Background: Estrogens prevent bone loss in part by preventing osteocyte apoptosis.
Results: Anti-apoptotic effects of 17β-estradiol in osteocytes require NO/cGMP-mediated stimulation of Akt and Akt- and cGMP-dependent protein kinase (PKG)-dependent phosphorylation of BAD.
Conclusion: PKG types I and II serve independent anti-apoptotic functions in 17β-estradiol-treated osteocytes, converging on BAD.
Significance: These novel mechanisms of 17β-estradiol-mediated bone protection provide a rationale for developing NO/cGMP-based therapies for osteoporosis.
Keywords: Apoptosis, Cyclic GMP (cGMP), Estrogen, Nitric oxide, Osteocyte
Abstract
Estrogens promote bone health in part by increasing osteocyte survival, an effect that requires activation of the protein kinases Akt and ERK1/2, but the molecular mechanisms involved are only partly understood. Because estrogens increase nitric oxide (NO) synthesis and NO can have anti-apoptotic effects, we examined the role of NO/cGMP signaling in estrogen regulation of osteocyte survival. Etoposide-induced death of MLO-Y4 osteocyte-like cells, assessed by trypan blue staining, caspase-3 cleavage, and TUNEL assays, was completely prevented when cells were pre-treated with 17β-estradiol. This protective effect was mimicked when cells were pre-treated with a membrane-permeable cGMP analog and blocked by pharmacological inhibitors of NO synthase, soluble guanylate cyclase, or cGMP-dependent protein kinases (PKGs), supporting a requirement for NO/cGMP/PKG signaling downstream of 17β-estradiol. siRNA-mediated knockdown and viral reconstitution of individual PKG isoforms demonstrated that the anti-apoptotic effects of estradiol and cGMP were mediated by PKG Iα and PKG II. Akt and ERK1/2 activation by 17β-estradiol required PKG II, and cGMP mimicked the effects of estradiol on Akt and ERK, including induction of ERK nuclear translocation. cGMP induced BAD phosphorylation on several sites, and experiments with phosphorylation-deficient BAD mutants demonstrated that the anti-apoptotic effects of cGMP and 17β-estradiol required BAD phosphorylation on Ser136 and Ser155; these sites were targeted by Akt and PKG I, respectively, and regulate BAD interaction with Bcl-2. In conclusion, 17β-estradiol protects osteocytes against apoptosis by activating the NO/cGMP/PKG cascade; PKG II is required for estradiol-induced activation of ERK and Akt, and PKG Iα contributes to pro-survival signaling by directly phosphorylating BAD.
Introduction
Skeletal integrity and maintenance of bone mass require continuous bone remodeling through resorption by osteoclasts and new bone formation by osteoblasts. Normal aging and estrogen deficiency are associated with progressive bone loss and increased bone fragility; both conditions are characterized by decreasing osteoblast numbers and increased apoptosis of osteoblasts and mature osteocytes (1–4). Estrogens prevent bone loss by prolonging the life span of osteoblasts and osteocytes while shortening osteoclast survival (2–4). Previous work has shown that 17β-estradiol protects osteoblasts and osteocytes from apoptosis by activating c-Src and the extracellular signal-responsive protein kinases (ERK-1/2) via a plasma membrane-bound estrogen receptor; these effects do not require nuclear localization or DNA binding of the estrogen receptor but nuclear translocation of ERK (5–7).
In a variety of cell types, including osteoblasts, estrogens increase NO synthesis through transcriptional and post-transcriptional regulation of endothelial NO synthase (8–11). Estrogen-induced NO synthesis has also been demonstrated in vivo (12, 13). Among other effects, NO activates soluble guanylate cyclase, generating cGMP, which in turn regulates cGMP-dependent protein kinases (PKGs)2 and phosphodiesterases (14). The PKG I gene (prkg1) encodes two splice variants differing in the N-terminal ∼100 amino acids, PKG Iα and Iβ, that are largely cytosolic enzymes, whereas the PKG II gene (prkg2) encodes a membrane-bound enzyme (15). PKGs I and II differ in their tissue distribution, but both genes are expressed in osteoblasts and osteocytes (15, 16). We recently showed that stimulation of osteoblasts and osteocytes by fluid shear stress increases NO and cGMP levels, activating PKG II, which leads to Src and ERK activation, induction of fos family genes, and increased osteoblast proliferation (16, 17). PKG II-null mice show defective Src and ERK signaling in osteoblasts and decreased c-fos expression in bone (17); these mice also exhibit dwarfism caused by defective chondroblast differentiation (18).
NO/cGMP signaling has been implicated in regulating apoptosis in different cell types (19, 20). Fluid shear stress-induced NO production or treatment with NO donors protect osteocytes and osteoblasts from tumor necrosis factor (TNF)-α-induced apoptosis, but the downstream targets of NO are unclear (21, 22). NO donors counteract estrogen deficiency-induced osteopenia in ovariectomized rats and show promise in ameliorating osteoporosis in post-menopausal women (23–26). Experiments in endothelial NO synthase-deficient mice suggest that at least some of the bone-protective effects of estrogens are mediated by the NO pathway (27, 28). We, therefore, decided to study the role of NO/cGMP signaling in 17β-estradiol regulation of osteocyte survival.
EXPERIMENTAL PROCEDURES
Reagents
17β-Estradiol, referred to subsequently as estradiol, and etoposide were from Sigma-Aldrich. Phospho-ERK-1 (pTyr204), total ERK-1/2, α-tubulin, and β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Akt (pSer473), total Akt, cleaved caspase-3, phospho-BAD (pSer112, pSer136, or pSer155), and total BAD antibodies were from Cell Signaling Technology (Beverly, MA), and the HA-epitope antibody was from Roche Applied Science (Indianapolis, IN). The cGMP agonist 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP) and antagonist 8-(4-chlorophenylthio)-β-phenyl-1,N2-ethenoguanosine-3′,5′-cyclic phosphothioate, Rp isomer (Rp-8-pCPT-PET-cGMPS) were from Biolog, Inc., Bremen, Germany. The NOS inhibitor N-nitro-l-arginine methyl ester (l-NAME), the soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), and the NO-releasing drug 2,2′-hydroxynitrosohydrazino)bis-ethanamine (DETA-NONOate) were from Cayman Chemical Co. (Ann Arbor, MI). LY294002 was from Calbiochem/EMD (San Diego, CA). HA-tagged BAD constructs were a gift from Dr. Tom Chittenden (ImmunoGen) (29).
Cell Culture
The murine osteocyte-like cell line MLO-Y4 was a gift from Dr. Lynda Bonewald (University of Missouri, Kansas City) and was maintained on collagen-coated plates in α-minimal essential medium (α-MEM) supplemented with 2.5% enriched calf serum and 2.5% heat-inactivated fetal bovine serum (30). For most experiments, cells were incubated overnight in phenol red-free α-MEM with 0.1% charcoal-stripped fetal bovine serum prior to estradiol stimulation. MC3T3 cells were from the American Tissue Culture Collection, and primary osteoblasts were isolated from the calvariae of 3- to 5-day-old mice and cultured as described before (17).
