Background: Withaferin A (WFA) is a vimentin-targeting inhibitor that has potent anti-proliferative activity.
Results: WFA protects against corneal fibrosis by down-regulating injury-induced vimentin to exert epithelial cell cycle arrest and inhibit myofibroblast expression, which is a mechanism closely mimicked in vimentin-deficient mice during injury healing.
Conclusion: Vimentin is a novel fibrosis target.
Significance: Ocular fibrotic conditions that overexpress vimentin could be treatable with WFA.
Keywords: Cell Cycle, Cytoskeleton, Drug Discovery, Fibrosis, Pharmacogenetics, Desmin, Skp2, Tissue Repair, Vimentin, Withaferin A
Abstract
The type III intermediate filaments (IFs) are essential cytoskeletal elements of mechanosignal transduction and serve critical roles in tissue repair. Mice genetically deficient for the IF protein vimentin (Vim−/−) have impaired wound healing from deficits in myofibroblast development. We report a surprising finding made in Vim−/− mice that corneas are protected from fibrosis and instead promote regenerative healing after traumatic alkali injury. This reparative phenotype in Vim−/− corneas is strikingly recapitulated by the pharmacological agent withaferin A (WFA), a small molecule that binds to vimentin and down-regulates its injury-induced expression. Attenuation of corneal fibrosis by WFA is mediated by down-regulation of ubiquitin-conjugating E3 ligase Skp2 and up-regulation of cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1. In cell culture models, WFA exerts G2/M cell cycle arrest in a p27Kip1- and Skp2-dependent manner. Finally, by developing a highly sensitive imaging method to measure corneal opacity, we identify a novel role for desmin overexpression in corneal haze. We demonstrate that desmin down-regulation by WFA via targeting the conserved WFA-ligand binding site shared among type III IFs promotes further improvement of corneal transparency without affecting cyclin-dependent kinase inhibitor levels in Vim−/− mice. This dissociates a direct role for desmin in corneal cell proliferation. Taken together, our findings illuminate a previously unappreciated pathogenic role for type III IF overexpression in corneal fibrotic conditions and also validate WFA as a powerful drug lead toward anti-fibrosis therapeutic development.
Introduction
Traumatic injury, in particular alkali burns to the eye, can cause irreversible loss of vision due to corneal opacification from fibrosis, which often necessitates corneal transplantation as a means of restoring vision. When coupled with the lack of effective therapeutic modalities and an inadequate medical infrastructure, corneal rehabilitation for millions of people in underdeveloped nations remains a vital challenge (1). Reflecting this insidious enigma, the leading cause of worldwide blindness, second only to cataract, is from corneal pathology where fibrosis is a central binding mechanism of refractive failure. Corneal haze, considered a reversible form of refractive aberration, is also a complication from corneal disease and injuries and also can present in 2–5% of subjects after surgical and laser vision corrective procedures (2). Because procedures such as LASIK (laser in situ keratomileusis) have become very popular, crossing the 10 million people mark in the United States alone, concerns over corneal failure to heal in patients who have had vision corrective surgery have also gained recent importance (3). Considering the unique requirement for transparency (4), the molecular underpinnings of how scar-free healing can be promoted to restore visual acuity continue to remain a vital challenge for corneal transplantation, rehabilitation, and refractive surgery.
The type III intermediate filaments (IFs)2 are a family of highly homologous cytoskeleton proteins that are widely conserved from humans to cold-blooded fish (5). These proteins mechanically integrate external influences with cellular biochemical processes and govern many critical aspects of cell structure, cell division, cell differentiation, apoptosis, and cell movement, acting together with the actin and microtubule cytoskeletal elements to regulate functions of a plethora of cellular proteins (6–8). These IFs are expressed widely (e.g. in mesenchymal cells such as fibroblasts, muscle and endothelial cells, elsewhere in leukocytes, and in astrocytes and macroglia of the central nervous system (CNS)). The genetic knockouts of IFs, while revealing that they are not essential for development or reproduction (9, 10), have drawn more recent attention to their functions in tissue repair and stress response (11, 12). Vimentin is the prototypic Type III IF protein that is widely studied because of its involvement in wound healing, fibrosis, angiogenesis, tumor cell differentiation, migration, and metastasis (13–18). Vimentin plays a critical role in wound repair by providing activated wound fibroblasts during transition to the myofibroblastic phenotype with force generation required for tissue contraction (19). Notwithstanding, vimentin-deficient (Vim−/−) mice display deficits in physiological wound closure from delayed myofibroblast activation and defective collagen contraction (20), but these mice are otherwise physiologically normal (9). Vimentin expression in endothelial cells also helps to form anchoring structures to assist leukocyte extravasation from vasculature, and hence Vim−/− mice are severely compromised in leukocyte transmigration, which is important for immune activation during infection (21). Because vimentin is also the sole IF expressed in vascular endothelial cells, neovascularization responses in Vim−/− mice are impaired (12, 16). Despite previous studies showing that vimentin is overexpressed in the cornea during injury and fibrosis (22, 23), its precise role in regulating corneal injury repair has remained largely unexplored.
The small molecule withaferin A (WFA) is a pluripotent natural product (24, 25), which was recently discovered to target vimentin (16). WFA covalently binds soluble tetrameric vimentin at its single cysteine residue that is present in the conserved rod 2B domain (16). Taking advantage of the targeting by WFA of this conserved cysteine residue found in type III IFs, we recently demonstrated that WFA, in addition to targeting vimentin in the retina, also binds to glial fibrillary acidic protein (GFAP) and down-regulates GFAP expression in reactive Müller cells to block retinal gliosis (26, 27). Thus, expanding the broad clinical usefulness of this newly discovered type III IF targeting drug, others have also exploited vimentin's in vivo druggability by WFA to illustrate tumor blockade through down-regulation of vimentin (15, 28) and also to demonstrate protection against bacterial meningitis via vascular targeting of vimentin (15, 28). Here we report a novel finding using a mouse model of alkali injury that vimentin and the related IF desmin are coordinately overexpressed during corneal fibrosis. We advance an important discovery that Vim+/+ mice treated with WFA recapitulate several hallmark features of Vim−/− mice in their protection from corneal fibrosis. Finally, we reveal a novel role for desmin in corneal refractive aberration, which we have unveiled in Vim−/− mice, demonstrating that the targeting by WFA of this second IF promotes further restoration of corneal transparency.
EXPERIMENTAL PROCEDURES
General Methods
WFA was purchased from a commercial vendor (Chromadex, Santa Ana, CA), and WFA-Bt synthesis has been described previously (29). All animal experiments were conducted in accordance with the Declaration of Helsinki, and procedures were approved by Institutional Animal Care and Use Committees of the University of Kentucky and University of Connecticut Health Center.
Cell Culture
Wild-type, Skp2−/−, and p27−/− Mouse Embryonic Fibroblasts (MEFs) were cultured in DMEM containing 10% FBS, 100 mm sodium pyruvate, 10 mm non-essential amino acids, 200 mm l-glutamine, and 50 mm β-mercaptoethanol as described previously (30) and used before passage 5. Corneal fibroblasts were derived from primary cultures of keratocytes isolated from corneas of New Zealand White rabbits by collagenase digestion and cultured in minimal essential medium containing 10% FBS and penicillin and streptomycin (100 units/ml) as described previously (31). Cells were used before passage 8.
Three-dimensional Model of Human Desmin Tetramer Fragment
The homology modeling of the desmin three-dimensional tetramer model and molecular docking for the WFA ligand binding was carried out essentially as described previously (16).
Cell Cycle Analysis
MEFs from wild-type mice and p27Kip1−/− and Skp2−/− were grown to confluence, growth-arrested by serum starvation for 48 h, and treated with vehicle or WFA in the presence of serum for 21 h for cell cycle analysis as described previously (24).
In Vivo Corneal Injury Model
The corneal alkali injury model in Vim−/− and Vim+/+ in 129 Svev background has been described in detail previously (16). Injured mice were treated with vehicle (DMSO) or WFA (2 mg/kg solubilized in DMSO) on the day of injury and every subsequent day by intraperitoneal injection for a period of 7 or 14 days.
Immunofluorescence
Using procedures previously described (16), whole-eye sections from Vim+/+ and Vim−/− mice were prepared by cryosectioning. Slides were probed with the following primary antibodies, diluted in a background-reducing buffer (Dako North America): anti-rabbit vimentin (1:200; Abcam), anti-mouse desmin (1:200; Dako), anti-mouse α-SMA (1:100; Dako), anti-mouse Skp2 (S-phase kinase-associated protein-2) (1:50; Abcam), anti-rabbit p27Kip1 (1:50; Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)), anti-rabbit vimentin (1:100; H-84, Santa Cruz Biotechnology, Inc.), anti-rat monoclonal E-cadherin (1:1000; Abcam), and anti-rabbit TGF-β2 (1:50; Santa Cruz Biotechnology, Inc.) overnight at 4 °C. After washing, sections were probed with the respective secondary antibodies conjugated to Alexa Fluor 488 or 545 (Invitrogen) for 45 min at room temperature and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 mg/ml in 0.1 m PBS) for 10 min. Digital images were acquired on a Nikon TE2000 microscope at 30× magnification and also on an Olympus IX81 fluorescence microscope equipped with MetaMorph software. Images were assembled using Adobe Photoshop Software. For vimentin tissue staining, samples were washed more extensively (30 h) at 4 °C to improve the quality and resolution of specific staining in fibroblasts from injured corneas due to very high expression of antigen. Thus, uninjured control samples processed in parallel required a lower threshold setting for imaging due to their relatively low levels of vimentin. In related studies, deconvolution software (MetaMorph) was also employed to assess the filamentous staining of vimentin in corneal sections. The measurement of activated p65/Rel A was assessed by its nuclear localization, which was scored by counting the numbers of nuclei (DAPI staining) in the epithelium from representative tissue sections of each treatment group (n = 8) that showed also nuclear co-staining with antibody to p65/RelA. The data were graphed as the percentage of epithelial cells showing nucleus-localized p65/RelA-positive expression.
TGF-β Treatment
Vim+/+ and Vim−/− fibroblasts (16) were cultured at low density (40% confluence) in 8-well chamber slides (Nalgene, Nunc, NY). After cell attachment, cell cultures in triplicate wells were incubated in medium containing 0.2% serum alone, or with serum plus 5 ng/ml TGF-β1 (R&D Systems) in the presence of vehicle (DMSO) or 500 nm WFA. The medium with each of these treatments was replaced every 2 days for a treatment period of 6 days. Cells were fixed in methanol and processed for immunostaining for α-SMA expression. Representative frames from triplicate experimental samples were assessed for numbers of cells positive for α-SMA staining. DAPI counterstaining was employed to provide a total count of cells in each frame.
Immunoblotting of Protein Extracts
Corneal full-thickness buttons were minced in ice-cold lysis buffer (5 mm NaF, 1 mm PMSF, 1 mm DTT, 20 mm HEPES, pH 7.5, 1 mm EDTA, 400 mm NaCl, 1 mm EGTA, 0.1% Nonidet P-40), to which was added protease inhibitor mixture (Roche Applied Science). SDS was subsequently added to a final concentration of 2% (w/v), and lysates were flash-frozen and thawed three times, passed through a G-25 syringe needle multiple times, vortexed, and finally centrifuged for 10 min at 14,000 rpm to sediment debris. The supernatant representing total proteins was collected, and an equal amount of protein (Bio-Rad protein assay) was fractionated by SDS-PAGE. For IF-poor (detergent-soluble) protein extractions, corneal tissue was extracted using a modified lysis buffer (5 mm NaF, 1 mm PMSF, 50 mm Tris, pH 7.5, 1 mm EDTA, 150 mm NaCl, 1% Nonidet P-40), to which was added protease inhibitor mixture (Roche Applied Science). The supernatant representing soluble proteins after centrifugation was adjusted with SDS-Laemmli sample buffer prior to SDS-PAGE analysis. Western blotting was performed as described previously (16). Protein blots were probed with anti-mouse desmin (1:400; Dako), anti-mouse α-SMA (1:400; Dako), anti-rabbit vimentin (1:400; V9, Santa Cruz Biotechnology, Inc.), anti-mouse Skp2 (1:200; Santa Cruz Biotechnology, Inc.), anti-mouse p27Kip1 (1:200; Santa Cruz Biotechnology, Inc.), anti-mouse p21Cip1 (1: 100; Santa Cruz Biotechnology, Inc.), anti-rabbit cyclin E (1:200; Abcam), anti-rabbit TKT (1:1000; a gift from Dr. Joram Piatigorsky (NEI, National Institutes of Health), anti-annexin II (1:200; H50, Santa Cruz Biotechnology, Inc.), anti-rabbit β-actin (1:1000; Abcam), and anti-GAPDH (1:1000; Santa Cruz Biotechnology, Inc.). Blots were reprobed as described previously (16, 26). Blots were scanned, and band intensities were quantified using National Institutes of Health ImageJ software and normalized to GAPDH or β-actin levels.
