection (OR 0.5, 95% CI 0.4–0.6) as those who did not undergo radiation therapy.
TABLE 2.
Demographic and clinical predictors of undergoing surgical treatment for nonmalignant intracranial meningioma in 12,053*
| Variable | Surgical Treatment (partial or total) |
Observation (biopsy or no surgery) |
OR for Resection (95% CI) |
|---|---|---|---|
| age category | |||
| less than 20 | <1% (34) | <1% (21) | 10.5 (6.1–18.1) |
| 20–39 | 11% (614) | 5% (305) | 12.5 (10.4–14.9) |
| 40–59 | 48% (2579) | 28% (1841) | 9.3 (8.1–10.7) |
| 60–79 | 35% (1901) | 40% (2660) | 5.0 (4.3–5.7) |
| ≥80 | 5% (245) | 28% (1853) | reference |
| sex | |||
| male | 50% (1526) | 50% (1497) | 1.4 (1.3–1.5) |
| female | 43% (3847) | 58% (5251) | reference |
| race | |||
| white | 45% (4302) | 55% (5299) | reference |
| black | 38% (509) | 62% (815) | 0.8 (0.7–0.9) |
| other | 47% (562) | 53% (634) | 1.1 (1.0–1.2) |
| marital status | |||
| single | 50% (947) | 50% (939) | reference |
| married | 51% (3274) | 49% (3174) | 1.0 (0.9–1.1) |
| divorced/widowed/separated | 30% (953) | 70% (2235) | 0.4 (0.4–0.5) |
| unknown | 33% (199) | 67% (400) | 0.6 (0.5–0.7) |
| year of diagnosis | |||
| 2004 | 46% (1287) | 54% (1537) | 1.3 (1.2–1.4) |
| 2005 | 47% (1442) | 53% (1623) | 1.4 (1.2–1.5) |
| 2006 | 45% (1415) | 55% (1721) | 1.3 (1.1–1.4) |
| 2007 | 40% (1229) | 60% (1867) | reference |
| size of tumor (quartile) | |||
| <17 mm | 6% (322) | 30% (1976) | reference |
| 18–27 mm | 12% (660) | 22% (1443) | 2.4 (2.1–2.8) |
| 28–41 mm | 22% (1173) | 14% (940) | 6.4 (5.6–7.4) |
| ≥42 mm | 28% (1531) | 9% (598) | 11.8 (10.3–13.6) |
| unknown | 31% (1687) | 26% (1723) | 5.3 (4.7–6.0) |
| side of tumor | |||
| midline | 38% (438) | 62% (719) | reference |
| left | 45% (2348) | 55% (2891) | 1.1 (0.9–1.3) |
| right | 45% (2389) | 45% (2934) | 1.4 (1.0–1.3) |
| bilateral | 57% (157) | 43% (117) | 1.8 (1.4–2.3) |
| unknown | 35% (41) | 65% (77) | 0.8 (0.6–1.2) |
| radiation treatment | |||
| any radiation | 6% (313) | 11% (767) | 0.5 (0.4–0.6) |
| no radiation | 94% (5025) | 88% (5954) | reference |
| unknown | <1% (35) | <1% (27) | 1.5 (0.9–2.5) |
Two hundred thirty-one patients with unknown surgical treatment information were not included. Bolded values indicate statistical significance (p < 0.05). Reference = reference group used to calculate the odds ratio.
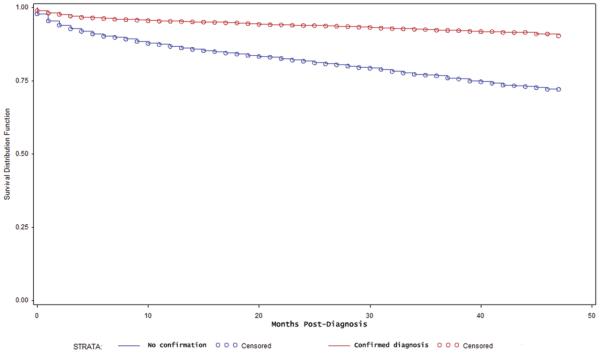
Overall survival after diagnosis of meningioma was analyzed separately for those patients with histological confirmation of the diagnosis and those without confirmation. For patients with a confirmed diagnosis (6737), the 1- and 3-year survival estimates were 95.4% (95% CI 94.8%–95.9%) and 92.4% (95% CI 91.5%–93.1%), respectively. For patients without histological confirmation (5547 cases), the 1- and 3-year survival estimates were 86.8% (95% CI 85.8%–87.7%) and 76.7% (95% CI 75.2%–78.2%), respectively. Figure 1 contains the Kaplan-Meier survival estimates according to histological confirmation of the diagnosis. There was a significant difference in the overall postdiagnosis survival (p < 0.01, log-rank test) in these 2 groups.