DNA and siRNA Transfections and RT-PCR
MLO-Y4 cells were transiently transfected in 6-well dishes at 60–80% confluency using 5 μl of Lipofectamine 2000TM (Invitrogen) and a total of 1 μg of DNA (0.25 μg of BAD expression vector) or 100 pmol of siRNA in 1 ml of serum-containing α-MEM. Experimental treatments occurred 48 h post-transfection for DNA-transfected cells and 24–48 h post-transfection for siRNA-transfected cells. siRNA oligoribonucleotides were from Qiagen. The siRNA target sequence for PKG Iα/β was 5′-CCGGACAUUUAAAGACAGCAA-3′ (PKG I), and for PKG II 5′-CTGCTTGGAAGTGGAATACTA-3′ (PKG IIa) and 5′-CCGGGTTTCTTGGGGTAGTCAA-3′ (PKG IIb). mRNA knockdown and protein depletion were quantified by real-time RT-PCR and Western blotting, respectively. Quantitative RT-PCR was performed, and PKG mRNA levels were normalized to gapdh mRNA levels as described (16). PCR primer sequences for PKG I were (forward) 5′-GTCACTAGGGATTCTGATGTATGA-3′ and (reverse) 5′-AGAATTTCCAAAGAAGATTGCAAA-3′. The PKG II primers were (forward) 5′-GTGACACAGCGCGGTTGTT-3′ and (reverse) 5′-TGGGAATGGAAAAGGACAAC-3′.
Measurement of Cell Death
MLO-Y4 cells received the indicated pharmacological inhibitor or vehicle for 1 h prior to receiving 100 nm estradiol, 100 μm 8-pCPT-cGMP, or additional vehicle for 1 h. Subsequently, cells were treated with 50 μm etoposide for 8 h, which induces apoptosis by forming a ternary complex with topoisomerase II and DNA, preventing DNA religation, and causing double-stranded DNA breaks. A minimum of 200 cells for each condition was examined by trypan blue uptake, and etoposide-induced cell death was calculated by subtracting the percentage of trypan blue-positive cells in untreated cells from the percentage in the etoposide-treated samples. Apoptosis was quantified by TUNEL staining after 6 h of etoposide treatment, using the DeadEndTM Colorimetric TUNEL System according to the manufacturer's instructions (Promega, Madison, WI). Apoptotic cells were also visualized by immunofluorescence staining with an antibody against cleaved caspase-3. With both assays, a minimum of 100 cells from three randomly selected fields were assessed for each condition, and the percentage of apoptotic cells was calculated by subtracting the percentage of TUNEL- or cleaved caspase-3-positive cells in untreated control cultures from the percentage in the etoposide-treated samples. All microscopic results were confirmed by an independent observer, who counted samples in a blinded fashion. Cleaved caspase-3 levels were also assessed by Western blotting.
NO Production Assay
NO production was measured based on nitrite and nitrate accumulation in the medium using a two-step colorimetric assay kit (Active Motif), as described (16).
In Vitro Phosphorylation of BAD
293T cells were transiently transfected with vectors encoding HA-tagged wild-type or mutant BAD constructs. After 24 h, cells were lysed in 50 mm Tris-HCl, pH 7.5, 137 mm NaCl, 1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, and protease inhibitors. BAD proteins were isolated by immunoprecipitation with anti-HA-agarose beads (Sigma-Aldrich) and incubated in the presence of 5 μCi of [γ-32P]ATP and purified catalytic domain of PKG I. Phosphorylation was analyzed by using SDS-PAGE and autoradiography.
Western Blot Analyses
Western blots were developed with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence as described (31). The bar graphs were generated by densitometry scanning using ImageJ software.
Statistical Analyses
Data were analyzed by using one-way analysis of variance with a Bonferroni post-test. A p value of <0.05 was considered statistically significant. Bar graphs are shown as the mean ± S.E. of at least three independent experiments. All other data are shown as representative results of at least three independent experiments.
RESULTS
Anti-apoptotic Effects of Estradiol in Osteocytes and Osteoblasts Are Mediated by NO/cGMP/PKG
In the first set of experiments, we asked if the anti-apoptotic effects of estradiol on osteocytes require signaling through the NO/cGMP/PKG pathway. In MLO-Y4 osteocyte-like cells, 100 nm estradiol increased NO production 2- to 3-fold, and this increase was completely blocked by l-NAME (supplemental Fig. S1A). Estradiol also induced phosphorylation of vasodilator-stimulated phosphoprotein at Ser259, a preferred PKG phosphorylation site, indicating that estradiol activates the NO/cGMP/PKG pathway (supplemental Fig. S1B).
When MLO-Y4 cells were serum-starved or treated with etoposide, the number of trypan blue-positive, dead cells increased by 5.8% and 8.4%, respectively, over basal levels observed in untreated cells in full growth medium (Fig. 1, A and B, first bar). These results are similar to those reported by others (6, 7). We used TUNEL staining to identify early apoptotic cells containing fragmented DNA and found that etoposide increased the number of apoptotic cells by 12.6% (Fig. 1, C (right panel) and D, show typical TUNEL stains). When cells were pre-treated with 100 nm estradiol prior to serum starvation or etoposide exposure, the number of trypan blue- or TUNEL-positive cells was reduced to near basal levels, similar to previous reports (6, 7) (Fig. 1, A–C). Pre-treating cells with 100 μm 8-pCPT-cGMP mimicked the protective effects of estradiol (Fig. 1, A–D).
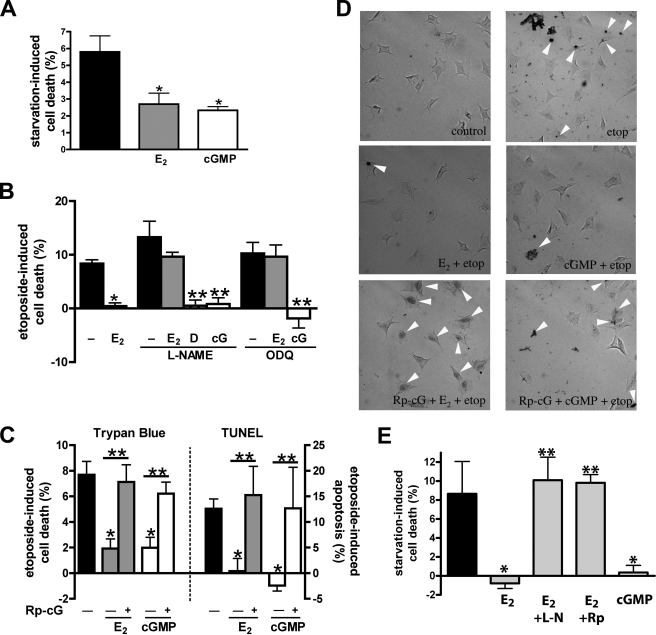
FIGURE 1.
