Abstract
Disease severity in asthma can be classified as mild, moderate or severe based upon the frequency of symptoms or the severity of airflow obstruction. This review will focus on the treatment of youths greater than 12 years of age and adults with moderate persistent asthma. Moderate asthmatics may have daily symptoms that cause some limitation with normal daily activities and require use of a rescue inhaled short-acting beta2-agonist inhaler or experience nocturnal awakenings secondary to asthma that occur more than once per week. Furthermore, spirometry may reveal airflow obstruction with a reduction in FEV1 to between 60% and 80% of predicted. Although inhaled corticosteroids (ICS) are the primary controller medication used to modify symptoms in moderate asthmatics, additional controller medications, such as inhaled long-acting beta2-agonists (LABA), leukotriene receptor antagonists (LTRA) or theophylline, are often needed to obtain optimal disease control. While the addition of an inhaled LABA to an ICS is very effective at improving disease control in moderate asthma, concerns have arisen over the safety of LABAs, in particular the risk of asthma-related death. Therefore, consideration may be given to initially adding a LTRA, rather than a LABA, to ICS when asthma symptoms are not adequately controlled by ICS alone. Furthermore, individualization of medication regimens, treatment of co-morbid conditions, and patient education are crucial to optimizing compliance with therapy, improving disease control, and reducing the risk of exacerbations. Lastly, the development of new asthma treatments, perhaps based upon personalized medicine, may revolutionize the future treatment of moderate asthma.
Keywords: asthma, inhaled corticosteroids, long-acting beta2-agonists, leukotriene modifiers, leukotriene receptor antagonists, theophylline
Asthma is a chronic inflammatory disease of the airway that is highly prevalent in both children and adults. The World Health Organization estimates that approximately 245 million individuals suffer from asthma worldwide, while the Centers for Disease Control reported that asthma prevalence in the United States was 8.2% in 2009, which represents approximately 24.6 million individuals.1,2 The Expert Panel 3 Report (EPR-3) from the National Asthma Education and Prevention Program (NAEPP) of the National Heart, Lung, and Blood Institute (NHLBI) defines asthma as the presence of “variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation,” which are commonly triggered by exposure to allergens, irritants or cold air, as well as by viral infections or exercise.3 The clinical manifestations of asthma are typified by intermittent symptoms of cough, wheeze, chest tightness or dyspnea, that often occur in individuals with a personal or family history of allergy and/or other common diseases, such as gastroesophageal reflux or chronic rhinosinusitis. Asthma symptoms and physiologic evidence of airflow obstruction are caused by underlying airway inflammation (cellular infiltration by activated T helper lymphocytes, eosinophils, neutrophils and mast cells with associated edema), airway remodeling (increased airway secretions, airway epithelial cell and smooth muscle hyperplasia, epithelial cell desquamation, goblet cell hyperplasia and collagen deposition), and smooth muscle constriction.4
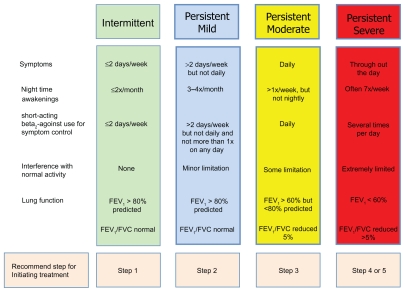
Characterization of a patient’s asthma severity is a critical guide for the initiation of asthma treatment and the determination of future risk of deterioration. Disease severity can be classified as mild, moderate or severe based upon the frequency of asthma symptoms or the severity of airflow obstruction (Fig. 1).3 While the majority of asthmatics have mild disease, those with moderate or severe asthma have more frequent symptoms that are difficult to control.5,6 This review will focus on the treatment of youths greater than 12 years of age and adult patients with moderate persistent asthma. Moderate asthmatics have daily symptoms or nocturnal awakenings secondary to asthma that occur more than once per week, but not nightly. On spirometry, moderate asthmatics have an FEV1 between 60% and 80% of predicted, and their FEV1/FVC ratios are generally reduced by 5% or less when compared with predicted values. In addition, they may require daily use of an inhaled short-acting beta2-agonist (SABA) for control of their asthma symptoms and also experience some limitation with normal daily activities.
Figure 1.
NHLBI National Asthma and Education and Prevention Program Expert Panel 3 Report classification of asthma severity in youths ≥ 12 years of age and adults.
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health, US. Department of Health and Human Services. 2007.
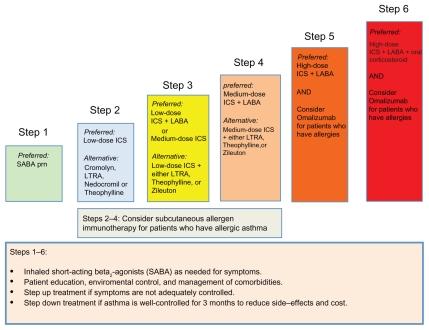
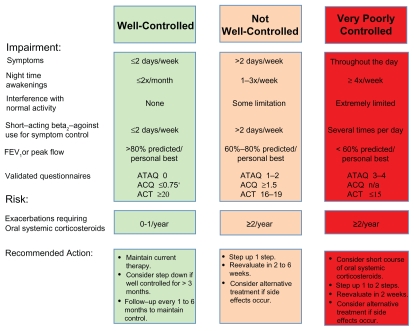
The EPR-3 recommends a step care approach that matches the intensity of treatment to disease severity and adequacy of control of asthma symptoms. As shown in Figures 1 and 2, the EPR-3 proposes that moderate asthmatics initiate treatment at Step 3 or 4.3 The objective of treatment is to decrease disease severity and improve control while providing optimally dosed medications with the fewest possible side effects. Furthermore, treatment is directed at decreasing the risk of future exacerbations, as well as reducing the loss of lung function over time. The specific benchmarks for improvement of symptoms are defined as a decrease in the use of short-acting beta2-agonists (SABA), a reduction in daytime and nocturnal asthma symptoms, an improvement in ability to conduct normal activities, and personal satisfaction with the level of asthma control (Fig. 3). By periodically monitoring disease severity through patient visits and spirometry, the health care provider can determine the level of control and risk of future exacerbations within each patient and adjust therapy accordingly.
