Abstract
Objectives
To characterize challenges experienced during stages of female-to-male sex transition and investigate associations between transition-specific measures of psychosocial stress, nocturnal decline in ambulatory blood pressure (amBP), and change in C-reactive protein (CRP) levels.
Methods
For this biocultural study, 65 healthy transmen who were using testosterone (T) therapy participated in interviews to assess transition-specific stress experience. They provided perceived stress scores (PSS), self-esteem scores, 24-hour amBP measures, salivary samples for T levels, and a blood spot for CRP levels. Psychosocial stress was examined in relation to amBP and CRP using linear regression while adjusting for age, BMI, and smoking.
Results
There were no differences in mean levels of BP in association with stage of transition. Men reporting stress associated with being “out” as transgender had significantly diminished nocturnal decline in systolic and diastolic amBP compared to men who did not report such stress. The associations remained significant when examined among men in stages 1 and 2 (≤3 yrs on T) but not among men in stage 3 (>3yrs on T) of transition. Men reporting stress related to “passing” as someone born male had higher CRP levels than those who did not report such stress. The association remained significant when examined among men in stages 2 and 3 (>.5–3yrs on T).
Conclusions
Measures of stress that captured individuals’ experiences of gender liminality were associated with diminished nocturnal decline in amBP and increased levels of CRP. There are significant differences between men grouped into different stages of the transition process.
Keywords: Stress, amBP, CRP, liminality, transition
Health disparities, including higher rates of mental or physical illness, are found among members of minority or marginalized groups (Williams and Mohammed 2009) including people who identify as lesbian, gay, bisexual or transgender (LGBT) (Huebner and Davis 2007). Transgender men (transmen) are people who were assigned a female sex designation at birth and who have a male gender identity. Many transmen pursue medical transition, enabling them to deliberately induce changes to their own phenotype through the use of exogenous T therapy and surgical procedures. These phenotypic changes help to align their internal male gender identities with their physical gender presentation. In a recent report from the National Institute of Health, the specific health needs of transgender individuals were identified as a priority research area due to the population’s unique health experiences, particularly chronic stress faced by sexual and gender minorities as a result of stigmatization (IOM 2011). For transgender people, the threat of violence and/or discrimination due to social stigma represents a real and daily threat; a study of transgender people in the United States found that 37% had experienced economic discrimination and 60% had experienced harassment or violence due to their gender presentation (Lombardi et al., 2001).
Though it is recognized that transitioning from one sex to another is a stressful experience, relatively little is known about the physiological manifestations of psychosocial stress or the implications for health among transmen. The process of medically transitioning from one sex to another within a North American social context, where sex is in large part viewed as both immutable and binary, provides a unique opportunity for insights into chronic stress effects related to a transition in phenotype and social identity. Psychosocial stress associated with the experience of social stigma or discrimination is known to function as a potential contributor to disparities in health through stress effects on behavioral, metabolic, and hormonal mechanisms. Chronic psychosocial stress has been shown to result in increased risk for cardiovascular diseases and CVD-related mortality (Matthews and Gump, 2002) with evidence that a diminished decline in nocturnal blood pressure (Ohkubo et al., 2002) as well as chronic inflammation (Danesh et al., 2000; Wilson et al., 2006) play key roles linking stress to CVD-related morbidity and mortality.
Research aimed at understanding the mechanisms by which psychosocial stress gets “into the body” leading to increases in disease risk, is central to understanding the link between social inequality and social disparities in health (Miller et al., 2009). A number of studies have shown that stress that is chronic in occurrence or chronic in its effects, leads to increased risk for negative cardiovascular outcomes such as coronary heart disease (Esler et al., 2008; Miller and Blackwell 2006; Segerstrom and Miller 2004). As an acute phase protein released in response to increased cytokine production, CRP has been found to increase in response to acute injuries or infection and also to acute and chronic psychosocial stress (Hansel et al., 2001; Steptoe et al., 2007 for review). While inflammation is clearly beneficial during an acute (particularly physical) stressor, extended periods of inflammation have been linked to increased risk for CVD through processes such as atherosclerosis and hypertension (Virdis et al., 2007). As even slight elevations in CRP have been found to be predictive of CVD, CRP measured using high sensitivity assays is a useful measure of low-level inflammatory activity and CVD risk (McDade et al., 2006; Ridker et al., 2000). In terms of nocturnal decline in amBP, there is evidence that even a minor diminishment in nocturnal decline in amBP is associated with increased disease risk; for each 5% decrease in the decline in nocturnal amBP there is a 20% to 30% increase in cardiovascular related morbidity and/or mortality (Ohkubo et al., 2002; Verdecchia et al., 1994).
The purpose of the study presented here was to utilize amBP measures and CRP levels in order to examine the physiological effects of self-reported perceived stress and transition-specific stress experience during the process of medical transition among 65 transmen undergoing T therapy. A pilot study was conducted during the summer of 2008 where, during in-depth interviews, nine transmen described their experience of sex transition. Using the themes that emerged during these interviews, the present study design aimed to contextualize the challenges that are faced during sex transition and to examine stress through both psychological and biological measures.
Hormonally Induced Changes and Transition-Stress
Stressors associated with sex transition are multifaceted as hormonally induced changes in phenotype are tightly bound with gender presentation and social identity. The objective of T therapy for transmen is to initiate changes in secondary sex characteristics so as to enable others to socially recognize them as male. In order to access T therapy, transmen generally must obtain a letter stating they meet the criteria for a diagnosis of gender identity disorder (GID) (American Psychiatric Association [DSM-IV-TR], 2000) from a licensed mental health professional who then refers them to a medical provider willing to prescribe cross-sex hormones (Meyer et al., 2005). As many feel that the GID diagnosis is itself stigmatizing, some clinics are beginning to follow informed consent models of care, which may not require a diagnosis of GID to access hormone treatments (Lev 2009).
Since the half-life of testosterone in blood is approximately 70 minutes, it is necessary for HRT to be administered regularly, in order for male secondary sex characteristics to be maintained. The two most commonly prescribed forms of injectable testosterone esters for use by transmen in the United States are testosterone enanthate (Delatestryl) and testosterone cypionate (Depo-Testosterone). Both forms function similarly and are suspended in a lipid base such as sesame oil, which slows the rate of metabolization. Multiple administration routes for T are available including weekly or biweekly intramuscular or subcutaneous injections or daily applications of transdermal creams. While appropriateness of T dosage is assessed using serum levels of circulating T aiming to achieve levels that approximate normal male physiologic production (4–9mg/daily = 290–900 ng/dl), satisfactory masculinization through the development of secondary sex characteristics and not lab values are the true target of therapy (Gorton et al., 2005). Hormonal replacement dosages used by transmen are usually 50–150mg per week depending on surgical status (dosage can be lower post-oopherectomy), age, and individual response and peak levels of testosterone are generally reached within 48 hours following injection while trough levels occur the day before next injection. The aim of transdermal application is to achieve a relatively consistent level of circulating T.