Estradiol protects MLO-Y4 osteocytic cells and primary murine osteoblasts from serum starvation- or etoposide-induced apoptosis through NO/cGMP/PKG signaling. A, MLO-Y4 cells were treated with vehicle (black bar), 100 nm estradiol (E2, gray bar), or 100 μm 8-pCPT-cGMP (cGMP, white bar) and serum-starved for 18 h. Starvation-induced cell death was measured by trypan blue uptake as described under “Experimental Procedures.” B, MLO-Y4 cells were treated with 4 mm l-NAME or 10 μm ODQ for 1 h as indicated, and then received vehicle, 100 nm estradiol, 3 μm DETA-NONOate (D), or 100 μm 8-pCPT-cGMP (cG) for 1 h, followed by an 8-h exposure to 50 μm etoposide. Etoposide-induced cell death was measured by trypan blue uptake as in A. C, MLO-Y4 cells were treated with 100 μm Rp-8-CPT-PET-cGMPS (Rp-cG) for 1 h as indicated (+), followed by 100 nm estradiol (gray bars) or 100 μm cGMP (white bars) for 1 h; cells were then exposed to etoposide for 8 h (trypan blue, left graph) or 6 h (TUNEL assay, right graph). D, representative images of TUNEL staining; cells were treated as in C. E, primary murine osteoblasts received vehicle (black bar), estradiol (gray bars), or cGMP (white bar) and were serum-starved for 18 h as in A. Some cells were pre-treated with vehicle, 4 mm l-NAME (l-N) or 100 μm Rp-8-CPT-PET-cGMPS (Rp) for 1 h prior to receiving estradiol as indicated. Cell death was measured by trypan blue uptake. A–C and D: *, p < 0.05 compared with apoptotic stimulus alone; **, p < 0.05 for the comparison between the presence and absence of pharmacological inhibitor.
Estradiol could no longer protect cells from etoposide-induced death when NO synthesis was inhibited by l-NAME; in contrast, DETA-NONOate provided protection in the presence of l-NAME (Fig. 1B). Inhibition of soluble guanylate cyclase by ODQ also blocked the protective effects of estradiol, but 8-pCPT-cGMP protected osteocytes from etoposide-induced cell death in the presence of either l-NAME or ODQ (Fig. 1B). These data indicate estradiol signals through NO/cGMP to protect osteocytes from apoptosis and are consistent with l-NAME and ODQ specifically inhibiting NO and cGMP synthesis, respectively, rather than enhancing osteocyte death nonspecifically. l-NAME by itself slightly increased etoposide-induced cell death, but this did not reach statistical significance (Fig. 1B). Inhibition of cGMP-dependent protein kinases with Rp-8-pCPT-PET-cGMPS prevented the pro-survival effects of both estradiol and 8-pCPT-cGMP, suggesting that osteocyte protection by estradiol and cGMP requires PKG (Fig. 1C summarizes results for trypan blue and TUNEL staining, and Fig. 1D shows typical TUNEL stains).
To determine if similar anti-apoptotic effects of estradiol and cGMP occur in osteoblastic cells, we studied murine primary osteoblasts and immortalized MC3T3 pre-osteoblast-like cells. We found that estradiol and cGMP protected the primary osteoblasts and MC3T3 cells from death induced by serum starvation or TNF-α, respectively, and that the protective effect of estradiol required signaling through NO/cGMP/PKG (Fig. 1E and supplemental Fig. S1C).
PKG Iα and PKG II Are Independently Required for Estradiol- and cGMP-mediated Protection from Apoptosis
To determine which PKG isoform was involved in preventing the anti-apoptotic effects of estradiol and cGMP, we used an siRNA approach. MLO-Y4 cells were transfected with siRNAs targeting either the common C-terminal region of PKG Iα and Iβ, or PKG II, with an siRNA targeting green fluorescent protein (GFP) serving as a control. Quantitative RT-PCR showed that PKG I mRNA levels were reduced by 71% within 24 h in cells transfected with PKG I siRNA, whereas PKG II mRNA levels were unaffected. Cells transfected with siRNA targeting PKG II showed a 76% reduction in PKG II mRNA levels with no change in PKG I mRNA (Fig. 2A). Expression of PKG I or PKG II protein was reduced correspondingly in PKG I or PKG II siRNA-transfected cells (supplemental Fig. S2A and C).
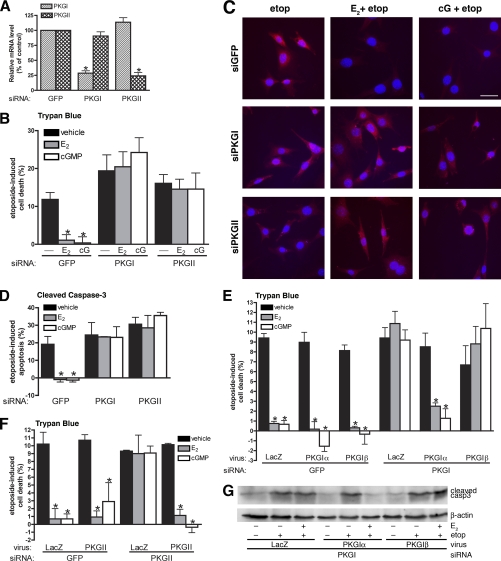
FIGURE 2.
PKG Iα and PKG II are independently required for estradiol- and cGMP-mediated protection against apoptosis. MLO-Y4 cells were transfected as described under “Experimental Procedures” with siRNA targeting GFP (control), PKG I, or PKG II. A, mRNA levels of PKG I (striped bars) and PKG II (cross-hatched bars) were measured by quantitative RT-PCR 24 h after transfection and normalized to gapdh mRNA levels as described under “Experimental Procedures.” PKG mRNA levels in GFP siRNA-transfected cells were considered 100%; *, p < 0.05 compared with GFP control. B, cells were pretreated with vehicle (black bars), 100 nm estradiol (E2, gray bars) or 100 μm 8-pCPT-cGMP (cGMP, white bars) for 1 h, followed by an 8-h treatment with 50 μm etoposide. Etoposide-induced cell death was measured by trypan blue uptake as described under “Experimental Procedures.” C and D, cells were treated as in B but cells were fixed, permeabilized, and stained for cleaved caspase-3 to mark apoptotic cells. Nuclei were counterstained with Hoechst 33342. D shows the percentage of cells staining positive for cleaved caspase-3. E, 24 h after GFP or PKG I siRNA transfection, cells were infected with adenovirus expressing β-galactosidase (LacZ, control), siRNA-resistant PKG Iα, or PKG Iβ for 24 h. Cells were treated with vehicle (black bars), estradiol (gray bars), or cGMP (white bars) for 1 h prior to exposure to etoposide for 8 h; cell death was quantified as in B. F, cells were treated as in E, except for transfection with PKG II-specific siRNA and subsequent infection with an adenovirus expressing siRNA-resistant PKG II. G, cells were transfected with siRNA targeting PKG I, were infected with the indicated virus, and were treated with vehicle or estradiol prior to etoposide exposure as in E. Cell lysates were analyzed by Western blotting with an antibody specific for cleaved caspase-3; a β-actin-specific antibody was used as a loading control. B–E: *, p < 0.05 compared with control cells treated with vehicle and then exposed to etoposide.
In control siRNA-transfected MLO-Y4 cultures, etoposide increased the number of trypan blue-positive cells by 12% (Fig. 2B) and the number of apoptotic cells containing cleaved caspase-3 by 20% (Fig. 2, C and D). The effects of etoposide were abrogated by estradiol or 8-pCPT-cGMP, similar to results obtained for trypan blue and TUNEL assays in untransfected cells. In cells transfected with either PKG I- or PKG II-specific siRNA, etoposide increased cell death regardless of whether estradiol or 8-pCPT-cGMP was present, indicating necessary and non-redundant roles for both PKG isoforms in estradiol- and cGMP-induced protection from cell death (Fig. 2B shows etoposide-induced cell death by trypan blue staining, and Fig. 2, C and D, show immunofluorescence staining for cleaved caspase-3).