Figure 2.
Stepwise approach for managing asthma in youths ≥ 12 years of age and adults as recommended by the NHLBI National Asthma and Education and Prevention Program Expert Panel 3 Report.
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health, US. Department of Health and Human Services. 2007.
Figure 3.
Assessing asthma control and adjustment of therapy in youths ≥ 12 years of age and adults as recommended by the NHLBI National Asthma and Education and Prevention Program Expert Panel 3 Report.
Notes: *ACQ values of 0.76 to 1.4 are indeterminate regarding well-controlled asthma.
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health, US. Department of Health and Human Services. 2007.
Abbreviations: ACQ, Asthma Control Questionnaire©; ACT, Asthma Control Test™; ATAQ, Asthma Therapy Assessment Questionnaire©.
Controller Medications for Moderate Persistent Asthma
The mainstay of therapy for moderate asthma is the chronic administration of controller medications that modify disease severity by reducing airway inflammation and bronchoconstriction in patients with persistent disease. The EPR-3 recommends that inhaled corticosteroids (ICS) be utilized as the main controller medication to which adjunctive controller medications can be added. These include inhaled long-acting beta2-agonists (LABAs), leukotriene modifiers, or theophylline.3 The addition of allergen immunotherapy can also be considered for patients who have allergic asthma. In addition, patients are provided with short-acting beta-agonists (SABA) to be used as needed to provide bronchodilation for symptomatic relief.
Inhaled corticosteroids
The mainstay of treatment for moderate persistent asthma among youths over the age of twelve and adults are inhaled corticosteroids (ICS).3 Corticosteroids, via interactions with glucocorticoid receptors, are highly effective for the treatment of asthma based upon their broad anti-inflammatory effects, which are mediated via the suppressed transcription of pro-inflammatory genes, termed trans-repression, as well as the activation of many anti-inflammatory genes, termed trans-activation.7 The clinical benefits of ICS therapy in asthma include improvements in disease severity, asthma control, quality of life, pulmonary function, airway hyperresponsiveness, disease exacerbations, hospitalizations and death.3 Furthermore, ICS reduce the number of airway inflammatory cells (eosinophils, mast cells and T cells), as well as mucus production and hypersecretion, airway edema, and vascularization.4,8 Therefore, all patients with moderate asthma should receive treatment with ICS.
A variety of ICS, either alone (Table 1) or in combination inhalers with LABAs (Table 2), are available for use in the United States. The potency of individual preparations differ based upon glucocorticoid receptor binding affinity, particle size, and the inhaler device used to deliver the medication.9 Therefore, it is important for clinicians to ensure that therapeutic doses are prescribed. The advantage of ICS over oral corticosteroid therapy is a reduction in systemic side-effects due to site-directed delivery of the medication directly to the lung. However, only 10% to 20% of the inhaled medication is delivered to the airway.10,11 The remainder is deposited in the oropharynx or swallowed and absorbed by the gastrointestinal tract, where most of the corticosteroid is converted to an inactive metabolite by “first pass metabolism” in the liver. Local side effects that may result from deposition of the ICS in the oropharynx and larynx include oropharyangeal candidiasis (thrush), dysphonia, cough, and throat irritation.10 Perioral dermatitis and tongue hypertrophy have also been reported.12 Local side-effects may be decreased by use of a spacer device, when possible, and by rinsing the mouth after use. Dose-dependent systemic toxicities of ICS occur when corticosteroids are absorbed from the lung or from the fraction that is not metabolized in the liver.8 The long-term systemic side effects that may impact adults when high doses of ICS are administered include osteoporosis, cataract formation and adrenal suppression and crisis.8 In the elderly, skin changes and easy bruising may also be seen.10,13 In children, high doses of inhaled corticosteroids can be associated with a dose-related, short-term decrease in growth velocity, which does not appear to result in a substantial decrease in adult height, thereby suggesting the presence of compensatory mechanisms.10 Systemic toxicities, however, are less likely with medium-dose ICS that are typically utilized for the treatment of moderate persistent asthma. Furthermore, medium-dose ICS may improve disease control and thereby decrease the need for oral corticosteroids bursts, which are more likely to result in corticosteroid-induced osteopenia.8
Table 1.
