Background: Let-7c is a microRNA down-regulated in prostate cancer.
Results: Let-7c suppresses androgen receptor expression by targeting its transcription via c-Myc. Suppression of AR by let-7c leads to decreased cell proliferation and tumor growth.
Conclusion: Let-7c suppresses androgen receptor expression.
Significance: Our study demonstrates that let-7c plays an important role in regulation of androgen signaling and prostate cancer proliferation.
Keywords: Androgen Receptor, MicroRNA, Nuclear Receptors, Oncogene, Prostate Cancer
Abstract
Castration-resistant prostate cancer continues to rely on androgen receptor (AR) expression. AR plays a central role in the development of prostate cancer and progression to castration resistance during and after androgen deprivation therapy. Here, we identified miR-let-7c as a key regulator of expression of AR. miR-let-7c suppresses AR expression and activity in human prostate cancer cells by targeting its transcription via c-Myc. Suppression of AR by let-7c leads to decreased cell proliferation of human prostate cancer cells. Down-regulation of Let-7c in prostate cancer specimens is inversely correlated with AR expression, whereas the expression of Lin28 (a repressor of let-7) is correlated positively with AR expression. Our study demonstrates that the miRNA let-7c plays an important role in the regulation of androgen signaling in prostate cancer by down-regulating AR expression. These results suggest that reconstitution of miR-let-7c may aid in targeting enhanced and hypersensitive AR in advanced prostate cancer.
Introduction
Prostate cancer (PCa)3 is the most commonly occurring malignancy and the second highest cause of cancer-related mortality in men in the United States. The majority of patients with advanced PCa respond initially to androgen deprivation therapy, but relapse because of the growth of castration-resistant prostate cancer (CRPC) cells. Significant efforts have been focused on understanding the mechanisms involved in the development and progression of CRPC. Although the levels of androgen are reduced to castration levels, the AR is still expressed, and AR target genes such as prostate-specific antigen (PSA) are activated. AR activation in CRPC may occur by a variety of mechanisms that alter the sensitivity or specificity of AR, including AR mutations, alternative splicing, amplification, alterations of coregulators, sensitization by growth factors and cytokines, and response to intracrine androgens (1, 2). The study of factors that regulate expression and activation of the AR will enhance our understanding of the mechanisms leading to castration resistance and will play a key role in devising therapeutic strategies to combat this lethal cancer.
MicroRNAs (miRNAs) are endogenous, small, non-coding RNAs that interfere with protein expression either by inducing cleavage of their specific target mRNAs or by inhibiting their translation. MiRNAs have been shown to regulate a rapidly increasing list of complex biological processes, including differentiation, cell cycle, apoptosis, and metabolism (3). Their emerging roles as tumor suppressors or oncogenes present novel diagnostic and therapeutic opportunities (4, 5). Let-7 encodes an evolutionarily conserved family of 13 homologous miRNAs located in genomic locations frequently deleted in human cancers (6). Let-7 expression was observed to be down-regulated in localized PCa tissues relative to benign peripheral zone tissue (7, 8). There is a significant association between loss of let-7 expression and development of poorly differentiated and aggressive cancers (9). Let-7 members have been shown to regulate expression levels of oncogenes like HMGA2 (10), RAS (11), and Myc (12), along with genes involved in cell cycle and cell division regulation. Thus, let-7 shows promise as a molecular marker in certain cancers and as a potential therapeutic agent in cancer treatment. Lin28, a highly conserved RNA-binding protein and a master regulator of let-7 miRNA processing, binds to the terminal loops of the precursors of let-7 family miRNAs and blocks their processing into mature miRNAs (13, 14). Lin28 also derepresses c-Myc by repressing let-7, and c-Myc transcriptionally activates Lin28 (15, 16). This Lin28/let-7/c-Myc loop may play an important role in the deregulated miRNA expression signature observed in many cancers (17).