To ensure these results were not from off-target effects of the siRNAs, we expressed each PKG isoform in siRNA-transfected cells using adenoviral vectors encoding siRNA-resistant PKG Iα, PKG Iβ, or PKG II. Reconstituting PKG Iα in PKG I-depleted cells, and PKG II in PKG II-depleted cells, restored the ability of estradiol and 8-pCPT-cGMP to reverse etoposide-induced cell death; however, reconstituting PKG Iβ in PKG I-depleted cells had no effect (Fig. 2, E and F, show viral infection of PKG I- and PKG II-depleted cells, respectively, with adenovirus encoding LacZ serving as a negative control; supplemental Fig. S2A and C shows PKG expression from the viral vectors). Similar results were obtained when apoptotic cells were analyzed by Western blotting or immunofluorescence staining for cleaved caspase-3 (Fig. 2G shows a representative Western blot). Infection of PKG I-depleted MLO-Y4 cells with adenoviral vectors encoding PKG Iα or Iβ resulted in similar levels of PKG I protein measured by Western blotting with an antibody specific for the common C terminus of both PKG I isoforms (supplemental Fig. S2A), and cGMP treatment of these cells produced similar levels of PKG I activity, as shown by phosphorylation vasodilator-stimulated phosphoprotein on Ser259 (supplemental Fig. S2B). We conclude that estradiol and cGMP require both PKG Iα and PKG II to protect osteocytes from apoptosis and that PKG Iβ cannot substitute for PKG Iα in this function.
Estradiol-induced Akt and ERK Activation in Osteocytes Is Mediated by NO/cGMP/PKG II, and Akt Is Necessary for the Anti-apoptotic Effect of Estradiol
We found that estradiol rapidly induced both Akt and ERK phosphorylation on activating sites in MLO-Y4 cells, and pharmacological inhibition of NO synthase, soluble guanylate cyclase, or PKG largely prevented both effects (Fig. 3A). Treating cells with 8-CPT-cGMP mimicked the effects of estradiol on Akt and ERK phosphorylation, with similar results obtained in primary murine osteoblasts and MC3T3 cells (data not shown). Using the above-described siRNAs, we determined that only PKG II, but not PKG I, was required for Akt and ERK activation by estradiol (Fig. 3B). Reconstitution of PKG II by adenoviral infection restored both Akt and ERK phosphorylation in PKG II-depleted cells (Fig. 3C).
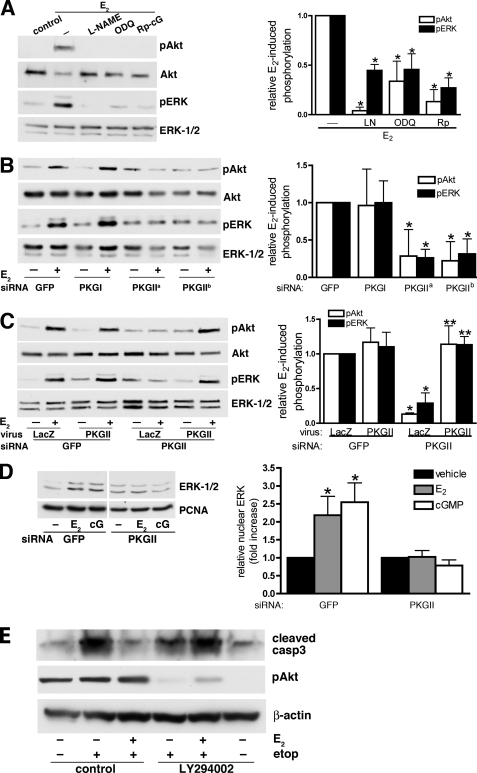
FIGURE 3.
Estradiol-induced Akt and ERK activation in osteocytes is mediated by NO/cGMP and PKG II. A, serum-starved MLO-Y4 cells were treated with 4 mm l-NAME, 10 μm ODQ, or 100 μm Rp-8-CPT-PET-cGMPS (Rp-cG) for 1 h and then stimulated with 100 nm estradiol (E2) for 5 min as indicated. Akt and ERK phosphorylation were assessed by Western blotting using antibodies specific for Akt phosphorylated on Ser473 (pAkt) or ERK-1 phosphorylated on Tyr204 (pERK); loading was assessed using antibodies recognizing Akt or ERK-1/2 irrespective of phosphorylation status. Relative amounts of pAkt (white bars) or pERK (black bars) were quantified by scanning densitometry. In the bar graph on the right, the amounts of pAkt or pERK in cells treated with estradiol alone were assigned a value of 1; *, p < 0.05 compared with cells treated with estradiol alone. B, MLO-Y4 cells were transfected with siRNAs specific for GFP, PKG I, or PKG II (PKG IIa and PKG IIb targeted two different sequences in PKG II). At 24 h post-transfection, cells were serum-starved for 12 h and stimulated with 100 nm estradiol for 5 min. In the bar graph, pAkt and pERK levels in estradiol-treated cells transfected with GFP siRNA were assigned a value of 1; *, p < 0.05 compared with GFP siRNA-transfected cells. C, 24 h after GFP or PKG II siRNA transfection, MLO-Y4 cells were incubated for 10 h with adenoviruses encoding LacZ or siRNA-resistant PKG II as indicated, and then serum-starved for 12 h. Cells were treated with 100 nm estradiol for the last 5 min. In the bar graph, pAkt and pERK levels in estradiol-treated cells transfected with GFP siRNA and infected with LacZ virus were assigned a value of 1; *, p < 0.05 compared with cells transfected with GFP siRNA and infected with LacZ virus; **, p < 0.05 compared with cells transfected with PKG II siRNA and infected with LacZ virus. D, MLO-Y4 cells were transfected with GFP- or PKG II-specific siRNAs, and were treated with vehicle, 100 nm estradiol, or 100 μm 8-pCPT-cGMP for 1 h. Cell homogenates were fractionated by differential centrifugation, and the nuclear fraction was analyzed by Western blotting using antibodies specific for ERK-1/2 and proliferating cell nuclear antigen (PCNA). In the bar graph, the amount of ERK found in vehicle-treated cells was assigned a value of 1; *, p < 0.05 compared with vehicle-treated cells. E, MLO-Y4 cells were treated with vehicle or 10 μm LY294002 for 1 h; cells then received 100 nm estradiol (E2) or additional vehicle for 1 h, followed by exposure to 50 μm etoposide for 8 h as indicated. Lysates were analyzed by Western blotting for cleaved caspase-3 (upper panel), Akt phosphorylated on Ser473 (middle panel), or β-actin (lower panel).
