Abstract
Fluorination of fluorophores can substantially enhance their photostability and improve spectroscopic properties. To facilitate access to fluorinated fluorophores, bis(2,4,5-trifluorophenyl)methanone was synthesized by treatment of 2,4,5-trifluorobenzaldehyde with a Grignard reagent derived from 1-bromo-2,4,5-trifluorobenzene, followed by oxidation of the resulting benzyl alcohol. This hexafluorobenzophenone was subjected to sequential nucleophilic aromatic substitution reactions, first at one or both of the more reactive 4, 4′ fluorines, and second by cyclization through substitution of the less reactive 2, 2′ fluorines, using a variety of oxygen, nitrogen, and sulfur nucleophiles, including hydroxide, methoxide, amines, and sulfide. This method yields symmetrical and asymmetrical fluorinated benzophenones, xanthones, acridones, and thioxanthones, and provides scalable access to known and novel precursors to fluorinated analogues of fluorescein, rhodamine, and other derivatives. Spectroscopic studies revealed that several of these precursors are highly fluorescent, with tunable absorption and emission spectra, depending on the substituents. This approach should allow access to a wide variety of novel fluorinated fluorophores and related compounds.
INTRODUCTION
Substitution of hydrogen with fluorine is extensively employed in medicinal chemistry to alter the binding of small molecules to biological targets and modulate metabolic reactivity.1,2 Fluorination can also alter the photophysical properties of compounds.3,4 Fluorination of fluorescein (1, Figure 1) at the 2′- and 7′-positions yields Oregon Green (2),5 a bright fluorophore with decreased phenol pKa (4.8 for 2 versus 6.3–6.86 for 1), improving fluorescence in acidic aqueous environments, and enhancing photostability. Although a limited number of fluorinated fluorophores have been reported, modification of other dyes7,8 including Tokyo Green9 (3) to yield Pennsylvania Green (4),10,11 and tetramethylrhodamine (5) to yield fluorinated rhodamines (e.g. 6)12 can confer beneficial photophysical properties. However, the synthesis and isolation of single isomers of fluorinated fluorophores such as 5-carboxy-Oregon Green5 is challenging and costly using existing methods.
Figure 1.
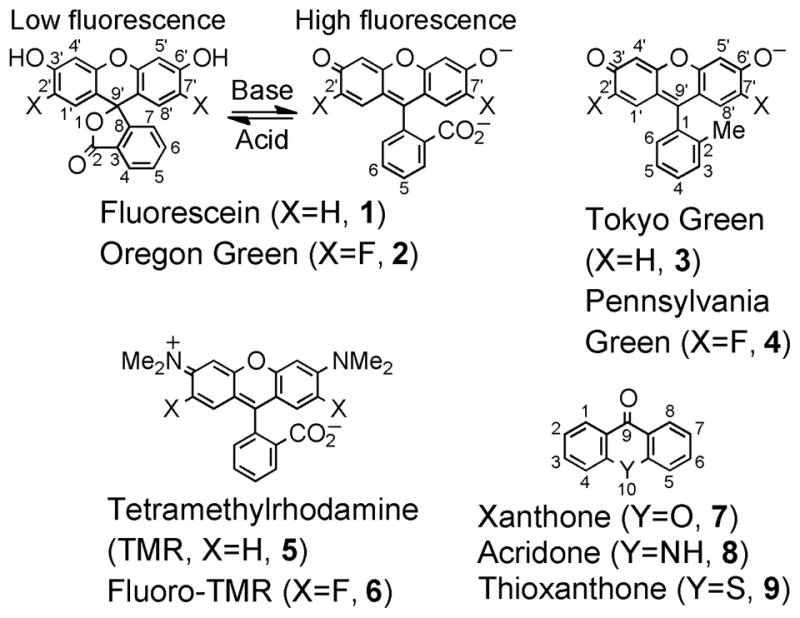
Structures of fluorinated fluorophores and related compounds.
To facilitate access to fluorinated fluorophores, we sought to develop a practical method to synthesize diverse fluorinated xanthone (7), acridone (8), and thioxanthone (9) precursors. Many of these compounds are biologically active,13–25 some are highly fluorescent,26 and the addition of Grignard reagents to protected xanthones has been used to prepare 4-carboxy-Tokyo Green,9 a fluorescent reporter of kinase activity,27 4-carboxy-Penn Green,10 Tokyo Magenta,28 and other rhodamine and fluorescein analogues.29–32 Methods for synthesis of derivatives of 7–9 have advanced over the past decade,13,33–41 but only a few reports27,42–44 describe derivatives with fluorine at the 2 and 7 positions and nitrogen or oxygen at carbons 3 and 6. Because existing syntheses of fluorinated xanthones and similar compounds are lengthy and low yielding, relatively little is known about their cognate photophysical and biological properties.
RESULTS AND DISCUSSION
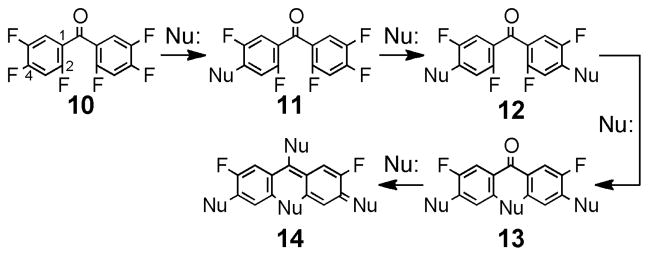
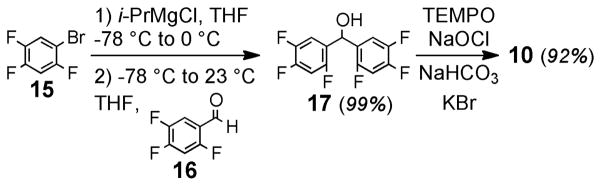
As shown in Scheme 1, we postulated that repeated SNAr reactions of the novel benzophenone 10 might allow access to other fluorinated benzophenones (11–12), as well as xanthones, acridones, and thioxanthones (13), as precursors to more highly conjugated fluorophores (14).45 The high selectivity observed for the sequential addition of multiple nucleophiles to cyanuric chloride46 and pentafluoropyridine47 through a similar mechanism offers precedent for this strategy. To evaluate this hypothesis, we synthesized 10 in a two-pot process involving magnesium-halogen exchange of inexpensive 1-bromo-2,4,5-trifluorobenzene (15) followed by addition to 2,4,5-trifluorobenzaldehyde (16) to generate the alcohol (17) in nearly quantitative yield (Scheme 2). Oxidation with TEMPO free radical and sodium hypochlorite48 produced benzophenone 10 in excellent overall yield. These reactions were scalable, and multiple grams of 10 could be produced in a single run.
Scheme 1.
A general synthesis of fluorinated benzophenones, xanthones, acridones, thioxanthones, and derivatives involving iterative addition of nucleophiles (Nu:) to bis(2,4,5-trifluorophenyl)methanone (10).
Scheme 2.
Synthesis of bis(2,4,5-trifluorophenyl)methanone (10).
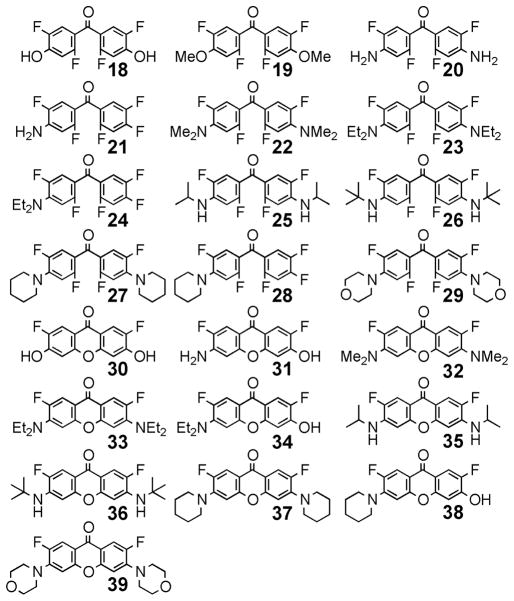
Using the strategy outlined in Scheme 1, novel fluorinated benzophenone derivatives (18–29, shown in Figure 2) were prepared from 10 with a variety of oxygen and nitrogen-derived nucleophiles in good to excellent yields. As summarized in Table 1, heating of 10 in aqueous KOH/DMSO at 80 °C efficiently substituted both fluorines at the 4, 4′-positions with hydroxyl groups to afford 18 (Table 1, entry 1). Sodium methoxide added to 10 at room temperature in nearly quantitative yield to produce 19 (entry 2).
Figure 2.
Structures of fluorinated benzophenones and xanthones derived from 10.
Table 1.
Reagents and conditions for synthesis of benzophenones 18–29 and xanthones 30–39.
| Entry | Nucleophile | Solvent | Temp (°C) | Time (h) | Yield (%) | Product (reactant) |
|---|---|---|---|---|---|---|
| 1 | KOH | DMSO/H2O | 80 | 12 | 92 | 18 (10) |
| 2 | NaOMe | MeOH | 26 | 12 | 99 | 19 (10) |
| 3 | NH4OH | DMSO/H2O | 110c | 12 | 93 | 20 (10) |
| 4 | NH4OH | DMSO/H2O | 35 | 12 | 75 | 21 (10) |
| 5 | DMF/KOHa | H2O | 60 | 12 | 78 | 22 (10) |
| 6 | HNEt2 | —b | 90c | 12 | 85 | 23 (10) |
| 7 | HNEt2 | —b | 26 | 12 | 70 | 24 (10) |
| 8 | H2NiPr | —b | 60c | 3 | 93 | 25 (10) |
| 9 | H2NtBu | —b | 46 | 12 | 82 | 26 (10) |
| 10 | Piperidine | THF | 26 | 12 | 91 | 27 (10) |
| 11 | Piperidine | THF | 26 | 3 | 76 | 28 (10) |
| 12 | Morpholine | THF | 26 | 12 | 81 | 29 (10) |
| 13 | KOH | H2O | 200c | 3 | 99 | 30 (10) |
| 14 | KOH | H2O | 110 | 48 | 99 | 30 (10) |
| 15 | KOH | DMSO/H2O | 150c | 12 | 80 | 31 (21) |
| 16 | KOH | DMSO/H2O | 150c | 12 | 85 | 32 (22) |
| 17 | KOH | DMSO/H2O | 170c | 12 | 85 | 33 (23) |
| 18 | KOH | DMSO/H2O | 150c | 12 | 95 | 34 (24) |
| 19 | KOH | DMSO/H2O | 150c | 16 | 92 | 35 (25) |
| 20 | KOH | DMSO/H2O | 150c | 12 | 90 | 36 (26) |
| 21 | KOH | DMSO/H2O | 170c | 12 | 92 | 37 (27) |
| 22 | KOH | DMSO/H2O | 150c | 12 | 95 | 38 (28) |
| 23 | KOH | DMSO/H2O | 170c | 12 | 83 | 39 (29) |
Dimethylamine derives from decomposition of DMF.
Reaction was performed neat.
Reaction performed in a sealed tube.