Tissue Fixation and Transmission Electron Microscopy
Corneas from two representative mice per group were used. Corneas were collected immediately after mouse sacrifice, fixed immediately in 3.5% glutaraldehyde, 4% paraformaldehyde in 0.1 m cacodylate buffer, pH 7.2, for 1.5 h at 4 °C by immersion. Tissue pieces were cut in fixative and then washed in 0.1 m cacodylate with 5% sucrose four times for 15 min each and postfixed with 1% OsO4 in buffer for 1.5 h at 4 °C. After washing in 0.1 m cacodylate buffer, they were dehydrated in a graded manner in ethanol (from 50% through 100%) at 4 °C, followed by final incubation in absolute ethanol at room temperature. Tissues were placed in propylene oxide at room temperature for 30 min, followed by infiltration with 50% Epon Araldite with accelerator-50% propylene oxide overnight. The propylene oxide-resin mixture was poured off, and tissue was placed in fresh 100% resin for 2 h. Tissues were embedded in BEEM® capsules for 48 h at 60 °C. Thin sections (70–80 nm) were obtained on a Reichert Ultracut E microtome and examined on a Philips Tecnai Biotwin 12 transmission electron microscope. Representative images from 35–50 frames/cornea were collected for analysis. A second set of corneal embedded sections were cut on a Leica UC7 ultramicrotome, mounted on 200-mesh copper/rhodium grids, and stained with 6% methanolic uranyl acetate for 4 min and Sato's lead for 4 min and examined on a Hitachi H-7650 transmission electron microscope.
Isolation of WFA-Bt-binding Proteins by Affinity Chromatography
The WFA-Bt ligand binding studies in early passage (passage between 5 and 6) rabbit corneal fibroblasts were performed as reported previously for endothelial cells and astrocytes (16, 26). In brief, fibroblast cultures plated at 60–70% confluence were incubated with 5 μm WFA or vehicle for 30 min. WFA-Bt (5 μm) was subsequently added to the medium, and cells were incubated for 2 h. Protein extracts were prepared in buffer A (5 mm Tris, pH 7.6, 50 mm NaF, 1% Triton X-100, 5 mm EGTA) supplemented with a proteinase inhibitor mixture (Roche Applied Science). An equal amount of proteins was precleared on agarose beads (Sigma) to remove nonspecific binding proteins. The beads were then centrifuged, and precleared cell lysates were repeatedly loaded three times on columns containing NeutrAvidin-agarose beads (Pierce) to maximize immobilization of biotinylated proteins. After extensive washing with ice-cold loading buffer A, bound biotinylated proteins were eluted in Laemmli gel loading buffer containing β-mercaptoethanol, subjected to SDS-PAGE on 10% polyacrylamide gels, and transferred to PVDF membrane for Western blot analysis. Blots were probed with anti-mouse vimentin (1:400; V9, Santa Cruz Biotechnology, Inc.), and anti-rabbit annexin II (1:200; H50, Santa Cruz Biotechnology, Inc.).
WFA Ligand Binding Studies with Recombinant Tetrameric Desmin and Liquid Chromatography-Mass Spectrometric (LC-MS) Analysis
Binding studies with WFA were performed essentially as described before for vimentin (16) and GFAP (26). Briefly, purified soluble recombinant human desmin (32) was incubated with vehicle or 5 μm WFA for 1 h at 37 °C. Protein samples were subjected to tryptic digestion, and LC-electron spray ionization-MS-MS analysis was performed on a ThermoFinnigan LTQ linear ion trap mass spectrometer (ThermoFinnigan, San Jose, CA). Resulting MS-MS spectra were searched against proteins in the Swiss-Prot data base with the X!Tandem search engine (see the Global Proteome Machine Web site). The assignment of fragment ions in the MS-MS of the modified and unmodified peptides was obtained with the X!Tandem protein database search engine, allowing for a variable modification of +470 Da for the WFA adduct.
Statistical Analysis
The statistical analysis to compare the percentile curves of the clarity data employed three different but related non-parametric test procedures (the Kolmogorov-Smirnov test, the Cramer-von Mises test, and Kuiper's test). All three procedures led to the same conclusion, with p values agreeing up to 4 digits. The computations were performed using SAS version 9.2.
RESULTS
Corneal Fibrotic Switch Is Blocked by Genetic and Pharmacological Deficiency of Vimentin
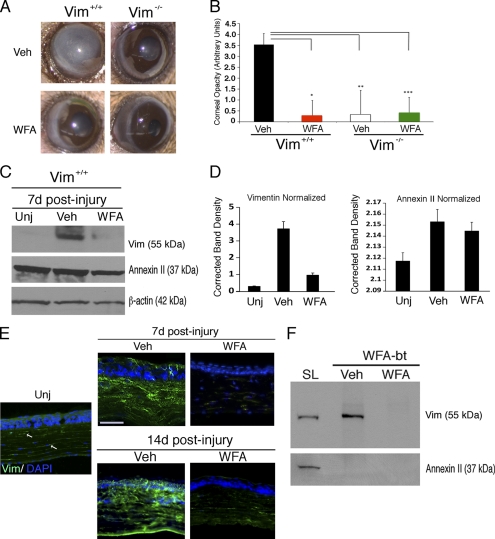
Several independent experiments described here and below indicate that the corneal fibrotic switch can be attenuated by causation of vimentin deficiency. Exploiting the modified alkali injury model (33), we show in injured corneas of Vim+/+ mice that by postinjury day 14 (d14) corneal transparency is lost due to heightened fibrosis (Fig. 1A). Surprisingly, in Vim−/− mice subjected in parallel to alkali injury, there is remarkable improvement in corneal healing, and these mice regain very significant levels of corneal transparency (Fig. 1A, right). We were prompted to ask whether use of a complementary approach to pharmacologically down-regulate vimentin would mimic the phenotype of genetic deficiency of vimentin. We exploited the vimentin-targeting small molecule WFA (16, 26) and show that in d14 injured Vim+/+ mice, treatment with 2 mg/kg WFA significantly restored corneal clarity to an extent similar to that observed in d14 healing Vim−/− mice (Fig. 1, A and B). In comparison, WFA effects on Vim−/− mice at d14 promoted levels of healing similar to those of vehicle-treated Vim−/− mice (Fig. 1B). To address whether this novel corneal reparative healing mechanism exerted in vimentin deficiency was recapitulated due to the efficacy of WFA in reducing injury-induced vimentin, we analyzed corneal tissues first by Western blot analysis (Fig. 1C). We show that injury-induced vimentin expression by d7 in Vim+/+ corneas was potently inhibited by WFA treatment. This WFA targeting activity is specific to type III IFs because annexin II, an unrelated cytoskeleton-associated protein reported also to bind WFA in cancer cells (34), was not down-regulated by drug treatment (Fig. 1D). Our findings were further corroborated by immunohistochemical analysis that showed that injury-induced expression of vimentin occurs in both corneal epithelium and in stromal cells, which was potently down-regulated by WFA at d7. Corneas of d14 Vim+/+ mice expressed higher levels of vimentin than at d7, and again, WFA potently down-regulated vimentin in both epithelium and stroma (Fig. 1E). To clarify whether vimentin-overexpressing stromal fibroblasts had invaded the epithelium, we also co-stained corneal sections with the epithelial marker E-cadherin. There were a remarkable number of filamentous vimentin-positive stromal cells found to have invaded the corneal epithelium at sites showing discontinuity of E-cadherin staining. Corneas from WFA-treated Vim+/+ mice at d14 revealed an absence of this phenotype, and stromal expression of vimentin was also greatly reduced (supplemental Fig. S1). Next, we investigated whether vimentin down-regulation is due to direct covalent binding by WFA, because previous findings we made in vascular endothelial cells and brain astrocytes showed that soluble tetrameric vimentin is targeted (16, 26). Employing a cell culture model, we differentiated naive primary cultures of corneal keratocytes to fibroblasts (35) and performed in vivo ligand binding studies to identify the target(s) of WFA. Cells were incubated with the cell-permeable biotinylated WFA analog (WFA-bt) in the presence and absence of free unconjugated WFA, and soluble proteins were affinity-isolated by streptavidin chromatography, boiled in 2-mercaptoethanol Laemmli buffer, and fractionated by gel electrophoresis (26, 29). Gel blots, when probed sequentially with vimentin and annexin II antibody, revealed that WFA-bt only formed a covalent adduct with vimentin and not with annexin II, and this binding was competed in vivo by excess WFA (Fig. 1F). Taken together, our findings corroborate that the in vivo pharmacological down-regulation of injury-induced vimentin by WFA affords protection against corneal fibrosis, a phenotype that we show is corroborated in genetic deficiency of vimentin.
FIGURE 1.
Control of vimentin expression favors corneal clarity in the alkaline burn injury model. Vim+/+ and Vim−/− mice were subjected to corneal chemical injury with limbal and corneal epithelial cell debridement and treated daily with DMSO (vehicle; Veh) or 2 mg/kg/day WFA by intraperitoneal injection for 7 and 14 days. A, representative images at d14 of Vim+/+ and Vim−/− whole eyes show dramatic reduction of corneal opacity in mice that have vimentin expression down-regulated either pharmacologically (WFA) or genetically (Vim−/−). B, quantification of corneal opacity by biomicroscopy at d14 using an opacity scale of 0 (clear) to 4 (opaque) (n = 8 samples/group). C, vimentin expression is down-regulated pharmacologically by WFA at d7 as shown by Western blot analysis of corneal buttons from uninjured (Unj), injured vehicle-treated (Veh), and WFA-treated Vim+/+ mice. Blots were probed sequentially with antibodies against vimentin (clone V9) and annexin II, and β-actin was used as loading control. Data are representative of two independent experiments (n = 4 mice/group). D, densitometric quantification of vimentin and annexin II normalized to β-actin using ImageJ software. E, immunofluorescence staining of vimentin expression (green) in thin tissue sections from corneas of uninjured, DMSO-treated, and WFA-treated Vim+/+ mice at d7 and d14. The image of the uninjured sample is enhanced compared with DMSO and WFA samples to reveal the low level of vimentin staining in corneal stromal keratocytes (white arrows). Nuclei are stained with DAPI (blue). Bars, 150 μm. Data are representative of two independent experiments (n = 4 mice/group). F, affinity isolation of WFA-bt-binding proteins from rabbit corneal fibroblasts. Corneal keratocytes were differentiated in vitro to wound fibroblasts and preincubated with DMSO (vehicle) or with 5 μm WFA for 30 min, and subsequently, both treatment groups were incubated with 5 μm WFA-bt for 2 h. Soluble protein lysates (SL) were obtained and subsequently purified over NeutrAvidin affinity columns and Western blotted by probing for vimentin and annexin II. A small amount of soluble lysate was included in parallel to demonstrate the presence of vimentin and annexin II in corneal cells. Error bars, S.D.
