Abstract
Here, we examined the role of the extracellular Ca2+-sensing receptor (CaSR) in the control of colonic epithelial cell proliferation in vivo and changes in β-catenin triggered by CaSR stimulation in human colonic epithelial cells in vitro. The in vivo studies, using a novel Casr intestinal-specific knock-out mouse, indicate that the genetic ablation of the Casr leads to hyperproliferation of colonic epithelial cells, expansion of the proliferative zone, changes in crypt structure, and enhanced β-catenin nuclear localization. The in vitro results indicate that stimulation of the CaSR, by Ca2+ or by the calcimimetic R-568, produced a striking and time-dependent decrease in the phosphorylation of β-catenin at Ser-552 and Ser-675, two amino acid residues that promote β-catenin transcriptional activity. The reduced phosphorylation of β-catenin coincided with a decline in its nuclear localization and a marked redistribution to the plasma membrane. Furthermore, CaSR stimulation promoted a down-regulation of β-catenin-mediated transcriptional activation. These studies demonstrate that signaling pathways emanating from the CaSR control colonic epithelial cell proliferation in vivo and suggest that the mechanism involves regulation of β-catenin phosphorylation.
Keywords: β-Catenin, Calcium, Colorectal Cancer, G Protein-coupled Receptor (GPCR), Transgenic Mice, Extracellular Calcium-sensing Receptor
Introduction
Signaling pathways activated by heterotrimeric GTP-binding (G) protein-coupled receptors (GPCRs)2 expressed in the gastrointestinal (GI) tract play a critical role in the regulation of multiple functions of the digestive system, including cell proliferation, differentiation, inflammation, and promotion of colorectal cancer (CRC) (1–8). In particular, the canonical Wingless (Wnt)/β-catenin signaling pathway, which is activated after Wnt binds its receptor complex consisting of the GPCR Frizzled and a co-receptor (9), has emerged as a key regulator of genes that control intestinal cell proliferation and differentiation (10–19). It is also recognized that β-catenin signaling is regulated via Wnt-independent pathways, including phosphorylation of Ser-552 and Ser-675 (20–22). Despite the fundamental importance of the β-catenin pathway in normal and abnormal regulation of the GI tract, including pathogenesis like inflammatory bowel diseases and CRC, the signaling and cross-talk mechanisms involved remain incompletely understood.
The extracellular Ca2+-sensing receptor (CaSR), a member of the C family of heptahelical GPCRs, was originally cloned from parathyroid chief cells (23). Inactivating and activating mutations of the CaSR in humans (24) and genetic disruption of the CaSR gene in mice (25) established that the CaSR functions in the control of Ca2+ homeostasis. The CaSR is also present in many other tissues not directly involved in the control of Ca2+ homeostasis (26), including both surface and crypt epithelial cells in rodent and human colons (27). Interestingly, the expression of the CaSR is greatly reduced or completely lost in CRC (28–30), suggesting that signaling pathways activated by this receptor negatively control cellular proliferation and CRC development. Indeed, we demonstrated previously that CaSR stimulation inhibits the proliferation of cells derived from normal human colon mucosal epithelium and from human colon carcinomas (31). Furthermore, CaSR stimulation suppresses β-catenin-mediated transcriptional activity in colon carcinoma-derived cell lines (32, 33). However, the precise cause-effect relationship of these in vitro observations is not clear. Furthermore, no data are available indicating whether CaSR signaling regulates the proliferation of epithelial cells in the intact colon.
The results presented here show, for the first time, that genetic ablation of the Casr leads to hyperproliferation of colonic epithelial cells, enhanced β-catenin nuclear localization, expansion of the proliferative zone, and changes in crypt architecture. Mechanistic studies with cells derived from normal human colon mucosal epithelium demonstrate that CaSR stimulation promotes a decrease in the phosphorylation of β-catenin at Ser-552 and Ser-675 which coincided with its redistribution to the plasma membrane and β-catenin-mediated transcriptional down-regulation. Overall, these studies indicate that CaSR signaling negatively controls colonic epithelial cell proliferation in vivo and suggest that this occurs through a mechanism that involves β-catenin phosphorylation.
EXPERIMENTAL PROCEDURES
Generation and Genotyping of Conditional Casr Knock-out Mice
Mice with knock out of Casr genes specifically in intestinal epithelial cells were generated by breeding Casrflox/flox mice (34) with transgenic mice expressing Cre-recombinase under the control of the villin 1 promoter (Vil-cre; Jackson Laboratory). We routinely bred male homozygous KO (vilCre/Casrflox/flox) mice, which carry the Cre transgene and both floxed-CaSR alleles, with female Casrflox/flox that carry two floxed-CaSR alleles but no Cre transgene, to produce the cohorts of vilCre/Casrflox/flox and Casrflox/flox mice used in this study. Mouse genotypes were determined by PCR analyses of genomic DNAs from tail snips with primers for the Cre transgene (Cre-1, GCAAAACAGGCTCTAGCGTTCG; Cre-2, CTGTTTCACTATCCAGGTTACGG), which amplified an ∼500-bp cDNA and the P3U (TGTGACGGAAAACATACTGC) and P3L (CGAGTACAGGCTTTGATGC) primer set for the loxP sequence at the 3′ end of exon 7, which amplified a 133-bp DNA fragment from wild-type alleles and a 167-bp DNA from floxed Casr alleles (34). To verify tissue-specific gene excision, genomic DNA was isolated from the tissues specified and was then subjected to PCR analysis with the P4 (CCTCGAACATGAACAACTTAATTCGG)/P3L (CGAGTACAGGCTTTGATGC) primer set, which amplified a 284-bp DNA fragment from the Casr gene allele after the excision of exon 7 (34). Homozygous vilCre/Casrflox/flox mice were born in the expected Mendelian ratios, and they are grossly normal compared with their control Casrflox/flox littermates. All mice were maintained under standard conditions with free access to food and water under protocols approved by the Animal Care Subcommittee, San Francisco Department of Veterans Affairs Medical Center.
Tissue Fixation, Immunohistochemistry, and Morphometry of Conditional Casr Knock-out Mice Colon
Tissue fixation was performed as described previously (34) in animals killed at 4–6 weeks of age. Fixed colons of male and female mice were embedded in paraffin blocks, and 4-μm sections were cut and stained with hematoxylin & eosin. For immunohistochemistry, deparaffinized sections were used. Unmasking was carried out by steaming the sections for 20 min. After inhibition of endogenous peroxidase with hydrogen peroxide and incubation with normal goat serum, a primary monoclonal rat anti-mouse Ki-67 antibody was applied. After washing, a secondary biotinylated goat anti-rat IgG antibody was applied, and the sections were then incubated with avidin/biotinylated horseradish peroxidase. Development was done with diaminobenzidine nickel or 3-amino-9-ethylcarbazole substrate kits for peroxidase (SK 4100 and SK 4200; Vector Laboratories). Sections were then counterstained with hematoxylin and mounted routinely (35). Hematoxylin & eosin-stained histological sections were analyzed to determine the effect of CaSR expression deficiency on tissue architecture. Briefly, 20 full-length, longitudinally cut crypts from five Casr KO and five control littermates were analyzed for crypt height (micrometers) and number of cells per crypt height. Cross-section of crypts (20/mouse) was used to determine the average crypt diameter (micrometers) and circumference (in number of cells). These data were used to calculate cell size (crypt height in micrometers/crypt height in cell number) and estimate the total cells per crypt (mean cells per crypt column × mean crypt circumference). Data from KO and control littermates were represented as mean ± S.E. and compared by an unpaired Student's t test. To examine the nuclear content of β-catenin, cross-sections of distal colon crypts from three Casr KO mice and three control littermates were stained with a rabbit primary antibody against β-catenin followed by secondary biotinylated goat anti-rabbit IgG antibody and avidin/biotinylated horseradish peroxidase (see above) and counterstaining with hematoxylin. Images acquired using a 2048 × 2048 active pixel Spot Pursuit CCD Camera (Diagnostic Instruments) were recombined into RG (β-catenin) and B (hematoxylin) color planes and analyzed using the imaging software SP2 version 4.0 (Carl Zeiss Microimaging). A region of interest (ROI) was used to define the nuclear compartment, and the average pixel intensity of β-catenin (RG channels) and hematoxylin (B channel) signals from each ROI was analyzed using SigmaPlot version 9 (Systat Software). Values represent the mean pixel intensity (MI) ± S.E. of β-catenin/hematoxylin, and they were compared using Student's t test.