A wide variety of primary and secondary amines generated similar SNAr adducts in good to excellent yields (Table 1, entries 3 – 12). Piperidine and morpholine exhibited particularly high reactivity towards 10, and dilution was necessary to prevent formation of tri- and tetrasubstituted products. As listed in Table 1, either monosubstituted or disubstituted fluorinated benzophenones derived from ammonia, diethylamine and piperidine (Table 1, entries 3, 4, 6, 7–10, 11) were obtained in >70% yield by adjusting reaction concentrations and temperatures, offering additional opportunities for structural diversification.
Symmetrical and asymmetrical xanthones (30–39) were readily accessed by addition of hydroxide to fluorinated benzophenones using the conditions listed in Table 1. Although previous routes10,27 to 30, a valuable precursor for preparation of fluorophores such as Pennsylvania Green, required five to eight steps and achieved only modest overall yields, heating benzophenone 10 with aqueous NaOH either in a sealed tube at 200 °C for 3 h or at reflux for 48 h provided 30 in nearly quantitative yield (Table 1, entries 13 and 14). This latter method was scalable, and multiple grams of 30 could be prepared. Aminobenzophenones (20–29) were cleanly converted to symmetric and asymetric xanthones via hydroxide-mediated SNAr reactions with DMSO as a cosolvent (Table 1, entries 15–23).
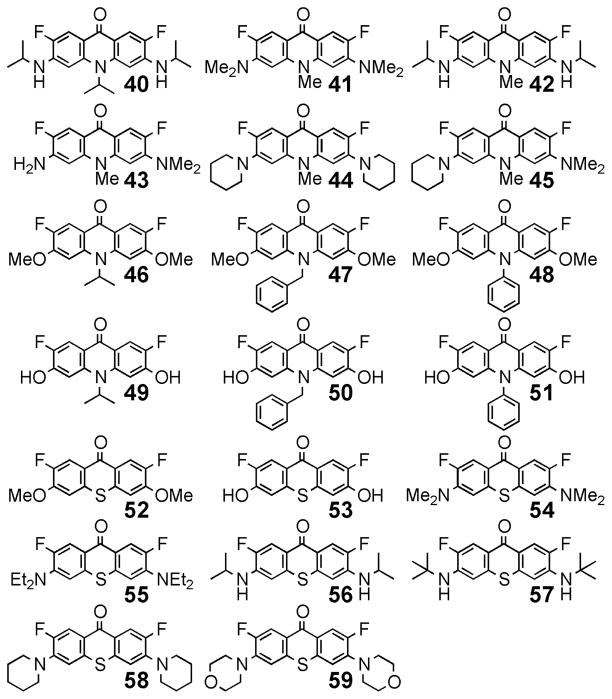
Cyclization with amine nucleophiles converted fluorinated benzophenones to fluorinated acridones (40–48, Figure 3). The conditions for synthesis of 40–48 are summarized in Table 2. Heating of 10 in DMF containing KOH provided N, N-dimethylacridone 41 in 93% yield (Table 2, entry 2). Decomposition of DMF was responsible for generation of the dimethylamine nucleophile. Using the same reaction conditions, acridones 42–45 were synthesized from benzophenones 25, 21, 27, and 28 in > 90% yield. Dimethoxyacridones 46, 47, and 48, produced by heating 19 with the corresponding amines, required subsequent treatment with NaH, or heating in higher-boiling dimethylacetamide, for completion. Attempts to demethylate 46–48 to generate 49–51 with NaSEt or Na2S cleaved only one of the two methyl groups at 130 °C. Further heating (170 °C) also cleaved the N-linked alkyl group of 46 and 47. However, reflux with BBr3 in 1,2-dichloroethane provided 49–51 in high yield (Table 2).
Figure 3.
Structures of fluorinated acridone and thioxanthone products.
Table 2.
Reagents and conditions for synthesis of acridones 40–51 and thioxanthones 52–59.
| Entry | Nucleophile | Solvent | Temp (°C) | Time (h) | Yield (%) | Product (reactant) |
|---|---|---|---|---|---|---|
| 1 | H2NiPr | —b | 100c | 12 | 45 | 40 (10) |
| 2 | DMF/KOHa | DMF/H2O | 150c | 6 | 93 | 41 (10) |
| 3 | DMF/KOHa | DMF/H2O | 80 | 12 | 95 | 42 (25) |
| 4 | DMF/KOHa | DMF/H2O | 150c | 12 | 93 | 43 (21) |
| 5 | DMF/KOHa | DMF/H2O | 150c | 4 | 96 | 44 (27) |
| 6 | DMF/KOHa | DMF/H2O | 150c | 12 | 91 | 45 (28) |
| 7 | H2NiPr | —b | 100c | 12 | 83 | 46 (19) |
| 8 | H2NBn | —b | 100 | 12 | 86 | 47 (19) |
| 9 | H2NPh | —b | 130 | 12 | 72 | 48 (19) |
| 10 | —e | Cl(CH2)2Cl | reflux | 12 | 83 | 49 (46) |
| 11 | —e | Cl(CH2)2Cl | reflux | 12 | 91 | 50 (47) |
| 12 | —e | Cl(CH2)2Cl | reflux | 12 | 79 | 51 (48) |
| 13 | Na2S | DMA | 26 | 12 | 92 | 52 (19) |
| 14 | Na2S | DMA | 90 | 12 | 74 | 53 (19) |
| 15 | Na2S | DMA | 70 | 12 | 91 | 54 (22) |
| 16 | Na2S | DMA | 70 | 12 | 90 | 55 (23) |
| 17 | Na2S | DMA | 70 | 12 | 85 | 56 (25) |
| 18 | Na2S | DMA | 70 | 12 | 89 | 57 (26) |
| 19 | Na2S | DMA | 70 | 12 | 85 | 58 (27) |
| 20 | Na2S | DMA | 70 | 12 | 94 | 59 (29) |
Dimethylamine derives from decomposition of DMF.
Reaction was performed neat.
Reaction performed in a sealed tube.
BBr3 was employed for demethylation.
To the best of our knowledge, 2,7-difluorinated thioxanthones have not been previously reported. To access these compounds, Na2S was employed to synthesize 52–59 (Figure 3) as summarized in Table 2. By controlling the temperature and concentration of Na2S, dimethoxybenzophenone 19 was converted into either 52 or 53 (Table 2, entries 13 and 14). Additionally, diaminobenzophenones 22, 23, 25, 26, 27, and 29 reacted with Na2S at 70 °C in greater than 85% yield (Table 2, entries 15–20).
Absorbance (18–59) and fluorescence emission spectra (30–59) were acquired on selected compounds. Values for λmax, molar extinction coefficient (Σ), determined for compounds with quantum yield (Φ) > 0.2, and Φ relative to diphenylanthracene (Φ = 0.95 in EtOH) and anthracene (Φ = 0.27 in EtOH) are listed in Table 3. Absorbance and emission spectra of 30–59 are provided in the Supporting Information. Among these compounds, substituents modulated absorbance by up to 77 nm and emission by up to 71 nm, with Stokes shifts of >100 nm in some cases. The ability to readily access multigram quantities of these precursors via iterative SNAr should have broad utility for the synthesis of diverse fluorinated fluorophores and related molecular probes.
Table 3.
Spectral properties of compounds in ethanol (18–51 and 53–59) or DMSO (52). Values not determined are indicated by –.
| Compd | Abs. λmax (nm) | ε (M−1 cm−1) | Fluor. λmax(nm) | Φ |
|---|---|---|---|---|
| 18 | 306 | – | – | – |
| 19 | 301 | – | – | – |
| 20 | 340 | – | – | – |
| 21 | 335 | – | – | – |
| 22 | 358 | – | – | – |
| 23 | 369 | – | – | – |
| 24 | 361 | – | – | – |
| 25 | 357 | – | – | – |
| 26 | 348 | – | – | – |
| 27 | 354 | – | – | – |
| 28 | 350 | – | – | – |
| 29 | 342 | – | – | – |
| 30 | 369 | 13300 @ 369 nm | 439 | 0.26 |
| 31 | 366 | 39200 @ 366 nm | 425 | 0.44 |
| 32 | 378 | – | 446 | < 0.1 |
| 33 | 389 | – | 452 | < 0.1 |
| 34 | 379 | – | 438 | < 0.1 |
| 35 | 370 | 36200 @ 370 nm | 422 | 0.41 |
| 36 | 368 | 48600 @ 368 nm | 418 | 0.31 |
| 37 | 369 | – | 457 | < 0.1 |
| 38 | 373 | 15300 @ 373 nm | 445 | 0.23 |
| 39 | 344 | – | 448 | < 0.1 |
| 40 | 376 | 36800 @ 376 nm | 423 | 0.32 |
| 41 | 384 | – | 441 | < 0.1 |
| 42 | 377 | 56700 @ 377 nm | 421 | 0.31 |
| 43 | 369 | – | 432 | < 0.1 |
| 44 | 368 | – | 455 | < 0.1 |
| 45 | 364 | – | 443 | < 0.1 |
| 46 | 383 | – | 425 | < 0.1 |
| 47 | 381 | – | 416 | < 0.1 |
| 48 | 378 | – | 424 | < 0.1 |
| 49 | 379 | – | 438 | < 0.1 |
| 50 | 381 | – | 431 | < 0.1 |
| 51 | 377 | – | 454 | < 0.1 |
| 52 | 316 | – | 416 | < 0.1 |
| 53 | 377 | – | 465 | < 0.1 |
| 54 | 382 | – | 477 | < 0.1 |
| 55 | 393 | – | 475 | < 0.1 |
| 56 | 377 | – | 446 | < 0.1 |
| 57 | 373 | – | 432 | < 0.1 |
| 58 | 377 | – | 487 | < 0.1 |
| 59 | 361 | – | 481 | < 0.1 |
EXPERIMENTAL SECTION
Optical spectroscopy
Quantum yields (Φ) of the highly emissive compounds 30, 31, 35, 36, 38, 40, 42 in ethanol were quantified by the method of Williams.49 Quantum yields for other compounds were determined to be < 0.1 by comparison. Diphenylanthracene (Φ = 0.95 in ethanol50) and anthracene (Φ = 0.27 in ethanol51) were used as standards, and data from these measurements is provided in the Supporting Information. Molar extinction coefficients (ε) of 30, 31, 35, 36, 38, 40, 42 in ethanol were determined by linear least squares fitting of Beer’s Law plots of absorbance versus concentration, and data from these measurements is provided in the Supporting Information.
Synthesis
In addition to specific methods described below, general procedures A–F were used to access structurally related compounds.
General Procedure A
A mixture of bis(2,4,5-trifluorophenyl)methanone (10, 2.00 g, 6.9 mmol) and the corresponding amine (73.3 mmol) was heated in a sealed tube. The reaction mixture was cooled and volatiles were removed in vacuo. If impurities were evident, compounds were purified by column chromatography (5% EtOAc in hexanes) to provide viscous oils that typically crystallized when washed with hexanes.
General Procedure B
A mixture of bis(2,4,5-trifluorophenyl)methanone (10, 1.00 g, 3.45 mmol), the corresponding amine (10.1 mmol), and THF (10.0 mL) were stirred for 12 h at 26 °C. The reaction mixture was concentrated in vacuo. If impurities were evident, compounds were purified by column chromatography (5% EtOAc in hexanes) to give viscous oils that typically crystallized upon standing.