Corneal Myofibroblast and TGF-β Activation Is Attenuated by Vimentin Deficiency
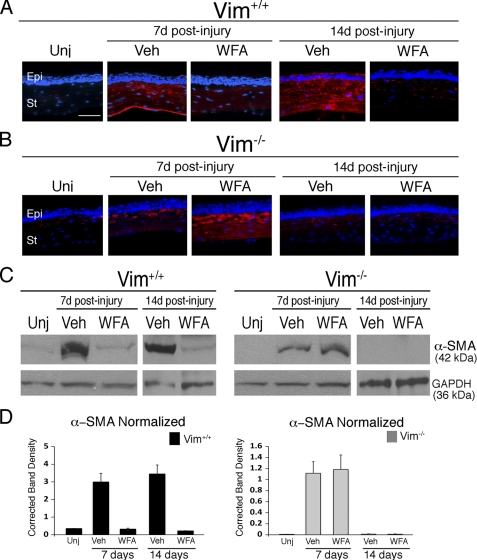
Myofibroblast activation through acquisition of α-SMA expression in wound fibroblasts is considered a major phenotypic switch that drives corneal fibrosis (36). Also, in our alkali burn model, α-SMA expression is up-regulated in the stromal cells of injured Vim+/+ mice (Fig. 2A), and its increased expression is time-dependent, reaching high levels at d14 (Fig. 2A). WFA significantly down-regulates α-SMA expression, being especially effective at d14 (Fig. 2A). Interestingly, injured Vim−/− corneas also showed early increased production of α-SMA at d7; however, this induced expression is not sustained and became devoid of detectable α-SMA expression by d14 in both vehicle and WFA-treated Vim−/− samples (Fig. 2B). Western blot analysis confirmed the staining results showing complete down-regulation of α-SMA expression in Vim−/− corneas at d14 (Fig. 2, C and D).
FIGURE 2.
Corneal fibrosis is mediated by vimentin. Vim+/+ and Vim−/− mice were subjected to corneal alkali injury and treated daily with vehicle (Veh) or 2 mg/kg/day WFA by intraperitoneal injection for 7 and 14 days. A and B, immunofluorescence staining of α-SMA (red) in repairing corneas of Vim+/+ (A) and Vim−/− (B) mice treated with vehicle or WFA. Nuclei were stained with DAPI (blue). Epi, epithelium; St, stroma. Bar, 150 μm. Data are representative of two independent experiments (n = 8/group). C, immunoblot analysis of α-SMA expression in corneal tissues from uninjured and injured Vim+/+ and Vim−/− mice at d7 and d14 treated with vehicle or WFA; GAPDH was used as loading control. D, densitometric quantification of α-SMA expression normalized to GAPDH. Error bars, S.D.
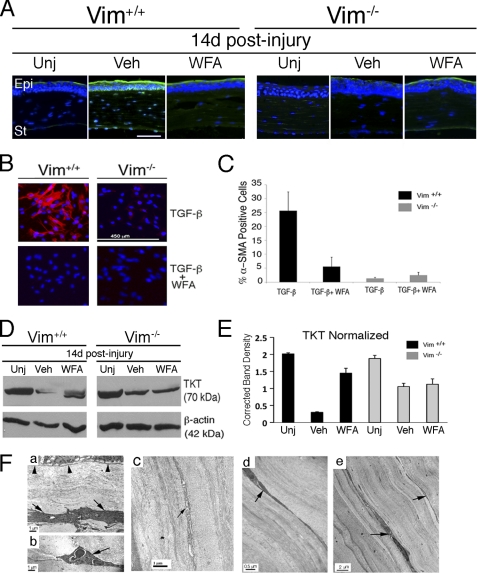
Given the well documented role for TGF-β in corneal fibrosis as a stimulator of myofibroblast transformation and mediator of α-SMA expression (36, 37), we next investigated TGF-β expression levels in injured Vim+/+ and Vim−/− corneas. We found that TGF-β was appreciably increased in the epithelium and stroma of injured Vim+/+ cornea, and WFA treatment potently down-regulates its expression to a level similar to that of the uninjured cornea (Fig. 3A). WFA treatment also potently reduced TGF-β expression in Vim+/+ corneas (Fig. 3A). On the contrary, in d14 injured Vim−/− mouse corneas, low levels of TGF-β were found in the epithelium and largely undetected in the corneal stroma (Fig. 3A). This epithelial low expression pattern was maintained in Vim−/− mice treated with WFA. To learn whether vimentin expression is necessary for TGF-β regulation, fibroblasts from Vim+/+ and Vim−/− mice were investigated. We found that Vim+/+ cells stimulated with TGF-β induced α-SMA expression by 25-fold over control, which was reduced significantly by WFA down to 5-fold over controls (Fig. 3, B and C; p < 0.05). On the contrary, Vim−/− cells responded weakly to TGF-β, resulting in only 1.3-fold mean increased expression of α-SMA (p = 0.0171), and the antagonism of WFA to this induction was not significant (p = 0.1586). Thus, we were curious whether recovery of corneas from fibrotic injury displayed other biomarkers associated with transparency, such as maintenance of tissue transketolase (TKT) (38). Western blot analysis revealed that TKT expression in d14 injured Vim+/+ corneas was reduced by 7-fold from the levels found in uninjured corneas, and WFA treatment restored TKT expression to almost control levels. In Vim−/− corneas, injury resulted in only 40% reduction of TKT expression compared with uninjured controls, and WFA did not produce any change (Fig. 3, D and E). To further compare the healing characteristics of Vim+/+ and Vim−/− corneas, we investigated their ultrastructure by transmission electron microscopy (Fig. 3F). Corneas from d14 Vim+/+ mice showed the increased presence of myofibroblasts that revealed extensive rough endoplasmic reticuli and enlarged cytoplasm (Fig. 3F, a), whereas the samples from uninjured Vim+/+ corneas showed scant rough endoplasmic reticuli characteristic of normal corneal keratocytes (Fig. 3F, c). We also found polymorphonuclear neutrophils (PMNs) in injured Vim+/+ corneas (Fig. 3F, b). WFA treatment also reduced the numbers of PMNs detected. Monocytes/macrophages were rarely detected at d14 in Vim+/+ corneas (data not shown), which suggests that the CD11b+ cells detected by immunostaining are mostly PMNs. Comparison of the fibrillary structure of collagen and fibril spacing in uninjured Vim+/+ and Vim−/− mice showed no remarkable differences (data not shown). Importantly, corneal epithelium in injured Vim+/+ mice at d14 was often observed to be thin and remarkably conjunctivalized, as shown by significant numbers of globlet cells (supplemental Fig. S2). In comparison, the epithelium of injured Vim−/− mice had acquired corneal cell characteristics similar to that of uninjured mice, which was similar also after WFA treatment in injured Vim+/+ and Vim−/− mice at d14 (supplemental Fig. S2). Collectively, these findings reveal a critical role for vimentin in myofibroblast activation that enables the corneal fibrotic switch to occur, which can be pharmacologically attenuated by WFA or impaired when vimentin is absent. Thus, vimentin deficiency results in both restoration of corneal epithelial characteristics and stromal healing.
FIGURE 3.
WFA down-regulates TGF-β expression in injured corneas. A, Vim+/+ and Vim−/− mice were subjected to corneal alkali injury and treated daily with vehicle (Veh) or 2 mg/kg/day WFA by intraperitoneal injection for 14 days. Shown is immunofluorescence staining of TGF-β2 (green) in uninjured (Unj) and injured corneas of Vim+/+ and Vim−/− mice. Nuclei were stained with DAPI (blue). Data are representative of two independent experiments (n = 8/group). B, differentiation of Vim+/+ and Vim−/− fibroblasts to myofibroblasts with TGF-β treatment in the presence and absence of 500 nm WFA. Expression of α-SMA expression (red) was assessed by immunofluorescence staining and counterstained with DAPI to mark nuclei (blue). C, percentage of cells expressing α-SMA to total number of cell nuclei from three replicates. Data are representative of two independent experiments (n = 9/group). D, Western blot analysis of corneal tissues from uninjured and injured d14 Vim+/+ and Vim−/− mice treated with vehicle or WFA. Blots were probed with polyclonal antibody to TKT followed by β-actin. E, densitometric quantification of TKT normalized to β-actin using ImageJ. F, transmission electron microscopy of mouse corneas. Vim+/+ and Vim−/− mice were subjected to corneal alkali injury and treated daily with vehicle or 2 mg/kg/day WFA for 14 days. Transmission electron microscopy images of corneas from vehicle-treated Vim+/+ mice reveal well differentiated myofibroblasts having an abundance of rough endoplasmic reticuli (a, arrow). The epithelial basement membrane appears to be intact (a, arrowheads). The presence of PMNs is also observed in vehicle samples (b, arrow). In comparison, corneas of uninjured Vim+/+ mice reveal a typical staining pattern for keratocytes (c, arrow). Corneas of Vim+/+ injured mice treated with WFA reveal mostly keratocytes and wound fibroblasts (d, arrow), and myofibroblasts (not shown) were only rarely found. Injured Vim−/− corneas also contain keratocytes/fibroblasts, which were similarly observed in injured corneas of Vim−/− mice treated with WFA (d and e, arrows). Error bars, S.D.
Vimentin Expression Supports Inflammatory Cell Recruitment in Injured Corneas
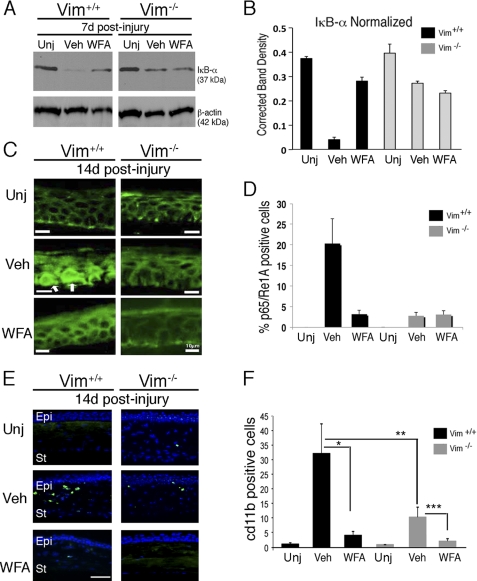
Alkali injury to the cornea induces an inflammatory cascade driven by NF-κB transcription factor activation, where its pharmacological inhibition has been shown to also improve corneal burn recovery in mice (39). We inquired whether the in vivo efficacy of WFA upon corneal injury is likewise also due to its potent NF-κB inhibitory activity (24, 40) that is potentially mediated by vimentin. Injured Vim+/+ corneas assessed at d7 showed reduction of IκB-α levels, whereas Vim+/+ and Vim−/− mice treated with WFA as well as vehicle-treated Vim−/− mice exhibited restoration of IκB-α in their corneas to ∼75% of the levels that were found in uninjured tissues (Fig. 4, A and B). These results were further confirmed by immunostaining corneal tissues at d14, where we found that p65/RelA was nucleus-localized in injured Vim+/+ corneas, whereas p65/RelA expression in all other injured corneas was found to be mostly cytoplasmic (Fig. 4C). Quantification of nuclear expression of p65/RelA in epithelium (overlap with DAPI; data not shown) revealed 20-fold induction in injured Vim+/+ corneas over uninjured corneas (p < 0.0001), which was reduced to 4-fold by WFA treatment (p < 0.0001). Injury to Vim−/− corneas produced only a 4-fold induction over uninjured corneas (p < 0.001), and this induced nuclear expression was not altered by WFA activity (p = 0.2295) (Fig. 4D). These data provide evidence that vimentin regulates NF-κB activation in the alkali injury model.
FIGURE 4.