Suggested dosing of inhaled corticosteroids for asthma therapy in adults and adolescents (≥ 12 years).
| Total daily corticosteroid dose | |||||||
|---|---|---|---|---|---|---|---|
| Generic name | Trade name | Inhaler type | Dosage forms | Dosing frequency | Low | Medium | High |
| Beclomethasone | QVAR® | HFA | 40 mcg 80 mcg |
Twice daily | 80–160 mcg/day | 240–320 mcg/day | 480–640 mcg/day |
| Budesonide | Pulmicort Flexhaler® |
DPI | 90 mcg 180 mcg |
Twice daily | 180–360 mcg/day | 540–720 mcg/day | 1080–1440 mcg/day |
| Ciclesonide | Alvesco® | MDA | 80 mcg 160 mcg |
Twice daily | 160 mcg/day | 320 mcg/day | 640 mcg/day |
| Fluticasone | Flovent® Diskus |
DPI | 50 mcg 100 mcg 250 mcg |
Twice daily | 200 mcg/day | 400 mcg/day | 500–1000 mcg/day |
| Fluticasone | Flovent® HFA |
HFA | 44 mcg 110 mcg 220 mcg |
Twice daily | 88–176 mcg/day | 220–440 mcg/day | 880–1760 mcg/day |
| Mometasone | Asmanex® Twisthaler® |
DPI | 220 mcg | Once or twice daily | 220 mcg/day | 440 mcg/day | 880 mcg/day |
Abbreviations: inh, inhalation; HFA, hydrofluoroalkane inhaler; MDA, metered dose aerosol; DPI, dry powder inhaler.
Table 2.
Suggested dosing of combined inhaled corticosteroids and long-acting beta2-agonists (LABAs) for asthma therapy in adults and adolescents (≥ 12 years).
| Combination inhaler | Inhaler type | Dose | Maximum # of daily inhalations (regardless of dose) | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Budesonide/formoterol (Symbicort®) | MDI | 80 mcg/4.5 mcg (2 inh twice daily) | 160 mcg/4.5 mcg (2 inh twice daily) | 4 inh | |
| Fluticasone/salmeterol (Advair®) diskus | DPI | 100 mcg/50 mcg (one inh twice daily) | 250 mcg/50 mcg (one inh twice daily) | 500 mcg/50 mcg (one inh twice daily) | 2 inh |
| Fluticasone/salmeterol (Advair®) HFA | HFA | 45 mcg/21 mcg (2 inh twice daily) | 115 mcg/21 mcg (2 inh twice daily) | 230 mcg/21 mcg (2 inh twice daily) | 4 inh |
| Mometasone/formoterol (Dulera®) | MDI | 100 mcg/5 mcg (2 inh twice daily) | 200 mcg/5 mcg (2 inh twice daily) | 4 inh | |
Abbreviations: inh, inhalation; DPI, dry powder inhaler; HFA, hydrofluoroalkane inhaler; MDI, metered dose inhaler.
Long-acting beta2-agonists
β2-agonists induce bronchodilation via interacting with β2 adrenergic receptors to increase cAMP with resultant airway smooth muscle relaxation.3 β2-agonist medications can be classified by their duration of action. The effects of short-acting β2-agonists (SABAs), such as albuterol, persist for 4 to 6 hours, whereas the effects of long-acting β2-agonists (LABAs), such as salmeterol and formoterol, last for 10 to 12 hours.14 Therefore, SABAs are recommended as a quick relief medication for asthma symptoms, whereas LABAs are considered a controller medication that attenuate bronchoconstriction, but do not modify underlying airway inflammation. Consistent with this concept, the addition of an inhaled LABA to an ICS has been shown to improve lung function, decrease symptoms and reduce exacerbations in asthmatics who are not well controlled on low or medium doses of ICS.3 The EPR-3 recommends that patients with moderate persistent asthma may be treated with a combination of low-dose (Step 3) or medium dose (Step 4) ICS plus a LABA. Combination inhalers facilitate delivery of the maximal recommended daily dose of LABA with varying doses of ICS (Table 2). The pharmacodynamics of inhaled salmeterol and formoterol differ. Inhaled salmeterol has an onset of action of between 30 and 48 minutes with a peak effect at 3 hours.15 It has a half-life of 5.5 hours and undergoes hepatic metabolism by CYP3A4. In contrast, inhaled formoterol has a rapid onset of action within 3 minutes of administration, with 80% of its peak effect evident within 15 minutes.16 The half-life of formoterol in powder form is approximately 10 to 14 hours, while nebulized formoterol has a half-life of approximately 7 hours. It undergoes hepatic metabolism via direct glucuronidation and O-demethylation by multiple cytochrome P450 enzymes. Arformoterol and indacaterol are new LABAs that have been approved by the US. Food and Drug Administration (FDA) for use in COPD, but not for the treatment of asthma.