General Procedure C
A mixture of the corresponding diaminoxanthone (21 – 29, 1.0 mmol), aqueous KOH (10 M, 1.5 mL, 15.0 mmol), and DMSO (1.5 mL) were heated in a sealed tube. After the time indicated, the reaction mixtures were cooled and transferred to ice water (150 mL). The resulting precipitate was collected by vacuum filtration and washed with water (3 × 100 mL). If impurities were evident, products were purified by washing with an organic solvent or by column chromatography.
General Procedure D
The corresponding benzophenone (10, 21, 25, 27, or 28, 1.0 mmol) was dissolved in a solution of DMF (2.0 mL, 25.9 mmol) and aqueous KOH (10 M, 2.0 mL, 20.0 mmol). This mixture was heated in a sealed tube and at 150 °C for the time indicated, cooled, and transferred to ice water (100 mL). The resulting precipitate was filtered, washed with water (3 × 50 mL) and washed with either diethyl ether or acetone to afford the product.
General Procedure E
The corresponding dimethoxyacridone (46 – 48, 0.10 mmol) was dissolved in 1,2-dichloroethane (4.0 mL) and treated with BBr3 in CH2Cl2 (0.57 mL of a 0.7 M solution, 0.40 mmol). The reaction mixture was heated to reflux for 12 h, cooled to room temperature, and quenched with methanol (1.00 mL). The product was concentrated and purified by column chromatography (0% to 3 % MeOH in CH2Cl2).
General Procedure F
A solution of diaminoxanthone (19, 22, 23, 25 – 27, 29, 1.0 mmol) in DMA (5.0 mL), was vigorously purged with Ar for 30 min followed by treatment with finely ground Na2S (0.375 g, 5.0 mmol). The resulting yellow-orange slurry was heated to 70 °C for 12 h with stirring. The hot reaction mixture was poured into ice-cold water (150 mL), and the precipitate was collected by vacuum filtration. The crude yellow solid was air dried and further purified by column chromatography or by washing with an organic solvent to give the corresponding thioxanthone.
Bis(2,4,5-trifluorophenyl)methanone (10)
To a solution of 17 (400 mg, 1.37 mmol) in CH2Cl2 (12 mL) was added KBr (33.2 mg, 0.28 mmol), TEMPO (11.0 mg, 0.07 mmol, 5 mol%), and saturated aqueous NaHCO3. The biphasic mixture was vigorously stirred and aqueous NaOCl (6.0 mL, 0.7 M) was added. The resulting bright orange mixture was stirred for 3 h, and the orange color dissipated. The colorless biphasic layers were separated, the aqueous layer was extracted with CH2Cl2 (2 × 12 mL), and the combined organic fractions were dried over anhydrous Na2SO4 and concentrated to give a crude yellow solid. The solid was purified elution through a plug of silica gel (10% EtOAc in hexanes). The filtrate was concentrated to yield 10 as a colorless solid (370 mg, 92%). mp 80–81 °C; 1H NMR (CDCl3, 400 MHz) δ 7.57 (qd, J = 8.8, 6.4 Hz, 2H), 6.95 (qd, J = 9.2, 6.4 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 184.7 (s), 156.8 (dd, J = 254.7, 9.8 Hz), 153.3 (ddd, J = 260.0, 14.5, 12.2 Hz), 147.1 (ddd, J = 248.1, 12.7, 3.1 Hz), 123.2 (d, J = 14.9 Hz), 118.8 (d, J = 20.1 Hz), 111.6 – 98.1 (m); IR (thin film) 3073, 1667, 1623, 1509, 1437, 1420, 1333, 1293, 1208, 1138, 889 cm−1; HRMS (ESI) m/z 291.0263 (M+H+, C13H5F6O requires 291.0245).
Bis(2,4,5-trifluoro-phenyl)methanol (17)
To a solution of 1-bromo-2,4,5-trifluorobenzene (2.34 mL, 20.0 mmol) in THF (30 mL) at −78 °C was added i-PrMgCl (1.3 M in Et2O, 16.1 mL, 21.0 mmol), dropwise. The resulting pale yellow solution was stirred at −78 °C for 10 min, was warmed to 4 °C, and was maintained at 4 °C for 1 h. This solution was cooled again to −78 °C and treated with 2,4,5-trifluorobenzaldehyde (2.55 mL, 22.0 mmol). This mixture was allowed to slowly warm to ambient temperature (23 °C). After stirring at room temperature for 3 h, the reaction mixture was slowly quenched with saturated aqueous NH4Cl solution (20 mL). The resulting phases were separated, the aqueous fraction was extracted with Et2O (2 × 30 mL), the combined organic phases were dried over anhydrous Na2SO4, and the solution was concentrated to give a crude oil that was purified by flash chromatography (5% EtOAc in hexanes) to provide pure 17 (5.74 g, 99%). mp 81 – 83 °C 1H NMR (CDCl3, 400 MHz) δ 7.27 (qd, J = 10.0, 6.4 Hz, 2H), 6.95 (qd, J = 9.2, 6.4 Hz, 2H), 6.29 (d, J = 3.2 Hz, 1H), 2.46 (d, J = 3.2Hz, 1H); 13C NMR (126 MHz, CDCl3) δ 154.6 (ddd, J = 246.9, 9.3, 2.8 Hz), 151.5 – 148.5 (m), 148.4 – 145.2 (m), 125.4 (d, J = 15.7 Hz), 116.5 – 114.1 (m), 105.8 (dd, J = 27.4, 21.1 Hz), 62.8 (s); IR (thin film) 3364, 1631, 1513, 1430, 1337, 1200, 1147, 1096 cm−1; HRMS (ESI) m/z 291.0263 (M-H, C13H5F6O requires 291.0245).
Bis(2,5-difluoro-4-hydroxyphenyl)methanone (18)
A mixture of 10 (3.0 g, 10.3 mmol), aqueous KOH (10 M, 10 mL, 100 mmol), and DMSO (10 mL) was heated to 80 °C for 12 h. This solution was transferred to a mixture of concentrated aqueous HCl (15 mL) and ice (200 g) to generate a fine precipitate that was collected by vacuum filtration. This colorless precipitate was washed with cold water (3 × 100 mL) and dried overnight (16 h) under high vacuum to provide pure 18 (2.71 g, 92%). mp 170–171 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.40 (brs, 2H), 7.45 (dd, J = 10.8, 7.0 Hz, 2H), 6.83 (dd, J = 11.6, 7.0 Hz, 2H); 13C NMR (126 MHz, DMSO-d6) δ 184.7 (s), 157.1 (d, J = 249.8 Hz), 150.6 (t, J = 13.5 Hz), 147.4 (d, J = 239.6 Hz), 117.4 (dd, J = 15.1, 4.9 Hz), 117.2 (dd, J = 21.5, 3.9 Hz), 104.9 (dd, J = 27.3, 3.0 Hz); IR (film) 3162, 3071, 2964, 1621, 1514, 1303, 1199, 1143, 789 cm−1; HRMS (ESI) m/z 285.0161 (M-H, C13H5F4O3 requires 285.0175).
Bis(2,5-difluoro-4-methoxyphenyl)methanone (19)
A mixture of 10 (2.00 g, 6.8 mmol) and methanol (28 mL) was treated with NaOMe in methanol (5.4 M, 5.50 mL, 29.6 mmol), dropwise. This mixture was stirred for 12 h, and water (300 mL) was added. A colorless precipitate was collected by vacuum filtration, washed with water (2 × 150 mL), and dried under high vacuum to provide of pure 19 (2.11 g, 99%). mp 158–160 °C; 1H NMR (400 MHz, 10% CD3OD in CDCl3) δ 7.47 (dd, J = 10.8, 7.0 Hz, 2H), 6.70 (dd, J = 11.2, 7.0 Hz, 2H); 13C NMR (126 MHz, 10% CD3OD in CDCl3) δ 185.5 (s), 157.9 (d, J = 252.5 Hz), 152.3 (dd, J = 12.8, 10.6 Hz), 148.4 (dd, J = 244.5, 2.2 Hz), 120.4 – 118.0 (m), 117.1 (dd, J = 21.4, 3.7 Hz), 103.6 – 99.7 (m), 56.6 (s); IR (film) 3066, 2951, 1663, 1621, 1513, 1346, 1145, 794 cm−1; HRMS (ESI) m/z 337.0474 (M+Na+, C15H10F4O3Na requires 337.0464).
Bis(4-amino-2,5-difluorophenyl)methanone (20)
Bis(2,4,5-trifluorophenyl)methanone (10, 3.00 g, 10.3 mmol) was dissolved in DMSO (10.0 mL) and treated with concentrated aqueous NH4OH (10.0 mL, 145 mmol). This mixture was stirred at 110 °C for 12 h (sealed tube), followed by addition to ice water (400 mL). A yellow precipitate was collected by vacuum filtration, washed with water (3 × 150 mL), and dried under high vacuum for 12 h to provide pure 20 (2.73 g 93%). mp 180–182 °C; 1H NMR (400 MHz, 10% CD3OD in CDCl3) δ 7.22 (dd, J = 11.2, 7.0 Hz, 2H), 6.37 (dd, J = 11.2, 7.0 Hz, 2H); 13C NMR (10% CD3OD in CDCl3) δ 187.7 (s), 160.1 (d, J = 250.1 Hz), 148.2 (d, J = 236.3 Hz), 142.6 (dd, J = 15.2, 12.7 Hz), 118.0 (dd, J = 21.0, 3.7 Hz), 117.2 (d, J = 13.7 Hz), 104.9 – 101.4 (m); IR (film) 3343, 3087, 1627, 1599, 1523, 1459, 1362, 1314, 1371, 750 cm−1; HRMS (ESI) m/z 307.0450 (M+Na+, C13H8F4N2ONa requires 307.0470).
(4-amino-2,5-difluorophenyl)-(2,4,5-trifluorophenyl)methanone (21)
Bis(2,4,5-trifluorophenyl)methanone (10, 1.00 g, 3.43 mmol) in DMSO (2.50 mL) was treated with concentrated aqueous NH4OH (2.50 mL, 36.3 mmol). This mixture was stirred at 35 °C for 12 h, diluted with ice-cold water (100 mL), and extracted with EtOAc (3 × 50 mL). The combined organic fractions were dried over anhydrous Na2SO4 and concentrated in vacuo to give a crude yellow oil that was purified by column chromatography (10% to 20% EtOAc in hexanes) to provide pure 21 (0.738 g, 75%). mp 134–136 °C; 1H NMR (400 MHz, CDCl3) δ 7.39–7.60 (m, 2H), 7.00 (ddd, J = 15.6, 6.0, 3.6 Hz, 1H), 6.43 (dd, J = 11.6, 6.8 Hz, 2H), 4.45 (s, 2H); 13C NMR (126 MHz, CDCl3) δ 184.9 (s), 159.4 (d, J = 252.3 Hz), 155.9 (ddt, J = 252.5, 9.9, 2.5 Hz), 152.2 (ddd, J = 257.0, 14.5, 12.2 Hz), 147.0 (d, J = 239.1 Hz), 148.1 – 145.5 (m), 141.7 (dd, J = 15.2, 12.9 Hz), 127.4 – 123.0 (m), 119.7 – 117.0 (m), 116.8 (dd, J = 21.5, 4.0 Hz), 115.1 (dd, J = 13.4, 5.5 Hz), 106.1 (dd, J = 28.3, 21.1 Hz), 102.2 (dd, J = 28.8, 3.5 Hz); IR (film) 3489, 3343, 3218, 1661, 1593, 1511, 1456, 1425, 1331, 1249, 1144, 900, 787 cm−1; HRMS (ESI) m/z 286.0276 (M-H, C13H5F5NO requires 286.0291).