Some of the characteristic changes that can occur during T administration include a deepening of the voice, increased growth of body hair, male pattern hair loss, increased musculature and libido, changes in skin tone and texture, shifts in body fat distribution, and physiological changes that can affect metabolism, mood, energy levels, and sleep quality. Surgeries - in particular, double mastectomies or “top surgery” – together with the changes produced through hormone therapy combine to create the socially recognized gender “cues” that dramatically affect how individuals are perceived and treated.
A recent meta-analysis reports several studies with findings that indicate that cross-sex hormonal intervention improves quality of life (QOL) and overall happiness among individuals who were diagnosed with GID (Murad et al., 2010). Similar findings were reported from a recent study of QOL among transgender men (Colton Meier et al., 2011), though when compared to the general population in the U.S. transmen show diminished mental health related QOL (Newfield et al., 2006). These findings indicate that though the physical changes associated with T administration are desirable, experiencing dramatic changes in one’s own body and social relations can at least initially increase perceived stress. Current research also suggests that the mental health and well-being of LGBT people is impacted by personal and social acceptance of both sexual orientation and gender identity (Grant et al., 2011; DHHS 2010). Because T initiates a slow process of physical change that occurs over the course of years, transmen who are early in transition may be susceptible to negative social consequences due to their liminal gender position of being perceived by others as “ambiguous” or failing to conform to the binary social categories of female and male. Liminality is itself characterized as falling “betwixt and between,” and people who occupy liminal positions are often viewed as contradictory in the sense that they elude social categorization (Turner 1967). The stress experience of being continuously categorized by others as female or as ambiguously sexed, can be exacerbated by delayed access to either hormones or surgical procedures. These factors, which contribute to the duration of time in which one remains in a liminal category relative to gender presentation, can contribute to the stress experience of transition.
The concept of stress, defined as a perception of demands with which an individual is unable to cope, has provided a useful framework within which to examine how individuals respond to environmental or psychosocial challenges (Lazarus 2000). The stress that is experienced during the process of transition, which in many ways mirrors the experience of adolescent puberty, obviously varies between transmen as do the stressors that they endure and their psychological and physiological response to those stressors. Stress researchers generally acknowledge that cultural context and social factors play a role in both exposure to and appraisal of stressors, thereby influencing an individual’s physiological and psychological reaction (Ice and James 2007). The application of a biocultural approach to psychosocial stress has enabled an examination that asks how “psychological processes mediate the effect of culture on individual bodies” (Hruschka et al., 2005:4). Subjective experiences are key to understanding the way that the body responds to its cultural surroundings.
Research examining the effects of psychosocial stress on stress physiology and health provides an important contribution to understanding the factors that may ameliorate or increase risk for CVD in particular. Through the use of multiple measures of perceived stress and physiological stress response, this study aims to enable us to better understand the physiological and psychological manifestations of stress associated with a physical transformation that influences social identity.
Methods
Participant recruitment
The participants in this study were sixty-five healthy transgender men recruited from western MA, Boston, MA, and southern Vermont. As transgender men represent a relatively hard to reach population, recruitment efforts focused on online list-serves, area support groups, social networks, and Facebook announcements. Efforts to earn the trust of members of the trans community included attendance at monthly support group meetings and transgender focused conferences as well as individual meetings with “elder” members of the community and other transgender health researchers. Numerous phone calls and/or in person meetings often preceded consent to participate and many potential participants conveyed their inability to commit to participate due to the stress they were experiencing in their lives as a consequence of transitioning. This fact is reflected in the lower number of participants who had been on T therapy for less than six months compared to those later in transition (Table 1).
Table 1.
Selected demographic and psychosocial stress characteristics across stages of transition (N=65)
| Characteristic Mean (SD) |
Total Sample N=65 |
Stage 1 N=14 |
Stage 2 N=26 |
Stage 3 N=25 |
|---|---|---|---|---|
| Agea* | 31.76 (9.1) | 29.95(9.7) | 28.7(7.5) | 35.9(9.1) |
| Yrs on Ta** | 3.27(3.3) | .24 (.16) | 1.67 (.77) | 6.6 (3.1) |
| BMI kg/m2 | 30.21 (6.66) | 30.55 (7.21) | 30.84 (6.27) | 29.38 (6.94) |
| % who smoke | 20% | 7% | 27% | 20% |
| Perceived Stress Scores (PSS)a* | 16.44 (6.73) N=63 |
17.57 (6.75) | 18.79 (6.45) N=24 |
13.56 (6.12) |
| Self-Esteem Scores | 21.78 (5.14) N=63 |
20.79 (4.83) | 21.46 (4.96) N=24 |
22.64 (5.52) |
| Reports stress related to being “out” as transgender | 44% | 57% | 46% | 32% |
| Reports stress related to “passing” | 22% | 43% | 19% | 13% |
ANOVA comparison across all three groups with Tukey post hoc tests,
p<0.05,
p<0.01
Eligibility criteria required that each participant was over 18 years old, was assigned a female sex designation at birth but expressed a male gender identity, and was using T therapy as part of their transition from female to male. Participants were ineligible for participation if they were currently taking medication for any cardiovascular or immune related conditions.
Due to the social stigma and risks endured by transgendered individuals in our society, extraordinary care was taken to insure that study participants’ identities were protected; interviews were conducted in LGBT sensitive settings or the participant’s own home and consent forms were provided and discussed with initials signed into a notebook instead of on the forms themselves. Both research aims and protocol were approved by the IRB for research involving human subjects at the University of Massachusetts Amherst.
Data Collection
The procedures used to collect data for this study were structured around individual participants’ T administration schedule. Interviews and anthropometric measures were conducted either in the participant’s home, in private meeting rooms at area LGBT health clinics, or in private offices at UMass Amherst.
Extensive semi-structured interviews (2–4 hour) were conducted with each participant. All interviews and measures were conducted in a private setting with no one else present and were digitally recorded. Psychometric scales and surveys with demographic and biobehavioral data were distributed via email or on paper prior to meeting and were collected in person during the interview.