Although the addition of an inhaled LABA to an ICS is very effective at improving disease control, concerns have arisen over the safety of LABAs. Prior to its approval by the FDA, the Serevent® Nationwide Surveillance Study noted an increase in risk of asthma-related death in patients treated with salmeterol as compared with albuterol.17 Based upon this study, as well as reports to the FDA of serious asthma exacerbations and deaths in patients treated with salmeterol, the manufacturer conducted the Salmeterol Multicenter Asthma Research Trial (SMART), which showed a small but significant increase in asthma-related deaths (1 in 700 patient-years) in individuals receiving salmeterol without concurrent ICS over 28 weeks.18,19 The safety concern of increased risk of asthma-related deaths associated with the use of LABAs prompted the FDA to conduct a comprehensive review of the benefits and risks of LABAs for the treatment of asthma, which resulted in a black box warning that contraindicates LABA monotherapy for the treatment of asthma.17 The FDA also recommended against LABA use in patients whose asthma is well-controlled with low-dose or medium-dose ICS alone. In addition, adjunctive LABA therapy use should be discontinued, if possible, once asthma control is achieved and maintained by an asthma controller medication, such as ICS. If LABA therapy is utilized, it should be provided as a combination inhaler with ICS, never as a separate LABA inhaler, in order to minimize the potential of overuse. More recently, in April of 2011, the FDA updated their recommendation stating that five prospective clinical trials will be conducted among all age groups to determine whether LABAs, when used concurrently with ICS, are safe for the treatment of asthma.20 These studies are expected to conclude in 2017 and hopefully will provide definitive data regarding the safety of LABAs. In the interim, it has been suggested that LABA use be restricted to patients in whom other adjunctive controller medications, such as leukotriene modifiers, do not provide adequate asthma control.19
Leukotriene modifiers
Leukotriene modifiers are another class of adjunctive controller medication that can be combined with ICS for the management of moderate persistent asthma. Cysteinyl leukotrienes (leukotriene C4, D4 and E4) are pro-inflammatory lipid mediators that are released by mast cells, eosinophils and basophils to induce airway smooth muscle contraction, tissue edema, eosinophil migration and increased airway secretions.3,4,21 The pathway that generates cysteinyl leukotrienes requires the conversion of the precursor fatty acid, arachidonic acid, to leukotriene A4 by 5-lipoxygenase (5-LO), which is then converted to leukotriene C4 or leukotriene B4.21 Leukotriene C4 can then be metabolized further to generate leukotriene D4 and leukotriene E4, which play important roles in allergen-mediated airway inflammation. Leukotriene modifiers either antagonize the ability of cysteinyl leukotrienes to interact with the cysteinyl leukotriene receptor, CystLT1, or inhibit the enzymatic activity of 5-LO.
Two leukotriene receptor antagonists (LTRA) are in clinical use in the United States, montelukast ( Singulair®) and zafirlukast (Accolate®) (Table 3). Both are administered in pill form. Montelukast is more commonly utilized for asthma treatment based upon its once daily dosing that does not need to be coordinated with meals.22 In contrast, zafirlukast requires twice daily dosing. Since the bioavailability of zafirlukast is decreased 40% by food, it should be taken either 1 hour before or 2 hours after meals.23 Montelukast has a half-life of up to 5.5 hours, a duration of action that exceeds 24 hours, and undergoes hepatic metabolism by CYP3A4 and 2C9.24 Zafirlukast has a half life of ten hours, is highly protein bound to albumin, and undergoes hepatic metabolism by CYP2C9.23 LTRAs are typically well- tolerated with few associated side-effects that occur in less than 2% of recipients, such as angioedema, anaphylaxis, dizziness, gastrointestinal symptoms and transaminitis.4,22 Post-marketing case reports to the FDA have suggested an association between all leukotriene modifying agents, but primarily montelukast, with suicide and neuropsychiatric events ( suicidal behavior, depression, insomnia) that resulted in the issuance of a safety alert.25 Subsequent reviews of this association, which were requested by the FDA, did not find an increase in suicidal ideation.22,26 Based upon this possible association, it is recommended that caution be utilized when administering leukotriene modifiers to patients with suicidal ideation or psychiatric symptoms. Finally, zafirlukast and montelukast have been associated with the development of Churg-Strauss syndrome (CSS), an eosinophilic vasculitis and granulomatosis that can be associated with severe asthma.4,27 Although this association was initially thought to reflect an unmasking of underlying CSS caused by a reduction in oral corticosteroid therapy, a recent review of 181 reported cases of drug-induced CSS in the FDA Adverse Event Reporting System showed that a LTRA was a suspect medication in 90% of cases.28 Furthermore, in the majority of cases, CSS could not be explained by either corticosteroid withdrawal or pre-existing CSS. This suggests that LTRA therapy might have a direct role in the pathogenesis of CSS.
Table 3.
| Generic name | Trade name | Medication type | Dosage | Frequency | Modify for meals |
|---|---|---|---|---|---|
| Montelukast | Singulair® | LTRA | 10 mg | Once daily | No |
| Zafirlukast | Accolate® | LTRA | 20 mg | Twice daily | 1 hr before or 2 hrs after meals |
| Zileuton | Zyflo CR® | 5-LO | 1200 mg | Twice daily | Within 1 hr after morning and evening meals |
| Zileuton | Zyflo® | 5-LO | 600 mg | Four times/day | No |
Abbreviations: 5-LO, 5-lipoxygenase inhibitor; LTRA, leukotriene receptor antagonist.