Bis(4-(dimethylamino)-2,5-difluorophenyl)methanone (22)
Bis(2,4,5-trifluorophenyl)methanone (10, 291 mg, 1.00 mmol) was dissolved in DMF (2.00 mL, 25.9 mmol), treated with aqueous KOH (10 M, 2.00 mL, 20.0 mmol), and stirred at 60 °C for 12 h. This mixture was cooled to room temperature (23 °C), transferred to ice water (50 mL), extracted with EtOAc (3 × 20 mL), and the combined organic fractions were washed with water (2 × 30 mL). The organic layer was dried over anhydrous Na2SO4, concentrated in vacuo, and purified by flash chromatography (5% to 10% EtOAc in hexanes) to provide pure 22 (265 mg, 78%) as a yellow solid. mp 120–121 °C; 1H NMR (400 MHz, CDCl3) δ 7.34 (dd, J = 14.1, 7.0 Hz, 2H), 6.39 (dd, J = 12.8, 7.0 Hz, 2H), 3.01 (s, 12H); 13C NMR (126 MHz, CDCl3) δ 185.2 (s), 158.2 (d, J = 249.8 Hz), 148.9 (d, J = 240.7 Hz), 144.7 (s), 120.4 – 116.9 (m), 117.1 – 115.6 (m), 106.9 – 99.3 (m), 42.1 (d, J = 6.1 Hz); IR (film) 2954, 2807, 1619, 1526, 1446, 1359, 1121, 782 cm−1; HRMS (ESI) m/z 341.1259 (M+H+, C17H17F4N2O requires 341.1277).
Bis(4-(diethylamino)-2,5-difluorophenyl)methanone (23)
Using General Procedure A at 90 °C for 12 h, bis(2,4,5-trifluorophenyl)methanone 10 (1.00 g, 3.45 mmol), and diethylamine (7.0 mL, 71.0 mmol) afforded 23 (1.07 g, 85%). mp 60–62 °C; 1H NMR (400 MHz, CDCl3) δ 7.34 (dd, J = 12.4, 5.6 Hz, 2H), 6.54 (dd, J = 12.8, 5.6 Hz, 2H), 4.50 (d, J = 3.6 Hz, 2H), 1.46 (s, 18H); 13C NMR (126 MHz, CDCl3) δ 184.9 (s), 158.4 (d, J = 249.6 Hz), 148.3 (d, J = 239.2 Hz), 142.5 (s), 118.22 (dd, J = 25.9, 5.2 Hz), 116.4 – 115.5 (m), 102.7 (d, J = 28.7 Hz), 46.0 (d, J = 5.7 Hz), 13.6 – 12.5 (m); IR (film) 2976, 2934, 1618, 1523, 1443, 1394, 1356, 1279, 1234, 1192, 1077, 779 cm−1; HRMS (ESI) m/z 397.1877 (M+H+, C21H25F4N2O requires 397.1903).
(4-(diethylamino)-2,5-difluorophenyl)-(2,4,5-trifluorophenyl)methanone (24)
Using General Procedure A at 26 °C for 12 h, bis(2,4,5-trifluorophenyl)methanone (10, 2.60 g, 6.90 mmol), and diethylamine (1.02 mL, 10.3 mmol), afforded 24 (1.65 g, 70%) after column chromatography (5% to 10% EtOAc in hexanes). mp 74–78 °C; 1H NMR (400 MHz, CDCl3) δ 7.35–7.50 (m, 2H), 6.96 (ddd, J = 15.7, 6.2, 3.4 Hz, 1H), 6.32 (dd, J = 14.1, 7.2 Hz, 2H), 3.41 (q, J = 6.2 Hz, 2H), 1.23 (t, J = 6.2 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 184.3 (s), 162.6 – 156.0 (m), 155.6 (ddt, J = 252.5, 9.9, 2.5 Hz), 149.4 – 146.1 (m), 147.9 – 145.2 (m),146.6 (d, J = 239.1 Hz), 141.4 (dd, J = 15.2, 12.9 Hz), 127.2 – 122.7 (m), 118.4 (d, J = 4.5 Hz), 116.4 (dd, J = 21.5, 4.0 Hz), 114.9 (dd, J = 13.4, 5.5 Hz), 105.9 (dd, J = 28.4, 21.1 Hz), 103.0 – 99.5 (m), 46.3 (d, J = 6.3 Hz), 13.1 (d, J = 1.8 Hz); IR (film) 3067, 2980, 2938, 1650, 1607, 1528, 1511, 1424, 1392, 1329, 1280, 1131, 1076, 887, 791 cm−1; HRMS (ESI) m/z 366.0889 (M+Na+, C17H14F5NONa requires 366.0893).
Bis(2,5-difluoro-4-(isopropylamino)phenyl)methanone (25)
Using General Procedure A, 10 (2.0 g), isopropylamine (6.00 mL, 73.3 mmol), and heating in a sealed tube at 60 °C for 3 h, provided pure 25 (2.32 g, 92%). mp 91– 92 °C; 1H NMR (400 MHz, CDCl3) δ 7.35 (dd, J = 12.0, 5.2 Hz, 2H), 6.30 (dd, J = 12.4, 5.6 Hz, 2H), 4.32 (d, J = 5.2 Hz, 2H), 3.66 (hept, J = 6.8 Hz, 2H), 1.30 (s, J = 6.4 Hz, 12H); 13C NMR (126 MHz, CDCl3) δ 185.4 (s), 159.2 (d, J = 249.5 Hz), 146.7 (d, J = 235.7 Hz), 140.9 (dd, J = 14.0, 12.2 Hz), 115.7 (dd, J = 21.7, 4.8 Hz), 114.8 – 113.2 (m), 98.4 – 96.9 (m), 44.2 (s), 22.6 (s); IR (film) 3433, 3366, 2972, 2934, 1625, 1607, 1534, 1456, 1368, 1280, 1156, 907, 727 cm−1; HRMS (ESI) m/z 391.1402 (M+Na+, C19H20F4N2ONa requires 391.1409).
Bis(4-(tert-butylamino)-2,5-difluorophenyl)methanone (26)
Using General Procedure A, 10 (500 mg, 1.72 mmol), t-butylamine (7.00 mL, 71.0 mmol), and heating at 46 °C for 12 h, afforded 26 (560 mg, 82%). mp 135–137 °C; 1H NMR (400 MHz, CDCl3) δ 7.34 (dd, J = 12.4, 5.6 Hz, 2H), 6.54 (dd, J = 12.8, 5.6 Hz, 2H), 4.50 (d, J = 3.6 Hz, 2H), 1.46 (s, 18H); 13C NMR (126 MHz, CDCl3) δ 184.3 (s), 157.5 (d, J = 249.0 Hz), 146.2 (d, J = 235.6 Hz), 139.2 (s), 114.2 (d, J = 4.5 Hz), 113.4 – 112.4 (m), 99.5 – 97.6 (m), 50.3 (s), 28.3 (s); IR (film) 3436, 2979, 1626, 1534, 1462, 1371, 1288, 1211, 796 cm−1; HRMS (ESI) m/z 397.1877 (M+H+, C21H25F4N2O requires 397.1903).
Bis(2,5-difluoro-4-(piperidin-1-yl)phenyl)methanone (27)
Using General Procedure B, 10 (1.00 g, 3.45 mmol), piperidine (1.0 mL, 10.1 mmol), and THF (6.00 mL), and a reaction time of 12 h, afforded 27 (1.28 g, 91%). mp 141–143 °C; 1H NMR (400 MHz, CDCl3) δ 7.33 (dd, J = 13.3, 6.4 Hz, 2H), 6.53 (dd, J = 12.4, 5.5 Hz, 2H), 3.17 (t, J = 5.2 Hz, 8H), 1.72 (p, J = 5.7 Hz, 8H), 1.61 (p, J = 5.4 Hz, 4H); 13C NMR (126 MHz, CDCl3) δ 185.6 (s), 158.0 (d, J = 251.1 Hz), 150.4 (dd, J = 242.6, 1.6 Hz), 145.7 (s), 119.5 – 117.9 (m), 118.3 – 116.5 (m), 105.1 (s), 51.0 (d, J = 5.0 Hz), 25.8 (s), 24.1 (s); IR (film) 2937, 2854, 2821, 1614, 1508, 1437, 1385, 1255, 1164, 1123, 782 cm−1; HRMS (ESI) m/z 421.1877 (M+H+, C23H25F4N2O requires 421.1903).
(2,5-difluoro-4-(piperidin-1-yl)phenyl)(2,4,5-trifluorophenyl)methanone (28)
Using General Procedure B, 10 (640 mg, 2.20 mmol), piperidine (0.24 mL, 2.40 mmol), THF (10 mL), and a reaction time of 3 h, afforded 28 (600 mg, 76%). mp 71–73 °C; 1H NMR (400 MHz, CDCl3) δ 7.35–7.52 (m, 2H), 6.96 (ddd, J = 15.7, 6.1, 3.5 Hz, 1H), 6.50 (dd, J = 13.1, 7.0 Hz, 1H), 3.24 (t, J = 5.2 Hz, 4H), 1.71 (p, J = 5.4 Hz, 8H), 1.65 (p, J = 5.2 Hz, 4H); 13C NMR (125 MHz, CDCl3) δ 184.8 (s), 159.0 (d, J = 315.0 Hz), 154.2 (dd, J = 163.8, 12.5 Hz), 150.3 (dd, J = 312.5, 12.3 Hz), 149.5 (d, J = 225.3 Hz), 146.9 (dd, J = 325.3, 9.8 Hz), 146.5 – 146.8 (m), 122.2 – 122.7 (m), 118.3 (d, J = 23.7 Hz), 117.4 (dd, J = 31.6, 5.1 Hz), 116.0 – 116.5 (m), 106.1 (dd, J = 35.5, 26.4 Hz), 104.9 (dd, J = 35.3, 5.0 Hz), 50.8 (s), 25.7 (s), 24.1 (s); IR (film) 3070, 2940, 2856, 1665, 1614, 1509, 1438, 1256, 1126, 878, 758 cm−1; HRMS (ESI) m/z 356.1067 (M+H+, C18H15F5NO requires 356.1074).
Bis(2,5-difluoro-4-morpholinophenyl)methanone (29)
Using General Procedure B, 10 (1.00 g, 3.45 mmol), morpholine (0.75 mL, 8.62 mmol), THF (8 mL), and a reaction time of 12 h, afforded 29 (1.18 g, 81%). mp 189–191 °C; 1H NMR (400 MHz, CDCl3) δ 7.37 (dd, J = 6.7, 5.6 Hz, 2H), 6.54 (dd, J = 12.0, 5.2 Hz, 2H), 3.86 (t, J = 4.6 Hz, 8H), 3.21 (t, J = 4.6 Hz, 8H); 13C NMR (125 MHz, CDCl3) δ 185.3 (s), 158.0 (d, J = 314.7 Hz), 150.5 (d, J = 305.0 Hz), 144.7 (d, J = 12.1 Hz), 119.2 – 119.8 (m), 117.7 (d, J = 30.6 Hz), 105.1 (d, J = 34.4 Hz), 66.6 (s), 49.9 (s); IR (film) 2960, 2916, 2861, 1620, 1509, 1267, 1170, 1115, 782 cm−1; HRMS (ESI) m/z 447.1300 (M+Na+, C21H20F4N2O3Na requires 447.1308).