Anthropometric Measures
Height was measured with a free-standing portable anthropometer. Body weight was measured using a portable digital scale (Tanita, model BC-550T). Body mass index (BMI) was then calculated from weight (kg)/height(m2).
Ambulatory Blood Pressure (amBP) Measures and Nocturnal Decline
Ambulatory systolic (amSBP) and diastolic (amDBP) blood pressures were measured for a 24-hour period with measures taken every 20 minutes while awake and every 30 minutes while asleep (as tolerated) using the Oscar2 ambulatory monitor, which uses an oscillometric technique and has been validated for use in clinical research (Jones et al., 2004).
Each participant was fitted with a monitor following the completion of height and weight measures. Participants then wore the monitor during the interview to guarantee tolerance and address any complications. The monitor was calibrated using back-to-back measures with a mercury column and stethoscope and was considered accurate when the two readings agreed to within 5mmHg. If calibration readings were inaccurate, a second monitor was used following calibration. One monitor was recalibrated by the manufacturer following two inaccurate calibration attempts. Participants were fully trained in how to remove/turn off the monitor as well as how to reapply the monitor if they chose to put it back on. Each was encouraged to only wear the monitor insofar as they did not feel that it was increasing their sense of stress and to call with any concerns or questions at any time of day or night. Participants were also asked to keep a diary in which they recorded the times at which they went to bed and got up.
Mean amSBP and amDBP were calculated from waking and sleeping measures for the entire sample and for each stage of transition. The percentage of decline in nocturnal amSBP was calculated as: (mean daytime amSBP –mean nighttime amSBP) × 100/mean daytime amSBP (Metoki et al., 2006). The percentage of decline in nocturnal amDBP was calculated using the same method.
Sampling for measurement of C-Reactive Protein (CRP)
CRP levels were measured from participants who reported no symptoms of illness or injuries in the last two weeks. Drops of blood were collected on standardized Whatman filter paper (#903), following a single finger prick using a sterile disposable lancet. Samples were dried for 24-hours at room temperature and then frozen in a lab grade freezer until shipped to the Laboratory for Human Biology Research at Northwestern University. Concentrations of CRP (mg/L) were determined using enzyme linked immunosorbent assay (ELISA) protocols (McDade et al., 2004; McDade 2007).
Sampling for measurement of testosterone (T)
T levels were measured from salivary samples collected from participants using the “passive-drool” technique, using a straw and polypropylene vial (Ice 2007). Participants were provided with collection materials and written and verbal instructions regarding salivary collection procedures. Participants administered exogenous T using either weekly or biweekly injections or daily transdermal application of T gel or cream. Participants who used injection techniques were instructed to collect salivary samples for “peak” T levels within 48 hours of an injection (Gorton et al., 2005). Participants who applied transdermal gel were instructed to collect salivary samples for peak T approximately 4 hours following application. Samples were labeled with the date and collection time and then refrigerated until retrieved by the investigator. Samples were then frozen in a lab-grade freezer until being shipped to the University of Dresden, Germany for analysis. Analysis was conducted using a chemiluminescence immunoassay assay (CLIA) with a lower sensitivity of 1.8 pg/ml (IBL-International; Hamburg, Germany). Intra and interassay coefficients of variation were below 10%.
Assessment of perceived stress and self-esteem
In order to evaluate perceived stress levels, participants filled out Cohen’s Perceived Stress Scale (PSS) (1983) which is the most commonly used measure of perceived stress and has been shown to be predictive of a number of health outcomes (Ice 2007). Answers to the 10-item PSS were summed for each participant, yielding scores that ranged from 0 (low perceived stress) to 40 (high perceived stress). Rosenberg’s Self Esteem Scale (1965) was used to assess individual levels of self-esteem. Answers to the 10-item Self Esteem Scale were summed for each participant, yielding scores that ranged from 0 (low self esteem) to 30 (high self esteem).
Stages of Transition
Stages of transition were developed a priori based on characteristics associated with T administration over time as well as qualitative data obtained during the pilot study. These three stages of transition are based on duration of time on T because while there is individual variation in how bodies respond to T, on average these stages correlate with certain changes that take place that have relevance for transmen.
Stage 1 includes men on T for ≤ 6 months,
Stage 2 includes men on T for >6 mos – 3 years, and
Stage 3 includes men on T for > 3 years.
Assessment of transition-specific stress
During the in-person interview, participants were asked to answer a number of questions about their transition experience, social identity, and personal relationships.
Stress associated with “Passing”
Stress experience related to “passing” as someone who was born male was assessed through a number of questions from the in-person interview. These questions included: Do people refer to you as “she” or use your old name on a regular basis? If yes, does it bother you? Is the issue of “passing” a source of stress for you?
A categorical variable was then created to reflect the answers to these questions. Individuals who answered “yes” or described that for them the issue of “passing” was a source of stress were coded 1 (yes) for “passing stress.” Those who live socially comfortably and consistently as male or who did not describe experiencing any stress related to “passing” were coded 0 (no) for “passing stress.”
Stress associated with being “out” as transgender
Stress associated with being “out” as transgender was assessed through a number of questions from the in-person interview. Initially, participants were asked: Is being out a source of stress or anxiety for you? Potential answers included: never, sometimes, most of the time, always and were coded 0–3. In order to clarify and contextualize the meaning of participant’s answers, follow-up questions included: Are there people with whom or situations in which being known as trans makes you feel uncomfortable? Are there people in your life who know you’re trans who you wish didn’t know? Why? Participants were then placed into 2 groups: those who described frequent stress about being “out” (1) and those with infrequent or no stress about being “out” (0).
Methods of Analysis
Prior to data analysis, participants were categorized into stages based upon their duration of time on T as described above. CRP and T levels were log transformed in order to normalize the distribution prior to analysis.
Characteristics of this sample of transmen and differences between men in different stages of transition were assessed using demographic, anthropometric, psychological, and physiological variables. Comparisons were conducted across stages of transition using analysis of variance (ANOVA) with Tukey post hoc tests and chi-square tests. Two-way main effects ANOVA were conducted to control for stage of transition when assessing the effect of transition-related stress on physiologic stress measures. Age, BMI, and smoking status were entered as covariates. T-tests were used to examine differences between stressed/non-stressed groups.