cDNA Constructs and Luciferase Reporter Vectors
A vector containing a β-catenin human cDNA (NM_001904) was obtained from Origene (SC107921). Site-directed mutagenesis of this β-catenin cDNA was used to generate one BamHI site 7 nucleotides upstream of the first nucleotide of the initiation codon and another BamHI site immediately downstream of the last nucleotide of the stop codon. The cDNA encoding β-catenin was isolated by BamHI digestion and subcloned into the BamHI site of pDsRed-Express-C1 (BD Biosciences). The obtained construct, pRFP-β-catenin, was verified by DNA sequence analysis, and the product of expression was analyzed by Western blotting using a β-catenin monoclonal antibody (BD Transduction Laboratories).
The firefly luciferase reporter vector of β-catenin-mediated transcriptional activation M50 Super 8× TOPFlash (36) and the control plasmid M51 Super 8× FOPFlash, which has mutant TCF/LEF binding sites, were obtained from Addgene (plasmids 12456 and 12457, respectively). The constitutive luciferase activity generated by the Renilla luciferase reporter vector phRGTK (Promega) was used to normalize for transfection efficiency (37).
Cell Culture, Western Blotting, and Indirect Immunofluorescence
NCMiCaSR is an immortalized cell line derived from normal human colon mucosal epithelium with functional APC and β-catenin proteins (38) that was established and maintained as described previously (31). This cell line expresses the CaSR under the control of a tetracycline-inducible promoter (31). Western blot analysis was performed as reported (31, 39), and the signals detected with a luminescent image analyzer were normalized by total β-catenin and α-tubulin and represent the results of three independent experiments. Indirect immunofluorescence was performed as reported previously (31, 39) using rabbit anti-β-catenin Ser(P)-552 and a mouse monoclonal anti-total β-catenin as primary antibodies and Alexa Fluor 488 chicken anti-mouse IgG and Alexa Fluor 568 goat anti-rabbit IgG as secondary antibodies. Images were acquired with a 2048 × 2048 active pixel Spot Pursuit CCD Camera, and ROI corresponding to the nuclear and plasma membrane (cell periphery) were defined using the imaging software SP2 version 4.0. Quantification of the MI of the Red (Ser(P)-552 β-catenin) and Green (β-catenin) channels in the ROI was also determined using the imaging SP2 version 4.0 software. Values represent the MI ± S.E., and they were compared using Student's t test.
Transient Transfection of cDNAs Encoding RFP-β-Catenin and Luciferase Reporter Vectors
For live NCMiCaSR cell analysis, cells were plated onto 15-mm No. 1 round glass coverslips inside 33-mm dishes at 1 × 105 cells/dish and transfected 18–20 h later with 0.4 μg/dish pRFP-β-catenin or pRFP (pDsRed-Express-C1). Transfections were carried out in Opti-MEM using Lipofectamine Plus according to the manufacturer's suggested conditions (Invitrogen). After 5 h, the transfection mixture was replaced with DMEM containing 10% FBS and supplemented with CaCl2 to a final concentration of 1.4 mm, with or without 0.5 μg/ml doxycycline to induce CaSR expression. The transfected cells were incubated 18 h before real-time imaging, a time we previously found optimum to detect very low levels of fluorescent-tagged protein expression (39).
For report vector assays, NCMiCaSR cells were plated in 33-mm dishes at 1 × 105 cells/dish and co-transfected as indicated above with 1 μg of pTOPFlash/0.2 μg phRGTK/dish or 1 μg of pFOPFlash/0.2 μg of phRGTK per dish. Five hours later, the transfection mixture was replaced with DMEM containing 10% FBS and supplemented with CaCl2 to a final concentration of 1.4 mm, 5 mm, or 100 nm R-568 and 1.4 mm CaCl2 without or with 0.5 μg/ml doxycycline to induce CaSR expression. The reporter activity of the vectors was assayed 24 h after transfection using a Turner Design luminometer and a dual luciferase assay system as recommended by the manufacturer (Promega). The luciferase activity values are the result of three independent experiments and correspond to (pTOPFlash/phRGTK-pFOPFlash/phRGTK) ± S.E. The obtained values were compared using Student's t test.
Real-time Imaging of RFP-β-Catenin Intracellular Distribution
To maintain a constant temperature of 37 °C during the experimental procedures, NCMiCaSR cells grown on the 15-mm glass coverslips were mounted in a perfusion chamber (RC-25; Warner Instrument Corporation) in an epifluorescence microscope (31). The cells were perfused with a saline solution containing 138 mm NaCl, 4 mm NaHCO3, 0.3 mm Na2HPO4, 5 mm KCl, 0.3 mm KH2PO4, 1.4 mm CaCl2, 0.5 mm MgCl2, 0.4 mm MgSO4, 5.6 mm d-glucose, 20 mm HEPES, pH: 7.4, that was preheated at 37 °C by a SH-27B solution in-line heater (Warner Instrument Corporation). To stimulate the CaSR, the CaCl2 concentration in the perfusion solution was raised to 5 mm during the indicated times. Images were captured as described previously (39). Quantification of RFP-β-catenin plasma membrane was determined as described above.
Antibodies and Kinase Inhibitors
Antibodies were obtained from GE Healthcare (horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG), BD Transduction (anti-β-catenin mouse monoclonal antibody), Invitrogen (Alexa Fluor 488 chicken anti-mouse IgG and Alexa Fluor 568 goat anti-rabbit IgG), Cell Signaling Technology (rabbit anti-Ser(P)-552-β-catenin, rabbit anti-Ser(P)-675-β-catenin, and rabbit anti-β-catenin), DakoCytomation (monoclonal rat anti-mouse Ki-67 antigen, clone TEC-3), and Vector Laboratories (biotinylated goat anti-rat IgG). The PKA and Akt inhibitors, KT 5720 and GSK 690693, respectively, were obtained from Tocris Bioscience.
RESULTS
Generation of Intestinal-specific Deficient Casr Mouse
The phenotype of a generalized Casr KO (Casr−/−) mouse is characterized by markedly elevated serum Ca2+ and parathyroid hormone levels, parathyroid hyperplasia, bone abnormalities, retarded growth, premature death, and incomplete gene excision (25, 34). Therefore, that animal model is inadequate to examine the role of CaSR signaling in the proliferation of colonic epithelial cells in vivo. Accordingly, we used a recently developed floxed Casr mouse (34) to delete specifically the Casr in the GI tract by crossing mice with loxP-flanked exon 7 of the Casr (Casrflox/flox) with mice expressing Cre-recombinase under the control of the mouse villin 1 promoter (vilCre). Villin, an actin-bundling protein, is expressed in every cell of the intestinal epithelium from duodenum through colon (40).