2,7-Difluoro-3,6-dihydroxy-xanthen-9-one (30)
A mixture of 10 (4.46 g, 15.4 mmol) and aqueous KOH (10 M, 30 mL, 0.300 mol) was heated to reflux for 48 h. During this time, 10 slowly dissolved to give a bright yellow-orange solution. This hot solution was poured onto acidic ice (20 mL of 12 M HCl and 200 g of ice) and was allowed to stand for 3 h. The resulting colorless slurry was filtered via vacuum and the filtrate was washed with cool water (3 × 100 mL), ethanol (2 × 50 mL), and dried under high vacuum to yield pure colorless difluoroxanthone 30 (3.91 g, 96%). Xanthone 30 had spectral properties identical to those previously reported.27
3-amino-2,7-difluoro-6-hydroxy-9H-xanthen-9-one (31)
Using General Procedure C, 21 (1.17 g, 4.07 mmol) and heating at 150 °C for 12 h, afforded 31 (726 mg, 80%). mp 247–250 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.69 (d, J = 10.9 Hz, 1H), 7.56 (d, J = 11.3 Hz, 1H), 7.04 (d, J = 7.0 Hz, 1H), 6.67 (d, J = 7.2 Hz, 1H), 6.55 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 172.7 (s), 154.0 (s), 152.8 (s), 151.3 (d, J = 14.8 Hz), 149.2 (d, J = 85.9 Hz), 147.2 (d, J = 83.7 Hz), 144.1 (d, J = 15.9 Hz), 112.8 (d, J = 5.5 Hz), 110.9 (d, J = 19.9 Hz), 109.7 (d, J = 19.8 Hz), 109.0 – 108.2 (m), 104.7 (d, J = 2.5 Hz), 100.1 (d, J = 4.5 Hz); IR (film) 3369, 3221, 2986, 1614, 1481, 1288, 773 cm−1; HRMS (ESI) m/z 262.0291 (M-H, C13H6F2NO3 requires 262.0316).
3,6-bis(dimethylamino)-2,7-difluoro-9H-xanthen-9-one (32)
Using General Procedure C, 22 (2.00 g, 5.05 mmol), and heating to 150 °C for 12 h, afforded 32 (1.61 g, 85%). mp 211–213 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.59 (d, J = 14.0 Hz, 2H), 6.76 (d, J = 7.6 Hz, 2H), 3.03 (s, 12H); 13C NMR (126 MHz, DMSO-d6) δ 172.4 (s), 153.2 (s), 150.7 (s), 149.5 – 144.7 (m), 111.4 (d, J = 6.7 Hz), 110.8 (d, J = 23.7 Hz), 103.1 (d, J = 4.1 Hz), 41.8 (d, J = 6.0 Hz); IR (film) 3450, 2922, 2850, 2798, 1615, 15221, 1447, 1371, 1332, 1257, 1132, 777 cm−1; HRMS (ESI) m/z 341.1087 (M+Na+, C17H16F2N2O2Na requires 341.1078).
3,6-bis(diethylamino)-2,7-difluoro-9H-xanthen-9-one (33)
Using General Procedure C, 23 (1.50 g, 3.78 mmol), and heating at 170 °C for 12 h, afforded 33 (1.20 g, 85%). mp 108–109 °C; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 14.4 Hz, 2H), 6.31 (d, J = 7.3 Hz, 2H), 3.42 (q, J = 7.0 Hz, 8H), 1.23 (t, J = 7.0 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 173.0 (s), 152.9 (s), 148.9 (d, J = 219.2 Hz), 142.5 (d, J = 10.5 Hz), 111.1(d, J = 24.9 Hz), 110.8 (d, J = 7.1 Hz), 101.5 (s), 45.1 (d, J = 5.9 Hz), 11.9 (d, J = 1.5 Hz); IR (film) 2976, 2934, 1615, 1519, 1457, 1274, 1246, 1071, 773 cm−1; HRMS (ESI) m/z 375.1857 (M+H+, C21H25F2N2O2 requires 375.1884).
3-(diethylamino)-2,7-difluoro-6-hydroxy-9H-xanthen-9-one (34)
Using General Procedure C, 24 (1.00 g, 2.92 mmol), and heating at 150 °C for 12 h, afforded 34 (884 mg, 95%). mp 299–300 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.41 (brs, 1 H), 7.71 (d, J = 10.8 Hz, 1H), 7.59 (d, J = 12.8 Hz, 1H), 7.00 (d, J = 7.0 Hz, 1H), 6.80 (d, J = 7.3 Hz, 1H), 3.43 (q, J = 6.5 Hz, 4H), 1.17 (t, J = 6.5 Hz, 6H); 13C NMR (126 MHz, DMSO-d6) δ 172.6 (s), 153.6 (s), 152.9 (s), 151.6 (s), 151.5 (s), 149.9 (d, J = 84.3 Hz), 148.0 (d, J = 84.6 Hz), 143.3 (d, J = 10.1 Hz), 112.8 (d, J = 5.4 Hz), 111.8 – 110.31 (m), 110.3 (s), 104.6 (d, J = 2.6 Hz), 102.4 (s), 45.7 (d, J = 5.9 Hz), 12.8 (s); IR (film) 3069, 2975, 2733, 1618, 1578, 1475, 1398, 1275, 1213, 1080, 775 cm−1; HRMS (ESI) m/z 318.0916 (M-H, C17H14F2NO3 requires 318.0942).
2,7-difluoro-3,6-bis(isopropylamino)-9H-xanthen-9-one (35)
Using General Procedure C, 25 (2.50 g, 6.79 mmol), and heating at 150 °C for 16 h, afforded 35 (2.15 g, 92%). mp 238–239 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.54 (d, J = 12.0 Hz, 2H), 6.64 (d, J = 7.2 Hz, 2H), 6.45 (dd, J = 2, 8.0 Hz, 2H), 3.76 (hept, J = 6.4 Hz, 2H), 1.22 (d, J = 6.4 Hz, 12H); 13C NMR (126 MHz, DMSO-d6) δ 172.3 (s), 154.2 (s), 147.7 (d, J = 129.2 Hz), 142.0 (s), 141.8 (s), 110.8 – 106.0 (m), 96.9 (d, J = 3.8 Hz), 43.5 (s), 21.7 (s); IR (film) 3435, 2392, 3968, 2837, 1645, 1610, 1531, 1461, 1296, 1016 cm−1; HRMS (ESI) m/z 347.1592 (M+H+, C19H21F2N2O2 requires 347.1571).
3,6-bis(tert-butylamino)-2,7-difluoro-9H-xanthen-9-one (36)
Using General Procedure C, 26 (1.00 g, 2.52 mmol), and heating at 150 °C for 12 h, afforded 36 (848 mg, 90%). mp 289–291 °C; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 12.0 Hz, 2H), 6.75 (d, J = 6.7 Hz, 2H), 4.60 (d, J = 4.9 Hz, 2H), 1.48 (s, 18H); 13C NMR (126 MHz, CDCl3) δ 174.3 (s), 154.1 (s), 148.8 (d, J = 239.0 Hz), 140.8 (d, J = 13.0 Hz), 110.1 (d, J = 6.7 Hz), 109.4 (d, J = 21.7 Hz), 99.1 (d, J = 2.2 Hz), 51.4 (s), 29.2 (s); IR (film) 3451, 2972, 1623, 1527, 1491, 1294, 1217, 887, 821 cm−1; HRMS (ESI) m/z 375.1897 (M+H+, C21H25F2N2O2 requires 375.1884).
2,7-difluoro-3,6-di(piperidin-1-yl)-9H-xanthen-9-one (37)
Using General Procedure C, 27 (840 mg, 2.00 mmol), and heating to 170 °C for 12 h, afforded 37 (733 mg, 92%). mp 200–201 °C; 1H NMR (400 MHz, 10% CD3OD in CDCl3) δ 7.45 (d, J = 13.2 Hz, 2H), 6.58 (d, J = 6.8 Hz, 2H), 2.99 (t, J = 4.8 Hz, 8H), 1.49 (p, J = 4.8 Hz, 8H), 1.41 (hept, J = 4.8 Hz, 4H); 13C NMR (125 MHz, 10% CD3OD in CDCl3) δ 175.2 (s), 153.9 (s), 151.9 (d, J = 252.0 Hz), 147.3 (d, J =10.1 Hz), 113.5 (d, J = 6.3 Hz), 111.0 (d, J = 23.9 Hz), 105.4 (d, J = 3.8 Hz), 51.1 (d, J = 5.0 Hz), 25.6 (s), 24.0 (s); IR (film) 2931, 1619, 1461, 1234, 1130, 776 cm−1; HRMS (ESI) m/z 421.1714 (M+Na+, C23H24F2N2O2Na requires 421.1704).
2,7-difluoro-3-hydroxy-6-(piperidin-1-yl)-9H-xanthen-9-one (38)
Using General Procedure C, 28 (710 mg, 2.01 mmol), and heating at 150 °C for 12 h, afforded 38 (630 mg, 95%). mp 230–231 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 2H), 7.73 (d, J = 10.8 Hz, 1H), 7.64 (d, J = 13.3 Hz, 1H), 7.03 (d, J = 6.9 Hz, 1H), 7.01 (d, J = 6.9 Hz, 1H), 3.21 (t, J = 5.4 Hz, 1H), 1.65 (p, J = 5.2 Hz, 1H), 1.58 (p, J = 5.4 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 172.9 (s), 152.6 (d, J = 161.3 Hz), 155.0 (d, J = 155.0 Hz), 152.1 (s), 150.1 (s), 149.7 (s), 147.7 (s), 146.5 (d, J = 10.1 Hz), 113.1 – 112.5 (m), 111.0 (d, J = 20.2 Hz), 110.7 (d, J = 23.5 Hz), 105.9 (s), 104.6 (s), 50.5 (d, J = 5.0 Hz), 25.3 (s), 23.6 (s); IR (film) 3126, 2936, 1625, 1587, 1484, 1296, 1090, 832, 778 cm−1; HRMS (ESI) m/z 330.0925 (M-H, C18H14F2NO3 requires 330.0942).
2,7-difluoro-3,6-dimorpholino-9H-xanthen-9-one (39)
Using General Procedure C, 29 (1.00 g, 2.36 mmol), and heating at 170 °C for 12 h, afforded 39 (787 mg, 83%). mp 236–237 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 13.0 Hz, 2H), 6.80 (d, J = 6.8 Hz, 2H), 3.90 (t, J = 5.0 Hz, 8H), 3.26 (t, J = 5.0 Hz, 8H); 13C NMR (125 MHz, CDCl3) δ 13C NMR (126 MHz, CDCl3) δ 174.4 (s), 153.6 (s), 151.9 (d, J = 245.9 Hz), 146.1 (d, J = 10.6 Hz), 114.9 (d, J = 7.2 Hz), 112.0 (d, J = 23.5 Hz), 105.5 (d, J = 3.0 Hz), 66.6 (s), 50.1 (d, J = 4.8 Hz); IR (film) 2921, 2863, 1619, 1473, 1259, 1198, 1123, 1030, 900, 777 cm−1; HRMS (ESI) m/z 403.1490 (M+H+, C21H21F2N2O4 requires 403.1469).