Previous work has shown that the anti-apoptotic effects of estradiol require transient nuclear localization of ERK (6). We found that cGMP induced nuclear translocation of ERK in a PKG II-dependent manner, again mimicking the effect of estradiol (Fig. 3D shows a Western blot of nuclear extracts; supplemental Fig. S3 shows nuclear ERK by immunofluorescence staining). To determine whether the anti-apoptotic effects of estradiol in osteocytes also require signaling through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, we used the PI3K inhibitor LY294002. PI3K generates phosphatidylinositol-3,4,5-triphosphate necessary for Akt recruitment to the plasma membrane and activation by upstream kinases (32). LY294002 did not increase apoptosis, but it prevented estradiol from blocking etoposide-induced apoptosis in MLO-Y4 cells, as shown by the levels of etoposide-induced caspase-3 cleavage (Fig. 3E; at this 9-h time point, Akt activation by estradiol had already subsided, and LY294002 inhibited basal Akt phosphorylation). We conclude that NO/cGMP activation of PKG II is necessary for estradiol-induced Akt and ERK activation and that, in addition to ERK (6), PI3K/Akt are necessary for estradiol to protect osteocytes from apoptosis.
Anti-apoptotic Effects of Estradiol and cGMP Require BAD Phosphorylation on Ser136 and Ser155, but Not Ser112
The pro-apoptotic family member BAD sensitizes cells to apoptosis by binding and inactivating anti-apoptotic Bcl-2 family members. BAD function is regulated by phosphorylation: Ser112 or Ser136 phosphorylation enables binding of 14-3-3 proteins and subsequent phosphorylation of Ser155 to fully disassociate BAD from the anti-apoptotic Bcl-2 family member (33, 34). To determine the role of BAD phosphorylation in the anti-apoptotic effects of estradiol and cGMP, we used mutant BAD constructs containing serine to alanine substitutions in each of the three phosphorylation sites (29). Etoposide-treated osteocytes expressing a BAD S112A mutant showed similar protection by estradiol and 8-pCPT-cGMP as cells expressing wild-type BAD; etoposide induced 12.7% and 13.6% cell death in wild-type and BAD S112A-expressing cells, respectively, which was reduced to basal levels by estradiol or 8-pCPT-cGMP (Fig. 4A). However, etoposide-treated cells expressing BAD S136A or BAD S155A continued to show a high percentage of cell death despite treatment with estradiol or 8-pCPT-cGMP (Fig. 4A). Similar results for the S155A mutant BAD were obtained when probing for cleaved caspase-3 (Fig. 4B). Transfection efficiency of MLO-Y4 cells was ∼60–70% (data not shown), and all three BAD mutants were expressed at levels slightly less than wild-type BAD (Fig. 4A). Thus, BAD phosphorylation on Ser136 and Ser155 is crucial for the effects of estradiol and cGMP on osteocyte apoptosis.
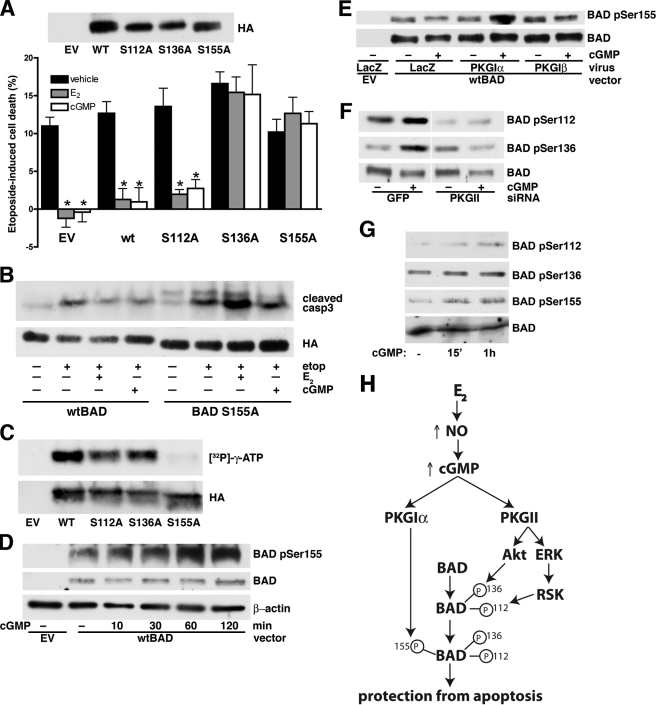
FIGURE 4.
The anti-apoptotic effects of estradiol and cGMP require BAD phosphorylation on Ser136 and Ser155. A, MLO-Y4 cells were transfected with empty vector (EV) and HA epitope-tagged wild-type or mutant BAD constructs as indicated. At 48 h post-transfection, cells were treated with vehicle (black bars), 100 nm estradiol (E2, gray bars), or 100 μm 8-pCPT-cGMP (cGMP, white bar) for 1 h prior to etoposide exposure for 8 h. Etoposide-induced cell death was measured by trypan blue uptake as described under “Experimental Procedures.” The upper panel shows expression of wild-type and mutant BAD proteins in MLO-Y4 cells. *, p < 0.05 compared with vehicle-treated cells. B, MLO-Y4 cells were transfected with wild-type (wt) or mutant BAD (S155A) and treated as in A. Cells were lysed and analyzed by Western blotting with an antibody specific for cleaved caspase-3; BAD expression was shown using an anti-HA antibody. Mutant BAD S155A migrates slightly faster in SDS-PAGE than the wild-type protein. C, cell lysates from 293T cells transfected with empty vector, wild-type, or mutant BAD constructs were incubated with anti-HA antibody coupled to agarose beads, and the immunoprecipitated proteins were subjected to in vitro phosphorylation with purified PKG I in the presence of [γ-32PO4]ATP as described under “Experimental Procedures.” D, MLO-Y4 cells were transfected with empty vector or wild-type BAD as indicated. At 24 h after transfection, cells were infected with PKG Iα adenovirus titered to increase PKG levels by ∼3-fold above endogenous levels to allow for phosphorylation of the transfected BAD. Twenty-four hours later, cells were treated with 100 μm cGMP for the indicated times. BAD phosphorylation on Ser155 was detected using a phospho-specific antibody (upper panel). The middle panel shows the amount of transfected BAD (endogenous BAD was below the limit of detection), and β-actin served as a loading control (lower panel). E, MLO-Y4 cells were transfected with wild-type BAD as in D, but were infected with adenovirus expressing LacZ, PKG Iα, or PKG Iβ, and 24 h later were treated with 100 μm 8-pCPT-cGMP for 1 h. Note that endogenous PKG in LacZ-infected cells is not sufficient for phosphorylation of overexpressed BAD. F, MLO-Y4 cells were transfected with siRNA targeting GFP or PKG II, transfected with wild-type BAD, and infected with PKG Iα virus as described in D; they were treated with 100 μm 8-pCPT-cGMP for the last 1 h. G, primary murine osteoblasts were serum-starved for 16 h and treated with 100 μm 8-pCPT-cGMP for the indicated time. Endogenous BAD phosphorylation was assessed with phospho-specific antibodies. H, model showing estradiol-induced NO/cGMP signaling converging on BAD phosphorylation through activation of PKG Iα and PKG II for protection from etoposide-induced apoptosis. BAD phosphorylation on Ser136 (by Akt, downstream of PKG II) and on Ser155 (by PKG Iα) are sequential, and both phosphorylation events are required for the anti-apoptotic effects of estradiol and cGMP. BAD phosphorylation on Ser112 is mediated by ribosomal S6 kinase downstream of ERK (40), but was not necessary for estradiol- and cGMP-mediated protection from apoptosis.