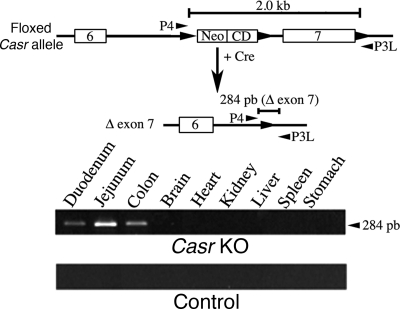
PCR analysis of genomic DNA extracted from vilCre/Casrflox/flox (Casr KO mouse) and control littermates (animals with two floxed Casr alleles and no vilCre transgene) using the P4/P3L primers exemplifies the successful excision of exon 7 of the Casr mouse as demonstrated by the detection of a PCR product of 284 bp only in the Casr KO mouse (Fig. 1). The excision of exon 7, which renders a nonfunctional CaSR (34), was restricted to intestinal cells and did not occur in other organs where this receptor is profusely expressed such as stomach and kidney (41–43). To confirm the ablation of Casr gene further, we performed quantitative PCR on RNA extracted from intestinal epithelia, and the results showed knockdown of CaSR RNA expression in the homozygous Casr KO mice versus control littermates (see supplemental Fig. 1).
FIGURE 1.
Mouse tissue-specific deletion of Casr. To verify tissue-specific gene excision, genomic DNA isolated from Casr KO and control littermates of specified tissues was subjected to PCR analysis with the P4 (CCTCGAACATGAACAACTTAATTCGG)/P3L (CGAGTACAGGCTTTGATGC) primer set, which amplified a 284-bp DNA fragment from the Casr gene allele after the excision of exon 7.
Characterization of Colonic Crypt Proliferation and Morphometry in Intestinal-specific Deficient Casr Mouse
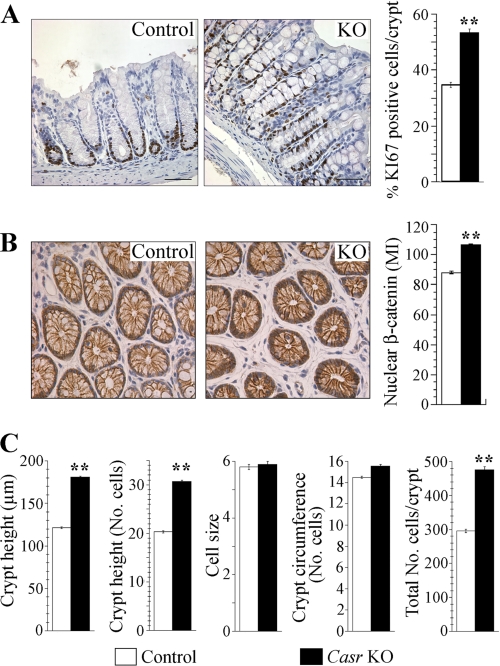
If CaSR signaling negatively regulates the proliferation of normal epithelial cells in the colonic crypts, the genetic ablation of this receptor should lead to an increase in their rate of proliferation. As a first step to examine this prediction, colon distal sections of Casr KO and control littermates were fixed and processed for immunohistochemistry using a Ki-67 antibody, a widely used marker of cellular proliferation (44). Results obtained after examining colonic crypts (at least 20 crypts/animal) of Casr KO (n = 5) and control littermates (n = 5) showed that the proportion of proliferating cells in the Casr KO was markedly higher than in control animals. Specifically, the percentage of proliferating cells in Casr KO mice (mean ± S.E.) (52.76 ± 1.3) was 54% higher than in control animals (34.27 ± 0.9) with a statistically significant increase (p < 0.0001). Representative histological sections showing Ki-67 staining indicate that the proliferating crypt cells in the Casr KO mice extended well beyond the transit-amplifying region in the mid-crypt (Fig. 2A).
FIGURE 2.
Proliferation analysis of colon sections of Casr KO mice. A, colons fixed and processed for immunocytochemistry using a Ki-67 antibody as described under “Experimental Procedures.” Ki-67-positive cells were quantified in a sample size of 5 mice/group with at least 20 crypts examined per mouse. Data from KO and control littermates were represented as mean of the percentage of Ki-67-positive cells ± S.E. and compared by unpaired Student's t test (**, p < 0.0001). Scale bars, 10 μm. B, quantification of nuclear β-catenin in colonocytes of Casr KO mice. The same level cross-sections of colonic crypts were processed for immunocytochemistry using a β-catenin rabbit antibody as described under “Experimental Procedures.” Quantification was performed as described under “Experimental Procedures” in a sample size of 3 mice/group with at least 60 cells examined per animal. Values represent the MI of nuclear β-catenin ± S.E., and they were compared using unpaired Student's t test (**, p < 0.0001). C, morphometric analysis of Casr KO colonic crypts. Full-length, longitudinally cut crypts (at least 20 per mouse) from 5 Casr KO and 5 control littermates were analyzed for crypt height (micrometers) and number of cells per crypt height. Cross-section of crypts (20/mouse) was used to determine the average crypt diameter (micrometers) and circumference (in number of cells). These data were used to calculate cell size (crypt height in micrometers/crypt height in cell number) and estimate the total cells per crypt (mean cells per crypt column × mean crypt circumference). Data from KO and control littermates are represented as mean ± S.E. and compared using unpaired Student's t test (**, p < 0.0001).
The binding of the Wnt ligand to its receptor promotes the stabilization and nuclear translocation of β-catenin, a key intestinal regulator of genes involved in the control of cell proliferation and differentiation (45, 46). In view of the results presented in Fig. 2A indicating that CaSR signaling negatively regulates proliferation, we examined whether the genetic ablation of the Casr enhanced the nuclear localization of β-catenin. Representative images of β-catenin staining in a cross-section of colon distal crypts obtained from equivalent locations suggested that the absence of Casr enhanced the nuclear localization of β-catenin (Fig. 2B). Quantitative analysis of the nuclear content of β-catenin of at least 180 colonocytes of Casr KO mice (n = 3) and 180 colonocytes of control littermates (n = 3) indicated that the relative concentration of β-catenin in Casr KO (mean ± S.E.) (106.1 ± 0.57) was 20% higher than in control animals (88.2 ± 0.64) with a statistically significant increase (p < 0.0001) (Fig. 2B).
In the GI tract normal cell numbers are maintained by balancing rates of cell proliferation, differentiation, migration, and apoptosis. If increases in proliferative rates are not precisely balanced by the rate of cell removal (e.g. by increase in apoptosis) we should expect a change in the size of the crypts. To examine whether Casr deficiency promoted a change in the colonic crypt architecture, as suggested by the results presented in Fig. 2A, we performed a quantitative morphometric analysis of the size and total number of epithelial cells in the crypts (47). Accordingly, we measured crypt height (in micrometers and cell number) and crypt circumference (in micrometers and cell number) in histological sections of Casr KO mice and control littermates. The data presented in Fig. 2C indicate that absence of Casr promotes a dramatic increase in the crypt height and in the total number of cells per crypt that is statistically significant without any changes in cell size (see also supplemental Table 1). Thus, these results show, for the first time, that the genetic ablation of the Casr leads to in vivo hyperproliferation of colonic epithelial cells, expansion of the proliferative zone, changes in crypt structure, and to an increase of nuclear β-catenin content.