Hierarchical linear regression analyses were performed to test associations between Cohen’s Perceived Stress Scores (PSS), Rosenberg’s Self-Esteem Scores, the presence or absence of stress related to “passing” and being “out,” and physiologic stress measures including nocturnal decline in amBP and levels of CRP. “Passing” stress and stress about being “out” were entered into separate models as categorical variables. Each model included age, BMI, and smoking status as covariates. Because significant associations have been found in some studies between T and CRP (Gooren and Giltay 2008; Maturana et al., 2008), additional models for analyses of CRP included peak T levels. Results were considered statistically significant if p<.05. Statistical analyses were performed using SPSS v.16.0 for the Mac.
Results
A total of 65 transmen participated in in-person interviews. Of those, 63 filled out the perceived stress scale (PSS) and self-esteem scale. Table 1 shows selected demographic and psychosocial stress characteristics across the three stages of transition. Men in stage 2 were significantly younger than men in stage 3. Men in stage 2 reported significantly higher perceived stress scores (PSS) than men in stage 3. Nearly half of the total sample reported stress concerning being “out” with no significant differences across transition stages. A higher percentage of men in stage 1 reported experiencing stress related to “passing” when compared to men in later stages (ns, p=.08). The majority of the total sample (N=65) self-identified as Caucasian (74%) with 11% identifying as Jewish, 6% as Latino, 5% as African American, 3% as Asian, and 3% as having a mixed racial/ethnic background. Of the 44 participants who provided amBP measures while they were sleeping, 40 were of European descent and 4 were of non-European descent.
Sixty-three participants provided amBP readings while they were awake and 44 of those were able to tolerate amBP monitoring during sleep. The mean number of total readings for all of the participants was 44 (SD 16.5) with a mean of 35 (12.5) measures taken while awake and 13 (4.0) while asleep. Mean ambulatory amSBP/amDBP values for the total sample were 130 (11)/78 (8) while awake and 108 (10)/59 (7) mmHG while asleep. The mean percentage of decline in nocturnal amSBP/amDBP was −16.16 (5.69)/−23.14 (6.23).
Sixty-one participants provided blood spots for analysis of CRP. Two cases with CRP >8.6, a cut-off point used to indicate the presence of active infection, were excluded from analyses (Mcdade et al., 2011). Mean amBP, nocturnal decline in amBP and CRP levels were not significantly different when comparing men across different stages of transition (Table 2). ANOVA analysis revealed no significant differences in stress variables between racial/ethnic groups. T-tests revealed no significant differences in nocturnal decline in amBP among study participants of non-European and those of European descent (not shown).
Table 2.
Selected biological characteristics across stages of transition (N=65)
| Characteristic Mean (SD) |
Total Sample | <.5yrs on T N=14 |
>.5–3yrs on T N=26 |
>3yrs on T N=25 |
|---|---|---|---|---|
| Peak T levels pg/ml | 367.5 (184.4) n=42 |
320.3 (125.1) n=8 |
437.5 (197.2) n=17 |
319.7 (180.7) n=17 |
| Peak T levels (log) | 2.51 (.22) | 2.48 (.15) | 2.60 (.21) | 2.44 (.24) |
| CRP levels | 1.50 (2.75) n=59 |
1.01 (1.3) | 1.61 (3.05) N=22 |
1.68 (3.12) |
| CRP levels (log) ELISA units | −.23 (.55) n=59 |
−.29 (.50) | −.20 (.56) n=22 |
−.21 (.58) |
| amSBP awake | 130 (11) n=63 |
128 (9) n=14 |
129 (10) n=25 |
131(13) n=24 |
| amDBP awake | 78 (8) n=63 |
78 (6) n=14 |
78 (8) n=25 |
7 (10) n=24 |
| amSBP asleep | 108 (10) n=44 |
109 (7) n=10 |
108 (8) n=17 |
107(12) n=17 |
| amDBP asleep | 59 (7) n=44 |
60 (7) n=10 |
59 (6) n=17 |
59 (8) n=17 |
| % dip wake to sleep amSBP | −16.16 (5.69) n=44 |
−14.80 (5.56) n=10 |
−16.35 (5.59) n=17 |
−16.76 (6.04) n=17 |
| % dip wake to sleep amDBP | −23.14 (6.23) n=44 |
−20.30 (4.6) n=10 |
−23.82 (5.64) n=17 |
−24.12 (7.37) n=17 |
No significant differences between groups
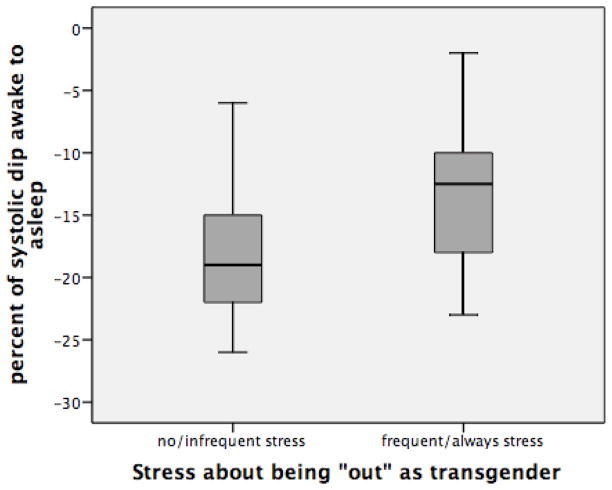
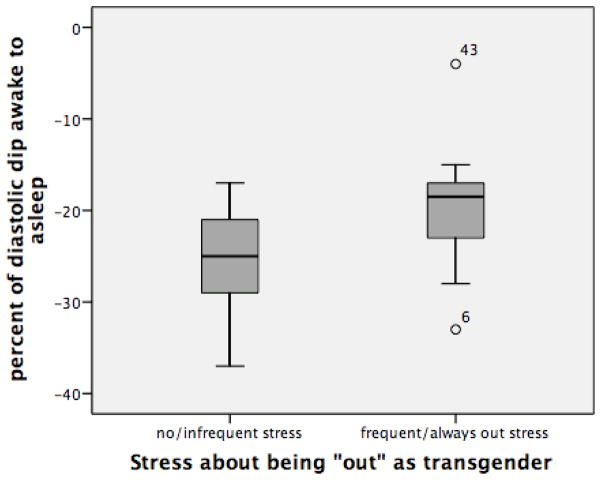
Men who reported experiencing stress about being “out” (n=18) had significantly less of a nocturnal decline in amSBP/amDBP (p<.01) (−13.17%/−20%) than men who did not report experiencing stress about being “out” (−18.23%/−25.31%) (n=26) (Figures 1a and 1b). There are no significant differences in age or BMI when comparing these two groups.