Zileuton is the only 5-lipoxygenase inhibitor currently approved for treatment of asthma in the United States. It is orally administered and requires dosing either 4 times daily for the regular formulation or twice daily for the controlled release formulation.22 Zileuton undergoes gastrointestinal and hepatic metabolism via CYP1A2, 2C9, and 3A4.29 The safety profile of zileuton is similar to LTRAs with the exception of transaminitis, which occurs in 4.4% of cases, but is typically not associated with jaundice or liver failure.30 Therefore, liver function tests should be monitored monthly during the first three months of treatment and every 2 to 3 months during the first year, as well as periodically in subsequent years.22,30 Other side effects that have been associated with zileuton include headache, gastrointestinal symptoms, myalgias, leukopenias, sleep disorders and behavior changes.22
The EPR-3 recommends the addition of a LTRA to low-dose ICS as Step 3 therapy or medium-dose ICS as Step 4 therapy (Fig. 2). Results of randomized clinical trials have suggested that LABAs are superior to LTRAs as add-on therapy based upon their ability to decrease symptoms and exacerbations when asthma symptoms are not adequately controlled with ICS alone.22,31,32 Recently, a community-based, multicenter trial compared the addition of a LTRA or LABA when patients were inadequately controlled by ICS.32 This “real world” study followed patients for 2 years and showed that LTRAs were equivalent to LABAs as add-on therapy for a diverse group of primary care patients who were not adequately controlled with ICS alone, using the miniAQLQ as the primary outcome measure. Furthermore, treatment with a LTRA was equivalent to an ICS as first-line controller medication. When these results are considered in the context of the uncertainty regarding the safety of LABAs for the treatment of asthma, clinicians may favor the addition of a LTRA, rather than a LABA, as initial step-up therapy when patients are persistently symptomatic on ICS alone.33 The ease of daily oral administration of LTRAs (montelukast), as well as their favorable safety profile and effectiveness for treatment of allergic rhinitis are additional reasons for clinicians to consider initial step-up therapy with LTRA in patients who are persistently symptomatic despite ICS. Furthermore, patients with aspirin-sensitive asthma, exercise-induced asthma and those with asthma exacerbations secondary to viral upper respiratory tract infections may be more likely to benefit from leukotriene modifier therapies.22
Theophylline
The xanthine derivative, theophylline, is an orally administered, non-selective phosphodiesterase inhibitor that mediates airway smooth muscle relaxation by increasing cAMP levels, and may also possess mild anti-inflammatory activities.3,34 EPR-3 recommends sustained release theophylline as an alternative, but not preferred, controller medication that can be added when asthma symptoms are not adequately controlled by ICS.3 Consistent with this recommendation, addition of theophylline to low-dose ICS has been shown to be as effective as increasing the dose of ICS for improving control of asthma symptoms.35,36 However, comparison of step-up therapy with theophylline to LABAs has shown that LABAs (ie, salmeterol) are superior in improving morning and evening peak expiratory flow rates, as well as in reducing the use of rescue medication, while no differences were noted in improving FEV1.36,37 Step-up therapy with the addition of theophylline, however, is associated with a higher incidence of central nervous system and gastrointestinal side effects. Few studies have directly compared step-up therapy with theophylline to LTRAs.36,38,39
Although theophylline has been used to treat asthma for over 70 years, its role has been limited by its narrow therapeutic range, variable inter-patient pharmacokinetics, multiple drug interactions and common side effects.34,36 Theophylline has a half-life of 8.7 hours in non-smoking adults that is dependent upon age, cardiac and liver function, lung disease and smoking history. Theophylline undergoes hepatic metabolism via CYP1A2, CYP2E1, and CYP3A4 to produce active metabolites, including caffeine and 3-methylxanthine.40 Monitoring of serum theophylline concentrations is required to ensure that toxic levels are avoided by maintaining serum levels in the range of 10 to 20 mcg/mL.41 Theophylline-associated toxicities are more significant than other commonly used medications for moderate asthma and include gastrointestinal (vomiting, abdominal pain), cardiac (ventricular arrhythmias), neurologic (seizures), musculoskeletal (tremors), and metabolic (hypokalemia, hyperglycemia) toxicities. Another limitation regarding the use of theophylline is the presence of multiple drug interactions with common medications that can result in theophylline-associated toxicities due to increased serum drug levels (Table 4). Taken together, theophylline is not utilized as a preferred adjunctive step-up controller medication to ICS when asthma is poorly controlled.3 Instead, step-up therapy with theophylline is typically reserved for patients in whom inhaled medications can not be adequately utilized and other adjunctive step-up medications have not been effective.
Table 4.