β-Catenin Ser-552 Phosphorylation Regulation by CaSR and Akt
The preceding results demonstrating a striking increase in colonic epithelial cell proliferation and enlargement of the crypt in the Casr KO mouse prompted studies to elucidate the mechanism(s) involved. Given the pivotal role of the canonical Wnt/β-catenin signaling pathway in promoting intestinal epithelial proliferation and differentiation (45, 46) and the enhanced nuclear accumulation of β-catenin in Casr KO mice, we hypothesized that a cross-talk between CaSR and β-catenin was taking place in colonic epithelial cells. Phosphorylation of β-catenin plays a fundamental role in its regulation. For example, the sequential phosphorylation of β-catenin in its N-terminal domain at Ser-45 by casein kinase 1 and Thr-41/Ser-37/Ser-33 by glycogen synthase kinase 3 targets β-catenin for proteosomal degradation. In contrast, β-catenin phosphorylation at Ser-552 and Ser-675 promotes its dissociation from cell-cell contacts, nuclear translocation, and transcriptional activity up-regulation (20–22). Accordingly, we examined whether CaSR signaling affected β-catenin Ser-552 phosphorylation in human colon-derived epithelial NCMiCaSR cells (31). This cell line was established by infecting NCM-460 cells, an immortalized cell line derived from normal human colon mucosal epithelium (48), with a retrovirus encoding the human CaSR under the control of a Tet promoter (31).
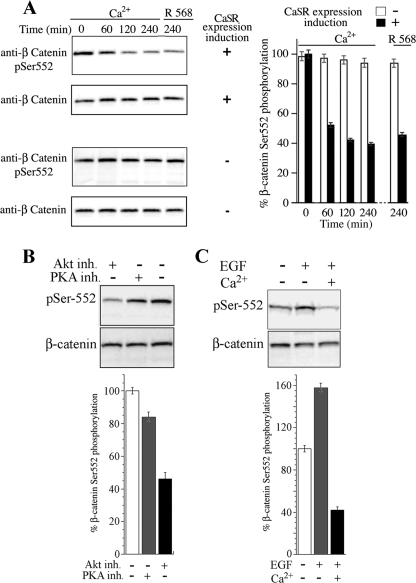
NCMiCaSR cells incubated for 16 h with or without 0.1 μg/ml doxycycline, a member of the tetracycline antibiotics group, were challenged with 5.0 mm Ca2+ and lysed at the indicated times. Lysates were analyzed by Western blotting using an antibody that specifically detects the phosphorylation of Ser-552 in β-catenin (supplemental Fig. 2). The results presented in Fig. 3A show that Ca2+ promoted a time-dependent decrease in Ser-552 phosphorylation only in the cells expressing the CaSR. To substantiate that this effect was mediated by CaSR signaling, the cells were challenged with the calcimimetic R-568, a specific CaSR allosteric activator (49). As Fig. 3A shows, stimulation of the CaSR with 100 nm R-568, in medium without Ca2+ supplementation, also promoted a marked decrease in the phosphorylation of β-catenin at Ser-552. Both Ca2+ and R-568 stimulation of the CaSR reduced the level of β-catenin Ser-552 phosphorylation in colonic epithelial cells to similar levels, i.e. ≈40% (Fig. 3). No significant changes in the level of β-catenin Ser-552 phosphorylation were detected in NCMiCaSR cells exposed to 5 mm Ca2+ or 100 nm R-568 in the absence of CaSR expression or in the expression level of β-catenin as result of CaSR stimulation (Fig. 3A). No changes were detected in the level of β-catenin phosphorylation at Ser-45, Thr-41, Ser-37, or Ser-33 in NCMiCaSR cells exposed to 5 mm Ca2+ or 100 nm R-568 (data not shown). Thus, these results indicate that the CaSR stimulation activates a novel signaling pathway that inhibits β-catenin phosphorylation at Ser-552 in human colon-derived epithelial cells.
FIGURE 3.
CaSR-mediated β-catenin Ser-552 phosphorylation in colonic epithelial cells. A, NCMiCaR cells incubated without (−) or with (+) the CaSR inducer doxycycline (0.1 μg/ml) and challenged with 5 mm Ca2+ or 100 nm R-568 in a background of 1.4 mm Ca2+. Cells were lysed at the indicated times and analyzed by Western blotting using rabbit antibodies against Ser(P)-552 (pSer552) and total β-catenin. Signals were detected with a luminescent image analyzer and quantified as described under “Experimental Procedures.” Error bars, S.D. B, Akt-mediated β-catenin Ser-552 phosphorylation in colonic epithelial cells. NCMiCaSR cells were incubated with 2.5 μm GSK 690693 or with 3.0 μm KT 5720 for 4 h and lysates analyzed by Western blotting using rabbit antibodies against Ser(P)-552 and total β-catenin. Signals were detected with a luminescent image analyzer and quantified as described under “Experimental Procedures.” C, Akt-mediated β-catenin Ser-552 phosphorylation inhibition by the CaSR. NCMiCaSR cells incubated with (+) doxycycline (0.1 μg/ml) were stimulated with 5 mm Ca2+ for 4 h and then challenged with EGF (10 ng/ml) for 10 min. Cells were lysed and lysates examined by Western blotting using rabbit antibodies against Ser(P)-552 and total β-catenin. Signals were detected with a luminescent image analyzer and quantified as described under “Experimental Procedures.”
The amino acid sequence of β-catenin surrounding Ser-552 is a consensus site for Akt, and experimental evidence indicates that Akt leads to the phosphorylation of this residue (20). Furthermore, a recent study implicated β-catenin phosphorylation at Ser-552 via PI3K/Akt as a major mechanism potentiating Wnt signaling in inflammation-induced dysplastic transformation in the colon (50). Although these recent findings indicate that Akt phosphorylates β-catenin at Ser-552 in the setting of inflammation, the role of Akt in CaSR-induced β-catenin dephosphorylation at Ser-552 is unknown. Furthermore, recent results using breast and prostate cancer cell lines suggested that modulation of the Akt signaling pathway does not change β-catenin transcriptional activation (51). Accordingly, we determined whether Akt mediates β-catenin Ser-552 phosphorylation in colon-derived epithelial cells. NCMiCaSR cells were incubated with 2.5 μm Akt kinase inhibitor GSK 690693 (52, 53) for 4 h, and lysates were analyzed by Western blotting using the β-catenin Ser(P)-552 antibody. The results presented in Fig. 3B show that GSK 690693 promoted a >50% inhibition in the phosphorylation of β-catenin Ser-552. In contrast, inhibition of PKA using the selective inhibitor KT 5720 (54–56) only induced a 15% inhibition of Ser-552 phosphorylation (Fig. 3B), even at a concentration as high as 30 μm (data not shown).
Tyrosine kinase receptors ligands, like EGF, are potent activators of Akt. If CaSR signaling interferes with Akt, we expected that CaSR stimulation should inhibit Akt-mediated Ser-552 phosphorylation in response to EGF. Therefore, NCMiCaSR cells were stimulated with 5 mm Ca2+ for 4 h and then challenged with EGF (10 ng/ml) for 10 min and lysates examined by Western blotting using the β-catenin Ser(P)-552 antibody. As Fig. 3C shows, EGF promoted a >50% increase over the basal level of β-catenin Ser-552 phosphorylation that was completely abrogated in response to CaSR stimulation. No reduction in β-catenin Ser-552 phosphorylation in response to EGF stimulation was detected after Ca2+ challenge when the CaSR expression was not induced (data not shown). Taken together, these results indicate that Akt is a major player in β-catenin Ser-552 phosphorylation in colon-derived epithelial and that Akt-mediated Ser-552 phosphorylation is inhibited by CaSR signaling.