In this study, we show that let-7c, one of the members of the let-7 family, antagonizes AR expression by degradation of c-Myc. Let-7c-mediated decrease in AR expression and activation led to inhibition of proliferation of PCa cells. Down-regulation of Let-7c expression in prostate cancer clinical specimens is inversely correlated, whereas expression of Lin28 is positively correlated, with AR expression. Our results imply that AR signaling can be regulated by let-7c in PCa by decreasing survival and proliferation of tumor cells.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
Human prostate cancer cell lines were obtained from the ATCC. Antibodies against c-Myc, AR (441) and actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Lin28 antibodies were obtained from Abcam (San Francisco, CA). SYBR Green iQ Supermix was from Bio-Rad.
Western Blot Analysis
Cells were lysed in high-salt buffer as described earlier (18). Western blots were probed with the indicated primary antibodies, and the chemiluminescence was detected by ECL (GE Healthcare).
Measurement of PSA
PSA levels were measured in the culture supernatants using ELISA (United Biotech, Inc., Mountain View, CA) according to the manufacturer's instructions.
Luciferase Assays
LNCaP cells were transfected with pGL3-PSA6.0-Luc or pGL4-AR-prom-Luc (∼6 kb) reporters along with plasmids as indicated in the figures. Cell lysates were subjected to luciferase assays with the luciferase assay system (Promega).
Real-time Quantitative RT-PCR
LNCaP cells were transfected with the indicated plasmids or oligonucleotides, and qRT-PCR analyses were performed as described previously (19, 20). (Sequences of primers are listed in the supplemental methods.)
ChIP Assays
LNCaP cells were transfected with the indicated plasmids, and DNA-protein complexes were isolated after cross-linking with 1% formaldehyde. ChIP assays were performed as described previously (20) using primers spanning androgen responsive elements (AREs) in PSA and NKX3.1 promoters or Myc-binding sites in the AR promoter. Isotype-matched IgG was used as a control. (Primer sequences are listed in the supplemental methods.)
Human PCa Specimens
The human prostate cancer specimens were obtained with consent from patients who underwent radical prostatectomies at University of California Davis Medical Center. The protein extracts from human prostate specimens used for the analysis of Lin28 expression were described previously (21, 22).
Gene Expression Omnibus Analysis
Two separate datasets from the Gene Expression Omnibus National Center for Biotechnology Information gene expression and hybridization array data repository were screened independently for expression levels of Lin28, AR, and Myc. The first data set (GDS1439) (23) compared specimens of benign prostatic hyperplasia with clinically localized primary prostate cancer and with metastatic prostate cancer. The second dataset (GDS2547) (24) compared normal prostate specimens without any pathology, normal prostate adjacent to tumor, primary prostate tumor, and metastatic prostate cancer. Relative levels of expression of Lin28, AR, and Myc were determined by comparison of single channel counts and expressed as a percentage of the highest count in the data set (25). Significant differences between groups were determined by Microsoft Excel Tools.
Oncomine Analysis
A gene search of the Oncomine database for expression levels of Lin28, AR, and Myc was performed at a significance threshold of p ≤ 0.001 and data sets generated from three comparisons of normal prostate tissue with prostate carcinoma: Wallace_prostate (26), Yu_prostate (27), and Vanaja_prostate (28) were analyzed by the differential expression function of Oncomine.
Statistical Analyses
Data are shown as means ± S.D. Multiple group comparison was performed using Microsoft Excel Tools. p ≤ 0.05 was considered significant.