2,7-difluoro-10-isopropyl-3,6-bis(isopropylamino)acridin-9(10H)-one (40)
Bis(2,4,5-trifluorophenyl)methanone 10 (291 mg, 1.00 mmol) was dissolved in isopropylamine (3.00 mL, 36.7 mmol) and transferred to a sealed tube. The reaction was stirred at 100 °C for 12 h, and all volatiles were removed under reduced pressure. The residual oil was purified by flash chromatography to yield 40 (170 mg, 45%) as a white solid. mp 160–162 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 12.0 Hz, 2H), 6.56 (d, J = 8.0 Hz, 2H), 5.01 (hept, J = 7.0 Hz, 1H), 4.34 (brs, 2H), 3.75 (hept, J = 5.92 Hz, 2H), 1.75 (d, J = 7.0 Hz, 6H), 1.33 (d, J = 7.0 Hz, 12H); 13C NMR (126 MHz, CDCl3) δ 173.9 (s), 146.5 (d, J = 238.3 Hz), 140.1 (s), 138.9 (d, J = 13.7 Hz), 112.4 (d, J = 5.8 Hz), 109.7 (d, J = 19.5 Hz), 95.4 (s), 50.8 (s), 43.0 (s), 21.6 (s), 20.5 (s); IR (film) 3437, 3304, 2970, 2934, 1622, 1599, 1498, 1281 cm−1; HRMS (ESI) m/z 388.2204 (M+H+, C22H28F2N3O requires 388.2200).
3,6-bis(dimethylamino)-2,7-difluoro-10-methylacridin-9(10H)-one (41)
Using General Procedure D, 10 (291 mg, 1.00 mmol), a reaction time of 6 h, purification by washing with acetone, and removal of solvent in vacuo, afforded 41 (307 mg, 93%). mp 200–203 °C; 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 14.2 Hz, 2H), 6.51 (d, J = 6.5 Hz, 2H), 3.73 (s, 3H), 3.06 (s, 12H); 13C NMR (126 MHz, DMSO-d6) δ 172.8 (s), 149.3 (d, J = 241.6 Hz), 144.9 (d, J = 10.3 Hz), 140.4 (s), 113.5 (s), 111.3 (d, J = 22.3 Hz), 102.2 (d, J = 3.3 Hz), 42.0 (d, J = 5.5 Hz), 34.2 (s); IR (film) 2916, 1602, 1329, 1267, 750 cm−1; HRMS (ESI) m/z 332.1548 (M+H+, C18H20F2N3O requires 332.1574).
2,7-difluoro-3,6-bis(isopropylamino)-10-methylacridin-9(10H)-one (42)
Using General Procedure D, 25 (1.70 g, 4.62 mmol), heating to 80 °C for 12 h, and purification by washing with ether and removal of solvent in vacuo, afforded 42 (1.58 g, 95%) mp 232–233 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 12.2 Hz, 2H), 6.33 (d, J = 6.8 Hz, 2H), 4.33 (d, J = 4.0 Hz, 2H), 3.73 (hept, J = 6.6 Hz, 2H), 3.69 (s, 3H), 1.31 (d, J = 6.3 Hz, 2H); 13C NMR (126 MHz, DMSO-d6) δ 172.5 (s), 146.9 (d, J = 244.4 Hz), 141.3 (s), 140.8 (d, J = 13.9 Hz), 110.4 (d, J = 5.1 Hz), 109.3 (d, J = 18.9 Hz), 94.9 (d, J = 3.1 Hz), 43.1 (s), 34.3 (s), 21.9 (s); IR (film) 3405, 3302, 2970, 1623, 1596, 1505, 1284, 1034, 800 cm−1; HRMS (ESI) m/z 360.1866 (M+H+, C20H24F2N3O requires 360.1887).
3-amino-6-(dimethylamino)-2,7-difluoro-10-methylacridin-9(10H)-one (43)
Using General Procedure D, 21 (300 mg, 1.05 mmol), a reaction time of 12 h, and purification by washing with ether and removal of solvent in vacuo, afforded 43 (295 mg, 93%). mp 263–264 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.75 (d, J = 14.3 Hz, 1H), 7.71 (d, J = 11.7 Hz, 1H), 6.89 (d, J = 7.4 Hz, 1H), 6.80 (d, J = 7.6 Hz, 1H), 6.24 (s, 2H), 3.75 (s, 3H), 3.03 (s, 6H); 13C NMR (101 MHz, DMSO-d6) δ 173.3 (s), 149.8 (d, J = 200.6 Hz), 147.4 (d, J = 250.7 Hz), 145.1 (d, J = 10.3 Hz), 143.3 (s), 143.1 (s), 141.5 (s), 140.5 (s), 113.9 (d, J = 6.3 Hz), 111.8 (d, J = 22.3 Hz), 110.6 (d, J = 18.6 Hz), 102.5 (d, J = 3.3 Hz), 99.0 (d, J = 3.9 Hz), 42.5 (s), 34.4 (s); IR (film) 3322, 3176, 1619, 1493, 1309, 1256, 1026, 902, 777 cm−1; HRMS (ESI) m/z 304.1260 (M+H+, C16H16F2N3O requires 304.1261).
2,7-difluoro-10-methyl-3,6-di(piperidin-1-yl)acridin-9(10H)-one (44)
Using General Procedure D, 27 (1.50 g, 3.58 mmol), a reaction time of 4 h, and purification by washing the product with ether and removal of solvent in vacuo, afforded 44 (1.37 g, 93%). mp 243–244 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 12.1 Hz, 2H), 6.69 (d, J = 6.9 Hz, 2H), 3.74 (s, 3H), 3.21 (t, J = 5.2 Hz, 8H), 1.79 (p, J = 5.2 Hz, 8H), 1.64 (hept, J = 5.2 Hz, 4H); 13C NMR (126 MHz, CDCl3) δ 174.9 (s), 151.1 (d, J = 245.7 Hz), 146.5 (d, J = 10.4 Hz), 140.3 (s), 115.9 (d, J = 6.7 Hz), 112.6 (d, J = 22.5 Hz), 102.9 (d, J = 2.7 Hz), 51.5 (d, J = 4.5 Hz), 34.2 (s), 26.0 (s), 24.2 (s); IR (film) 2938, 2846, 1624, 1597, 1494, 1274, 1225, 1145, 783 cm−1; HRMS (ESI) m/z 412.2196 (M+H+, C24H28F2N3O requires 412.2200).
3-(dimethylamino)-2,7-difluoro-10-methyl-6-(piperidin-1-yl)acridin-9(10H)-one (45)
Using General Procedure D, 28 (355 mg, 1.00 mmol), a reaction time of 12 h, and purification by washing with ether and removal of solvent in vacuo, afforded 45 (338 mg, 91%). mp 227–229 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 12.6 Hz, 1H), 7.96 (d, J = 14.2 Hz, 1H), 6.65 (d, J = 6.9 Hz, 1H), 6.43 (d, J = 7.2 Hz, 1H), 3.67 (s, 3H), 3.19 (t, J = 5.2 Hz, 4H), 3.05 (s, 6H), 1.78 (p, J = 5.4 Hz, 4H), 1.65 (p, J = 5.4 Hz, 2H); 13C NMR (126 MHz, DMSO-d6) δ 173.6 (s), 151.3 (d, J = 138.6 Hz), 149.5 (d, J = 127.8 Hz), 146.1 (d, J = 10.4 Hz), 145.5 (d, J = 10.0 Hz), 141.1 (s), 141.0 (s), 115.5 (s), 114.2 (s), 111.9 (d, J = 22.5 Hz), 111.7 (d, J = 22.6 Hz), 104.7 (d, J = 2.6 Hz), 102.4 (d, J = 3.4 Hz), 51.5 (d, J = 4.7 Hz), 42.5 (d, J = 5.8 Hz), 34.7 (s), 26.0 (s), 24.2 (s); IR (film) 2836, 1626, 1601, 1503, 1280, 1011, 895 cm−1; HRMS (ESI) m/z 372.1889 (M+H+, C21H24F2N3O requires 372.1887).
2,7-difluoro-10-isopropyl-3,6-dimethoxyacridin-9(10H)-one (46)
A solution of 19 (100 mg, 0.32 mmol) in isopropylamine (3.00 mL, 36.6 mmol) was heated to 100 °C in a sealed tube for 12 h. This mixture was cooled and residual isopropylamine removed in vacuo. The resulting crude yellow oil was dissolved in THF (7.00 mL), treated with NaH (60%, 77.0 mg, 1.92 mmol), and heated to 60 °C for 12 h. The reaction mixture was cooled in an ice bath and carefully neutralized with saturated aqueous NaHCO3 (20 mL). Extraction with THF (3 × 20 mL), drying of the combined organic fractions over anhydrous Na2SO4, and removal of solvent in vacuo provided a crude yellow oil that was purified by column chromatography (CH2Cl2) to provide dimethoxyacridone 46 (88.4 mg, 83%). mp 158–159 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 11.2 Hz, 2H), 7.04 (d, J = 6.8 Hz, 2H), 5.09 (p, J = 7.2 Hz, 1H), 4.06 (s, 6H), 1.83 (d, J = 7.2 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 175.3 (s), 152.1 (d, J = 12.9 Hz), 148.5 (d, J = 245.9 Hz), 140.2 (s), 117.0 (d, J = 5.4 Hz), 112.8 (d, J = 18.9 Hz), 100.1 (s), 56.4 (s), 52.4 (s), 21.4 (s); IR (film) 2924, 2851, 1736, 1626, 1603, 1488, 1263, 1091, 796 cm−1; HRMS (ESI) m/z 334.1255 (M+H+, C18H18F2NO3 requires 334.1255).
10-benzyl-2,7-difluoro-3,6-dimethoxyacridin-9(10H)-one (47)
A solution of 19 (1.50 g, 4.78 mmol) in benzylamine (8.00 mL, 73.3 mmol) was heated to 100 °C for 12 h. This mixture was cooled and residual benzylamine removed by vacuum distillation (36 °C, 1 mm Hg). The resulting crude yellow oil was dissolved in THF (30 mL), treated with NaH (60%, 574 mg, 14.4 mmol), and heated to 60 °C for 12 h. The reaction mixture was cooled in an ice bath and carefully neutralized with saturated aqueous NaHCO3 (20 mL). The resulting biphasic mixture was extracted with THF (3 × 20 mL) and the combined organic fractions were dried over anhydrous Na2SO4 and concentrated to give a crude yellow oil that was purified by column chromatography (CH2Cl2) to provide dimethoxyacridone 47 (1.56 g, 86%). mp 205–206 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 11.2 Hz, 2H), 7.45-7.30 (m, 3H), 7.22 (d, J = 6.8 Hz, 2H), 6.93 (d, J = 6.8 Hz, 2H), 5.70 (s, 2H), 4.82 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 174.9 (s), 153.0 (d, J = 13.0 Hz), 148.7 (d, J = 246.0 Hz), 140.1 (s), 135.1 (s), 129.5 (s), 128.22 (s), 125.6 (s), 115.6 (d, J = 5.4 Hz), 112.5 (d, J = 19.1 Hz), 98.5 (s), 56.21 (s), 51.6 (s); IR (film) 2989, 2938, 1607, 1498, 1273, 1042 cm−1; HRMS (ESI) m/z 382.1278 (M+H+, C22H18F2NO3 requires 382.1255).