Fig. 1.
Kaplan-Meier survival estimate for patients with a histological confirmation of the diagnosis of nonmalignant meningioma (red) and without a histological confirmation (blue). Survival is displayed as months postdiagnosis.
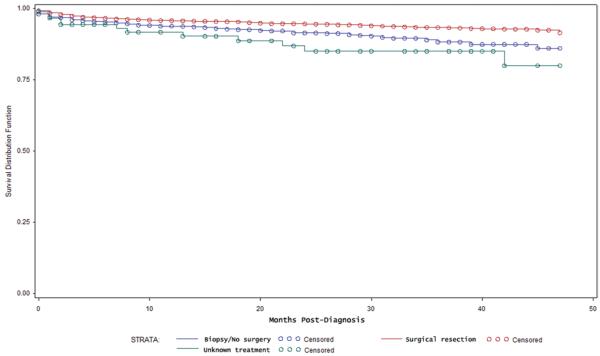
In cases involving a confirmed diagnosis of meningioma, postdiagnosis survival was evaluated in 5370 patients who underwent resection and compared with 1270 patients who underwent biopsy or no surgical intervention. Figure 2 contains the Kaplan-Meier survival estimates for these patients. There was a significant increase in overall survival associated with resection (p < 0.01, log-rank test). The estimated 3-year survival was lower in patients who underwent biopsy or no resection (88.3%, 95% CI 85.5%–90.6%) than in patients treated with resection (93.4%, 95% CI 92.5%–94.3%). The survival estimates for the patients with unknown data regarding type of treatment received are also shown in Fig. 2.
Fig. 2.
Kaplan-Meier survival estimate for patients with histologically confirmed meningioma according to type of treatment received: resection (red), biopsy/no resection (blue), unknown treatment (green) (p < 0.01, log-rank test). Survival is displayed as months postdiagnosis.
Univariate and multivariate Cox regression models were evaluated to determine the association of clinical and demographic variables with postdiagnosis survival. Separate models were used for patients with and without histological confirmation of a meningioma diagnosis. The unadjusted and adjusted hazard ratios with the 95% CIs associated with the variables are listed in Table 3. For patients with histological confirmation, on unadjusted analyses, increased hazard ratios for postoperative death were associated with male sex (HR 1.50, 95% CI 1.21–1.83), marital status of divorced/widowed/separated compared with single (HR 2.20, 95% CI 1.61–3.01), and larger tumor size (HR 1.72, 95% CI 1.05–2.83) for 42 mm or greater compared with tumors smaller than 17 mm. A decreased hazards ratio for postoperative death was associated with all age groups when compared with the 80 and older age group, unilateral compared with midline tumors (HR 0.52, 95% 0.38–0.69, for left compared with midline, and HR 0.47, 95% CI 0.35–0.64, for right compared with midline tumors), and for resection compared with biopsy or no surgical intervention (HR 0.61, 95% CI 0.48–0.76).
TABLE 3.