BAD Phosphorylation on Ser136 and Ser155 Is Mediated by Akt and PKG Iα, Respectively
Previous studies suggest that PKG Iα phosphorylates BAD on Ser155 (29, 34, 35). Using wild-type and mutant BAD constructs isolated from 293T cells, we confirmed that PKG I directly phosphorylated BAD Ser155, because alanine substitution for Ser155 completely prevented in vitro phosphorylation of BAD by PKG (Fig. 4C). Mutation of Ser112 or Ser136 slightly reduced 32PO4 incorporation, possibly because phosphorylation of these sites by other kinases normally enhances the efficiency of Ser155 phosphorylation by PKG, as has been shown for BAD Ser155 phosphorylation by cAMP-dependent protein kinase (29, 34). To examine the kinetics of BAD Ser155 phosphorylation in intact cells, we used a phospho-specific antibody (specificity of this antibody is shown in supplemental Fig. S4A). Detection of BAD phosphorylation in MLO-Y4 cells required transfection of wild-type BAD; cells were infected with the PKG Iα adenovirus to enhance PKG activity corresponding to the increase in substrate level. Using this system, we found that BAD Ser155 phosphorylation was increased within 10 min after adding 8-CPT-cGMP to MLO-Y4 cells and remained elevated at 2 h (Fig. 4D). Only adenoviral expression of PKG Iα, but not PKG Iβ, promoted cGMP-induced Ser155 phosphorylation of transfected BAD in intact cells (Fig. 4E).
Treating MLO-Y4 cells with cGMP also increased BAD phosphorylation on Ser112 and Ser136 (Fig. 4F); this was most likely through cGMP/PKG II-mediated activation of ERK and Akt (Fig. 3B), because BAD Ser112 and Ser136 are targets of ribosomal S6 kinase (acting downstream of ERK) and Akt, respectively (33). Consistent with this hypothesis, siRNA-mediated depletion of PKG II prevented BAD phosphorylation at Ser112 and Ser136 (Fig. 4F), and treatment with the phosphatidylinositol 3-kinase inhibitor LY294002 blocked Akt phosphorylation and prevented BAD phosphorylation at Ser136 (supplemental Fig. S4B). In primary murine osteoblasts, which express higher amounts of BAD, we found that 8-CPT-cGMP increased phosphorylation of endogenous BAD on Ser112, Ser136, and Ser155 (Fig. 4G). We conclude that PKG II is necessary for BAD Ser136 phosphorylation by Akt, allowing direct phosphorylation of Ser155 by PKG Iα (Fig. 4H).
DISCUSSION
Osteoporosis in post-menopausal women is a major public health issue (2). Besides inhibiting osteoclastic bone resorption, estrogens enhance bone formation by protecting osteoblasts and osteocytes from apoptosis (3, 7, 36). Increased numbers of apoptotic osteoblasts and osteocytes are observed in aging humans and after pharmacological induction of a hypo-estrogenic state by administration of gonadotropin-releasing hormone (2–5, 7). Similar findings are reported in animal models of aging or estrogen deficiency after gonadectomy and are reversible with estrogen administration (2–5, 7). The anti-apoptotic effects of estradiol in osteocyte-like cells are blocked by pharmacological or genetic inhibition of Src or ERK and require nuclear translocation of ERK as well as regulation of Bcl-2 family proteins (6, 7, 36, 37). In other cell types, estrogens activate both ERK and Akt through extranuclear actions of a membrane-bound receptor (38–40), and the anti-apoptotic actions of estrogens require signaling through Akt (40–42).
We found that estradiol protection of MLO-Y4 osteocyte-like cells from etoposide- or serum starvation-induced apoptosis required NO and cGMP synthesis and PKG activation. Similar results were obtained in serum-starved primary osteoblasts. cGMP mimicked the anti-apoptotic effects of estradiol, and PKG Iα and PKG II performed independent anti-apoptotic functions converging on BAD. Mutant BAD S136A and S155A constructs prevented estradiol- and cGMP-induced protection from cell death; the mutant proteins appeared to act in a dominant-negative fashion, likely by dimerizing with endogenous Bcl-2 or Bcl-XL (40). PKG Iα directly phosphorylated BAD on Ser155, whereas PKG II indirectly increased BAD phosphorylation on Ser136 by activating Akt. In other cell types, estradiol-induced Akt and ERK activation are dependent on Src (38, 39). We previously showed in osteoblasts and osteocytes that PKG II activates ERK via Src activation and that the membrane-bound enzyme is uniquely situated to stimulate Src through activation of an Shp-1/2 phosphatase complex (16, 17). Akt activation by PKG II in osteoblasts also requires Src.3 Akt is upstream of several pro-survival pathways, but one of its most prominent pro-survival effects is phosphorylation of BAD on Ser136, which is required for binding of 14-3-3 proteins and a pre-requisite for BAD phosphorylation on Ser155 and dissociation from Bcl-2 (34).
The NO/cGMP/PKG pathway can regulate apoptosis in a positive or negative fashion, depending on the cell type. For example, NO donors and cGMP analogs protect neuronal and myocardial cells from cell death induced by ischemia/reperfusion injury, growth factor withdrawal, or TNF-α exposure (19, 43, 44). In contrast, PKG activation promotes apoptosis in intestinal epithelial cells (45). We found that estradiol and cGMP protected MC3T3 osteoblast-like cells from TNF-α-induced apoptosis in a PKG-dependent fashion; other workers reported protection by NO donors, but the effect was not attributed to cGMP/PKG, because of lack of reversal by the PKG inhibitor KT5828, which has shown varied efficacy in other studies (22). MLO-Y4 osteocytes are protected from TNF-α-induced apoptosis by exposure to fluid shear stress; this effect is NO-dependent, but downstream effects of NO were not examined (21). While this report was in preparation, Wong and Fiscus described a role for cGMP/PKG I in protecting OP9 bone marrow stromal osteoprogenitor cells from spontaneous apoptosis (46).
Using siRNA-mediated depletion of PKG I and reconstitution with adenoviral vectors encoding either PKG Iα or Iβ, we found that only PKG Iα, but not Iβ, mediates the anti-apoptotic effects of cGMP, despite similar expression levels of both kinases and comparable activities toward the shared substrate vasodilator-stimulated phosphoprotein. There are few examples of PKG Iα-specific functions for which PKG Iβ cannot substitute. In PKG I-null mice, it appears that either PKG I isoform can rescue basic vascular and intestinal smooth muscle functions (47). However, due to their unique N-terminal dimerization/leucine zipper domain, PKG Iα and Iβ bind to some isoform-specific G-kinase interaction proteins, which may target the kinases to unique subcellular localizations and substrates. For example, PKG Iα specifically binds to the regulator of G-protein signaling and the myosin-targeting subunit of myosin phosphatase (48, 49), whereas PKG Iβ specifically binds to the inositol 1,4,5-trisphosphate receptor-associated G-kinase substrate and the transcriptional regulator TFII-I (50, 51). PKG Iα may be targeted to a subcellular compartment that favors phosphorylation of BAD Ser155.