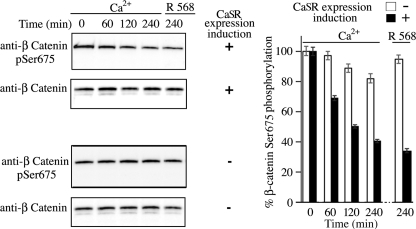
Next, we determined whether CaSR stimulation decreases the phosphorylation of β-catenin Ser-675 in colon-derived epithelial cells using a phospho-specific antibody (supplemental Fig. 2). As occurred with β-catenin Ser-552, the results indicated that Ca2+-mediated CaSR stimulation induced a time-dependent decrease in Ser-675 phosphorylation (Fig. 4) that was similar to that detected after challenging the cells with 100 nm R-568, i.e. ≈ 60% (Fig. 4). No significant change in the level of β-catenin phosphorylation at Ser-675 was detected in NCMiCaSR cells exposed to 5 mm Ca2+ or 100 nm R-568 in the absence of CaSR expression. In addition, no changes in the level of β-catenin phosphorylation at Ser-552 or Ser-675 were detected in the parental NCM-460 cells exposed to 5 mm Ca2+ or 100 nm R-568 (not shown). Thus, these results indicate that the CaSR stimulation activates a novel signaling pathway that promotes a decrease in β-catenin phosphorylation at Ser-552 and Ser-675 in human colon-derived epithelial cells.
FIGURE 4.
CaSR-mediated β-catenin Ser-675 phosphorylation. NCMiCaR cells incubated without (−) or with (+) doxycycline (0.1 μg/ml) were challenged with 5 mm Ca2+ or 100 nm R-568 in a background of 1.4 mm Ca2+, lysed at the indicated times, and analyzed by Western blotting using rabbit antibodies against Ser(P)-675 (pSer675) and total β-catenin. Signals were detected with a luminescent image analyzer and quantified as described under “Experimental Procedures.” Error bars, S.D.
β-Catenin Intracellular Distribution Regulation by CaSR
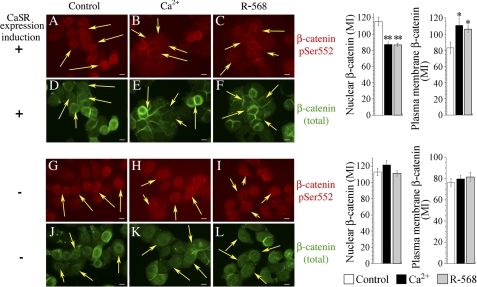
Recent evidence indicates that β-catenin phosphorylation is also involved in the regulation of its intracellular distribution (57). For example, phosphorylation at Ser-552 and Ser-675 has been proposed to drive β-catenin from the plasma membrane to the nuclear compartment by promoting its dissociation from cell-cell contacts (20, 21, 50). Accordingly, and based on the results presented in Figs. 3 and 4, we hypothesized that the decrease observed in β-catenin Ser-552 and Ser-675 phosphorylation after CaSR stimulation should promote the plasma membrane translocation of β-catenin. To test this hypothesis, NCMiCaSR cells expressing the CaSR were exposed to Ca2+ or challenged with the calcimimetic R-568 for 1 h and processed for indirect immunofluorescence as described previously (39). As illustrated by representative cells displayed in Fig. 5, the distribution of β-catenin phosphorylated at Ser-552 in unstimulated control cells expressing (A) or not (G) the CaSR was predominantly nuclear (arrows). In agreement with the results presented in Fig. 3A, the expression and stimulation of the CaSR, either by 5.0 mm Ca2+ (B) or by 100 nm R-568 (C), induced a decrease in the reactivity of the Ser(P)-552 antibody throughout the cell, with special emphasis in the nuclear compartment (arrows). Quantitative analysis of the nuclear content of β-catenin phosphorylated at Ser-552 in cells expressing the CaSR (n = 30) indicated that its relative content in control cells (mean ± S.E.) (115.73 ± 5.14) underwent a 25% reduction in response to Ca2+ (87.4 ± 3.89) or R-568 stimulation (86.5 ± 2.3) (Fig. 5). No changes in Ser-552 antibody reactivity or signal localization were observed in NCMiCaSR cells irrespective of the treatment, i.e. Ca2+ or R-568, when the CaSR expression was not induced (H and I). In agreement with these observations, quantitative analysis of the nuclear content of β-catenin phosphorylated at Ser-552 in cells that did not express the CaSR indicated that its relative content in control cells was not significantly affected despite Ca2+ or R-568 challenge (see also supplemental Table 2). Thus, these results further reinforced the conclusion that signaling pathways emanating from the CaSR are associated with a decrease in β-catenin Ser-552 phosphorylation.
FIGURE 5.
CaSR-mediated β-catenin Ser-552 phosphorylation and plasma membrane localization. NCMiCaR cells incubated without (−) or with (+) doxycycline (0.1 μg/ml) were challenged with 5.0 mm Ca2+ or 100 nm R-568 in a background of 1.4 mm Ca2+ for 1 h and processed for immunofluorescence using a rabbit Ser(P)-552 antibody and mouse monoclonal antibody anti-total β-catenin. The images displayed are representative of 90% of the population of cells. Scale bars, 10 μm. Quantification was performed as described under “Experimental Procedures.” The values represent the MI ± S.E., and they were compared using unpaired Student's t test (**, p < 0.005; *, p < 0.036).
To determine whether CaSR stimulation promotes the plasma membrane translocation of β-catenin, the cells were co-stained with a mouse monoclonal antibody that recognizes β-catenin independently of its phosphorylation (Fig. 5). This antibody revealed that β-catenin was not only present in the nucleus but also in the cytoplasm and plasma membrane in unstimulated control cells expressing or not the CaSR (D and J, respectively). In contrast, this antibody revealed that CaSR stimulation either by Ca2+ (E, arrows) or by the allosteric activator R-568 (F, arrows) promoted a reduction in the nuclear localization of β-catenin accompanied by a substantial redistribution to the plasma membrane as demonstrated by its prominent localization at intercellular plasma membrane junctions. Quantitative analysis of cells expressing the CaSR (n = 20) indicated that the relative content of β-catenin in the plasma membrane underwent an increase of >30% or 25% in response to Ca2+ (110.89 ± 8.1) or R-568 stimulation (105.8 ± 4.43), respectively, compared with control cells (83.14 ± 6.8) (Fig. 5). Importantly, no changes in β-catenin distribution were detected after Ca2+ or R-568 challenge when the CaSR expression was not induced (K and L, respectively). Quantitative analysis of cells that did not express the CaSR also indicated that Ca2+ or R-568 challenge did not significantly modify plasma membrane β-catenin compared with control cells (see also supplemental Table 2).
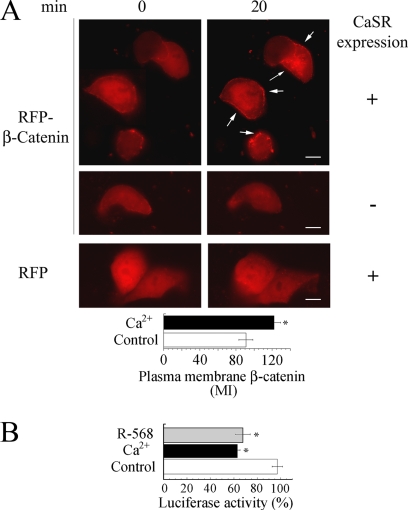
Further support for the observation that CaSR stimulation promotes the plasma membrane translocation of β-catenin was obtained by examining the intracellular distribution of a chimeric protein between the RFP from Discosoma sp. fused to the N terminus of β-catenin. As illustrated by the representative images in Fig. 6A, RFP-β-catenin expressed in unstimulated NCMiCaSR cells was distributed throughout the cytosol with very little fluorescent signal localized to the plasma membrane. Real-time imaging revealed that CaSR stimulation induced the translocation of RFP-β-catenin to the plasma membrane, causing a localized fluorescence at the cell periphery. Quantitative analysis of RFP-β-catenin translocated to the plasma membrane (n = 10) indicated an increase of >30% in response to Ca2+ stimulation (122 ± 7.2) compared with control cells (91.2 ± 7.7) (p < 0.01). No change in RFP-β-catenin intracellular distribution in response to 5 mm Ca2+ was detected in NCMiCaSR cells when the expression of the CaSR was not induced. Furthermore, no change in the intracellular distribution of the RFP moiety was detected in NCMiCaSR cells after Ca2+ challenging despite the expression of the CaSR. Therefore, our results demonstrate that the CaSR signaling is associated with a reduced phosphorylation of β-catenin that coincided with a decline in its nuclear localization and redistribution to the plasma membrane.