Univariate and multivariate Cox regression model analysis of clinical and demographic factors associated with postdiagnosis death hazards*
| Hazard Ratio |
||
|---|---|---|
| Variable | Unadjusted (95% CI) |
Adjusted (95% CI) |
| histologically confirmed diagnosis | ||
| age category (yrs) | ||
| <20 | 0.06 (0.01–0.46) | 0.05 (0.01–0.39) |
| 20–39 | 0.04 (0.02–0.07) | 0.04 (0.02–0.08) |
| 40–59 | 0.07 (0.06–0.10) | 0.08 (0.06–0.11) |
| 60–79 | 0.22 (0.17–0.28) | 0.23 (0.18–0.30) |
| ≥80 | reference | reference |
| sex | ||
| male | 1.50 (1.21–1.83) | 1.50 (1.20–1.85) |
| female | reference | reference |
| race | ||
| white | reference | |
| black | 1.31 (0.97–1.77) | NS |
| other | 0.80 (0.55–1.17) | NS |
| marital status | ||
| single | reference | reference |
| married | 0.92 (0.68–1.23) | 0.76 (0.56–1.03) |
| divorced/widowed/ separated |
2.20 (1.61–3.01) | 1.13 (0.81–1.57) |
| unknown | 1.86 (1.18–3.02) | 1.26 (0.78–2.03) |
| year of diagnosis | ||
| 2004 | 1.45 (1.03–2.03) | 1.49 (1.06–2.09) |
| 2005 | 0.99 (0.70–1.42) | 1.05 (0.74–1.50) |
| 2006 | 1.02 (0.71–1.46) | 1.03 (0.72–1.48) |
| 2007 | reference | reference |
| size of tumor (quartile) | ||
| <17 mm | reference | reference |
| 18–27 mm | 0.75 (0.41–1.37) | 0.71 (0.39–1.30) |
| 28–41 mm | 1.32 (0.79–2.21) | 1.03 (0.61–1.73) |
| ≥42 mm | 1.72 (1.05–2.83) | 1.29 (0.78–2.14) |
| unknown | 1.67 (1.02–2.73) | 1.37 (0.83–2.25) |
| side of tumor | ||
| midline | reference | reference |
| left | 0.52 (0.38–0.69) | 0.48 (0.36–0.66) |
| right | 0.47 (0.35–0.64) | 0.45 (0.33–0.60) |
| bilateral | 1.37 (0.86–2.18) | 1.28 (0.80–2.06) |
| unknown | 0.32 (0.08–1.31) | 0.29 (0.07–1.18) |
| treatment | ||
| radiation | 0.70 (0.44–1.12) | NS |
| no radiation | reference | NS |
| unknown | 0.32 (0.04–2.28) | NS |
| surgical treatment | ||
| partial/total resection | 0.61 (0.48–0.76) | 0.75 (0.59–0.95) |
| none/biopsy | reference | reference |
| unknown | 1.50 (0.83–2.73) | 1.09 (0.59–2.02) |
| unconfirmed diagnosis | ||
| age category | ||
| <20 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| 20–39 | 0.05 (0.02–0.13) | 0.5 (0.2–0.15) |
| 40–59 | 0.08 (0.06–0.11) | 0.11 (0.8–0.15) |
| 60–79 | 0.29 (0.25–0.34) | 0.34 (0.30–0.40) |
| ≥80 | reference | reference |
| sex | ||
| male | 1.3 (1.12–1.50) | 1.54 (1.31–1.80) |
| female | reference | reference |
| race | ||
| white | reference | reference |
| black | 1.23 (1.03–1.50) | 1.30 (1.08–1.57) |
| other | 0.83 (0.65–1.05) | 1.02 (0.79–1.30) |
| marital status | ||
| single | reference | reference |
| married | 0.68 (0.54–0.85) | 0.64 (0.51–0.80) |
| divorced/widowed/ separated |
1.72 (1.40–2.12) | 0.96 (0.77–1.20) |
| unknown | 0.92 (0.65–1.30) | 0.77 (0.54–1.10) |
| year of diagnosis | ||
| 2004 | 1.67 (0.93–1.47) | NS |
| 2005 | 1.03 (0.81–1.29) | NS |
| 2006 | 1.14 (0.91–1.43) | NS |
| 2007 | reference | NS |
| size of tumor (quartile) | ||
| <17 mm | reference | reference |
| 18–27 mm | 1.39 (1.06–1.58) | 1.17 (0.96–1.43) |
| 28–41 mm | 2.33 (1.90–2.84) | 1.80 (1.46–2.20) |
| ≥42 mm | 4.39 (3.53–5.46) | 3.28 (2.63–4.09) |
| unknown | 1.59 (1.32–1.91) | 1.51 (1.26–1.83) |
| side of tumor | ||
| midline | reference | NS |
| left | 1.10 (0.89–1.38) | NS |
| right | 0.92 (0.74–1.51) | NS |
| bilateral | 1.29 (0.79–2.10) | NS |
| unknown | 1.13 (0.64–2.02) | NS |
| treatment | ||
| radiation | 0.30 (0.22–0.41) | 0.46 (0.33–0.64) |
| no radiation | reference | reference |
| unknown | 0.72 (0.37–1.38) | 1.75 (0.65–4.69) |
NS = not significant. Bolded values indicate statistical significance (p < 0.05). Reference = reference group used to calculate the hazard ratio.