In conclusion, we identified PKG Iα and PKG II as important mediators of the anti-apoptotic effects of estradiol in osteocytes. These results help explain why ovariectomized endothelial NO synthase-deficient mice have a defective anabolic response to exogenous estrogens, l-NAME reduces estradiol-induced bone formation in intact mice, and NO donors alleviate ovariectomy-induced bone loss in rats (24, 27, 28, 52). Several epidemiological studies and clinical trials suggest that NO donors may be as effective as estrogens in preventing bone loss in post-menopausal women (25, 26, 53). However, current NO donors may have detrimental effects due to increased oxidative stress (54), and our results provide a rationale for using direct PKG or soluble guanylate cyclase activators as bone-protective agents.
Supplementary Material
Acknowledgment
We thank Dr. L. Bonewald for providing us with MLO-Y4 cells.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-AR051300 (to R. B. P.) and Training Grant T32-HL007261 (to N. M.).

This article contains supplemental Figs. S1–S4.
H. Rangaswami and R. Pilz, unpublished results.
- PKG
- cGMP-dependent protein kinase
- 8-pCPT-cGMP
- 8-(4-chlorophenylthio)guanosine-3′,5′-cyclic monophosphate
- gapd
- glyceraldehyde-3-phosphate dehydrogenase
- LacZ
- β-galactosidase
- L-NAME
- N-nitro-l-arginine methyl ester
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- DETA-NONOate
- 2,2′-hydroxynitrosohydrazino)bis-ethanamine
- Rp-8-pCPT-PET-cGMPS
- 8-(4-chlorophenylthio)-β-phenyl-1,N2-ethenoguanosine-3′,5′-cyclic monophosphorothioate (Rp isomer).
REFERENCES
- 1. Raisz L. G. (2005) Pathogenesis of osteoporosis. Concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manolagas S. C. (2010) From estrogen-centric to aging and oxidative stress. A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Almeida M., Han L., Martin-Millan M., Plotkin L. I., Stewart S. A., Roberson P. K., Kousteni S., O'Brien C. A., Bellido T., Parfitt A. M., Weinstein R. S., Jilka R. L., Manolagas S. C. (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 282, 27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomkinson A., Reeve J., Shaw R. W., Noble B. S. (1997) The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 82, 3128–3135 [DOI] [PubMed] [Google Scholar]
- 5. Almeida M., Martin-Millan M., Ambrogini E., Bradsher R., 3rd, Han L., Chen X. D., Roberson P. K., Weinstein R. S., O'Brien C. A., Jilka R. L., Manolagas S. C. (2010) Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERα. J. Bone Miner. Res. 25, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J. R., Plotkin L. I., Aguirre J. I., Han L., Jilka R. L., Kousteni S., Bellido T., Manolagas S. C. (2005) Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J. Biol. Chem. 280, 4632–4638 [DOI] [PubMed] [Google Scholar]
- 7. Kousteni S., Bellido T., Plotkin L. I., O'Brien C. A., Bodenner D. L., Han L., Han K., DiGregorio G. B., Katzenellenbogen J. A., Katzenellenbogen B. S., Roberson P. K., Weinstein R. S., Jilka R. L., Manolagas S. C. (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors. Dissociation from transcriptional activity. Cell 104, 719–730 [PubMed] [Google Scholar]
- 8. Armour K. E., Ralston S. H. (1998) Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology 139, 799–802 [DOI] [PubMed] [Google Scholar]
- 9. O'Shaughnessy M. C., Polak J. M., Afzal F., Hukkanen M. V., Huang P., MacIntyre I., Buttery L. D. (2000) Nitric oxide mediates 17β-estradiol-stimulated human and rodent osteoblast proliferation and differentiation. Biochem. Biophys. Res. Commun. 277, 604–610 [DOI] [PubMed] [Google Scholar]
- 10. Li L., Hisamoto K., Kim K. H., Haynes M. P., Bauer P. M., Sanjay A., Collinge M., Baron R., Sessa W. C., Bender J. R. (2007) Variant estrogen receptor-c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proc. Natl. Acad. Sci. U.S.A. 104, 16468–16473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stratton R. C., Squires P. E., Green A. K. (2010) 17β-Estradiol elevates cGMP and, via plasma membrane recruitment of protein kinase GIα, stimulates Ca2+ efflux from rat hepatocytes. J. Biol. Chem. 285, 27201–27212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo X., Razandi M., Pedram A., Kassab G., Levin E. R. (2005) Estrogen induces vascular wall dilation. Mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J. Biol. Chem. 280, 19704–19710 [DOI] [PubMed] [Google Scholar]
- 13. Cherney A., Edgell H., Krukoff T. L. (2003) NO mediates effects of estrogen on central regulation of blood pressure in restrained, ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R842–R849 [DOI] [PubMed] [Google Scholar]
- 14. Francis S. H., Busch J. L., Corbin J. D., Sibley D. (2010) cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hofmann F., Bernhard D., Lukowski R., Weinmeister P. (2009) Handbook of Experimental Pharmacology (Schmidt H. H. H. W., ed) Vol. 191, pp. 137–162, Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- 16. Rangaswami H., Marathe N., Zhuang S., Chen Y., Yeh J. C., Frangos J. A., Boss G. R., Pilz R. B. (2009) Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J. Biol. Chem. 284, 14796–14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rangaswami H., Schwappacher R., Marathe N., Zhuang S., Casteel D. E., Haas B., Chen Y., Pfeifer A., Kato H., Shattil S., Boss G. R., Pilz R. B. (2010) Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci. Signal. 3, ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfeifer A., Aszódi A., Seidler U., Ruth P., Hofmann F., Fässler R. (1996) Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 274, 2082–2086 [DOI] [PubMed] [Google Scholar]
- 19. Fiscus R. R. (2002) Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals 11, 175–190 [DOI] [PubMed] [Google Scholar]
- 20. Blaise G. A., Gauvin D., Gangal M., Authier S. (2005) Nitric oxide, cell signaling and cell death. Toxicology 208, 177–192 [DOI] [PubMed] [Google Scholar]
- 21. Tan S. D., Kuijpers-Jagtman A. M., Semeins C. M., Bronckers A. L., Maltha J. C., Von den Hoff J. W., Everts V., Klein-Nulend J. (2006) Fluid shear stress inhibits TNFα-induced osteocyte apoptosis. J. Dent. Res. 85, 905–909 [DOI] [PubMed] [Google Scholar]
- 22. Chae H. J., Chin H. Y., Lee G. Y., Park H. R., Yang S. K., Chung H. T., Pae H. O., Kim H. M., Chae S. W., Kim H. R. (2006) Carbon monoxide and nitric oxide protect against tumor necrosis factor-α-induced apoptosis in osteoblasts. HO-1 is necessary to mediate the protection. Clin. Chim. Acta 365, 270–278 [DOI] [PubMed] [Google Scholar]
- 23. Hukkanen M., Platts L. A., Lawes T., Girgis S. I., Konttinen Y. T., Goodship A. E., MacIntyre I., Polak J. M. (2003) Effect of nitric oxide donor nitroglycerin on bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Bone 32, 142–149 [DOI] [PubMed] [Google Scholar]