FIGURE 6.
Real-time analysis of β-catenin intracellular distribution in response to CaSR stimulation. A, NCMiCaR cells expressing or not the CaSR were transfected with a construct encoding RFP-β-catenin or RFP as indicated under “Experimental Procedures.” The intracellular distribution of RFP-β-catenin and RFP in response to 5 mm Ca2+-mediated CaSR stimulation was examined in real time with an epifluorescence microscope. Quantification was performed as described under “Experimental Procedures.” The values represent the MI ± S.E., and they were compared by unpaired Student's t test (*, p < 0.01). Scale bar, 10 μm. B, CaSR-mediated β-catenin transcriptional activity regulation. NCMiCaSR cells not expressing (Control) or expressing the CaSR were challenged with 5 mm Ca2+ or 100 nm R-568 and 1.4 mm Ca2+, and the luciferase activity was determined as described under “Experimental Procedures.” The luciferase activity values are the result of three independent experiments ± S.E., and they were compared using unpaired Student's t test (*p < 0.01).
CaSR Stimulation Down-regulates β-Catenin-mediated Transcriptional Activation
Because the transcription of β-catenin targeted genes requires its nuclear localization, we hypothesized that the plasma membrane translocation of β-catenin in response to CaSR stimulation should down-regulates β-catenin-mediated transcriptional activation. To examine this hypothesis, we determined the effect of CaSR stimulation on β-catenin transcriptional activity using the pTOPFlash reporter vector (36). As Fig. 6B shows, stimulation of NCMiCaSR cells with either 5 mm Ca2+ or 100 nm R-568 inhibited β-catenin-mediated transcription. Importantly, no inhibition in β-catenin-mediated transcription was detected under the same experimental conditions in the absence of CaSR expression. β-Catenin-mediated transcription was equivalent in NCMiCaSR cells expressing or not the CaSR in the presence of 1.4 mm Ca2+.
Concluding Remarks
A major physiological role of the CaSR, which was originally cloned from parathyroid chief cells (23), is to correct small changes in extracellular Ca2+ concentration by regulating parathyroid hormone secretion (24, 25). The fact that this GPCR recognizes other ligands (23) and that it is expressed in many tissues and organs not involved in the control of Ca2+ homeostasis, including the entire GI tract (34, 58, 59), suggests that the CaSR plays important roles in the regulation of other cellular functions. Nevertheless, no animal model was available to investigate this hypothesis.
The results presented in this study using a recently developed intestinal-specific deficient Casr mouse show that CaSR signaling regulates the proliferation of colonic intestinal epithelial cells in vivo. Specifically, we found that the absence of a functional CaSR is associated with hyperproliferation of epithelial cells in the intact colon, with enhanced β-catenin nuclear localization, and with a significant change in the crypt structure including up to a 50% height increase. We also found that in contrast to control littermates, the proliferative zone in the Casr KO mice extended beyond the base of the colonic crypts into the upper third of the colonic crypt. An increased rate of proliferation and a shift of the proliferative zone have both been shown to occur with increased frequency in patients at increased risk of developing CRC (60–63). A variety of dietary factors profoundly influence the proliferation and differentiation of intestinal epithelial cells. In this context, dietary Ca2+ has generated considerable attention because clinical trials support an inverse association between Ca2+ supplementation (1,200–2,000 mg/day) and the recurrence of colorectal adenoma (64–66). It is tempting to speculate that the reported chemopreventive properties of dietary Ca2+ are the result of the activation of antiproliferative pathways regulated by the CaSR in colonic epithelial cells.
The binding of the Wnt ligand to its receptor promotes the stabilization and nuclear translocation of β-catenin, a key intestinal regulator of genes involved in the control of cell proliferation and differentiation, including cyclin D1, c-Myc, and LGR5 (45, 46). The importance of this pathway in intestinal homeostasis is further emphasized by the fact that >90% of CRCs are associated with deregulated β-catenin signaling (67). Phosphorylation cascades that are dependent and independent of Wnt play a critical role in the control of β-catenin stability, intracellular distribution, and transcriptional activity (20–22, 45, 50, 68, 69), implying that β-catenin is a central point of convergence and integration in multiple signal transduction pathways. In support of this hypothesis, the results presented here indicate that an inhibitory cross-talk that regulates β-catenin phosphorylation exists between CaSR signaling and Akt in colon-derived epithelial cells.
When located at the plasma membrane, β-catenin interacts with E-cadherin, thereby blocking its nuclear localization, transcriptional activity, and the expression of canonical Wnt/β-catenin target genes. Thus, nuclear import/export of β-catenin represents a crucial step in regulating signaling competent β-catenin. Phosphorylation of β-catenin also plays an important role in its intracellular distribution by regulating its interaction with other proteins. In particular, β-catenin phosphorylation at Ser-552 and Ser-675 promotes its dissociation from cell-cell contacts, its nuclear translocation, and transcriptional activity (20, 21, 50). The results presented here show that CaSR stimulation is associated with a plasma membrane redistribution of β-catenin and with a down-regulation of its transcriptional activity. Although the precise mechanism mediating these effects needs further investigation, it is very likely that β-catenin Ser-552 and Ser-675 reduced phosphorylation in response to CaSR signaling affects its nuclear/cytoplasmic shuttling.
To our knowledge, this article provides the first in vivo evidence that CaSR signaling negatively controls colonic epithelial cells proliferation and suggest that this occurs by a mechanism that involves regulation of β-catenin Ser-552 and Ser-675 phosphorylation.
Supplementary Material
Acknowledgments
We thank Amgen for providing the calcimimetic R-568 and Richard T. Waldron for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants K22CA128883 (to O. R.), K22CA128883-03S1 (to O. R.), RO1-AG21353 (to W. C.), RO1AR050023 (to D. B.), RO1 AR055924 (to D. B.), RO1DK054793 (to D. B.), RO1DK56930 (to E. R.), RO1DK55003 (to E. R.), and P30DK41301 (to E. R.). This work was also supported by Veteran Affairs Merit Review (to D. B.) and Veteran Affairs Merit Review (to E. R.).

This article contains supplemental Figs. 1 and 2 and Tables 1 and 2.
- GPCR
- G protein-coupled receptor
- CaSR
- calcium-sensing receptor
- CRC
- colorectal cancer
- GI
- gastrointestinal
- MI
- mean pixel intensity
- RFP
- red fluorescent protein
- ROI
- region of interest
- Wnt
- Wingless.