Examples of common medications that can modify serum theophylline levels.40
| Medication class | Increase serum theophylline levels | Decrease serum theophylline levels |
|---|---|---|
| Asthma/allergy | Zafirlukast Zileuton |
|
| Antibiotics | Ciprofloxacin, Norfloxacin, Ofloxacin Isoniazid Macrolides (except azithromycin, telithromycin) Thiabendazole |
Protease inhibitors Rifampin |
| Anti-metabolites | Allopurinol Methotrexate |
|
| Cardiovascular | Amiodarone Mexiletene Propafenone |
|
| Neurologic | Barbiturates Carbamazepine Phenytoin |
|
| Miscellaneous | Cimetidine Disulfiram Estrogens Pentoxyfylline |
Allergen immunotherapy
The EPR-3 recommends that subcutaneous allergen immunotherapy (AIT) also be considered for patients who have persistent asthma if there exists a clear relationship between asthma symptoms and exposure to the offending allergen.3 AIT is typically utilized when patients have symptoms during at least a major portion of the year and are persistently symptomatic despite use of multiple controller medications. There is evidence that immunotherapy needs to be administered for prolonged periods, typically three to five years, to be effective.3,42,43 A recent update of a Cochrane review of 88 trials of injection allergen immunotherapy for asthma concluded that AIT reduces asthma symptoms and the use of asthma medications, as well as improves allergen-specific bronchial hyperreactivity, but does not have a consistent effect on lung function.43 Systemic reactions to AIT were frequent, occurring in one out of nine patients. Since subcutaneous AIT can be associated with severe and sometimes fatal allergic reactions, especially severe bronchoconstriction, therapy should only be administered in a health care provider’s office.3 Similarly, a Cochrane style meta-analysis of 25 trials of sublingual immunotherapy for asthma concluded that there was a significant reduction in asthma severity, but not asthma symptoms, which suggests that the magnitude of the effect is not very large.44 No severe reactions were noted in these studies, which represents an advantage of sublingual over subcutaneous immunotherapy. Potential mechanisms that mediate the effectiveness of AIT include induction of tolerance by allergen-specific regulatory T cells with resultant increases in allergen-specific IgG4 and decreases in Th2 cells, IgE and eosinophilia.45,46 Sublingual AIT may also decrease allergic responses via stimulating oral Langerhans cells.46 New forms of immunotherapy are currently under development which may advance further the role of AIT for the treatment of allergic disease.45
Putting it all Together: Management of Moderate Asthma
The EPR-3 recommends that patients with moderate persistent asthma initiate therapy at Step 3, which includes an ICS alone or in combination with an adjunctive controller medication.3 This recommendation is based upon the potent anti-inflammatory properties of ICS that attenuate the airway inflammation that underlies the pathogenesis of asthma. Thus, therapy for moderate persistent asthma may be initiated with either a medium dose ICS or a low-dose ICS in combination with either a LABA, LTRA or theophylline. Although combination therapy with an ICS plus a LABA had frequently been utilized as the medication of choice for this patient population, safety concerns regarding LABAs have supported the concept that LABAs be utilized with caution until definitive data are available regarding their effect on asthma-associated mortality.19 Therefore, clinicians and patients may opt to initiate therapy with either a medium dose ICS alone or a low-dose ICS in combination with a LTRA instead of an ICS in combination with a LABA. Furthermore, monotherapy with a LABA should never be utilized as a treatment for asthma. The addition of theophylline to a low-dose ICS is a non-preferred option due to the potential for toxicity and the need for monitoring of serum levels. Similarly, 5-LO inhibition with zileuton is a less desirable option due to more limited efficacy data and the need to monitor liver function tests.3
Patient preference also needs to be considered when devising an asthma treatment plan, as a significant proportion of asthmatics with sub-optimally controlled asthma are non-adherent with prescribed treatments.47 In particular, non-adherence rates with controller medications range from 30% to 70%, while approximately 20% may not acquire any controller medication at all.48 Inclusion of patient preferences in the decision making process regarding asthma treatment may improve adherence to treatment. Factors that may improve compliance include use of a combination inhaler as opposed to two single inhalers, as well as the use of oral agents as compared with inhalers, as it is easier to swallow a pill than to properly use an inhaler.32,49 Furthermore, improper inhaler technique is common and is associated with poor disease control and increased risk of hospitalization or emergency department visits, especially in patients who are older, have less education and have not received proper instruction by health care providers.50,51 Compliance may be improved by education about the disease and proper inhaler technique.50,52
Frequency of administration may also impact compliance, as it is easier to adhere to a once daily medication than one that requires repeated dosing.53 Similarly, compliance may be more difficult with medications that require periodic monitoring, such as zileuton or theophylline. Therefore, asthma management therapies need to be tailored to the preference of individual patients to increase adherence with prescribed treatments.
After therapy has been initiated, patients should be monitored at two to six week intervals to ensure that symptom control has been achieved.3 Patients are not well-controlled if they have asthma symptoms more frequently than twice a week, utilize a rescue short-acting beta2-agonist inhaler more than twice a week, have nocturnal awakenings more than twice per month, FEV1 or a peak flow less than 80% of predicted, or have evidence of poor control on a validated asthma questionnaire (Fig. 3). If asthma symptoms are not well-controlled, then therapy should be “stepped up” by either increasing the ICS dose from low dose to medium-dose or by the addition of a LTRA, LABA or theophylline to medium dose ICS (Fig. 2). Allergen immunotherapy may also be considered as an adjunct therapy in individuals with atopic asthma who are chronically exposed to the offending allergen. Additionally, the patient’s inhaler technique should be reviewed, as well as their compliance with therapy. Furthermore, an investigation to determine whether environmental factors, such as allergens, pollutants or irritants, or co-morbid conditions, such as gastrointestinal reflux or rhinosinusitis, are preventing adequate control of asthma symptoms. If these measures fail to improve asthma control, then therapy should be “stepped up” further to Steps 5 or 6 for severe asthma, as needed, which includes the options of anti-IgE therapy (omalizumab) and oral corticosteroids.
Once asthma symptoms are well-controlled for a period of 3 months, then consideration can be given to “stepping down” therapy, which is an equally important goal of asthma therapy in order to minimize side effects that can be associated with the long-term use of asthma controller medications. For example, although ICS have few side effects, systemic absorption occurs and can be associated with a reduction in bone growth in children.8,33 Furthermore, high dose ICS can be associated with an increased risk of cataracts and fractures in high-risk adults.8 Consistent with this approach, a recent community-based study of supervised step-down therapy confirmed that a significant reduction in ICS dose could be achieved without deterioration of airway inflammation or function.54 Given the recent safety concerns, consideration may be given to withdrawal of LABAs. However, stepping down to ICS monotherapy may be associated with poorer asthma control as compared to a reduction in dose of ICS with continuation of the LABA.55–57 Consideration might also be given to “stepping down” from other medications with potential side effects, such as theophylline or zileuton.