After multivariate adjustment, resection remained associated with a decreased hazards ratio for death compared with no surgery/biopsy (HR 0.75, 95% CI 0.59–0.95). Decreasing patient age as well as unilateral compared with midline tumors also remained associated with decreased hazard ratios for death. Male sex remained associated with an increased hazard ratio for death (HR 1.50, 95% CI 1.20–1.85). Size of the tumor and marital status were no longer significantly associated with the hazards ratio after multivariate adjustment.
For patients without histological confirmation, on unadjusted analyses, young age at diagnosis was also associated with a reduced hazard for postdiagnosis death. Likewise, the patients who underwent radiation treatment had lower death hazards compared with those that did not under radiation treatment (HR 0.30, 95% CI 0.22–0.41). An increase in death hazards was associated with male gender (HR 1.30, 95% CI 1.12–1.50), black compared with white race (HR 1.30, 95% CI 1.08–1.57), as well as increasing quartile of tumor size (HR 4.39, 95% CI 3.53–5.46 for tumors 42 mm or greater compared with tumors less than 17 mm). Married patients had lower death hazards compared with single patients (HR 0.68, 95% CI 0.54–0.85), while divorced, widowed, or separated patients had increased death hazards compared with single patients (HR 1.72, 95% CI 1.40–2.12).
Similar results were obtained after multivariate adjustment. Decreasing patient age and radiation treatment remained associated with decreased hazards for postdiagnosis death (HR 0.46, 95% CI 0.33–0.64, for radiation treatment). Male sex (HR 1.54, 95% CI 1.31–1.80), black compared with white individuals (HR 1.30, 95% CI 1.08–1.57), and increasing quartile of tumor size remained associated with increased risk of death hazards. Married patients also had decreased death risk compared with single patients (HR 0.64, 95% CI 0.51–0.80); however, divorced, widowed, or separated patients no longer had increased risk of death compared with single patients.
Finally, 1- and 3-year estimates of postdiagnosis survival times were calculated for patients with confirmed meningioma according to sex and surgical treatment. As shown in Table 4, the 1- and 3-year survival estimates for all tumor sizes treated with resection were greater than 90%. For patients treated without resection, the survival estimates decreased, with nonsurgically treated male patients having the lowest overall survival.
TABLE 4.
One-year and 3-year survival estimates of survival according to sex and surgical treatment for patients with a confirmed diagnosis of nonmalignant intracranial meningioma
| Treatment | No. of Patients | 1-Year Survival (95% CI) | 3-Year Survival (95% CI) |
|---|---|---|---|
| female patients | |||
| resection | 3844 | 96.4% (95.7%–96.9%) | 94.0% (92.9%–94.8%) |
| no resection | 937 | 94.1% (92.2%–95.5%) | 91.1% (88.4%–93.2%) |
| male patients | |||
| resection | 1526 | 94.2% (92.8%–95.3%) | 91.8% (89.9%–93.4%) |
| no resection | 335 | 93.3% (89.7%–95.6%) | 81.1% (73.7%–86.5%) |
Discussion
Commencing in January of 2004, benign intracranial tumors were included in the SEER registry as a result of the Benign Brain Tumor Cancer Registries Amendment Act. This report represents the first modern population-based analysis of trends and outcomes for over 12,000 US patients with intracranial meningioma using the SEER data set. As such, this analysis provides data specific to the US population that were previously unavailable and may be more generalizable than previously published single-center or single-surgeon case series.
There were several notable findings from this analysis. First, 45% of patients in the SEER registry with a diagnosis of meningioma have histological confirmation of the diagnosis. Given that these radiographic diagnoses may have included tumors other than nonmalignant meningioma, these patients were considered separately for the survival analyses in this study. For patients in whom the diagnosis of nonmalignant meningioma was histologically confirmed, the overall postdiagnosis 1- and 3-year survival estimates were 95.4% and 92.4%, respectively. Patients who underwent resection had significantly increased overall survival than patients treated without resection. This finding of increased survival in resection-treated patients is consistent with the findings of the NCDB, in which a 5-year survival of 75.1% was estimated for patients who underwent resection compared with a 49.9% estimate in patients who did not undergo surgery.9 Although exact estimates were not provided for 1- and 3-year time points in the NCDB analysis, it appears that the survival rates from the current report are markedly higher, possibly representing a trend of improved outcomes since the 1980s.