WFA down-regulates expression of inflammatory markers during corneal repair. Vim+/+ and Vim−/− mice were subjected to corneal alkali injury and treated daily with vehicle (Veh) or 2 mg/kg/day WFA for 7 and 14 days. A, immunoblot analysis of corneal tissues from uninjured and injured Vim+/+ and Vim−/− mice at d7 treated with vehicle or WFA. B, densitometric quantification of IκB-α normalized to β-actin. C, immunolocalization of p65 RelA/NF-κB staining (green) in the epithelium of Vim+/+ and Vim−/− corneas at d14 showing nuclear localization of p65 (white arrows in the basal layer) in vehicle-treated Vim+/+ sample. Bar, 10 μm. D, percentage of cells showing p65/RelA staining in nuclei of epithelial cells was determined by comparing with DAPI staining (not shown). Data are representative of two independent experiments (n = 8/group). E, immunofluorescence staining of CD11b (green) in Vim+/+ and Vim−/− corneas at d14. Nuclei were counterstained with DAPI (blue). Epi, epithelium; St, stroma. Bar, 150 μm. Data are representative of two independent experiments (n = 8/group). F, numbers of CD11b+ cells detected in corneal tissue sections (n = 8/group). *, p = 0.0025; **, p = 0.010; ***, p = 0.0053. Error bars, S.D.
Alkali injury induces inflammation through recruitment of monocyte/macrophages and neutrophils that infiltrate into corneas, where increased numbers of CD11b+ neutrophils were associated with corneal opacity (41). We assessed corneas of Vim+/+ and Vim−/− mice at d14 (Fig. 4E), which is the peak period of inflammatory cell infiltration in this model (42). Alkali injury significantly increased CD11b+ cell numbers in Vim+/+ corneas compared with Vim−/− corneas, showing a 32-fold (p = 0.0015) versus a 10-fold (p = 0.01004) increase, respectively. WFA treatment reduced the numbers of CD11b+ cells infiltrating the injured corneas producing a 4.2-fold increase (p = 0.002525) in Vim+/+ versus a 2.2-fold increase (p = 0.005387) in Vim−/− corneas compared with their respective uninjured controls (Fig. 4F). This suggested that although vimentin deficiency significantly reduced the numbers of CD11b+ cells, WFA treatment in combination with vimentin deficiency resulted in the most potent inhibition of CD11b+ cell infiltration. Our result corroborates previous findings showing that deficiency in type III IFs severely attenuates CD11b+ infiltration into injured tissues of mice, as revealed in the retinal detachment model (43).
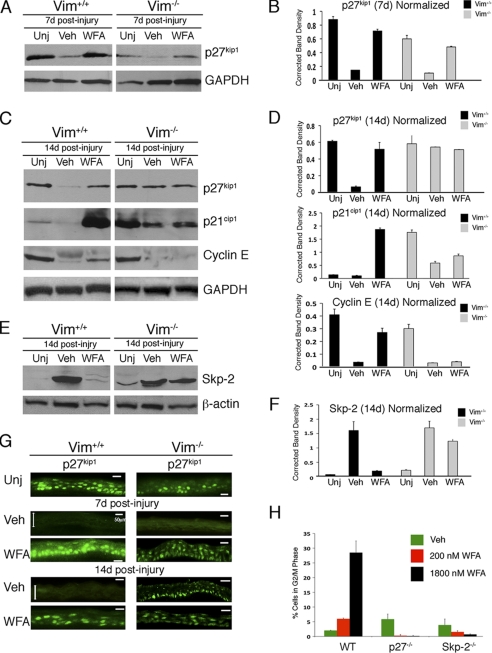
Corneal Epithelial Expression of p27Kip1 Is Restored by Genetic and Pharmacological Down-regulation of Vimentin
We next investigated whether the critical determinants of corneal healing are due to altered cell proliferation characteristics that are different between Vim+/+ and Vim−/− mice. The CKI p27Kip1 is critically implicated in regulation of epithelial regeneration in the injury-healing cornea (44, 45). We show by Western blots that p27Kip1 is potently down-regulated by injury at d7 and d14 in Vim+/+ corneas. WFA treatment at d7 and d14 restored p27Kip1 to levels similar to that of uninjured mice (Fig. 5, A–D). p27Kip1 expression levels were also reduced at d7 in Vim−/− corneas and restored to nearly normal levels by WFA treatment (Fig. 5, A and B). However, at d14, the expression of p27Kip1 differed between the two mouse lines; in the injured Vim+/+ corneas, p27Kip1 expression was persistently down-regulated, and WFA restored its expression back up to levels found in uninjured corneas (Fig. 5, C and D). On the other hand, in the injured Vim−/− corneas, p27Kip1 expression was fully recovered, and WFA treatment did not further alter its expression (Fig. 5, C and D). Immunostaining of tissues at d7 and d14 confirmed that nucleus-associated changes in p27Kip1 expression (overlap with DAPI staining; data not shown) were responsible for the alterations in protein analyzed by Western blotting and, importantly, that induced p27Kip1 expression was predominantly occurring at the basal cell layer of the epithelium, which is the site of mitosis (Fig. 5G) (46). Because p27Kip1 expression is tightly controlled through the ubiquitin proteasome pathway via the activity of E3 ubiquitin ligase Skp2 (30), we investigated next the expression of the other cell cycle targets of Skp2 by Western blotting. The CKI p21Cip1 was expressed at low levels in uninjured corneas and became nearly undetectable in injured Vim+/+ corneas at d14. WFA induced p21Cip1 expression by over 13-fold in d14 Vim+/+ healing corneas (Fig. 5, C and D). Interestingly, uninjured corneas of Vim−/− mice expressed 3.4-fold higher levels of p21Cip1 compared with uninjured Vim+/+ corneas. Consequently, in injured Vim−/− corneas, p21Cip1 levels declined by 7.5-fold, and WFA treatment did not alter this expression level. On the other hand, in Vim+/+ corneas, cyclin E expression was expressed at high basal levels in uninjured tissue and down-regulated by injury (Fig. 5, C and D). This expression was restored by WFA to levels almost reaching that of uninjured corneas in Vim+/+ mice. However, in injured Vim−/− corneas cyclin E was highly down-regulated with injury and remained down-regulated even after WFA treatment (Fig. 5, C and D). Finally, we investigated whether expression levels of Skp2 were also affected by WFA activity. Skp2 expression was highly induced in injured corneas of Vim+/+ mice, and WFA treatment potently down-regulated Skp2 expression to levels found in uninjured corneas (Fig. 5, E and F). There was a similar level of injury-induced expression of Skp2 in Vim−/− corneas at d14; however, WFA treatment reduced Skp2 expression by less than 50%. Taken together, these findings reveal that Skp2 expression is induced with injury in both Vim+/+ and Vim−/− corneas, but its down-regulation by WFA activity that is partly enabled in vimentin deficiency does not result in control of its targets p27Kip1, p21Cip1, and cyclin E in Vim−/− corneas.
FIGURE 5.
WFA cell cycle activity during corneal repair is mediated by vimentin. Vim+/+ and Vim−/− mice were subjected to corneal alkali injury and treated daily with vehicle (Veh) or 2 mg/kg/day WFA for 7 and 14 days. Corneal tissues were isolated, and equal amounts of protein extracts were subjected to Western blotting and probed sequentially with antibodies against p27Kip1, p21Cip1, and cyclin E (A and C). Blots from d14 samples were also probed with antibody to Skp2. E, densitometric quantification of proteins to GAPDH (B and D) and β-actin (F) was performed using ImageJ (National Institutes of Health). G, immunolocalization of p27Kip1 staining (green) in the epithelium of Vim+/+ and Vim−/− corneas at d7 and d14. Double arrowheads delimit the epithelium in injured (Veh) samples. Data are representative of two independent experiments (n = 8/group). Bar, 50 μm. H, WFA induces G2/M cell cycle arrest. Embryonic fibroblasts from wild-type (WT), p27Kip1-deficient, and Skp2-deficient mice were stimulated to proliferate in the presence of vehicle or WFA and subjected to flow cytometry for cell cycle analysis. Data are representative of two independent experiments. Error bars, S.D.
Because Skp2 deficiency causes sustained expression of p27Kip1 that results in decreased corneal epithelial cell proliferation (44), we next investigated whether the growth-inhibitory activity of WFA was dependent on the Skp2-p27 axis. We exploited a cell culture model using mouse embryonic fibroblasts (MEFs) from wild-type and Skp2- and p27Kip1-deficient mice (30). WFA induced potent G2/M cell cycle arrest in a dose-related manner in wild-type MEFs, but Skp2- and p27Kip1-deficient MEFs completely lose this growth inhibition at both low and high concentrations of WFA (Fig. 5H). Taken together, consistent with the major role of Skp2 in causing p27Kip1 degradation during G2-M progression (47), our findings identify the critical importance of the Skp2-p27Kip1 axis in the cell cycle-targeting effects of WFA.
A Reverse Chemical Genetic Approach Identifies Desmin as Second Binding Target of WFA
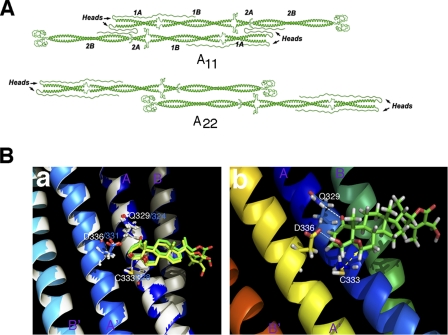
Type III IFs are highly homologous and share as much as 62% overall amino acid identity (32). Because WFA covalently binds to the highly conserved rod 2B domain of tetrameric vimentin (16) and the corresponding 2B domain of tetrameric GFAP (26), we postulated that WFA should similarly also bind to the third IF member, desmin, at its rod 2B region that is highly conserved with that of vimentin (16). Therefore, we developed a molecular model for the tetrameric 2B segment of desmin bound to WFA using the tetrameric A22 orientation (48), which was also used previously to develop molecular models for vimentin (16) and GFAP (26). Stable binding of WFA in the ligand-binding pocket of tetrameric desmin (i.e. between the antiparallel half-staggered coiled-coil dimers) was revealed by docking analysis of the molecular dynamics-simulated human desmin-WFA complex (Fig. 6). As shown with the vimentin-WFA and GFAP-WFA complexes (16), the invariant cysteine amino acid residue (Cys-333) in the desmin helix lies in close proximity to the C3 and C6 carbons of WFA (Fig. 6A), which facilitates a proper orientation for nucleophilic attack by Cys-333 on the electrophilic carbon centers of WFA (Fig. 6B). Having also identical residues in desmin that make contact with the ligand, Gln-329 can form a hydrogen bond with the C1-carbonyl group of WFA, and Asp-336 can hydrogen-bond with the C4-hydroxyl group of WFA. Therefore, we can superimpose the binding site of desmin-WFA on that of vimentin-WFA to reveal that the binding mode of WFA is nearly identical in both tetramer fragments (Fig. 6B). Sharing a high degree of conservation in a region of 44 amino acids between vimentin and desmin, this segment of the 2B region bound by WFA has also 70.5% identical amino acids and 84% similarity (supplemental Fig. S3). Last, the molecular mechanics/Poisson-Boltzmann surface area-calculated binding energies for the two complexes are found to be ΔGbind ∼ −17.3 and −17.4 kcal/mol for vimentin-WFA and desmin-WFA, respectively. To validate this molecular model, we also performed in vitro binding analysis with purified human recombinant tetrameric desmin and WFA. Mass spectrometric sequencing analysis of tryptic peptides showed that WFA binds covalently at the predicted Cys-333 residue, which was revealed by the addition of the molecular mass of WFA to this amino acid residue (supplemental Fig. S4), and these data are in agreement with our previous findings for vimentin and GFAP (16, 26).
FIGURE 6.
Molecular model of WFA-desmin. Atomic model of tetramers formed by head-to-tail dimers in the A11 and A22 configurations (A) that was assembled using a part of a graphic representation previously published (48). B, ribbon representation of the molecular dynamics-simulated desmin-WFA complex structure overlapped with vimentin-WFA complex. a, superposition of the desmin-WFA complex with the previously simulated vimentin-WFA complex (16) in which the ribbon structures of the desmin and vimentin are in white and blue, respectively. Amino acids that hydrogen-bond with ring A of the ligand are represented by their stick structures. b, ribbon structure of desmin-WFA showing hydrogen bonds between Gln-329 (Q329) and C1-ketone of WFA and between Asp-336 (D336) and the C4-hydroxyl group of WFA. The β-oriented 5,6-epoxide of WFA is positioned for nucleophilic attack by Cys-333 (C333) (yellow dotted arrow).