REFERENCES
- 1. Chell S., Kaidi A., Kadi A., Williams A. C., Paraskeva C. (2006) Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim. Biophys. Acta 1766, 104–119 [DOI] [PubMed] [Google Scholar]
- 2. Jin G., Ramanathan V., Quante M., Baik G. H., Yang X., Wang S. S., Tu S., Gordon S. A., Pritchard D. M., Varro A., Shulkes A., Wang T. C. (2009) Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J. Clin. Invest. 119, 2691–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin S., Wang D., Iyer S., Ghaleb A. M., Shim H., Yang V. W., Chun J., Yun C. C. (2009) The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 136, 1711–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rozengurt E. (2002) Neuropeptides as growth factors for normal and cancerous cells. Trends Endocrinol. Metab. 13, 128–134 [DOI] [PubMed] [Google Scholar]
- 5. Rozengurt E., Guha S., Sinnett-Smith J. (2002) Gastrointestinal peptide signalling in health and disease. Eur. J. Surg. Suppl. 23–38 [PubMed] [Google Scholar]
- 6. Rozengurt E., Sternini C. (2007) Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol. 7, 557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rozengurt E., Walsh J. H. (2001) Gastrin, CCK, signaling, and cancer. Annu. Rev. Physiol. 63, 49–76 [DOI] [PubMed] [Google Scholar]
- 8. Wilson C. H., McIntyre R. E., Arends M. J., Adams D. J. (2010) The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc(Min/+) mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene 29, 4567–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulte G. (2010) International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 62, 632–667 [DOI] [PubMed] [Google Scholar]
- 10. He T. C., Chan T. A., Vogelstein B., Kinzler K. W. (1999) PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99, 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. (1998) Identification of c-Myc as a target of the APC pathway. Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 12. Crawford H. C., Fingleton B. M., Rudolph-Owen L. A., Goss K. J., Rubinfeld B., Polakis P., Matrisian L. M. (1999) The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 18, 2883–2891 [DOI] [PubMed] [Google Scholar]
- 13. Gradl D., Kühl M., Wedlich D. (1999) The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol. Cell. Biol. 19, 5576–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann B., Gelos M., Siedow A., Hanski M. L., Gratchev A., Ilyas M., Bodmer W. F., Moyer M. P., Riecken E. O., Buhr H. J., Hanski C. (1999) Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. U.S.A. 96, 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roose J., Huls G., van Beest M., Moerer P., van der Horn K., Goldschmeding R., Logtenberg T., Clevers H. (1999) Synergy between tumor suppressor APC and the β-catenin-Tcf4 target Tcf1. Science 285, 1923–1926 [DOI] [PubMed] [Google Scholar]
- 16. Tetsu O., McCormick F. (1999) β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 [DOI] [PubMed] [Google Scholar]
- 17. Wielenga V. J., Smits R., Korinek V., Smit L., Kielman M., Fodde R., Clevers H., Pals S. T. (1999) Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am. J. Pathol. 154, 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koh T. J., Bulitta C. J., Fleming J. V., Dockray G. J., Varro A., Wang T. C. (2000) Gastrin is a target of the β-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J. Clin. Invest. 106, 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lickert H., Domon C., Huls G., Wehrle C., Duluc I., Clevers H., Meyer B. I., Freund J. N., Kemler R. (2000) Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development 127, 3805–3813 [DOI] [PubMed] [Google Scholar]
- 20. Fang D., Hawke D., Zheng Y., Xia Y., Meisenhelder J., Nika H., Mills G. B., Kobayashi R., Hunter T., Lu Z. (2007) Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J. Biol. Chem. 282, 11221–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taurin S., Sandbo N., Qin Y., Browning D., Dulin N. O. (2006) Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 281, 9971–9976 [DOI] [PubMed] [Google Scholar]
- 22. Taurin S., Sandbo N., Yau D. M., Sethakorn N., Dulin N. O. (2008) Phosphorylation of β-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 294, C1169–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown E. M., MacLeod R. J. (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 81, 239–297 [DOI] [PubMed] [Google Scholar]
- 24. Hendy G. N., D'Souza-Li L., Yang B., Canaff L., Cole D. E. (2000) Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum. Mutat. 16, 281–296 [DOI] [PubMed] [Google Scholar]
- 25. Ho C., Conner D. A., Pollak M. R., Ladd D. J., Kifor O., Warren H. B., Brown E. M., Seidman J. G., Seidman C. E. (1995) A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat. Genet. 11, 389–394 [DOI] [PubMed] [Google Scholar]
- 26. Quarles L. D. (2003) Extracellular calcium-sensing receptors in the parathyroid gland, kidney, and other tissues. Curr. Opin. Nephrol. Hypertens. 12, 349–355 [DOI] [PubMed] [Google Scholar]
- 27. Geibel J. P., Hebert S. C. (2009) The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu. Rev. Physiol. 71, 205–217 [DOI] [PubMed] [Google Scholar]
- 28. Kállay E., Bajna E., Wrba F., Kriwanek S., Peterlik M., Cross H. S. (2000) Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect. Prev. 24, 127–136 [PubMed] [Google Scholar]
- 29. Sheinin Y., Kállay E., Wrba F., Kriwanek S., Peterlik M., Cross H. S. (2000) Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J. Histochem. Cytochem. 48, 595–602 [DOI] [PubMed] [Google Scholar]
- 30. Hizaki K., Yamamoto H., Taniguchi H., Adachi Y., Nakazawa M., Tanuma T., Kato N., Sukawa Y., Sanchez J. V., Suzuki H., Sasaki S., Imai K., Shinomura Y. (2011) Epigenetic inactivation of calcium-sensing receptor in colorectal carcinogenesis. Mod. Pathol. 24, 876–884 [DOI] [PubMed] [Google Scholar]
- 31. Rey O., Young S. H., Jacamo R., Moyer M. P., Rozengurt E. (2010) Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J. Cell. Physiol. 225, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chakrabarty S., Radjendirane V., Appelman H., Varani J. (2003) Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of β-catenin/TCF activation. Cancer Res. 63, 67–71 [PubMed] [Google Scholar]
- 33. Wong N. A., Pignatelli M. (2002) β-Catenin: a linchpin in colorectal carcinogenesis? Am. J. Pathol. 160, 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang W., Tu C., Chen T. H., Bikle D., Shoback D. (2008) The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal. 1, ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu S. V., Rozengurt N., Yang M., Young S. H., Sinnett-Smith J., Rozengurt E. (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. U.S.A. 99, 2392–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. (2003) Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685 [DOI] [PubMed] [Google Scholar]
- 37. Rey O., Lee S., Park N. H. (2000) Human papillomavirus type 16 E7 oncoprotein represses transcription of human fibronectin. J. Virol. 74, 4912–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hope C., Planutis K., Planutiene M., Moyer M. P., Johal K. S., Woo J., Santoso C., Hanson J. A., Holcombe R. F. (2008) Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol. Nutr. Food Res. 52, S52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rey O., Young S. H., Cantrell D., Rozengurt E. (2001) Rapid protein kinase D translocation in response to G protein-coupled receptor activation: dependence on protein kinase C. J. Biol. Chem. 276, 32616–32626 [DOI] [PubMed] [Google Scholar]