2,7-difluoro-3,6-dimethoxy-10-phenylacridin-9(10H)-one (48)
A solution of 19 (100 mg, 0.320 mmol) in aniline (2.00 mL, 21.9 mmol) was heated to 130 °C for 12 h in a sealed tube. The reaction mixture was cooled and the residual aniline was removed by vacuum distillation (40 °C, 1 mm Hg). The resulting crude yellow oil was dissolved in DMA (3.5 mL) and heated at 170 °C for 12 h. The reaction mixture was cooled, diluted with water (30 mL), and extracted with THF (3 × 20 mL). The combined organic fractions were dried over anhydrous Na2SO4 and concentrated to give a crude yellow oil that was purified by column chromatography (CH2Cl2) to provide dimethoxyacridone 48 (84.5 mg, 72%). mp 256–257 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 11.6 Hz, 2H), 7.74–7.87 (m, 3H), 7.59 (d, J = 7.2 Hz, 2H), 6.17 (d, J = 7.2 Hz, 2H)), 3.62 (s, 6H); 13C NMR (126 MHz, DMSO-d6) δ 173.5 (s), 151.8 (d, J = 13.1 Hz), 148.0 (d, J = 244.2 Hz), 140.7 (s), 138.0 (s), 131.4 (s), 130.3 (s), 129.6 (s), 113.9 (d, J = 5.1 Hz), 111.0 (d, J = 18.7 Hz), 100.1 (s), 55.8 (s); IR (film) 3057, 2939, 1612, 1580, 1479, 1306, 1256, 1084, 823, 701 cm−1; HRMS (ESI) m/z 368.1098 (M+H+, C21H16F2NO3 requires 368.1098).
2,7-difluoro-3,6-dihydroxy-10-isopropylacridin-9(10H)-one (49)
Using General Procedure E, 46 (20.0 mg, 0.0601 mmol) afforded dihydroxylacridone 49 (15.2 mg, 83%). mp 172–173 °C; 1H NMR (400 MHz, CD3OD) δ 7.95 (d, J = 11.6 Hz, 2H), 7.29 (d, J = 7.2 Hz, 2H), 5.18 (p, J = 7.2 Hz, 2H), 1.75 (d, J = 7.2 Hz, 2H); 13C NMR (126 MHz, CD3OD) δ 177.2 (s), 152.4 (d, J = 15.4 Hz), 149.6 (d, J = 242.1 Hz), 142.1 (s), 116.7 (d, J = 5.1 Hz), 112.8 (d, J = 19.2 Hz), 105.2 (s), 53.6 (s), 20.9 (s); IR (film) 3357, 3075, 2977, 1701, 1627, 1542, 1490, 1448, 1406, 1277, 1199, 889, 770 cm−1; HRMS (ESI) m/z 304.0765 (M-H, C16H12F2NO3 requires 304.0785).
10-benzyl-2,7-difluoro-3,6-dihydroxyacridin-9(10H)-one (50)
Using General Procedure E, 47 (20.0 mg, 0.0526 mmol) afforded dihydroxyacridone 50 (17.0 mg, 91%). mp 247–249 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.92 (d, J = 11.2 Hz, 2H), 7.25–7.45 (m, 3H), 7.18 (d, J = 7.2 Hz, 2H), 6.97 (d, J = 7.2 Hz, 2H), 5.53 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 173.6 (s), 151.2 (s), 147.8 (d, J = 241.4 Hz), 140.3 (s), 135.6 (s), 129.0 (s), 127.4 (s), 125.7 (s), 113.8 (s), 111.6 (d, J = 18.9 Hz), 102.8 (s), 50.3 (s); IR (film) 3369, 3050, 3007, 1629, 1574, 1490, 1276, 750 cm−1; HRMS (ESI) m/z 352.0776 (M-H, C20H12F2NO3 requires 352.0785).
2,7-difluoro-3,6-dihydroxy-10-phenylacridin-9(10H)-one (51)
Using General Procedure E, 48 (20.0 mg, 0.0545 mmol) afforded dihydroxylacridone 51 (14.6 mg, 79%). mp 257–259 °C; 1H NMR (400 MHz, CD3OD) δ 8.10 (d, J = 11.2 Hz, 2H), 7.75–7.90 (m, 3H), 7.49 (d, J = 6.8 Hz, 2H), 6.36 (d, J = 7.2 Hz, 2H); 13C NMR (126 MHz, CD3OD) δ 174.4 (s), 154.1 (d, J = 15.5 Hz), 150.7 (d, J = 245.7 Hz), 143.3 (s), 140.1 (s), 132.6 (s), 131.6 (s), 130.6 (s), 113.6 (d, J = 6.2 Hz), 112.0 (d, J = 20.4 Hz), 104.8 (d, J = 2.4 Hz); IR (film) 3369, 3064, 2924, 1727, 1624, 1547, 1489, 1395, 1268, 1193, 893 cm−1; HRMS (ESI) m/z 338.0609 (M-H, C19H10F2NO3 requires 338.0629).
2,7-difluoro-3,6-dimethoxy-9H-thioxanthen-9-one (52)
Using General Procedure F, 19 (3.15 g, 10.0 mmol), 26 °C, and purification by washing with water (3 × 100 mL), methanol (3 × 50 mL), acetone (3 × 50 mL), and removal of solvent in vacuo, afforded 52 (2.74 g, 92%). mp >300 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 12.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 2H), 4.032 (s, 2H); IR (film) 3047, 2967, 1603, 1419, 1346, 1263, 1046, 894, 771 cm−1; HRMS (ESI) m/z 309.0407 (M+H+, C15H11F2O3S requires 309.0397).
2,7-difluoro-3,6-dihydroxy-9H-thioxanthen-9-one (53)
Using General Procedure F, 19 (1.58 g, 5.00 mmol), heating to 90 °C, and purification by column chromatography (1% to 3% MeOH in CH2Cl2), afforded 53 (1.04 g, 74%). mp >300 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.03 (d, J = 12.0 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 3.42 (brs, 2H); 13C NMR (126 MHz, DMSO) δ 175.7 (s), 150.5 (d, J = 243.2 Hz), 150.2 (d, J = 14.1 Hz), 133.4 (d, J = 1.9 Hz), 120.1 (d, J = 5.0 Hz), 115.3 (d, J = 19.5 Hz), 112.7 (d, J = 2.7 Hz); IR (film) 3393, 3064, 1617, 1553, 1502, 1413, 1391, 1303, 1287, 1182, 1114, 903 cm−1; HRMS (ESI) m/z 278.9924 (M-H, C13H5F2O3S requires 278.9927).
3,6-bis(dimethylamino)-2,7-difluoro-9H-thioxanthen-9-one (54)
Using General Procedure F, 22 (150 mg, 0.440 mmol), and purification by column chromatography (10% to 20% EtOAc in hexanes), afforded 54 (134 mg, 91%). mp 218–220 °C; 1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 15.1 Hz, 2H), 6.63 (d, J = 8.0 Hz, 2H), 3.02 (s, 12H); 13C NMR (126 MHz, CDCl3) δ 175.8 (s), 151.4 (d, J = 246.1 Hz), 142.7 (d, J = 10.0 Hz), 132.9 (s), 119.5 (d, J = 6.3 Hz), 114.8 (d, J = 23.5 Hz), 110.0 (d, J = 3.7 Hz), 41.1 (d, J = 6.0 Hz); IR (film) 2907, 2898, 2853, 1682, 1601, 1583, 1354, 1256, 1116, 722 cm−1; HRMS (ESI) m/z 335.1042 (M+H+, C17H17F2N2OS requires 335.1030).
3,6-bis(diethylamino)-2,7-difluoro-9H-thioxanthen-9-one (55)
Using General Procedure F, 23 (1.10 g, 2.77 mmol), and purification by column chromatography (10% to 20% EtOAc in hexanes), afforded 55 (973 mg, 90%). mp 145–147 °C; 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 15.6 Hz, 2H), 6.66 (d, J = 8.0 Hz, 2H), 3.40 (q, J = 7.0 Hz, 8H), 1.21 (t, J = 7.0 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 176.7 (s), 152.1 (d, J = 312.5 Hz), 141.8 (d, J = 9.9 Hz), 134.0 (d, J = 1.5 Hz), 119.7 (s), 116.3 (d, J = 24.3 Hz), 110.6 (s), 46.0 (d, J = 5.8 Hz), 13.0 (d, J = 1.5 Hz); IR (film) 2972, 2931, 2872, 1590, 1504, 1426, 1352, 1264, 1238, 1071, 901, 790 cm−1; HRMS (ESI) m/z 391.1662 (M+H+, C21H25F2N2OS requires 391.1656).
2,7-difluoro-3,6-bis(isopropylamino)-9H-thioxanthen-9-one (56)
Using General Procedure F, 25 (1.00 g, 2.71 mmol), and purification by column chromatography (CH2Cl), afforded 56 (834 mg, 85%). mp 164–165 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 12.8 Hz, 2H), 6.59 (d, J = 7.6 Hz, 2H), 4.41 (brs, 2H), 3.74 (hept, J = 6.4 Hz, 2H), 1.33 (d, J = 6.4 Hz, 12H); 13C NMR (126 MHz, CDCl3) 176.9 (s), 150.4 (d, J = 225.5 Hz), 139.3 (d, J = 41.6 Hz), 134.8 (s), 118.2 (s), 113.8 (d, J = 18.9 Hz), 104.9 (s), 44.0 (s), 22.6 (s); IR (film) 3435, 3322, 2969, 1610, 1592, 1515, 1426, 1287, 1326, 1026, 774 cm−1; HRMS (ESI) m/z 363.1338 (M+H+, C19H21F2N2OS requires 363.1343).
3,6-bis(tert-butylamino)-2,7-difluoro-9H-thioxanthen-9-one (57)
Using General Procedure F, 26 (300 mg, 0.762 mmol), and purification by column chromatography (10% to 20% EtOAc in hexanes), afforded 57 (263 mg, 89%). mp 199–201 °C; 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 13.2 Hz, 2H), 6.33 (d, J = 7.8 Hz, 2H), 4.57 (d, J = 4.6 Hz, 2H), 1.47 (s, 18H), 1.31 (d, J = 6.3 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 176.9 (s), 150.9 (d, J = 241.0 Hz), 139.2 (d, J = 12.2 Hz), 135.6 – 132.8 (m), 118.0 (d, J = 6.1 Hz), 113.6 (d, J = 21.3 Hz), 106.6 (s), 51.4 (s), 29.3 (s); IR (film) 3437, 2978, 1598, 1514, 1420, 1364, 1202 cm−1; HRMS (ESI) m/z 391.1649 (M+H+, C21H25F2N2OS requires 391.1656).