Figure 1.
Figure 1a: Percent of nocturnal decline in amSBP among men with and without stress about being “out” as transgender (p<.01).
Figure 1b: Percent of nocturnal decline in amDBP among men with and without stress about being “out” as transgender (p<.01).
The effect of experiencing stress associated with being “out” on the percentage of nocturnal decline in amBP was then assessed within each stage of transition, among men early in transition (<3yrs on T=stages 1 and 2 combined) and among the entire sample using separate linear regression models. Among the entire sample, when controlling for age, BMI, and smoking status, stress about being “out” was significantly associated with a diminished nocturnal decline in both amSBP (Beta .461, p<.01) and amDBP (Beta .468, p<.01) (Table 3). Table 4 shows the effects of stress related to being “out” among men early in transition (stages 1 and 2). Among these men, in addition to BMI, stress about being out was significantly associated with a diminished nocturnal decline in both amSBP(Beta .653, p<.01) and amDBP (Beta .681, p=.01).
Table 3.
Relationships between stress variables and physiological measures among the entire sample of transmen.
| % amSBP Dip (n=44) | % amDBP dip (n=44) | CRP (n=59) | ||||
|---|---|---|---|---|---|---|
| Standardized Beta | P value | Standardized Beta | P value | Standardized Beta | P value | |
| Perceived Stress Scores (PSS) | Model not significant | Model not significant | .064a | NSa | ||
| “Out” Stress | .461b | .003b | .468c | .003c | .064d | NSd |
| “Passing” Stressc | Model not significant | Model not significant | .327e | .004e | ||
Models included age, BMI, and smoking status as covariates.
BMI (Beta .488, p = .000), Adjusted R2 = .209, model significance = .002
Adjusted R2 = .193, model significance = .014
Adjusted R2 = .162, model significance = .027.
BMI (Beta .513, p = .000), Adjusted R2 = .223, model significance = .001
BMI (Beta .532, p = .000), Adjusted R2 = .379, model significance = .000
Table 4.
Relationships between stress variables and physiological measures among men early in transition (<3 years on T).
| % amSBP Dip (n=44) | % amDBP dip (n=44) | CRP (n=59) | ||||
|---|---|---|---|---|---|---|
| Standardized Beta | P value | Standardized Beta | P value | Standardized Beta | P value | |
| Perceived Stress Scores (PSS) | Model not significant | Model not significant | Model not significant | |||
| “Out” Stress | .653a | .000a | .681b | .001b | Model not significant | |
| “Passing” Stressc | Model not significant | Model not signficant | .336c | .039c | ||
Models included age, BMI, and smoking status as covariates. NS = models were not significant.
BMI (Beta .449, p=.010), Adjusted R2 = .488, model significance = .001
BMI (Beta .403, p=034), Adjusted R2 = .354, model significance = .008
BMI (Beta .413, p = .011), Adjusted R2 = .224, model significance = .019
The relationship between stress about being “out” and diminished nocturnal decline remained significant among men who were in stage 2 (amSBP=Beta .432, p<.05, amDBP=Beta .514, p<.05). The relationship was not significant when examined only among men in stage 1 or among men in stage 3. Results from 2-way main effects ANOVA showed that the between subjects effect of “out” stress significantly diminishes nocturnal decline in amSBP (F(1,35)=9.29, p<.01) and amDBP (F(1,35), p<.01) even when stage is taken into account.
As a measure of general stress, PSS was correlated with DBP while awake (r=.26, p<.05) and SBP while asleep (r=.317, p<.05) but the association was no longer significant when entered into a regression model which controlled for age, BMI, and smoking (Table 3). PSS was found significantly associated with nocturnal decline in amDBP (Beta .607, p<.05) only among men in stage 1 of transition when controlling for age, BMI, and smoking. PSS was not significantly associated with nocturnal decline in amBP when examined among the entire sample, men early in transition (combined stages 1 and stage 2) or men in stage 3. When examining the effect of PSS among men in stage 2, only age and BMI were significantly associated with nocturnal decline in amSBP.
Self-esteem was significantly correlated with nocturnal decline in amSBP (r=−.318, p<.05) and reflected that as self-esteem scores increased, the percentage of nocturnal decline also increased (becomes a larger negative number). The relationship remained significant when examined among men in stage 1 (Beta -.650, P<.05). Neither relationship remained significant when BMI, age, and smoking status were controlled for using linear regression models.
T-tests revealed significant differences in logCRP levels (p<.05) between men who reported stress associated with “passing” (n=13) and those who reported no stress associated with “passing” (n=46) (Figure 2). Figure 2 shows that among the entire sample, men who reported experiencing stress associated with “passing,” had significantly higher logCRP levels than men without “passing” stress. Bivariate correlations were significant between logCRP and log peak T levels (r = −.334, p=.041) (not shown).
Figure 2.
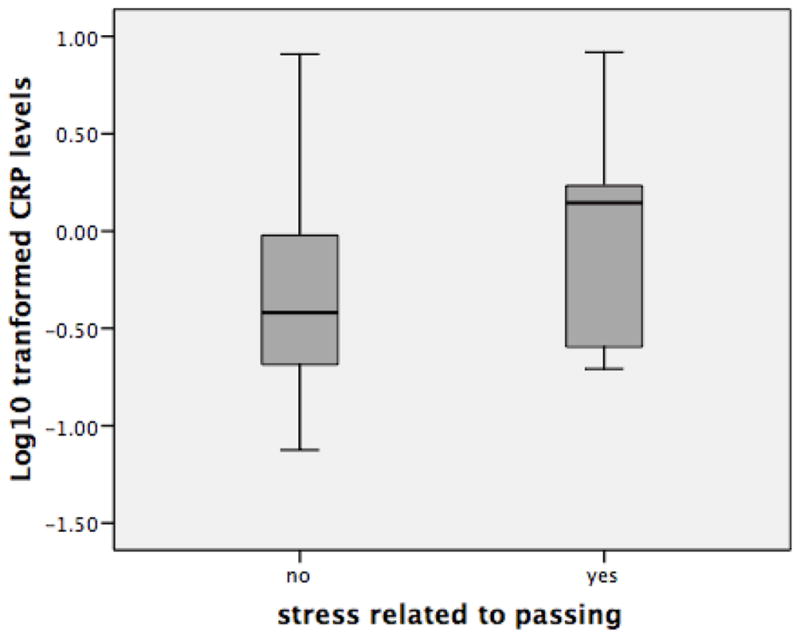
LogCRP level among men with and without stress about “passing”(p<.05).