Our analysis also identified clinical and demographic factors that are associated with selection for meningioma resection. As might be expected based on prior reports of increased surgical morbidity and mortality rates in the elderly, the age of the patient was strongly associated with treatment with resection.1,13 Younger patients had up to 15 times the odds ratio of undergoing resection than elderly patients. Interestingly, the sex and race of the patient were also associated with differences in the utilization of surgical treatment. Male patients were more likely than female patients to undergo resection. This finding is interesting given the noted 2-fold increase in meningioma prevalence for women and the fact that survival rates in women were higher than those in men. It is possible that this may be related to differences in age and tumor size at diagnosis that influenced selection for surgery (although these variables were controlled for in analyses). A higher percentage of male patients presented with tumors of the largest quartile than females (24% of males had tumors ≥ 42 mm compared with only 15% of females), whereas females had a higher percentage of individuals older than age 80 years at presentation (18% for females, 14% for males). Likewise, black patients were less likely to undergo resection than white patients. There were no obvious explanations for this finding because there were no differences in tumor size at diagnosis between white and black patients, and a larger percentage of white patients were of age 80 or greater (18% for white patients, 14% for black patients). As such, it is possible that this finding may be related to other factors such as variable access to health care in black patients compared with white patients, a finding that has been reported and explored for other brain tumors.11
The size and location of the meningioma were also significantly associated with the likelihood of undergoing resection as well as postdiagnosis survival. The smallest quartile of tumors (< 17 mm in diameter) represented 6% of surgically treated lesions compared with 30% of tumors that were not resected. Likewise, the largest quartile of meningiomas (≥ 42 mm in diameter) represented 28% of surgically treated tumors compared with 9% of tumors that were not resected. Interestingly, despite the increased likelihood of resection for larger tumors, there was no effect of tumor size on the adjusted death risk in patients with a confirmed meningioma diagnosis. Additionally, unilateral, nonmidline tumors were associated with decreased risk of postdiagnosis death, likely in part due to the involvement of venous sinuses in midline tumors. In prior analyses, tumor size and location has not been uniformly included in outcome analyses. In a single-institution series from the 1980s, tumors located in sites that allowed for complete excision were associated with increased probability of survival-free recurrence, although size was not analyzed.10 In another large single-institution series of 342 patients with benign meningiomas, lesion size was not associated with recurrence rates or survival, but in the NCDB lesion size was an independent predictor of mortality in multivariate analyses.7,9
There are several limitations of this analysis that must be considered when interpreting this our findings. First, the SEER data set only provides information on the first course of treatments for the tumor. Therefore, it is not possible to obtain information about tumor recurrence and subsequent treatments that may be important factors in long-term survival. Data on chemotherapy are not included, although it is extremely rare for a benign meningioma to be treated with such therapy after the initial diagnosis and, hence, is not likely of significant importance in these analyses. Second, there is very limited clinical information about the patients other than the details of the tumor. As such, it is not possible to stratify for medical comorbidites that may be important in the selection of a treatment strategy or overall survival. Additionally, our study involved a retrospective analysis and as such is subject to unbalanced confounders that may influence outcomes such as survival. Multivariate adjustment was used to help address this concern, but only limited clinical information is provided in the data set, and it is likely that there are important differences between patients selected for surgery that are not evident in the SEER data. Furthermore, given that this analysis is based on data obtained from coded clinical information derived from numerous sites, the potential for inaccurate coding exists. Finally, no details of the facility or provider of treatment are given for analysis. It is possible that treatment patterns and outcomes are influenced by the center providing care, and these factors could not be evaluated in this analysis.
Conclusions
This analysis represents the first modern population-based analysis of treatment patterns and outcomes in US patients with nonmalignant intracranial meningioma. We demonstrated that over 85% of patients survive 3 years after diagnosis and that resection is associated with improved survival. There also appear to be differences in the selection of patients for resection based on age, sex, race, and clinical features of the meningioma. We anticipate that these data will provide generalizable information useful to clinicians treating patients with nonmalignant intracranial meningiomas.
Acknowledgments
This work was supported by NIH R01 grant nos. CA109468, CA109461, CA109745, CA108473, and CA109475, as well as the Brain Science Foundation and the Meningioma Mommas.