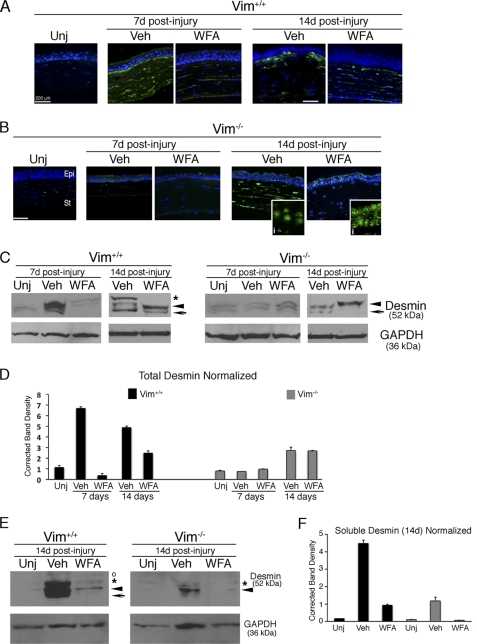
WFA Targets Desmin Expression in Vivo
We next investigated by immunohistochemical analysis whether desmin might be up-regulated during corneal fibrosis and thus become a potential target for WFA. Uninjured corneas did not show expression of desmin. In injured Vim+/+ corneas, desmin IFs were found overexpressed in conjunctivalized epithelium and stroma at d7, and this expression pattern was potently down-regulated by WFA treatment (Fig. 7A). Contrary to its abundant expression in injured Vim+/+ corneas, desmin IF expression in Vim−/− corneas at d7 was barely apparent in the stroma (Fig. 7B). WFA treatment potently down-regulated desmin also in Vim−/− corneas at d7 to levels that became undetectable. Desmin IF staining was increased in the corneas of injured Vim+/+ mice at d14, being strongly expressed in the anterior stroma and subepithelial region, which was potently down-regulated by WFA. Remarkably, in Vim−/− corneas, desmin IF staining became more strongly expressed in the stroma at d14, and lower levels of expression in the epithelium were noted as dots (Fig. 7B, inset). WFA treatment down-regulated desmin staining in the stroma, and only the epithelium showed an increased dotlike nucleus-associated staining pattern, suggesting IF polymer disassembly. Because the tissue staining for desmin reveals almost exclusively the abundant polymeric structures of this protein in that the less abundant soluble forms are undetected, we also performed Western blotting experiments to assess the effects of WFA on total and soluble desmin expression. Low levels of total desmin expression were observed as a doublet band in uninjured Vim+/+ corneas representing mural cells of preexisting limbal blood vessels. However, Vim+/+ corneas at d7 and d14 showed the presence of multiple bands indicating different desmin isoforms or post-translationally-processed variants. Injured Vim+/+ corneas also showed the greatest abundance (4–8-fold increase), and the number of desmin species, including a prominent 52 kDa upper band, most prominently increased at d14 (Fig. 7, C and D). WFA treatment potently abrogated expression of these desmin bands at d7 and also caused quantitative changes in the abundance of lower molecular weight species at d14 (Fig. 7D). On the other hand, Vim−/− uninjured corneas expressed similarly low levels of the two desmin variants as found in d7 corneas, and these desmin variants became increased by 2-fold at d14. Interestingly, the protein band profile of desmin variants in Vim+/+ mice treated with WFA showed a similar pattern to that of vehicle-treated Vim−/− corneas at d14, revealing also the striking absence of the prominent 52-kDa disease-associated desmin species. On the other hand, WFA-treated Vim−/− corneas at d14 revealed a single desmin variant/species that probably derives from the desmin dots observed in the epithelium. Because previous findings showed that WFA targets soluble tetrameric vimentin and GFAP that results ultimately in their insoluble forms being depolymerized in vivo (16, 26), we next investigated whether soluble desmin was targeted by WFA in vivo. Soluble (low salt-extracted) proteins (26) were isolated and Western blotted for desmin. The injury-induced soluble desmin isoform (52 kDa band) was most strikingly reduced by WFA in d14 Vim+/+ corneas, whereas in Vim−/− d14 corneas, both injury-induced soluble desmin bands were down-regulated by WFA (Fig. 7, E and F). Collectively, these findings reveal that desmin is differentially regulated in injured Vim+/+ compared with Vim−/− corneas, and furthermore, in vimentin deficiency, WFA potently targets injury-induced soluble desmin expression, resulting in IF disassembly.
FIGURE 7.
WFA down-regulates injury-induced desmin expression in healing corneas. Vim+/+ and Vim−/− mice were subjected to corneal alkali injury and treated daily with vehicle (Veh) or 2 mg/kg/day WFA for 7 and 14 days. A and B, temporal induction and localization of desmin (green) in repairing tissues of corneas from Vim+/+ (A) and Vim−/− (B) corneas from uninjured (Unj) and vehicle- and WFA-treated mice. Higher magnified images of Vim−/− corneal sections at d14 reveal a novel dotlike staining pattern for desmin in the epithelium (i) that appears nucleus-associated. Nuclei were stained with DAPI (blue). Epi, epithelium; St, stroma. Bar, 200 μm. Data are representative of two independent experiments (n = 8/group). C, total corneal tissue lysates were also prepared from d7 and d14 mice, subjected to Western blotting, and probed with desmin antibody. *, major 52-kDa protein species; arrowhead and arrow, lower molecular weight desmin species/variants differentially regulated. D, densitometric quantification of desmin in Vim+/+ and Vim−/− samples normalized to GAPDH. E, Western blot analysis of soluble desmin from corneas of d14 Vim+/+ and Vim−/− mice. *, major 52 kDa band; arrowhead and arrow, smaller sized desmin variants; open circle, a nonspecific band. F, densitometric quantification of soluble desmin in Vim+/+ and Vim−/− samples normalized to GAPDH. Error bars, S.D.
Desmin Targeting Attenuates Corneal Haze in Vimentin Deficiency
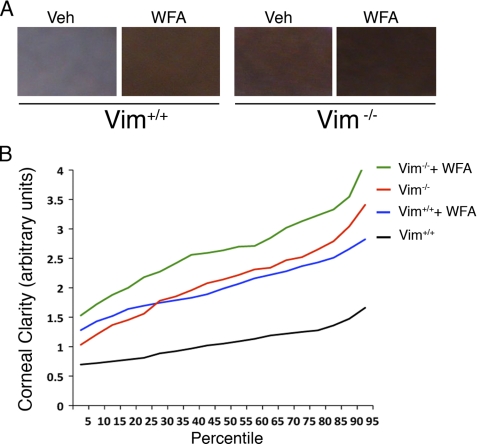
Finally, we wondered whether the targeting by WFA of desmin in vimentin deficiency produced an effect on the quality of corneal repair beyond the noticeable attenuation of corneal fibrosis. We hypothesized that overexpression of desmin in Vim−/− corneas at d14 could potentially alter refractive functions of the cornea via expression in wound fibroblasts. To afford a sensitive and objective quantitative method to measure corneal clarity in mice, we developed a computer-based algorithm to assess the level of corneal transparency. Slit lamp biomicroscopy affords visualization of iris structural details, enabling the examiner to score corneal transparency; thus, we premised that detection of pigment color of the iris using a digital method (49) could also be adopted as a surrogate measure of corneal transparency. Consequently, opacity from injury-induced fibrosis or haze that causes the cornea to whiten should alter the color spectrum captured in digital images. According to principles of color theory, white light is composed of equal representation of red, green, and blue (supplemental Fig. S5); therefore, corneal opacity that renders the cornea white in color represents a deviation from the equal mixing of red, green, and blue. The advantage of this color detection method is its digital sensitivity and that color interpretation is not left to human subjectivity (50). We coded the digital photographic images of mouse eyes and obtained in a blinded manner three representative equal sized rectangular segments (Fig. 8A) that were taken from non-overlapping regions of the cornea (avoiding the central pupil due to the underlying lens). The image collection library was analyzed to measure deviation from the brown color base line of uninjured eyes, which was valid because both Vim+/+ and Vim−/− mice in the 129 Svev strain have equivalent brown colored irises. The opacity values for injured vehicle- and WFA-treated groups were plotted as a function of their percentile distributions (Fig. 8B), which revealed three important results. First, injured Vim+/+ corneas that ranked even in the top 10th percentile for clarity came only as close as the lowest 80th percentile group of injured Vim−/− corneas (mean 1.47 versus 1.42, p < 0.008). This result confirmed our visual biomicroscopic assessment scoring data (Fig. 1B) that the Vim−/− mice have a significant advantage over Vim+/+ mice for healing such corneal injuries (mean 1.09 versus 2.13, p < 0.0001). Second, vehicle-treated Vim−/− mice showed a similar percentile rank distribution for corneal clarity as WFA-treated Vim+/+ mice (mean 2.04 versus 2.13, p = 0.1704), a finding also supported by visual scoring data revealing that the pharmacological down-regulation of vimentin had similar protective effects as genetic deficiency of vimentin. Last, and to our surprise, we found that restoration of corneal clarity was the greatest in Vim−/− corneas when mice were treated with WFA (mean 2.13 versus 2.67, p < 0.0001). Statistical comparison of percentile distributions for human scored data and computer-assisted image analysis was also performed (supplemental Table S1). This illustrated the greater sensitivity of the computer-assisted image method over human-scored data. Taken together, we have identified vimentin as a major driver of the proliferative mechanism of corneal fibrosis and identified that the underlying haze observed in injured corneas of Vim−/− mice is associated with overexpressed desmin. This desmin-related corneal haze that is also responsive to WFA treatment results in significant improvement of corneal clarity, which appears not to be directly associated with the cell cycle regulatory mechanism of WFA.
FIGURE 8.
Computer-aided imaging analysis of corneal transparency. A, representative images of injured (vehicle; Veh) and WFA-treated corneas of Vim+/+ and Vim−/− mice showing opacity that ranked at the 50th percentile for corneal clarity. B, corneal clarity values for Vim+/+ and Vim−/− mice treated with and without WFA (n > 150 images/group) were plotted as a function of their percentile rank distributions to reveal the trends in healing for each group. Maximal clarity scores are at 4.0.
DISCUSSION
The major finding of this study is that vimentin deficiency alters the fibrotic response to corneal alkali injury and instead engages a reparative healing mechanism to restore corneal clarity. We validate this novel discovery by complementing the genetic deficiency of vimentin with a pharmacological approach to cause vimentin down-regulation, and thus, we also illuminate vimentin as a novel druggable target for potential treatment of fibrotic conditions.
A remarkable conclusion drawn from this study is that the pharmacological down-regulation of vimentin by WFA in Vim+/+ mice broadly recapitulates the repair mechanism elicited in the genetic deficiency of vimentin in this corneal alkali injury model. In this respect, we already have shown that vimentin deficiency impairs corneal neovascularization in this alkali injury model (16). Additionally, we recently reported that retinal gliosis manifested in response to corneal alkali burns was similarly attenuated in Vim−/− mice and mediated by restoration of p27Kip1 expression in retinal ganglion astrocytes and glia, which mimicked the activity of WFA in the retina (26). Here, we identify that in addition to restoration of p27Kip1 expression in the healing corneal epithelium by WFA, the coordinate up-regulation of p21Cip1 expression may be critical to the mechanism of corneal reparative healing elicited by vimentin down-regulation. Considering that p27Kip1 null mice develop retinal gliosis and dysplasia even in the absence of an injury (47, 51), whereas the corneas of these mice do not display ectopic or inappropriate epithelial proliferation even after corneal scrape injuries (44), this suggests that CKIs in addition to p27Kip1 may be important for corneal mitotic arrest. This rationale is supported by the loss of corneal transparency in lumican-deficient mice that occurs, in part, through down-regulated p21Cip1 expression, which causes aberrant cell proliferation (52). Taken together, our findings underscore that a novel vimentin-mediated negative regulation of CKI expression is associated with the corneal fibrotic switch in traumatic injury healing that is blocked by suppression of vimentin expression.