- 40. Madison B. B., Dunbar L., Qiao X. T., Braunstein K., Braunstein E., Gumucio D. L. (2002) Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 [DOI] [PubMed] [Google Scholar]
- 41. Ray J. M., Squires P. E., Curtis S. B., Meloche M. R., Buchan A. M. (1997) Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J. Clin. Invest. 99, 2328–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vezzoli G., Soldati L., Gambaro G. (2009) Roles of calcium-sensing receptor (CaSR) in renal mineral ion transport. Curr. Pharm. Biotechnol. 10, 302–310 [DOI] [PubMed] [Google Scholar]
- 43. Brown E. M., Pollak M., Hebert S. C. (1995) Sensing of extracellular Ca2+ by parathyroid and kidney cells: cloning and characterization of an extracellular Ca2+-sensing receptor. Am. J. Kidney Dis. 25, 506–513 [DOI] [PubMed] [Google Scholar]
- 44. Holt P. R., Moss S. F., Kapetanakis A. M., Petrotos A., Wang S. (1997) Is Ki-67 a better proliferative marker in the colon than proliferating cell nuclear antigen? Cancer Epidemiol. Biomarkers Prev. 6, 131–135 [PubMed] [Google Scholar]
- 45. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 46. McDonald S. A., Preston S. L., Lovell M. J., Wright N. A., Jankowski J. A. (2006) Mechanisms of disease: from stem cells to colorectal cancer. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 267–274 [DOI] [PubMed] [Google Scholar]
- 47. Sinnett-Smith J., Rozengurt N., Kui R., Huang C., Rozengurt E. (2011) Protein kinase D1 mediates stimulation of DNA synthesis and proliferation in intestinal epithelial IEC-18 cells and in mouse intestinal crypts. J. Biol. Chem. 286, 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moyer M. P., Manzano L. A., Merriman R. L., Stauffer J. S., Tanzer L. R. (1996) NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev. Biol. Anim. 32, 315–317 [DOI] [PubMed] [Google Scholar]
- 49. Fox J., Lowe S. H., Petty B. A., Nemeth E. F. (1999) NPS R-568: a type II calcimimetic compound that acts on parathyroid cell calcium receptor of rats to reduce plasma levels of parathyroid hormone and calcium. J. Pharmacol. Exp. Ther. 290, 473–479 [PubMed] [Google Scholar]
- 50. Lee G., Goretsky T., Managlia E., Dirisina R., Singh A. P., Brown J. B., May R., Yang G. Y., Ragheb J. W., Evers B. M., Weber C. R., Turner J. R., He X. C., Katzman R. B., Li L., Barrett T. A. (2010) Phosphoinositide 3-kinase signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139, 869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ng S. S., Mahmoudi T., Danenberg E., Bejaoui I., de Lau W., Korswagen H. C., Schutte M., Clevers H. (2009) Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J. Biol. Chem. 284, 35308–35313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heerding D. A., Rhodes N., Leber J. D., Clark T. J., Keenan R. M., Lafrance L. V., Li M., Safonov I. G., Takata D. T., Venslavsky J. W., Yamashita D. S., Choudhry A. E., Copeland R. A., Lai Z., Schaber M. D., Tummino P. J., Strum S. L., Wood E. R., Duckett D. R., Eberwein D., Knick V. B., Lansing T. J., McConnell R. T., Zhang S., Minthorn E. A., Concha N. O., Warren G. L., Kumar R. (2008) Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase. J. Med. Chem. 51, 5663–5679 [DOI] [PubMed] [Google Scholar]
- 53. Rhodes N., Heerding D. A., Duckett D. R., Eberwein D. J., Knick V. B., Lansing T. J., McConnell R. T., Gilmer T. M., Zhang S. Y., Robell K., Kahana J. A., Geske R. S., Kleymenova E. V., Choudhry A. E., Lai Z., Leber J. D., Minthorn E. A., Strum S. L., Wood E. R., Huang P. S., Copeland R. A., Kumar R. (2008) Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 68, 2366–2374 [DOI] [PubMed] [Google Scholar]
- 54. Cabell L., Audesirk G. (1993) Effects of selective inhibition of protein kinase C, cyclic AMP-dependent protein kinase, and Ca(2+)-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurons. Int. J. Dev. Neurosci. 11, 357–368 [DOI] [PubMed] [Google Scholar]
- 55. Gadbois D. M., Crissman H. A., Tobey R. A., Bradbury E. M. (1992) Multiple kinase arrest points in the G1 phase of nontransformed mammalian cells are absent in transformed cells. Proc. Natl. Acad. Sci. U.S.A. 89, 8626–8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. (1987) K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 142, 436–440 [DOI] [PubMed] [Google Scholar]
- 57. Verheyen E. M., Gottardi C. J. (2010) Regulation of Wnt/β-catenin signaling by protein kinases. Dev. Dyn. 239, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Adams G. B., Chabner K. T., Alley I. R., Olson D. P., Szczepiorkowski Z. M., Poznansky M. C., Kos C. H., Pollak M. R., Brown E. M., Scadden D. T. (2006) Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 [DOI] [PubMed] [Google Scholar]
- 59. Geibel J., Sritharan K., Geibel R., Geibel P., Persing J. S., Seeger A., Roepke T. K., Deichstetter M., Prinz C., Cheng S. X., Martin D., Hebert S. C. (2006) Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc. Natl. Acad. Sci. U.S.A. 103, 9390–9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lipkin M., Blattner W. A., Gardner E. J., Burt R. W., Lynch H., Deschner E., Winawer S., Fraumeni J. F., Jr. (1984) Classification and risk assessment of individuals with familial polyposis, Gardner's syndrome, and familial non-polyposis colon cancer from [3H]thymidine labeling patterns in colonic epithelial cells. Cancer Res. 44, 4201–4207 [PubMed] [Google Scholar]
- 61. Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. (1987) Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology 92, 704–708 [DOI] [PubMed] [Google Scholar]
- 62. Bostick R. M., Fosdick L., Grandits G. A., Lillemoe T. J., Wood J. R., Grambsch P., Louis T. A., Potter J. D. (1997) Colorectal epithelial cell proliferative kinetics and risk factors for colon cancer in sporadic adenoma patients. Cancer Epidemiol. Biomarkers Prev. 6, 1011–1019 [PubMed] [Google Scholar]
- 63. Eastwood G. L. (1995) A review of gastrointestinal epithelial renewal and its relevance to the development of adenocarcinomas of the gastrointestinal tract. J. Clin. Gastroenterol. 21, S1–11 [PubMed] [Google Scholar]
- 64. Baron J. A., Beach M., Mandel J. S., van Stolk R. U., Haile R. W., Sandler R. S., Rothstein R., Summers R. W., Snover D. C., Beck G. J., Bond J. H., Greenberg E. R. (1999) Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N. Engl. J. Med. 340, 101–107 [DOI] [PubMed] [Google Scholar]
- 65. Grau M. V., Baron J. A., Sandler R. S., Wallace K., Haile R. W., Church T. R., Beck G. J., Summers R. W., Barry E. L., Cole B. F., Snover D. C., Rothstein R., Mandel J. S. (2007) Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J. Natl. Cancer Inst. 99, 129–136 [DOI] [PubMed] [Google Scholar]
- 66. Wei E. K., Wolin K. Y., Colditz G. A. (2010) Time course of risk factors in cancer etiology and progression. J. Clin. Oncol. 28, 4052–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walther A., Johnstone E., Swanton C., Midgley R., Tomlinson I., Kerr D. (2009) Genetic prognostic and predictive markers in colorectal cancer. Nat. Rev. Cancer 9, 489–499 [DOI] [PubMed] [Google Scholar]
- 68. He X. C., Yin T., Grindley J. C., Tian Q., Sato T., Tao W. A., Dirisina R., Porter-Westpfahl K. S., Hembree M., Johnson T., Wiedemann L. M., Barrett T. A., Hood L., Wu H., Li L. (2007) PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 39, 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vermeulen L., De Sousa E., Melo F., van der Heijden M., Cameron K., de Jong J. H., Borovski T., Tuynman J. B., Todaro M., Merz C., Rodermond H., Sprick M. R., Kemper K., Richel D. J., Stassi G., Medema J. P. (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.