Abbreviations used in this paper
- NCDB
National Cancer Database
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Disclosure Author contributions to the study and manuscript preparation include the following. Conception and design: both authors. Acquisition of data: Cahill. Analysis and interpretation of data: both authors. Drafting the article: both authors. Critically revising the article: both authors. Approved the final version of the paper on behalf of all authors: Claus. Statistical analysis: both authors. Administrative/technical/material support: Claus. Study supervision: Claus.
This article contains some figures that are displayed in color on line but in black and white in the print edition.
References
- 1.Bateman BT, Pile-Spellman J, Gutin PH, Berman MF. Meningioma resection in the elderly: nationwide inpatient sample, 1998-2002. Neurosurgery. 2005;57:866–872. doi: 10.1227/01.neu.0000179923.66729.87. [DOI] [PubMed] [Google Scholar]
- 2.Black P, Kathiresan S, Chung W. Meningioma surgery in the elderly: a case-control study assessing morbidity and mortality. Acta Neurochir (Wien) 1998;140:1013–1017. doi: 10.1007/s007010050209. [DOI] [PubMed] [Google Scholar]
- 3.Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197–205. doi: 10.1007/BF00165649. [DOI] [PubMed] [Google Scholar]
- 4.Central Brain Tumor Registry of the United States (CBTRUS) [Accessed March 29, 2011];CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004-2007. ( http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf)
- 5.Caroli M, Locatelli M, Prada F, Beretta F, Martinelli-Boneschi F, Campanella R, et al. Surgery for intracranial meningiomas in the elderly: a clinical-radiological grading system as a predictor of outcome. J Neurosurg. 2005;102:290–294. doi: 10.3171/jns.2005.102.2.0290. [DOI] [PubMed] [Google Scholar]
- 6.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neu rosurgery. 2005;57:1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 7.Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB, Jr, Rhoton AL. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427–436. doi: 10.1016/s0360-3016(97)00317-9. [DOI] [PubMed] [Google Scholar]
- 8.Feun LG, Raub WA, Jr, Landy HJ, Green B, Wolfson A, Markoe A. Retrospective epidemiologic analysis of patients diagnosed with intracranial meningioma from 1977 to 1990 at the Jackson Memorial Hospital, Sylvester Comprehensive Cancer Center: the Jackson Memorial Hospital Tumor Registry experience. Cancer Detect Prev. 1996;20:166–170. [PubMed] [Google Scholar]
- 9.McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88:831–839. doi: 10.3171/jns.1998.88.5.0831. [DOI] [PubMed] [Google Scholar]
- 10.Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee D, Zaidi HA, Kosztowski T, Chaichana KL, Brem H, Chang DC, et al. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010;145:247–253. doi: 10.1001/archsurg.2009.288. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Surveillance Research Program. Cancer Statistics Branch [Accessed March 29, 2011];Surveillance, Epidemiology, and End Results (SEER) Program: Seer Data (2004-2007) ( http://seer.cancer.gov/data/index.html)
- 13.Patil CG, Veeravagu A, Lad SP, Boakye M. Craniotomy for resection of meningioma in the elderly: a multicentre, prospective analysis from the National Surgical Quality Improvement Program. J Neurol Neurosurg Psychiatry. 2010;81:502–505. doi: 10.1136/jnnp.2009.185074. [DOI] [PubMed] [Google Scholar]
- 14.Preston-Martin S, Staples M, Farrugia H, Giles G. Primary tumors of the brain, cranial nerves and cranial meninges in Victoria, Australia, 1982-1990: patterns of incidence and survival. Neuroepidemiology. 1993;12:270–279. doi: 10.1159/000110328. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed March 29, 2011];Public Law 107-260: Benign Brain Tumor Cancer Registries Amendment Act. 2002 ( http://training.seer.cancer.gov/brain/non-malignant/history/public.html)
- 16.Sankila R, Kallio M, Jääskeläinen J, Hakulinen T. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland. Comparison of the observed and expected survival rates in a population-based se ries. Cancer. 1992;70:1568–1576. doi: 10.1002/1097-0142(19920915)70:6<1568::aid-cncr2820700621>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Sant M, Crosignani P, Bordo BM, Nicola G, Bianchi M, Berrino F. Incidence and survival of brain tumors: a population-based study. Tumori. 1988;74:243–252. doi: 10.1177/030089168807400301. [DOI] [PubMed] [Google Scholar]