Vimentin is a motile protein that exists in many dynamic native states, most noted by its abundant filamentous structures that adorn the cytoplasm of mesenchymal cells (53). Soluble tetrameric vimentin (54), only a small fraction of cellular vimentin, undergoes annealing to form intermediate unit-length filaments that become rapidly incorporated into long polymeric forms (7, 55). As cells initiate G1 cell cycle phase, both protein synthesis of vimentin and its incorporation into polymers occur (56). Investigating the cell cycle, we previously showed that WFA targets tetrameric vimentin in vitro and in vivo, resulting in G0/G1 cell cycle arrest in endothelial cells and astrocytes (16, 26). We have also shown that the pool of soluble tetrameric vimentin becomes depleted in a WFA concentration-dependent manner, where at the higher concentrations of WFA, filamentous vimentin collapse around the nucleus and ultimately their depolymerization was observed (16, 26). Whereas it is still unclear how WFA-targeted soluble vimentin affects this dynamic process in vivo, phosphorylation-dependent mechanisms that govern vimentin functions during cell cycle progression (57) may be affected by WFA (26). It is noteworthy that the dynamic reorganization of filamentous vimentin is also quite noticeable at prometaphase, where granular dotlike structures believed to be depolymerized IFs become plentiful (58). This enriched pool of soluble vimentin might also be targeted by WFA during G2 cell cycle progression to cause mitotic arrest. Importantly, our findings also identify that both Skp2 and p27Kip1 are required for the induction by WFA of G2/M cell cycle arrest as revealed in our study of MEFs from Skp2- and p27Kip1-deficient mice. Corroborating this mechanism, down-regulation of Skp2 expression was also induced by a related natural product, a withanolide (59), that contains the conserved steroidal backbone and critical pharmacophore moieties of WFA (29) shown to be important for binding to the 2B region of type III IFs (16, 26). This growth-inhibitory mechanism would be consistent with Skp2 expression becoming maximal at S and G2 phase (60), which also represents the critical time when p27Kip1 degradation by Skp2 occurs in G2 phase in order for progression into mitosis (47). Thus, identification that Skp2 and its target p27Kip1 are reciprocally responsive to the activity of WFA in injured corneas of Vim+/+ mice, but not in Vim−/− mice, links vimentin as a mediator of WFA activity on the critical Skp2-p27Kip1 cell cycle axis. Furthermore, because p21Cip1 and cyclin E, other targets of the E3 ligase activity of Skp2 (30, 61), also responded to WFA activity in a vimentin-mediated manner during injury healing, it is clear that vimentin targeting can have profound regulatory control on many critical cell cycle regulators. This is obviously important to therapeutic development efforts that recognize that suppressing Skp2 expression or interfering with its E3 ligase function has wide applications to diverse proliferative disorders and malignant conditions characterized by Skp2 overexpression (30, 62). Taken together, we believe that our findings illuminate vimentin as a novel druggable target for regulatory control over the critical Skp2-p27Kip1 axis and have also borne out WFA as an important chemical probe of this mechanism.
It is interesting that vimentin down-regulation by WFA results in the differential regulation of p21Cip1 compared with p27Kip1. This was noted in injured Vim+/+ corneas treated with WFA having much higher expression of p21Cip1 compared with p27Kip1. One possible explanation is that p21Cip1 becomes targeted for degradation in prometaphase by the anaphase-promoting complex/cyclosome (APC/Ccdc20) (63). Thus, if WFA activity were to cause APC/C down-regulation in injured Vim+/+ corneas, this would alleviate negative control over p21Cip1 to cause p21Cip1 levels to become elevated. In fact, WFA has been demonstrated to cause APC/C down-regulation and promote G2/M cell cycle arrest (64), which would suggest that this mechanism could be relevant to the activity of WFA in the cornea. Given the complexity of regulatory control of Skp2 and APC/C in exacting timed destruction of CKIs, recognition that TGF-β-induced cell cycle arrest occurs by limiting Skp2 expression at post-transcriptional levels via promoting its nuclear translocation and subsequent degradation by APC/Ccdh1 adds another level of regulation to CKI expression (15, 65). Whether APC/C is also affected by genetic deficiency of vimentin is not known, but this may be one possible reason for the basal levels of p21Cip1 being higher in Vim−/− corneas compared with Vim+/+ corneas. We postulate that Vim−/− corneas having reduced levels of TGF-β may alter the effectiveness of APC/Ccdh1 to regulate Skp2 and, hence, lose control over its nuclear targets in Vim−/− corneas. Furthermore, our data suggest that while the mechanism(s) governing induction of Skp2 expression with injury are apparently not affected in vimentin deficiency, a vimentin-independent pathway induced by WFA contributes to partial Skp2 down-regulation in injured Vim−/− corneas. We speculate that desmin targeting by WFA may be responsible for down-regulation of Skp2 expression in Vim−/− corneas. Thus, the differential regulation of CKIs in vimentin deficiency remains to be further investigated.
It has recently become recognized that Skp2 acts as a node governing the integration of signals from mechanical tension and growth factors to regulate cell proliferation (66). Such tension-related integration of signaling through Skp2 can impact fibrosis, which was demonstrated by therapeutic delivery of Skp2-targeting siRNAs to provide protection against fibrosis and reduce scarring in a glaucoma filtration surgery model (67). Also, Skp2-deficient mice are protected from renal fibrosis from p27Kip1 up-regulation in epithelial cells (68). However, in the cornea, desmin is not expressed after simple incision injuries that normally heal without induction of fibrosis (69). This may explain the clinical success of numerous types of scar-less surgical procedures being performed to correct for visual defects of the human cornea (70). However, expression of both vimentin and desmin is remarkably elevated in corneas that develop opacity, which was illustrated in an animal model of laser photo ablation that emulates the clinical outcomes of failure to heal in this popular type of laser vision corrective procedure (22). Hence, our finding of overproduced desmin in Vim+/+ fibrotic corneas and the persistence of its expression, although at significantly lower levels, in Vim−/− corneas that display residual opacity or “corneal haze” was compelling evidence to also investigate desmin as a potential corneal fibrotic mediator. We believe that increased cell stiffness produced by desmin IFs (71) could contribute to altering the viscoelasticity of the cornea (70). In this respect, thermal burn injury increases the stiffness of corneal tissue (72), suggesting that such fibrotic injuries that affect corneal viscoelasticity from edema and increasing intraocular pressure (73) may also be governed by type III IFs. Furthermore, our finding of desmin localization in injured Vim+/+ corneas at the subepithelial and anterior stroma suggests that the anterior region of alkali-injured Vim+/+ corneas is also under greater stress than elsewhere. It is intriguing that the anterior region of the cornea is also known to be stiffer than the posterior stroma (74). Although the role of desmin in viscoelastic properties of the corneas has not been investigated before, stress studies conducted in other viscoelastic tissues have identified that microarteries (resistant arteries) that express the greatest amounts of desmin are most affected, whereas large compliance arteries that have low levels of desmin are not affected when desmin is deficient (75). These resistant arteries are most affected because of their dependence on IFs for maintaining viscoelasticity, where desmin promotes transmission of contractile force to cell surface. Similar studies made in the bladder confirm such a role for desmin in viscoelasticity (76). Thus, it remains to be investigated whether the viscoelastic function of desmin is truly responsible for the corneal haze we observed in injured Vim−/− corneas.
On the other hand, the reduction of TGF-β, a profibrogenic stimulus for myofibroblast differentiation, in Vim−/− mice and in WFA-treated Vim+/+ mice points to a suppression of a fibrotic switch elicited in genetic and pharmacological deficiency of vimentin. That direct blockade of TGF-β (39) or antagonizing its signaling mechanism in corneal cells (77) inhibits α-SMA expression in corneal stroma and restores corneal clarity in alkali injured mice further supports the idea that this cytokine is relevant to therapeutic strategies being considered for treatment of corneal fibrotic disorders (37). In this respect, the regulatory control of TGF-β biogenesis and its activity is associated with mechanical forces exerted by stromal myofibroblasts on the extracellular matrix that facilitates a positive feedback loop, where mechanical tension in the stroma promotes and sustains the myofibroblast phenotype through activation of α-SMA expression (78). Overexpression of both vimentin and desmin in wound fibroblasts would provide this contractile force and also enable long distance communication within the mesenchyme of the repairing tissue enabled by polymeric IFs. Through such mechanical tension (79), myofibroblasts/fibroblasts can continuously sense the changing geometry of the corneal stroma as it remodels its injured tissues (36, 80). Indeed, vimentin expression is observed extended to the tips of the fibroblastic cellular processes, where these extensions connect over 100 μm in length across the cornea; injured Vim−/− corneas would have significant deficits in this tension-induced fibrotic positive feedback loop, which probably reflects the lower expression levels of α-SMA and TGF-β expression (81) and delayed expression of desmin. Moreover, suggesting a common function of these IFs in cell stiffness that contributes to pathology, increased expression of vimentin and GFAP in retinal glia contributes to glial scarring (82). In a similar vein, overexpression of vimentin with desmin may also contribute to corneal scarring due to increased stiffness of fibroblasts/myofibroblasts because cells that lack vimentin are known to lose their stiffness (19), which may be due to loss of IF-related nanomechanical functions (6).
Last, although vimentin and desmin are expressed in the fibrotic cornea, their respective roles are likely to be different. First, desmin expression is rarely observed in α-SMA-expressing myofibroblasts (data not shown). Second, desmin expression in injured Vim−/− corneas occurs at later stages of injury repair, when other fibrotic biomarkers were attenuated; this disassociates a role for desmin in fibrotic cell proliferation. Furthermore, overexpressed vimentin but not desmin is observed with corneal opacity in other corneal alkali burn injury models in rabbits (69). Although the reason for desmin not being up-regulated in rabbits is unclear presently, the critical role of vimentin overexpression in fibrosis is highlighted. On the other hand, our findings identifying TKT down-regulation in fibrotic corneas and its restoration by both genetic absence and pharmacological down-regulation of vimentin validate TKT loss as a biomarker of corneal fibrosis (38). This also identifies that TKT regulation in corneal fibrosis may be uncoupled from desmin-related refractive aberrations. In this respect, our findings would support the model in which TKT loss contributes significantly to increased refractive properties of myofibroblasts (83), suggesting that TKT-independent mechanisms unveiled in Vim−/− corneas may also contribute further to the quality of corneal repair.
In conclusion, our finding that alkali injured Vim−/− corneas engage in a reparative healing process leading to significant improvement in corneal refractive function came as a surprise because of reports of defective wound repair in Vim−/− mice (19, 20). Moreover, vimentin deficiency has also been shown to reduce epithelial cell migration (84). Importantly, these prior studies have investigated the role of vimentin in normal physiological tissue repair, where the contribution of vimentin's contractile forces to promote stromal tissue contraction is important to seal a wound with scar tissue (20, 70), whereas in the epithelium, cell migration is a requirement for resurfacing scrape injuries (84). Because embryonic tissue repair is also defective in Vim−/− mice (20), which is a situation where myofibroblasts are not involved (85), clearly it is the contextual nature of the injury that determines when a lack of vimentin expression can be either beneficial or detrimental to the outcome. In this corneal alkali injury model, stromal fibroblast/myofibroblast invasion into the corneal epithelium was also attenuated by pharmacological and genetic deficiency of vimentin. Thus, from a clinical treatment paradigm, traumatic corneal conditions that progress to fibrosis with IF overexpression would most likely benefit from down-regulation of vimentin and desmin. Our rationale appears to be consistent also with other traumatic injury models studied in the CNS, where the deficiencies of type III IFs have promoted regenerative healing (11). On the other hand, WFA does not show toxicity to sensitive neuroretinal tissues or cause lens cataracts (26, 27), and importantly, Vim−/− mice also do not develop cataracts developmentally or have an increased sensitivity to cataracts after being subjected to alkali injury.3 Thus, the recent finding of a vimentin mutation (E151K) in humans, which recapitulates the phenotype of the corresponding genetically engineered mutation in mouse vimentin, causing posterior lens cataracts, defines a novel dominant function of mutant vimentin that is cataract-specific (86). Because no other organ defect has been reported in this human patient or the corresponding mouse model, it is clear that type III IF-genetic mutations cause highly specific organ and tissue-specific disorders (87). The use of WFA to target these type III IFs diseases in mouse models could afford new insight into the treatment of this class of rare human genetic diseases. Taken together, the successful use of WFA to control angiogenesis (16), gliosis (26), and tumor growth and metastasis (28, 88, 89), underscores the broad therapeutic efficacy of this novel type III IF-targeting drug-like molecule and its potential for drug development.