The effect of experiencing stress associated with “passing” on logCRP levels was assessed among the entire sample, within each stage of transition, and among men early in transition (stages 1 and 2) using separate linear regression models. Log peak T levels were included in a second model to examine effects on logCRP.
When controlling for age, BMI and smoking, men who reported experiencing stress related to “passing” were more likely to have higher levels of logCRP than men who did not experience “passing” stress (Table 3). These findings were also significant among men in stage 2 (Beta .431, p<.05), men early in transition (combining stage 1 and 2) (Beta .336, p<.05) (Table 4) and men in stage 3 (Beta .388, p<.05) but not among men in stage 1. “Passing stress” was no longer significantly associated with CRP for any group of men after controlling for T levels (Tables 5 and 6). Results from 2-way main effects ANOVA show the between subjects effect of “passing” stress (p<.01) which, along with BMI (F(DF) p<.01) was significantly associated with elevated CRP levels even when stage is taken into account.
Table 5.
Relationship between stress about “passing” and logCRP levels when controlling for peak T levels among the entire sample of transmen.
| Outcome variable: logCRP | ||
|---|---|---|
| Stand. Beta | p-value | |
| Age | .195 | .079 |
| BMI | .673 | .000 |
| Smoker | −.153 | .181 |
| Peak T level (log) | −.211 | .071 |
| “passing” stress | .209 | .066 |
Adjusted R2 .577, model significance=.000
Table 6.
Relationship between stress about “passing” and logCRP levels when controlling for peak T levels among men early in transition (<3 years on T).
| Outcome variable: logCRP | ||
|---|---|---|
| Stand. Beta | p-value | |
| Age | .367 | .053 |
| BMI | .413 | .030 |
| Smoker | −.148 | .404 |
| Peak T level (log) | −.291 | .111 |
| “passing” stress | .186 | .322 |
Adjusted R2 .566, model significance=.013
PSS was not significantly associated with levels of logCRP when examined among the entire sample or within the separate stages of transition. When controlling for age, BMI and smoking, men in stage 1 who had lower scores on the self-esteem scale were more likely to have higher levels of logCRP than men with higher self-esteem scores (Beta −.559, p<.05).
Discussion
In this cross-sectional study of female to male sex transition, amBP measures and CRP levels were utilized in order to examine the physiological effects of self-reported general perceived stress and self-esteem alongside levels of transition-specific stress that were assessed through ethnographically derived measures. Overall, the general measure of stress (PSS) was found to be less effective at capturing psychosocial stress with physiological effects than measures of stress associated with being “out” as transgender and stress associated with “passing,” both of which more specifically reflect aspects of the transition experience. These findings have implications for this sample as well as for studies among other people for whom stress is experienced in association with changes in the body and social identity.
Though several field-based studies have found mean amBP to be elevated in response to perceived stress including stress while at work (Kario et al., 2002; James and Bovbjerg 2001), lifestyle incongruity (Bindon et al., 1997; Dressler 1991), and a lack of cultural consonance (Dressler and Bindon 2000), general perceived psychosocial stress was not associated with significant changes in mean amBP among this sample of transmen. Though there were significant differences in levels of PSS among men in different stages of transition, mean amBP levels for both waking and sleeping measures were also in normal range among men in all stages of transition despite a relatively high mean BMI for this sample.
In this study, transition-specific stress measures were associated with nocturnal decline in amBP and levels of CRP. Men who experienced stress associated with being “out” as transgender had a significantly diminished decline in nocturnal amBP and men who experienced stress associated with “passing” had significantly higher levels of CRP. These findings correspond with those of other researchers who have found associations between psychological factors and diminished nocturnal decline in amBP (Huang et al., 2011; Ituarte et al., 1999; Linden et al., 2008) and to studies finding increased levels of inflammation associated with perceived stress (Mcdade et al., 2006) and chronic stress associated with caregiving (Kiecolt-Glaser et al., 2003).
The findings in this study are consistent with a hypothesis linking elevated CRP with “social evaluative threat” (SET), characterized as threats to the social self that occur when an aspect of the self could be negatively judged by others (Dickerson et al., 2009; Dickerson et al., 2004). Increased levels of inflammatory molecules have been found in response to these specific types of stressors, as well as to psychosocial stressors that are both serious and chronic (Segerstrom and Miller 2004) prompting researchers to propose a model recognizing that chronic stress can result in immune dysregulation (Robles et al., 2005). In the North American context where this study took place, sex attribution into one of two categories occurs on a largely unconscious level and deviation from the binary categories of female/male can arguably be characterized as both serious and chronic and can also lead to interactions that are perceived as evaluative and/or threatening to the social self.
Studies involving nonhuman animals provide evidence that an inflammatory response to social threat may be adaptive. Inflammation would be beneficial in the context of social subordination where immunosuppression would be maladaptive for purposes of healing from a wound or infection (Sapolsky, 2004). Similarly, elevated levels of CRP have been linked to depressive symptoms and “sick behavior,” which could also function as an adaptation to reduce conflict (Dickerson et al., 2009; Everson-Rose et al., 2005).
Elevated levels of CRP and diminished nocturnal decline in amBP were each observed in response to transition-specific stressors among men in early stages (stages 1 and 2) of transition. Stress associated with being “out” as transgender was also significantly associated with nocturnal decline in amSBP and amDBP (Figures 1a and 1b) among men in stages 1 and 2. In this study, sex transition was viewed as a potentially stressful process in part due to its liminal nature – as a process during which time one’s gender identity and physical presentation may not conform to normative gender expectations. In a sense, stage 2 can be understood to include men in the most liminal of the 3 stages.
Though transition-specific stress related to being “out” was associated with a diminished nocturnal decline in amBP among men in the liminal stage, which could indicate increased risk for CVD (Ohkubo et al., 2002), the effect was not observed among men in stage 3, who had been on T for >3 years and still reported experiencing stress about being “out.” This finding is consistent with points to the potentially temporary nature of negative health effects associated with transition-related stress. When stage of transition was controlled for, stress associated with being “out” remained significantly associated with diminished decline in amBP. Stress associated with “passing” was not significantly associated with increased levels of CRP once peak T levels were added to the model.