- 24. Wimalawansa S. J., De Marco G., Gangula P., Yallampalli C. (1996) Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone 18, 301–304 [DOI] [PubMed] [Google Scholar]
- 25. Jamal S. A., Cummings S. R., Hawker G. A. (2004) Isosorbide mononitrate increases bone formation and decreases bone resorption in postmenopausal women. A randomized trial. J. Bone Miner. Res. 19, 1512–1517 [DOI] [PubMed] [Google Scholar]
- 26. Jamal S. A., Hamilton C. J., Eastell R., Cummings S. R. (2011) Effect of nitroglycerin ointment on bone density and strength in postmenopausal women. A randomized trial. JAMA 305, 800–807 [DOI] [PubMed] [Google Scholar]
- 27. Armour K. E., Armour K. J., Gallagher M. E., Gödecke A., Helfrich M. H., Reid D. M., Ralston S. H. (2001) Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142, 760–766 [DOI] [PubMed] [Google Scholar]
- 28. Grassi F., Fan X., Rahnert J., Weitzmann M. N., Pacifici R., Nanes M. S., Rubin J. (2006) Bone re/modeling is more dynamic in the endothelial nitric oxide synthase(−/−) mouse. Endocrinology 147, 4392–4399 [DOI] [PubMed] [Google Scholar]
- 29. Zhou X. M., Liu Y., Payne G., Lutz R. J., Chittenden T. (2000) Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J. Biol. Chem. 275, 25046–25051 [DOI] [PubMed] [Google Scholar]
- 30. Kato Y., Windle J. J., Koop B. A., Mundy G. R., Bonewald L. F. (1997) Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 12, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 31. Gudi T., Lohmann S. M., Pilz R. B. (1997) Regulation of gene expression by cyclic GMP-dependent protein kinase requires nuclear translocation of the kinase. Identification of a nuclear localization signal. Mol. Cell Biol. 17, 5244–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manning B. D., Cantley L. C. (2007) AKT/PKB signaling. Navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Danial N. N. (2008) BAD. undertaker by night, candyman by day. Oncogene 27 Suppl 1, S53-S70 [DOI] [PubMed] [Google Scholar]
- 34. Datta S. R., Katsov A., Hu L., Petros A., Fesik S. W., Yaffe M. B., Greenberg M. E. (2000) 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell 6, 41–51 [PubMed] [Google Scholar]
- 35. Johlfs M. G., Fiscus R. R. (2010) Protein kinase G type-Iα phosphorylates the apoptosis-regulating protein Bad at serine 155 and protects against apoptosis in N1E-115 cells. Neurochem. Int. 56, 546–553 [DOI] [PubMed] [Google Scholar]
- 36. Gohel A., McCarthy M. B., Gronowicz G. (1999) Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology 140, 5339–5347 [DOI] [PubMed] [Google Scholar]
- 37. Plotkin L. I., Aguirre J. I., Kousteni S., Manolagas S. C., Bellido T. (2005) Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J. Biol. Chem. 280, 7317–7325 [DOI] [PubMed] [Google Scholar]
- 38. Cheskis B. J., Greger J. G., Nagpal S., Freedman L. P. (2007) Signaling by estrogens. J. Cell Physiol. 213, 610–617 [DOI] [PubMed] [Google Scholar]
- 39. Levin E. R. (2011) Minireview. Extranuclear steroid receptors. Roles in modulation of cell functions. Mol. Endocrinol. 25, 377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alexaki V. I., Charalampopoulos I., Kampa M., Nifli A. P., Hatzoglou A., Gravanis A., Castanas E. (2006) Activation of membrane estrogen receptors induce pro-survival kinases. J. Steroid Biochem. Mol. Biol. 98, 97–110 [DOI] [PubMed] [Google Scholar]
- 41. Koga M., Hirano K., Hirano M., Nishimura J., Nakano H., Kanaide H. (2004) Akt plays a central role in the anti-apoptotic effect of estrogen in endothelial cells. Biochem. Biophys. Res. Commun. 324, 321–325 [DOI] [PubMed] [Google Scholar]
- 42. Patten R. D., Pourati I., Aronovitz M. J., Baur J., Celestin F., Chen X., Michael A., Haq S., Nuedling S., Grohe C., Force T., Mendelsohn M. E., Karas R. H. (2004) 17β-Estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ. Res. 95, 692–699 [DOI] [PubMed] [Google Scholar]
- 43. Kim Y. M., Chung H. T., Kim S. S., Han J. A., Yoo Y. M., Kim K. M., Lee G. H., Yun H. Y., Green A., Li J., Simmons R. L., Billiar T. R. (1999) Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling. J. Neurosci. 19, 6740–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Das A., Xi L., Kukreja R. C. (2008) Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3β. J. Biol. Chem. 283, 29572–29585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soh J. W., Mao Y., Kim M. G., Pamukcu R., Li H., Piazza G. A., Thompson W. J., Weinstein I. B. (2000) Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin. Cancer Res. 6, 4136–4141 [PubMed] [Google Scholar]
- 46. Wong J. C., Fiscus R. R. (2011) Essential roles of the nitric oxide (no)/cGMP/protein kinase G type-Iα (PKG-Iα) signaling pathway and the atrial natriuretic peptide (ANP)/cGMP/PKG-Iα autocrine loop in promoting proliferation and cell survival of OP9 bone marrow stromal cells. J. Cell Biochem. 112, 829–839 [DOI] [PubMed] [Google Scholar]
- 47. Weber S., Bernhard D., Lukowski R., Weinmeister P., Wörner R., Wegener J. W., Valtcheva N., Feil S., Schlossmann J., Hofmann F., Feil R. (2007) Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ. Res. 101, 1096–1103 [DOI] [PubMed] [Google Scholar]
- 48. Tang K. M., Wang G. R., Lu P., Karas R. H., Aronovitz M., Heximer S. P., Kaltenbronn K. M., Blumer K. J., Siderovski D. P., Zhu Y., Mendelsohn M. E., Tang M., Wang G. (2003) Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nature Medicine 9, 1506–1512 [DOI] [PubMed] [Google Scholar]
- 49. Surks H. K., Mochizuki N., Kasai Y., Georgescu S. P., Tang K. M., Ito M., Lincoln T. M., Mendelsohn M. E. (1999) Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Iα. Science 286, 1583–1587 [DOI] [PubMed] [Google Scholar]
- 50. Casteel D. E., Zhuang S., Gudi T., Tang J., Vuica M., Desiderio S., Pilz R. B. (2002) cGMP-dependent protein kinase I β physically and functionally interacts with the transcriptional regulator TFII-I. J. Biol. Chem. 277, 32003–32014 [DOI] [PubMed] [Google Scholar]
- 51. Schlossmann J., Ammendola A., Ashman K., Zong X., Huber A., Neubauer G., Wang G. X., Allescher H. D., Korth M., Wilm M., Hofmann F., Ruth P. (2000) Regulation of intracellular calcium by a signaling complex of IRAG, IP3 receptor, and cGMP kinase Iβ. Nature 404, 197–201 [DOI] [PubMed] [Google Scholar]
- 52. Samuels A., Perry M. J., Gibson R. L., Colley S., Tobias J. H. (2001) Role of endothelial nitric oxide synthase in estrogen-induced osteogenesis. Bone 29, 24–29 [DOI] [PubMed] [Google Scholar]
- 53. Wimalawansa S. J. (2007) Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Ann. N.Y. Acad. Sci. 1117, 283–297 [DOI] [PubMed] [Google Scholar]
- 54. Parker J. D. (2004) Nitrate tolerance, oxidative stress, and mitochondrial function. Another worrisome chapter on the effects of organic nitrates. J. Clin. Invest. 113, 352–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.