Conclusions
Moderate asthma is a common, but challenging, disease that is frequently managed by general practitioners, as well as specialists. Patients with moderate asthma can be difficult to manage and typically require more than one controller drug, as well as therapy for co-morbid conditions, such as environmental allergies, gastroesophageal reflux or rhinosinusitis. Involvement of health care providers in the individualization of medication regimens, as well as in patient education, are crucial to optimizing compliance with therapy and disease control. Health care providers also need to be continually aware of emerging data regarding the utilization and safety of controller medications that can significantly impact disease management. Lastly, the development of new asthma treatments, perhaps based upon personalized medicine, has the potential to revolutionize the future therapy of moderate asthma.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.World Health Organization. Available from: http://www.who.int/mediacentre/factsheets/fs307/en/index.html.
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United states, 2005–2009. National Health Statistics Reports. 2011:1–14. [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health, US. Department of Health and Human Services; 2007. [Google Scholar]
- 4.Fanta CH. Asthma. N Engl J Med. 2009;360:1002–14. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus SC. Mild persistent asthma: Is any treatment needed. J Allergy Clin Immunol. 2006;118:805–8. doi: 10.1016/j.jaci.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Levine SJ, Wenzel SE. Narrative review: The role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes. Ann Intern Med. 2010;152:232–7. doi: 10.1059/0003-4819-152-4-201002160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PJ. Glucocorticosteroids: Current and future directions. Br J Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoloff SW, Kelly HW. Updates on the use of inhaled corticosteroids in asthma. Curr Opin Allergy Clin Immunol. 2011;11:337–44. doi: 10.1097/ACI.0b013e328348a813. [DOI] [PubMed] [Google Scholar]
- 9.Kelly HW. Comparison of inhaled corticosteroids: An update. Ann Pharmacother. 2009;43:519–27. doi: 10.1345/aph.1L546. [DOI] [PubMed] [Google Scholar]
- 10.Saag KG, Furst DE, Barnes PJ. Major side effects of inhaled glucocorticoids. In: Bochner BS, Wood RA, editors. UpToDate. 19.2. 2011. [Google Scholar]
- 11.Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol. 1996;97:169–76. doi: 10.1016/s0091-6749(96)80217-x. [DOI] [PubMed] [Google Scholar]
- 12.Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: Current understanding and review of the literature. Chest. 2004;126:213–9. doi: 10.1378/chest.126.1.213. [DOI] [PubMed] [Google Scholar]
- 13.Roy A, Leblanc C, Paquette L, et al. Skin bruising in asthmatic subjects treated with high doses of inhaled steroids: Frequency and association with adrenal function. Eur Respir J. 1996;9:226–31. doi: 10.1183/09031936.96.09020226. [DOI] [PubMed] [Google Scholar]
- 14.Lemanske RF. Beta agonists in asthma: Controversy regarding chronic use. In: Bochner BS, editor. UpToDate. 19.2. 2011. [Google Scholar]
- 15.UpToDate. 19.2. 2011. Salmeterol: Drug information. [Google Scholar]
- 16.UpToDate. 19.2 Formoterol: Drug information. [Google Scholar]
- 17.Chowdhury BA, Dal Pan G. The fda and safe use of long-acting beta-agonists in the treatment of asthma. The New England Journal of Medicine. 2010;362:1169–71. doi: 10.1056/NEJMp1002074. [DOI] [PubMed] [Google Scholar]
- 18.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The salmeterol multicenter asthma research trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 19.Drazen JM, O’Byrne PM. Risks of long-acting beta-agonists in achieving asthma control. The New England Journal of Medicine. 2009;360:1671–2. doi: 10.1056/NEJMe0902057. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding labas to inhaled corticosteroids for treating asthma. The New England Journal of Medicine. 2011;364:2473–5. doi: 10.1056/NEJMp1104375. [DOI] [PubMed] [Google Scholar]
- 21.Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. The New England Journal of Medicine. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 22.Peters-Golden M. Agents affecting the 5-lipoxygenase pathway in the treatment of asthma. In: Wood RA, Bochner BS, editors. UpToDate. 19.2. 2011. [Google Scholar]
- 23.UpToDate. 19.2. 2011. Zafirlukast: Drug information. [Google Scholar]
- 24.UpToDate. 19.2. 2011. Montelukast: Drug information. [Google Scholar]
- 25.Schumock GT, Lee TA, Joo MJ, Valuck RJ, Stayner LT, Gibbons RD. Association between leukotriene-modifying agents and suicide: What is the evidence. Drug Saf. 2011;34:533–44. doi: 10.2165/11587260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Philip G, Hustad C, Noonan G, et al. Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124:691–6. e696. doi: 10.1016/j.jaci.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler ME, Garpestad E, Flier SR, et al. Pulmonary infiltrates, eosinophilia, and cardiomyopathy following corticosteroid withdrawal in patients with asthma receiving zafirlukast. Jama. 1998;279:455–7. doi: 10.1001/jama.279.6.455. [DOI] [PubMed] [Google Scholar]