The amBP results suggests that, while overall stress decreases the longer someone is on T, there are specific stressors that endure and have physiologic effects across stages of transition and among men who have been on T for more than 3 years. Qualitative interview data suggests that stress about being “out” occurs both among men who, early in transition, are concerned about informing others about their trans status for fear of rejection or discrimination but also among men later in transition, who tend to be living full lives as men. These men may desire to be “out” as transgender in order to have more people in their lives who understand their complex life history. An improved understanding of the stages of change could help to elucidate some of the observed differences in who experiences stress associated with “passing” and being “out” and how that stress manifests in the body.
While transition-specific stressors consistently predicted physiological responses among men in different stages of the transition process, in this study, the more general measure of perceived stress (PSS) was associated with nocturnal decline in amDBP only during the earliest stage of transition and was not associated with CRP. This finding may reflect that only men in stage 1 may experience generalized stress that can be captured sufficiently with the PSS. Similarly, among men in stage 1, higher self-esteem scores were associated with lower CRP indicating the possible amelioration of stress-effects that come about with the onset of T access/administration as well as the overall positive effect of higher self-esteem. On the other hand, general measures of PSS and self-esteem did not sufficiently capture the stress effects of being “out” or “passing” among men in later stages of transition. Particularly among men in the liminal stage of transition, the ethnographically-derived measures of stress better assessed the specific stress associated with physiological change. These findings suggest that psychosocial factors that are specific to the experience of sex transition, including stress associated with being “out” as transgender and stress associated with “passing” as male, are important predictors of health outcomes such as CVD risk, particularly when stage of transition is taken into account. They also reflect the importance of targeting efforts to reduce transition-specific stress and increase self-esteem among transmen during transition.
This study documents a number of important psychological and physiological manifestations of stress associated with the process of transition. But it is important to note that, when study participants were asked if they felt that transitioning was the right thing for them to do, 100% responded yes without qualification. Additionally, consistent with the aim of gender affirmation treatment (including testosterone therapy), several studies have found an increased quality of life and lower levels of depression, anxiety and stress among transmen who have received T therapy compared to those who have not (Colton Meier et al., 2011; Newfield et al., 2006). The findings presented here document a decline in stress over the course of transition and are consistent with prior research that identifies T therapy as an adaptive therapeutic option and key to the well-being of transmen.
Because of the cross-sectional design, small sample size, select nature of the sample population, and the lack of prevalence estimates of those undergoing female to male transition, caution should be used when extrapolating these findings to the general population. Additionally, this study was conducted predominantly among transmen living in urban and rural Massachusetts; as transition-specific stress response could reflect experiences that are in part contingent upon local, cultural interpretations of gender norms, future research of a longitudinal nature and among transmen living in different cultural environments would be worthwhile.
This research has relevance beyond the trans population to other populations and circumstances of physical change. The findings of this study indicate that stress associated with a changing body and social identity can result in increased disease risk, but also that the effect may be transient and most strongly evident in the midst of the most liminal stage of transition.
Acknowledgments
Funding Source: NSF Dissertation Improvement Grant #0751969 and a research grant from the Williams Institute supported this project. Thanks are also due to the Center for Population Research in LGBT Health at The Fenway Institute supported by NICHD under award number R21HD051178 for providing a safe and confidential space to conduct interviews.
I am grateful to all of the men who participated in the project. This project was supported by NSF 0751969 and a research grant from the Williams Institute. Thank you to the Center for Population Research in LGBT Health at The Fenway Institute supported by NICHD under award number R21HD051178 and to Tapestry Health Clinics for providing safe and private meeting rooms for conducting interviews. Thank you to Dr. Conall O’Clerigh from The Fenway Institute and Dr. Lynnette Sievert for assistance with this manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Revised 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Bindon JR, Knight A, Dressler WW, Crews DE. Social Context and Psychosocial Influences on Blood Pressure Among American Samoans. Am J Phys Anthropol. 1997;103:7–18. doi: 10.1002/(SICI)1096-8644(199705)103:1<7::AID-AJPA2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:386–396. [PubMed] [Google Scholar]
- Colton Meier SL, Fitzgerald KM, Pardo ST, Babcock J. The effects of hormonal gender affirmation treatment on mental health in female-to-male transsexuals. J Gay Lesb Mental Health. 2011;15(3):281–299. [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL. When the social self is threatened: Shame, Physiology, and Health. J Pers. 2004;72(6):1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Michael IR, Aziz N, Kemeny ME. Social-Evaluative Threat and Proinflammatory cytokine regulation. Psychol Sci. 2009;20(10):1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler WW. Social support, lifestyle incongruity, and arterial blood pressure in a southern black community. Psychosom Med. 1991;53:608–620. doi: 10.1097/00006842-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Dressler WW, Bindon JR. The health consequences of cultural consonance: Cultural dimensions of lifestyle, social support, and arterial blood pressure in an African American community. Am Anthropol. 2000;102:244–260. [Google Scholar]
- Esler M, Schwarz R, Alvarenga M. Mental Stress is a cause of cardiovascular diseases: from skepticism to certainty. Stress Health. 2008;24:175–180. [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Gooren LJG, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: Effects and risks of administration of androgens to females. J Sex Med. 2008;5:765–776. doi: 10.1111/j.1743-6109.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- Gorton R, Buth J, Spade D. Medical Therapy and Health Maintenance for Transgender Men: A Guide For Health Care Providers. Lyon-Martin Women’s Health Services; San Francisco, CA: 2005. [Google Scholar]
- Grant JM, Mottet LA, Tanis J, Harrison J, Herman JL, Keisling M. Injustice at Every Turn: A Report of the National Transgender Discrimination Survey. Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force; 2011. [Google Scholar]