Supplementary Material
Acknowledgments
We thank J. Ambati, M. Fannon, and A. Pearson for many useful scientific discussions. We also thank S. Crocker, C. Shaw, and A. Thomson for help with image scoring. We thank Jack Goodman (University of Kentucky mass spectrometry core center), Mary Gail Engle (University of Kentucky imaging facility), Maya Yankova (University of Connecticut Health Center electron microscopy facility), Glenn Florence (University of Kentucky animal housing facilities), and Greg Bauman (University of Kentucky flow cytometry core center) for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 EY016782 and R01 CA131059 (to R. M.) and R01 DA013930, R01 DA025100, and R01 DA021416 (to C.-G. Z.). This work was also supported in part by the John A. and Florence Mattern Solomon Endowed Chair in vision biology and eye diseases (to R. M.) and funds from Fight for Sight, Inc., and Kentucky Science and Engineering Foundation Grant KSEF-578-RDE-005 (to R. M.).

This article contains supplemental Figs. S1–S5 and Table 1.
P. Bargagna-Mohan, R. R. Paranthan, and R. Mohan, unpublished observations.
- IF
- intermediate filament
- WFA
- withaferin A
- WFA-Bt
- withaferin A-biotin
- Vim−/−
- vimentin-deficient
- α-SMA
- α-smooth muscle actin
- TKT
- transketolase
- MEF
- mouse embryonic fibroblast
- CKI
- cyclin-dependent kinase inhibitor
- GFAP
- glial fibrillary acidic protein
- PMN
- polymorphonuclear neutrophil
- APC/C
- anaphase-promoting complex/cyclosome
- d7 and d14
- postinjury day 7 and 14, respectively.
REFERENCES
- 1. Whitcher J. P., Srinivasan M., Upadhyay M. P. (2001) Corneal blindness. A global perspective. Bull. World Health Organ. 79, 214–221 [PMC free article] [PubMed] [Google Scholar]
- 2. Salomao M. Q., Wilson S. E. (2009) Corneal molecular and cellular biology update for the refractive surgeon. J. Refract. Surg. 25, 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taneri S., Weisberg M., Azar D. T. (2011) Surface ablation techniques. J. Cataract Refract. Surg. 37, 392–408 [DOI] [PubMed] [Google Scholar]
- 4. Qazi Y., Wong G., Monson B., Stringham J., Ambati B. K. (2010) Corneal transparency: genesis, maintenance and dysfunction. Brain Res. Bull. 81, 198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schaffeld M., Herrmann H., Schultess J., Markl J. (2001) Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris. Sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 80, 692–702 [DOI] [PubMed] [Google Scholar]
- 6. Herrmann H., Bär H., Kreplak L., Strelkov S. V., Aebi U. (2007) Intermediate filaments. From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 8, 562–573 [DOI] [PubMed] [Google Scholar]
- 7. Goldman R. D., Grin B., Mendez M. G., Kuczmarski E. R. (2008) Intermediate filaments. Versatile building blocks of cell structure. Curr. Opin. Cell Biol. 20, 28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helfand B. T., Chang L., Goldman R. D. (2004) Intermediate filaments are dynamic and motile elements of cellular architecture. J. Cell Sci. 117, 133–141 [DOI] [PubMed] [Google Scholar]
- 9. Colucci-Guyon E., Portier M. M., Dunia I., Paulin D., Pournin S., Babinet C. (1994) Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79, 679–694 [DOI] [PubMed] [Google Scholar]
- 10. Pekny M. (2001) Astrocytic intermediate filaments. Lessons from GFAP and vimentin knock-out mice. Prog. Brain Res. 132, 23–30 [DOI] [PubMed] [Google Scholar]
- 11. Pekny M., Pekna M. (2004) Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 204, 428–437 [DOI] [PubMed] [Google Scholar]
- 12. Lundkvist A., Reichenbach A., Betsholtz C., Carmeliet P., Wolburg H., Pekny M. (2004) Under stress, the absence of intermediate filaments from Müller cells in the retina has structural and functional consequences. J. Cell Sci. 117, 3481–3488 [DOI] [PubMed] [Google Scholar]
- 13. Pekny M., Johansson C. B., Eliasson C., Stakeberg J., Wallén A., Perlmann T., Lendahl U., Betsholtz C., Berthold C. H., Frisén J. (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 145, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plodinec M., Loparic M., Suetterlin R., Herrmann H., Aebi U., Schoenenberger C. A. (2011) The nanomechanical properties of rat fibroblasts are modulated by interfering with the vimentin intermediate filament system. J. Struct. Biol. 174, 476–484 [DOI] [PubMed] [Google Scholar]
- 15. Che X., Chi F., Wang L., Jong T. D., Wu C. H., Wang X., Huang S. H. (2011) Involvement of IbeA in meningitic Escherichia coli K1-induced polymorphonuclear leukocyte transmigration across brain endothelial cells. Brain Pathol. 21, 389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bargagna-Mohan P., Hamza A., Kim Y. E., Khuan, Abby Ho Y., Mor-Vaknin N., Wendschlag N., Liu J., Evans R. M., Markovitz D. M., Zhan C. G., Kim K. B., Mohan R. (2007) The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 14, 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satelli A., Li S. (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 68, 3033–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verardo M. R., Lewis G. P., Takeda M., Linberg K. A., Byun J., Luna G., Wilhelmsson U., Pekny M., Chen D. F., Fisher S. K. (2008) Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest. Ophthalmol. Vis. Sci. 49, 3659–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eckes B., Dogic D., Colucci-Guyon E., Wang N., Maniotis A., Ingber D., Merckling A., Langa F., Aumailley M., Delouvée A., Koteliansky V., Babinet C., Krieg T. (1998) Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907 [DOI] [PubMed] [Google Scholar]
- 20. Eckes B., Colucci-Guyon E., Smola H., Nodder S., Babinet C., Krieg T., Martin P. (2000) Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 113, 2455–2462 [DOI] [PubMed] [Google Scholar]
- 21. Nieminen M., Henttinen T., Merinen M., Marttila-Ichihara F., Eriksson J. E., Jalkanen S. (2006) Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 8, 156–162 [DOI] [PubMed] [Google Scholar]
- 22. Chaurasia S. S., Kaur H., de Medeiros F. W., Smith S. D., Wilson S. E. (2009) Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 89, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishizaki M., Zhu G., Haseba T., Shafer S. S., Kao W. W. (1993) Expression of collagen I, smooth muscle α-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest. Ophthalmol. Vis. Sci. 34, 3320–3328 [PubMed] [Google Scholar]
- 24. Mohan R., Hammers H. J., Bargagna-Mohan P., Zhan X. H., Herbstritt C. J., Ruiz A., Zhang L., Hanson A. D., Conner B. P., Rougas J., Pribluda V. S. (2004) Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 7, 115–122 [DOI] [PubMed] [Google Scholar]
- 25. Mishra L. C., Singh B. B., Dagenais S. (2000) Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha). A review. Altern. Med. Rev. 5, 334–346 [PubMed] [Google Scholar]
- 26. Bargagna-Mohan P., Paranthan R. R., Hamza A., Dimova N., Trucchi B., Srinivasan C., Elliott G. I., Zhan C. G., Lau D. L., Zhu H., Kasahara K., Inagaki M., Cambi F., Mohan R. (2010) Withaferin A targets intermediate filaments glial fibrillary acidic protein and vimentin in a model of retinal gliosis. J. Biol. Chem. 285, 7657–7669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paranthan R. R., Bargagna-Mohan P., Lau D. L., Mohan R. (2011) Mol. Vision 17, 1901–1908 [PMC free article] [PubMed] [Google Scholar]
- 28. Lahat G., Zhu Q. S., Huang K. L., Wang S., Bolshakov S., Liu J., Torres K., Langley R. R., Lazar A. J., Hung M. C., Lev D. (2010) Vimentin is a novel anti-cancer therapeutic target. Insights from in vitro and in vivo mice xenograft studies. PLoS One 5, e10105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Yokota Y., Bargagna-Mohan P., Ravindranath P. P., Kim K. B., Mohan R. (2006) Development of withaferin A analogs as probes of angiogenesis. Bioorg. Med. Chem. Lett. 16, 2603–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakayama K., Nagahama H., Minamishima Y. A., Matsumoto M., Nakamichi I., Kitagawa K., Shirane M., Tsunematsu R., Tsukiyama T., Ishida N., Kitagawa M., Nakayama K., Hatakeyama S. (2000) Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook J. R., Mody M. K., Fini M. E. (1999) Failure to activate transcription factor NF-κB in corneal stromal cells (keratocytes). Invest. Ophthalmol. Vis. Sci. 40, 3122–3131 [PubMed] [Google Scholar]
- 32. Herrmann H., Häner M., Brettel M., Ku N. O., Aebi U. (1999) Characterization of distinct early assembly units of different intermediate filament proteins. J. Mol. Biol. 286, 1403–1420 [DOI] [PubMed] [Google Scholar]
- 33. Amano S., Rohan R., Kuroki M., Tolentino M., Adamis A. P. (1998) Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 39, 18–22 [PubMed] [Google Scholar]
- 34. Falsey R. R., Marron M. T., Gunaherath G. M., Shirahatti N., Mahadevan D., Gunatilaka A. A., Whitesell L. (2006) Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat. Chem. Biol. 2, 33–38 [DOI] [PubMed] [Google Scholar]
- 35. West-Mays J. A., Cook J. R., Sadow P. M., Mullady D. K., Bargagna-Mohan P., Strissel K. J., Fini M. E. (1999) Differential inhibition of collagenase and interleukin-1α gene expression in cultured corneal fibroblasts by TGF-β, dexamethasone, and retinoic acid. Invest. Ophthalmol. Vis. Sci. 40, 887–896 [PubMed] [Google Scholar]
- 36. Jester J. V., Huang J., Barry-Lane P. A., Kao W. W., Petroll W. M., Cavanagh H. D. (1999) Transforming growth factor β-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 40, 1959–1967 [PubMed] [Google Scholar]
- 37. Tandon A., Tovey J. C., Sharma A., Gupta R., Mohan R. R. (2010) Role of transforming growth factor β in corneal function, biology, and pathology. Curr. Mol. Med. 10, 565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stramer B. M., Cook J.R., Fini M. E., Taylor A., Obin M. (2001) Induction of the ubiquitin-proteasome pathway during the keratocyte transition to the repair fibroblast phenotype. Invest. Ophthalmol. Vis. Sci. 42, 1698–1706 [PubMed] [Google Scholar]
- 39. Saika S., Miyamoto T., Yamanaka O., Kato T., Ohnishi Y., Flanders K. C., Ikeda K., Nakajima Y., Kao W. W., Sato M., Muragaki Y., Ooshima A. (2005) Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-κB, in treatment of corneal alkali burns in mice. Am. J. Pathol. 166, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bargagna-Mohan P., Ravindranath P. P., Mohan R. (2006) Small molecule antiangiogenic probes of the ubiquitin proteasome pathway. Potential application to choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 47, 4138–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oshima T., Sonoda K. H., Tsutsumi-Miyahara C., Qiao H., Hisatomi T., Nakao S., Hamano S., Egashira K., Charo I. F., Ishibashi T. (2006) Analysis of corneal inflammation induced by cauterisation in CCR2 and MCP-1 knockout mice. Br. J. Ophthalmol. 90, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ambati B. K., Joussen A. M., Kuziel W. A., Adamis A. P., Ambati J. (2003) Inhibition of corneal neovascularization by genetic ablation of CCR2. Cornea 22, 465–467 [DOI] [PubMed] [Google Scholar]