- 28.Bibby S, Healy B, Steele R, Kumareswaran K, Nelson H, Beasley R. Association between leukotriene receptor antagonist therapy and churg-strauss syndrome: An analysis of the fda aers database. Thorax. 2010;65:132–8. doi: 10.1136/thx.2009.120972. [DOI] [PubMed] [Google Scholar]
- 29.UpToDate. 19.2. 2011. Zileuton: Drug information. [Google Scholar]
- 30.Watkins PB, Dube LM, Walton-Bowen K, Cameron CM, Kasten LE. Clinical pattern of zileuton-associated liver injury: Results of a 12-month study in patients with chronic asthma. Drug Saf. 2007;30:805–15. doi: 10.2165/00002018-200730090-00006. [DOI] [PubMed] [Google Scholar]
- 31.Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011:CD003137. doi: 10.1002/14651858.CD003137.pub4. [DOI] [PubMed] [Google Scholar]
- 32.Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. The New England Journal of Medicine. 2011;364:1695–707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 33.Dahlen SE, Dahlen B, Drazen JM. Asthma treatment guidelines meet the real world. The New England Journal of Medicine. 2011;364:1769–70. doi: 10.1056/NEJMe1100937. [DOI] [PubMed] [Google Scholar]
- 34.Cosio BG, Soriano JB. Theophylline again? Reasons for believing. Eur Respir J. 2009;34:5–6. doi: 10.1183/09031936.00011309. [DOI] [PubMed] [Google Scholar]
- 35.Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O’Connor BJ, Barnes PJ. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. The New England Journal of Medicine. 1997;337:1412–8. doi: 10.1056/NEJM199711133372002. [DOI] [PubMed] [Google Scholar]
- 36.Hendeles L, Weinberger M. Theophylline use in asthma. In: Bochner BS, Wood RA, editors. UpToDate. 19.2. 2011. [Google Scholar]
- 37.Tee AK, Koh MS, Gibson PG, Lasserson TJ, Wilson AJ, Irving LB. Long-acting beta2-agonists versus theophylline for maintenance treatment of asthma. Cochrane Database Syst Rev. 2007:CD001281. doi: 10.1002/14651858.CD001281.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah AR, Sharples LD, Solanki RN, Shah KV. Double-blind, randomised, controlled trial assessing controller medications in asthma. Respiration. 2006;73:449–56. doi: 10.1159/000090898. [DOI] [PubMed] [Google Scholar]
- 39.Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–42. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 40.UpToDate. 19.2. 2011. Theophylline: Drug information. [Google Scholar]
- 41.Perry H. Theophylline poisoning. In: Ewald MB, Traub SJ, editors. UpToDate. 19.2. 2011. [Google Scholar]
- 42.Tabar AI, Arroabarren E, Echechipia S, Garcia BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011;127:57–63. 63 e51–3. doi: 10.1016/j.jaci.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010:CD001186. doi: 10.1002/14651858.CD001186. [DOI] [PubMed] [Google Scholar]
- 44.Calamita Z, Saconato H, Pela AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: Systematic review of randomized-clinical trials using the cochrane collaboration method. Allergy. 2006;61:1162–72. doi: 10.1111/j.1398-9995.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- 45.Stokes JR, Casale TB. Allergic rhinitis and asthma: Celebrating 100 years of immunotherapy. Curr Opin Immunol. 2011 doi: 10.1016/j.coi.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. quiz 28–19. [DOI] [PubMed] [Google Scholar]
- 47.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180:817–22. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–77. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoloff SW, Stempel DA, Meyer J, Stanford RH, Carranza Rosenzweig JR. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–51. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–8. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Delea TE, Stanford RH, Hagiwara M, Stempel DA. Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs*. Curr Med Res Opin. 2008;24:3435–42. doi: 10.1185/03007990802557344. [DOI] [PubMed] [Google Scholar]
- 52.Adams RJ, Weiss ST, Fuhlbrigge A. How and by whom care is delivered influences anti-inflammatory use in asthma: Results of a national population survey. J Allergy Clin Immunol. 2003;112:445–50. doi: 10.1067/mai.2003.1625. [DOI] [PubMed] [Google Scholar]
- 53.Price D, Robertson A, Bullen K, Rand C, Horne R, Staudinger H. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: A randomized open-label study. BMC Pulm Med. 2010;10:1. doi: 10.1186/1471-2466-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clearie KL, Jackson CM, Fardon TC, et al. Supervised step-down of inhaled corticosteroids in the community—an observational study. Respir Med. 2011;105:558–65. doi: 10.1016/j.rmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Hagiwara M, Delea TE, Stanford RH, Stempel DA. Stepping down to fluticasone propionate or a lower dose of fluticasone propionate/salmeterol combination in asthma patients recently initiating combination therapy. Allergy Asthma Proc. 2010;31:203–10. doi: 10.2500/aap.2010.31.3359. [DOI] [PubMed] [Google Scholar]
- 56.Bateman ED, Jacques L, Goldfrad C, Atienza T, Mihaescu T, Duggan M. Asthma control can be maintained when fluticasone propionate/salmeterol in a single inhaler is stepped down. J Allergy Clin Immunol. 2006;117:563–70. doi: 10.1016/j.jaci.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 57.Koenig SM, Ostrom N, Pearlman D, et al. Deterioration in asthma control when subjects receiving fluticasone propionate/salmeterol 100/50 mcg diskus are “stepped-down”. J Asthma. 2008;45:681–7. doi: 10.1080/02770900802168695. [DOI] [PubMed] [Google Scholar]