- Hansel A, Hong SZ, Camara RJA, von Kaenel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2001;35(1):115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Huang YL, Mai WY, Hu YZ, Wu YX, Song YB, Qiu RF, Dong YG, Kuang J. Poor sleep quality, stress status, and sympathetic nervous system activation in nondipping hypertension. Blood Press Monit. 2011;16(3):117–123. doi: 10.1097/MBP.0b013e328346a8b4. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Lende DH, Worthman CM. Biocultural Dialogues: Biology and Culture in Psychological Anthropology. Ethos. 2005;33(1):1–19. [Google Scholar]
- Huebner DM, Davis MC. Perceived Antigay Discrimination and Physical Health Outcomes. Health Psychol. 2007;26(5):627–634. doi: 10.1037/0278-6133.26.5.627. [DOI] [PubMed] [Google Scholar]
- Ice G. Measuring emotional and behavioral response. In: Ice GH, James GD, editors. Measuring Stress in Humans: A Practical Guide for the Field. Cambridge, UK: Cambridge University Press; 2007. pp. 60–93. [Google Scholar]
- Ice G, James GD. Conducting a field study of stress: general principles. In: Ice GH, James GD, editors. Measuring Stress in Humans: A Practical Guide for the Field. Cambridge, UK: Cambridge University Press; 2007. pp. 3–24. [Google Scholar]
- IOM (Institute of Medicine) The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: The National Academics Press; 2011. [PubMed] [Google Scholar]
- Ituarte PH, Kamarck TW, Thompson HS, Bacanu S. Psychosocial mediators of racial differences in nighttime blood pressure dipping among normotensive adults. Health Psychol. 1999;18:393–402. doi: 10.1037//0278-6133.18.4.393. [DOI] [PubMed] [Google Scholar]
- James GD, Bovbjerg DH. Age and perceived stress independently influence daily blood pressure levels and variation among women employed in wage jobs. Amer J Human Biology. 2001;13:268–274. doi: 10.1002/1520-6300(200102/03)13:2<268::AID-AJHB1038>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jones SC, Bilous M, Winship S, Finn P, Goodwin J. Validation of the Oscar2 oscillometric 24-hour ambulatory blood pressure monitor according to the International Protocol for the validation of blood pressure measuring devices. Blood Press Monit. 2004;9(4):219–223. doi: 10.1097/00126097-200408000-00007. [DOI] [PubMed] [Google Scholar]
- Kario K, James GD, Marion R, Ahmed M, Pickering PG. The influence of work-and home-related stress on the levels of diurnal variation of ambulatory blood pressure and neurohumoral factors in employed women. Hypertens Res. 2002;25(4):499–506. doi: 10.1291/hypres.25.499. [DOI] [PubMed] [Google Scholar]
- Kielcolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic Stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci, USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS. Towards Better Research on Stress and Coping. Am Psychol. 2000;55(6):665–673. doi: 10.1037//0003-066x.55.6.665. [DOI] [PubMed] [Google Scholar]
- Lev A. The ten tasks of the mental health provider: Recommendation for revision of the World Professional Association for Transgender Health’s Standards of Care. IJT. 2009;11(2):74–99. [Google Scholar]
- Linden W, Klassen K, Phillips M. Can psychological factors account for a lack of nocturnal blood pressure dipping? Ann Behav Med. 2008;36:253–258. doi: 10.1007/s12160-008-9069-0. [DOI] [PubMed] [Google Scholar]
- Lombardi EL, Wilchins RA, Priesing D, Malouf D. Gender violence: transgender experiences with violence and discrimination. J Homosex. 2001;42(1):89–101. doi: 10.1300/j082v42n01_05. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB. Chronic Work Stress and Marital Dissolution Increase Risk of Posttrial Mortality in Men:From the Multiple Risk Factor Intervention Trial. Arch Intern Med. 2002;162:309–315. doi: 10.1001/archinte.162.3.309. [DOI] [PubMed] [Google Scholar]
- Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. J Metabol. 2008;57:961–965. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and Behavioral Predictors of Inflammation in Middle-Aged and Older Adults: The Chicago Health, Aging, and Social Relations Study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. Measuring immune function: markers of cell-mediated immunity and inflammation in dried blood spots. In: Ice Gillian H, James Gary D., editors. Measuring Stress in Humans: A practical guide for the field. Cambridge University Press; Cambridge, UK: 2007. [Google Scholar]
- McDade TW, Lindau ST, Wroblewski K. Predictors of C-Reactive Protein in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2011;66(1):129–136. doi: 10.1093/geronb/gbq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic Significance for Stroke of a Morning Pressor Surge and a Nocturnal Blood Pressure Decline: The Ohasama Study. Hypertens. 2006;47:149–154. doi: 10.1161/01.HYP.0000198541.12640.0f. [DOI] [PubMed] [Google Scholar]
- Meyer W, III, Bockting W, Cohen-Kettenis P, Coleman E, DiCeglie D, Devor H, Gooren L, Hage JJ, Kirk S, Kuiper B, Laub D, Lawrence A, Manard Y, Patton J, Schaefer L, Webb A, Wheeler C. The Standards of Care for Gender Identity Disorders. 6th version. Harry Benjamin International Gender Dysphoria Association; 2005. [Google Scholar]
- Miller GE, Blackwell E. Turning Up the Heat: Inflammation as a Mechanism Linking Chronic Stress, Depression, and Heart Disease. Curr Dir Psychol Sci. 2006;15(6):269–272. [Google Scholar]
- Miller GE, Chen E, Cole SW. Health Psychology: Developing Biologically Plausible Models Linking the Social World and Physical Health. Annu Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Murad MH, Elamin MB, Garcia MZ, Mullan RJ, Murad A, Erwin PJ, Montori VM. Hormonal therapy and sex reassignment: a systematic review and meta-analysis of quality of life and psychosocial outcomes. Clin Endo. 2010;72:214–231. doi: 10.1111/j.1365-2265.2009.03625.x. [DOI] [PubMed] [Google Scholar]
- Newfield E, Hart S, Dibble S, Kohler L. Female-to-male transgender quality of life. Qual of Life Res. 2006;15:1447–1457. doi: 10.1007/s11136-006-0002-3. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori MK, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The Ohasama Study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rafai N. C-Reactive Protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Robles TF, Glaser R, Kiecolt-Glaser JK. Out of Balance: A new look at chronic stress, depression, and immunity. Curr Dir Psychol Sci. 2005;14(2):111–115. [Google Scholar]
- Rosenberg M. Society and the adolescent self image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Sapolsky R. Social status and health among humans and other animals. Annu Rev Anthropol. 2004;33:393–418. [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Turner V. The Forest of Symbols: Aspects of NDembu Ritual. Cornell University Press; Ithaca and London: 1967. [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2020. Companion Document for LGBT Health. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertens. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Plantinga Y, Taddei S, Salvetti A. C-Reactive Protein and Hypertension: Is there a causal relationship? Curr Pharm Des. 2007;13:1693–1698. doi: 10.2174/138161207780831365. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, Ryan MC, Boyle AC. The novel role of C-reactive protein in cardiovascular disease: Risk marker or pathogen. Int J Cardiol. 2006;106:291–297. doi: 10.1016/j.ijcard.2005.01.068. [DOI] [PubMed] [Google Scholar]