- 43. Nakazawa T., Takeda M., Lewis G. P., Cho K. S., Jiao J., Wilhelmsson U., Fisher S. K., Pekny M., Chen D. F., Miller J. W. (2007) Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Invest. Ophthalmol. Vis. Sci. 48, 2760–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshida K., Nakayama K., Nagahama H., Harada T., Harada C., Imaki J., Matsuda A., Yamamoto K., Ito M., Ohno S., Nakayama K. (2002) Involvement of p27KIP1 degradation by Skp2 in the regulation of proliferation in response to wounding of corneal epithelium. Invest. Ophthalmol. Vis. Sci. 43, 364–370 [PubMed] [Google Scholar]
- 45. Zieske J. D. (2000) Expression of cyclin-dependent kinase inhibitors during corneal wound repair. Prog. Retin. Eye Res. 19, 257–270 [DOI] [PubMed] [Google Scholar]
- 46. Kase S., Yoshida K., Ohgami K., Shiratori K., Ohno S., Nakayama K. I. (2006) Phosphorylation of p27KIP1 in the mitotic cells of the corneal epithelium. Curr. Eye Res. 31, 307–312 [DOI] [PubMed] [Google Scholar]
- 47. Nakayama K., Nagahama H., Minamishima Y. A., Miyake S., Ishida N., Hatakeyama S., Kitagawa M., Iemura S., Natsume T., Nakayama K. I. (2004) Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6, 661–672 [DOI] [PubMed] [Google Scholar]
- 48. Herrmann H., Aebi U. (2004) Intermediate filaments. Molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu. Rev. Biochem. 73, 749–789 [DOI] [PubMed] [Google Scholar]
- 49. Mackey D. A., Wilkinson C. H., Kearns L. S., Hewitt A. W. (2011) Classification of iris colour. Review and refinement of a classification schema. Clin. Experiment. Ophthalmol. 39, 462–471 [DOI] [PubMed] [Google Scholar]
- 50. Conway B. R., Chatterjee S., Field G. D., Horwitz G. D., Johnson E. N., Koida K., Mancuso K. (2010) Advances in color science. From retina to behavior. J. Neurosci. 30, 14955–14963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dyer M. A., Cepko C. L. (2000) Control of Müller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 3, 873–880 [DOI] [PubMed] [Google Scholar]
- 52. Vij N., Roberts L., Joyce S., Chakravarti S. (2004) Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis. Implications in the cornea. Exp. Eye Res. 78, 957–971 [DOI] [PubMed] [Google Scholar]
- 53. Yoon M., Moir R. D., Prahlad V., Goldman R. D. (1998) Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soellner P., Quinlan R. A., Franke W. W. (1985) Identification of a distinct soluble subunit of an intermediate filament protein. Tetrameric vimentin from living cells. Proc. Natl. Acad. Sci. U.S.A. 82, 7929–7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chou Y. H., Flitney F. W., Chang L., Mendez M., Grin B., Goldman R. D. (2007) The motility and dynamic properties of intermediate filaments and their constituent proteins. Exp. Cell Res. 313, 2236–2243 [DOI] [PubMed] [Google Scholar]
- 56. Moon R. T., Lazarides E. (1983) Synthesis and post-translational assembly of intermediate filaments in avian erythroid cells. Vimentin assembly limits the rate of synemin assembly. Proc. Natl. Acad. Sci. U.S.A. 80, 5495–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Skalli O., Chou Y. H., Goldman R. D. (1992) Cell cycle-dependent changes in the organization of an intermediate filament-associated protein. Correlation with phosphorylation by p34cdc2. Proc. Natl. Acad. Sci. U.S.A. 89, 11959–11963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Franke W. W., Schmid E., Grund C., Geiger B. (1982) Intermediate filament proteins in nonfilamentous structures. Transient disintegration and inclusion of subunit proteins in granular aggregates. Cell 30, 103–113 [DOI] [PubMed] [Google Scholar]
- 59. Chang H. C., Chang F. R., Wang Y. C., Pan M. R., Hung W. C., Wu Y. C. (2007) A bioactive withanolide Tubocapsanolide A inhibits proliferation of human lung cancer cells via repressing Skp2 expression. Mol. Cancer Ther. 6, 1572–1578 [DOI] [PubMed] [Google Scholar]
- 60. Hara T., Kamura T., Nakayama K., Oshikawa K., Hatakeyama S. (2001) Degradation of p27Kip1 at the G0-G1 transition mediated by a Skp2-independent ubiquitination pathway. J. Biol. Chem. 276, 48937–48943 [DOI] [PubMed] [Google Scholar]
- 61. Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25752–25757 [DOI] [PubMed] [Google Scholar]
- 62. Bond M., Wu Y. J. (2011) Proliferation unleashed. The role of Skp2 in vascular smooth muscle cell proliferation. Front. Biosci. 16, 1517–1535 [DOI] [PubMed] [Google Scholar]
- 63. Amador V., Ge S., Santamaría P. G., Guardavaccaro D., Pagano M. (2007) APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol. Cell 27, 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stan S. D., Zeng Y., Singh S. V. (2008) Nutr. Cancer 60, Suppl. 1, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hu D., Liu W., Wu G., Wan Y. (2011) Nuclear translocation of Skp2 facilitates its destruction in response to TGFβ signaling. Cell Cycle 10, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang X., Austin P. F., Niederhoff R. A., Manson S. R., Riehm J. J., Cook B. L., Pengue G., Chitaley K., Nakayama K., Nakayama K. I., Weintraub S. J. (2009) Mechanoregulation of proliferation. Mol. Cell Biol. 29, 5104–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang F., Qi L. X., Su Y., Yan Q. H., Teng Y. (2010) Inhibition of cell proliferation of Tenon's capsule fibroblast by S-phase kinase-interacting protein 2 targeting SiRNA through increasing p27 protein level. Invest. Ophthalmol. Vis. Sci. 51, 1475–1482 [DOI] [PubMed] [Google Scholar]
- 68. Suzuki S., Fukasawa H., Kitagawa K., Uchida C., Hattori T., Isobe T., Oda T., Misaki T., Ohashi N., Nakayama K., Nakayama K. I., Hishida A., Yamamoto T., Kitagawa M. (2007) Renal damage in obstructive nephropathy is decreased in Skp2-deficient mice. Am. J. Pathol. 171, 473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ishizaki M., Wakamatsu K., Matsunami T., Yamanaka N., Saiga T., Shimizu Y., Zhu G., Kao W. W. (1994) Dynamics of the expression of cytoskeleton components and adherens molecules by fibroblastic cells in alkali-burned and lacerated corneas. Exp. Eye Res. 59, 537–549 [DOI] [PubMed] [Google Scholar]
- 70. Dupps W. J., Jr., Wilson S. E. (2006) Biomechanics and wound healing in the cornea. Exp. Eye Res. 83, 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schopferer M., Bär H., Hochstein B., Sharma S., Mücke N., Herrmann H., Willenbacher N. (2009) Desmin and vimentin intermediate filament networks. Their viscoelastic properties investigated by mechanical rheometry. J. Mol. Biol. 388, 133–143 [DOI] [PubMed] [Google Scholar]
- 72. Tanter M., Touboul D., Gennisson J. L., Bercoff J., Fink M. (2009) High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans. Med. Imaging 28, 1881–1893 [DOI] [PubMed] [Google Scholar]
- 73. Macsai M. S. (2000) The management of corneal trauma. Advances in the past twenty-five years. Cornea 19, 617–624 [DOI] [PubMed] [Google Scholar]
- 74. Müller L. J., Pels E., Vrensen G. F. (2001) The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br. J. Ophthalmol. 85, 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lacolley P., Challande P., Boumaza S., Cohuet G., Laurent S., Boutouyrie P., Grimaud J. A., Paulin D., Lamazière J. M., Li Z. (2001) Mechanical properties and structure of carotid arteries in mice lacking desmin. Cardiovasc. Res. 51, 178–187 [DOI] [PubMed] [Google Scholar]
- 76. Scott R. S., Li Z., Paulin D., Uvelius B., Small J. V., Arner A. (2008) Role of desmin in active force transmission and maintenance of structure during growth of urinary bladder. Am. J. Physiol. Cell Physiol. 295, C324—C331 [DOI] [PubMed] [Google Scholar]
- 77. Saika S., Ikeda K., Yamanaka O., Flanders K. C., Nakajima Y., Miyamoto T., Ohnishi Y., Kao W. W., Muragaki Y., Ooshima A. (2005) Therapeutic effects of adenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab. Invest. 85, 474–486 [DOI] [PubMed] [Google Scholar]
- 78. Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 [DOI] [PubMed] [Google Scholar]
- 79. Petroll W. M., Vishwanath M., Ma L. (2004) Corneal fibroblasts respond rapidly to changes in local mechanical stress. Invest. Ophthalmol. Vis. Sci 45, 3466–3474 [DOI] [PubMed] [Google Scholar]
- 80. Hassell J. R., Birk D. E. (2010) The molecular basis of corneal transparency. Exp. Eye Res. 91, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao X. H., Laschinger C., Arora P., Szászi K., Kapus A., McCulloch C. A. (2007) Force activates smooth muscle α-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801–1809 [DOI] [PubMed] [Google Scholar]
- 82. Lu Y. B., Iandiev I., Hollborn M., Körber N., Ulbricht E., Hirrlinger P. G., Pannicke T., Wei E. Q., Bringmann A., Wolburg H., Wilhelmsson U., Pekny M., Wiedemann P., Reichenbach A., Käs J. A. (2011) Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 25, 624–631 [DOI] [PubMed] [Google Scholar]
- 83. Jester J. V., Petroll W. M., Cavanagh H. D. (1999) Corneal stromal wound healing in refractive surgery. The role of myofibroblasts. Prog. Retin. Eye Res. 18, 311–356 [DOI] [PubMed] [Google Scholar]
- 84. Rogel M. R., Soni P. N., Troken J. R., Sitikov A., Trejo H. E., Ridge K. M. (2011) Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 25, 3873–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hopkinson-Woolley J., Hughes D., Gordon S., Martin P. (1994) Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J. Cell Sci. 107, 1159–1167 [DOI] [PubMed] [Google Scholar]
- 86. Müller M., Bhattacharya S. S., Moore T., Prescott Q., Wedig T., Herrmann H., Magin T. M. (2009) Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum. Mol. Genet. 18, 1052–1057 [DOI] [PubMed] [Google Scholar]
- 87. Bär H., Mücke N., Ringler P., Müller S. A., Kreplak L., Katus H. A., Aebi U., Herrmann H. (2006) Impact of disease mutations on the desmin filament assembly process. J. Mol. Biol. 360, 1031–1042 [DOI] [PubMed] [Google Scholar]
- 88. Thaiparambil J. T., Bender L., Ganesh T., Kline E., Patel P., Liu Y., Tighiouart M., Vertino P. M., Harvey R. D., Garcia A., Marcus A. I. (2011) Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 129, 2744–2755 [DOI] [PubMed] [Google Scholar]
- 89. Maxwell S. A., Cherry E. M., Bayless K. J. (2011) Akt, 14-3-3ζ, and vimentin mediate a drug-resistant invasive phenotype in diffuse large B-cell lymphoma. Leuk. Lymphoma 52, 849–864 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.