2,7-difluoro-3,6-di(piperidin-1-yl)-9H-thioxanthen-9-one (58)
Using General Procedure F, 27 (400 mg, 1.00 mmol), and purification by column chromatography (10% to 20% EtOAc in hexanes), afforded 58 (352 mg, 85%). mp 205–206 °C; 1H NMR (400 MHz, CDCl3) δ 8.15 (d, J = 14.3 Hz, 2H), 6.87 (d, J = 7.7 Hz, 2H), 3.23 (t, J = 5.2 Hz, 8H), 1.75 (p, J = 5.2 Hz, 8H), 1.63 (hept, J = 5.2 Hz, 4H); 13C NMR (126 MHz, CDCl3) δ 177.1 (s), 153.9 (d, J = 238.1 Hz), 145.1 (d, J = 10.0 Hz), 133.9 (s), 122.0 (d, J = 6.5 Hz), 115.8 (d, J = 23.2 Hz), 113.3 (d, J = 3.0 Hz), 51.2 (d, J = 4.8 Hz), 25.8 (s), 24.2 (s); IR (film) νmax 2936, 2852, 1624, 1597, 1497, 1420, 1350, 1269, 1252, 1240, 1115, 750 cm−1; HRMS (ESI) m/z 415.1650 (M+H+, C23H25F2N2OS requires 415.1656).
2,7-difluoro-3,6-dimorpholino-9H-thioxanthen-9-one (59)
Using General Procedure F, 29 (213 mg, 0.500 mmol), and purification by column chromatography (15% to 30% EtOAc in hexanes), afforded 59 (195 mg, 94%). mp 275–276 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 14.2 Hz, 2H), 6.86 (d, J = 7.6 Hz, 2H), 3.90 (t, J = 4.7 Hz, 8H), 3.26 (t, J = 4.7 Hz, 8H); 13C NMR (126 MHz, CDCl3) δ 176.0 (s), 152.9 (d, J = 258.3 Hz), 143.1 (d, J = 9.9 Hz), 132.8 (d, J = 2.0 Hz), 122.0 (d, J = 6.7 Hz), 115.1 (d, J = 23.1 Hz), 112.1 (d, J = 2.8 Hz), 65.6 (s), 49.1 (d, J = 4.7 Hz); IR (film) 2990, 2923, 2887, 1602, 1499, 1426, 1271, 1122, 1006 cm−1; HRMS (ESI) m/z 441.1056 (M+Na+, C21H20F2N2O3SNa requires 441.1060).
Supplementary Material
Acknowledgments
We thank the NIH (R01 CA83831 and RC1 GM091086) for financial support. ZW thanks the NIH for an IRACDA postdoctoral fellowship.
Footnotes
Supporting Information. Methods and data used to determine quantum yields and molar extinction coefficients, and absorption, fluorescence emission, 1H NMR, and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Muller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.Hagmann WK. J Med Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 3.Lavis LD, Raines RT. ACS Chem Biol. 2008;3:142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funabiki K, Sugiyama N, Iida H, Jin JY, Yoshida T, Kato Y, Minoura H, Matsui M. J Fluor Chem. 2006;127:257–262. [Google Scholar]
- 5.Sun WC, Gee KR, Klaubert DH, Haugland RP. J Org Chem. 1997;62:6469–6475. [Google Scholar]
- 6.Lavis LD, Rutkoski TJ, Raines RT. Anal Chem. 2007;79:6775–6782. doi: 10.1021/ac070907g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JX, Diwu ZJ, Leung WY. Bioorg Med Chem Lett. 2001;11:2903–2905. doi: 10.1016/s0960-894x(01)00595-9. [DOI] [PubMed] [Google Scholar]
- 8.Spagnuolo CC, Massad W, Miskoski S, Menendez GO, Garcia NA, Jares-Erijman EA. Photochem Photobiol. 2009;85:1082–1088. doi: 10.1111/j.1751-1097.2009.00565.x. [DOI] [PubMed] [Google Scholar]
- 9.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. J Am Chem Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 10.Mottram LF, Boonyarattanakalin S, Kovel RE, Peterson BR. Org Lett. 2006;8:581–584. doi: 10.1021/ol052655g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mottram LF, Maddox E, Schwab M, Beaufils F, Peterson BR. Org Lett. 2007;9:3741–3744. doi: 10.1021/ol7015093. [DOI] [PubMed] [Google Scholar]
- 12.Mitronova GY, Belov VN, Bossi ML, Wurm CA, Meyer L, Medda R, Moneron G, Bretschneider S, Eggeling C, Jakobs S, Hell SW. Chem Eur J. 2010;16:4477–4488. doi: 10.1002/chem.200903272. [DOI] [PubMed] [Google Scholar]
- 13.Pinto MM, Sousa ME, Nascimento MS. Curr Med Chem. 2005;12:2517–2538. doi: 10.2174/092986705774370691. [DOI] [PubMed] [Google Scholar]
- 14.Watterson SH, Chen P, Zhao Y, Gu HH, Dhar TG, Xiao Z, Ballentine SK, Shen Z, Fleener CA, Rouleau KA, Obermeier M, Yang Z, McIntyre KW, Shuster DJ, Witmer M, Dambach D, Chao S, Mathur A, Chen BC, Barrish JC, Robl JA, Townsend R, Iwanowicz EJ. J Med Chem. 2007;50:3730–3742. doi: 10.1021/jm070299x. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito T, Tanaka T, Kobayashi E, Iinuma M, Nozawa Y. Bioorg Med Chem. 2004;12:5799–5806. doi: 10.1016/j.bmc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Schwaebe MK, Moran TJ, Whitten JP. Tetrahedron Lett. 2005:827–829. [Google Scholar]
- 17.Pouli N, Marakos P. Anticancer Agents Med Chem. 2009;9:77–98. doi: 10.2174/187152009787047699. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. Bioorg Med Chem. 2005;13:6064–6069. doi: 10.1016/j.bmc.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 19.Na Y. J Pharm Pharmacol. 2009;61:707–712. doi: 10.1211/jpp/61.06.0002. [DOI] [PubMed] [Google Scholar]
- 20.Pedro M, Cerqueira F, Sousa ME, Nascimento MS, Pinto M. Bioorg Med Chem. 2002;10:3725–3730. doi: 10.1016/s0968-0896(02)00379-6. [DOI] [PubMed] [Google Scholar]
- 21.Dodean RA, Kelly JX, Peyton D, Gard GL, Riscoe MK, Winter RW. Bioorg Med Chem. 2008;16:1174–1183. doi: 10.1016/j.bmc.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 22.Riscoe M, Kelly JX, Winter R. Curr Med Chem. 2005;12:2539–2549. doi: 10.2174/092986705774370709. [DOI] [PubMed] [Google Scholar]
- 23.Poondru S, Zhou S, Rake J, Shackleton G, Corbett TH, Parchment RE, Jasti BR. J Chrom B Biomed Sci Appl. 2001;759:175–178. doi: 10.1016/s0378-4347(01)00217-1. [DOI] [PubMed] [Google Scholar]
- 24.Corbett TH, Valeriote FA, Demchik L, Lowichik N, Polin L, Panchapor C, Pugh S, White K, Kushner J, Rake J, Wentland M, Golakoti T, Hetzel C, Ogino J, Patterson G, Moore R. Invest New Drugs. 1997;15:207–218. doi: 10.1023/a:1005875015011. [DOI] [PubMed] [Google Scholar]
- 25.Manfroni G, Paeshuyse J, Massari S, Zanoli S, Gatto B, Maga G, Tabarrini O, Cecchetti V, Fravolini A, Neyts J. J Med Chem. 2009;52:3354–3365. doi: 10.1021/jm801608u. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Hu M, Yao SQ. Org Lett. 2009;11:3008–3011. doi: 10.1021/ol9010344. [DOI] [PubMed] [Google Scholar]
- 27.Chen CA, Yeh RH, Lawrence DS. J Am Chem Soc. 2002;124:3840–3841. doi: 10.1021/ja017530v. [DOI] [PubMed] [Google Scholar]
- 28.Egawa T, Koide Y, Hanaoka K, Komatsu T, Terai T, Nagano T. Chem Commun. 2011;47:4162–4164. doi: 10.1039/c1cc00078k. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Yao SQ. Org Lett. 2009;11:405–408. doi: 10.1021/ol802700w. [DOI] [PubMed] [Google Scholar]
- 30.Gannon MK, Detty MR. J Org Chem. 2007;72:2647–2650. doi: 10.1021/jo062370x. [DOI] [PubMed] [Google Scholar]
- 31.Calitree BD, Detty MR. Synlett. 2010:89–92. [Google Scholar]
- 32.Lim SH, Wu L, Burgess K, Lee HB. Anticancer Drugs. 2009;20:461–468. doi: 10.1097/CAD.0b013e32832b7bee. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Larock RC. Org Lett. 2005;7:4273–4275. doi: 10.1021/ol0517731. [DOI] [PubMed] [Google Scholar]
- 34.MacNeil SL, Wilson BJ, Snieckus V. Org Lett. 2006;8:1133–1136. doi: 10.1021/ol053162e. [DOI] [PubMed] [Google Scholar]
- 35.Dubrovskiy AV, Larock RC. Org Lett. 2010;12:3117–3119. doi: 10.1021/ol101017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MM, Naidoo JM, Fernandes MA, Mmutlane EM, van Otterlo WA, de Koning CB. J Org Chem. 2010;75:8701–8714. doi: 10.1021/jo101873v. [DOI] [PubMed] [Google Scholar]
- 37.Gong J, Xie F, Chen H, Hu Y. Org Lett. 2010;12:3848–3851. doi: 10.1021/ol101496w. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L, Xie F, Cheng G, Hu Y. Angew Chem Int Ed. 2009;48:6520–6523. doi: 10.1002/anie.200902618. [DOI] [PubMed] [Google Scholar]
- 39.Gibson SL, Holt JJ, Ye M, Donnelly DJ, Ohulchanskyy TY, You Y, Detty MR. Bioorg Med Chem. 2005;13:6394–6403. doi: 10.1016/j.bmc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 40.Ahn YH, Lee JS, Chang YT. J Am Chem Soc. 2007;129:4510–4501. doi: 10.1021/ja068230m. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Burgess K. J Org Chem. 2008;73:8711–8718. doi: 10.1021/jo800902j. [DOI] [PubMed] [Google Scholar]
- 42.Lubenets EG, Gerasimova TN, Fokin EP. Zh Org Khim. 1971;7:805–812. [Google Scholar]
- 43.Eggins BR. Tetrahedron. 1975;31:1191–1194. [Google Scholar]
- 44.Dmitrieva LL, Babushkin VA, Kurov GN, Nechvold GV, Smirnov VI. Zh Prikl Khim. 1993;66:610–614. [Google Scholar]
- 45.Wu L, Burgess K. Org Lett. 2008;10:1779–1782. doi: 10.1021/ol800526s. [DOI] [PubMed] [Google Scholar]
- 46.Simanek EE, Chouai A. J Org Chem. 2008;73:2357–2366. doi: 10.1021/jo702462t. [DOI] [PubMed] [Google Scholar]
- 47.Cartwright MW, Convery L, Kraynck T, Sandford G, Yufit DS, Howard JAK, Christopher JA, Miller DD. Tetrahedron. 2010;66:519–529. [Google Scholar]
- 48.Anelli PL, Biffi C, Montanari F, Quici S. J Am Chem Soc. 1987;52:2559–2562. [Google Scholar]
- 49.Williams AT, Winfield SA. Analyst. 1983;108:1067–1071. [Google Scholar]
- 50.Morris JV, Mahaney MA, Huber JR. J Phys Chem. 1976;80:969–974. [Google Scholar]
- 51.Dawson WR, Windsor MW. J Phys Chem. 1968;72:3251–3260. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.