RESULTS
Let-7c Decreases Expression of AR
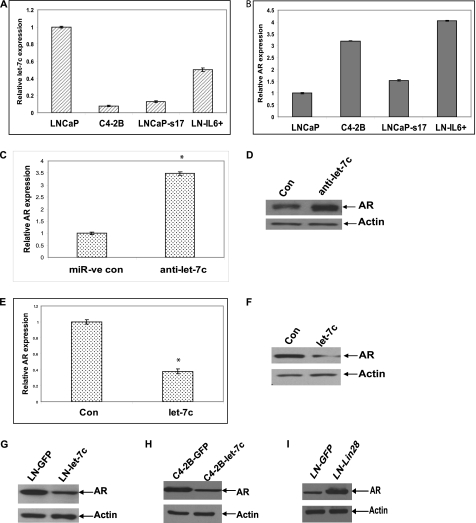
We initially determined let-7c expression in several prostate cancer cell lines, including androgen-sensitive and castration-resistant prostate cancer cells. Our data showed that the levels of let-7c were lower in the castration-resistant cell lines C4-2B, LNCaP-s17 (overexpressing IL-6), and LN-IL6+ (LNCaP chronically treated with IL-6), which express higher levels of AR compared with parental LNCaP cells that express lower levels of AR (Fig. 1, A and B) (29, 30), indicating an inverse relationship between AR and let-7c. Hence, we tested whether let-7c affects AR expression in PCa cells. Antisense oligonucleotides against let-7c (Ambion) were transfected into LNCaP cells, which express high levels of let-7c, and the expression level of AR was analyzed by qRT-PCR. Down-regulation of let-7c enhanced AR mRNA level ∼3.5-fold (Fig. 1C). Down-regulation of let-7c was confirmed by qRT-PCR (supplemental Fig. 1A). To confirm that the increase in AR mRNA results in an increase in AR protein, we analyzed whole cell lysates from LNCaP cells transfected with let-7c antisense oligos by Western blotting. The AR protein level was increased ∼70% when let-7c was down-regulated (Fig. 1D). These findings were further confirmed in LN-IL6+ cells, which express higher levels of AR but lower levels of let-7c compared with LNCaP cells. Overexpression of let-7c in LNCaP-IL6+ cells reduced the levels of AR mRNA (Fig. 1E) and protein expression (F). Overexpression of let-7c was confirmed by qRT-PCR (supplemental Fig. 1B). These results suggested that let-7c inhibits AR expression in PCa cells. Because the above results were obtained by transient overexpression of let-7c and to test whether stable expression of let-7c would exhibit similar effects, we generated LNCaP and C4-2B cells stably expressing let-7c and LNCaP cells stably expressing Lin28. Western blotting was performed to analyze protein levels of AR and Lin28. The results demonstrated that levels of AR were down-regulated in both LNCaP and C4-2B cells stably expressing let-7c, whereas AR expression was enhanced in LNCaP cells expressing Lin28 (Fig. 1, G, H, and I). Higher levels of expression of let-7c in C4-2B cells stably expressing let-7c were confirmed by qRT-PCR (supplemental Fig. 1C). Collectively, these data demonstrate that let-7c represses AR expression in prostate cancer cells.
FIGURE 1.
Let-7c suppresses AR expression. Relative expression levels of let-7c (A) and AR (B) were determined in LNCaP, C4-2B, LNCaP-s17, and LN-IL6+ cells by qRT-PCR. Results are presented as relative fold change compared to expression levels in LNCaP cells. C, LNCaP cells were transfected with let-7c antisense oligonucleotides, and AR mRNA levels were analyzed by qRT-PCR. Results are presented as relative fold change compared to expression levels in LNCaP cells transfected with control oligonucleotides. Data points represent mean ± S.D. of triplicate samples from two independent experiments. Error bars denote mean ± S.D. * denotes p ≤ 0.05. AR expression was increased when let-7c expression was down-regulated. D, Western blot analysis showing the increase in AR expression in LNCaP cells transfected with let-7c antisense. Actin is shown as loading control. E, LN-IL6+ cells were transfected with let-7c, and AR mRNA levels were analyzed by qRT-PCR. AR expression was reduced when let-7c was overexpressed. Con, control. * denotes p ≤ 0.05. F, Western blot analysis showing the down-regulation of AR expression in LN-IL6+ cells transfected with let-7c. G and H, AR levels were analyzed in LNCaP and C4-2B cells stably expressing let-7c by Western blotting. AR protein levels were reduced when let-7c was expressed. I, protein levels of AR were analyzed in LNCaP cells stably expressing Lin28 by Western blotting. AR expression was enhanced in cells expressing Lin28.
Let-7c Reduces AR Activity
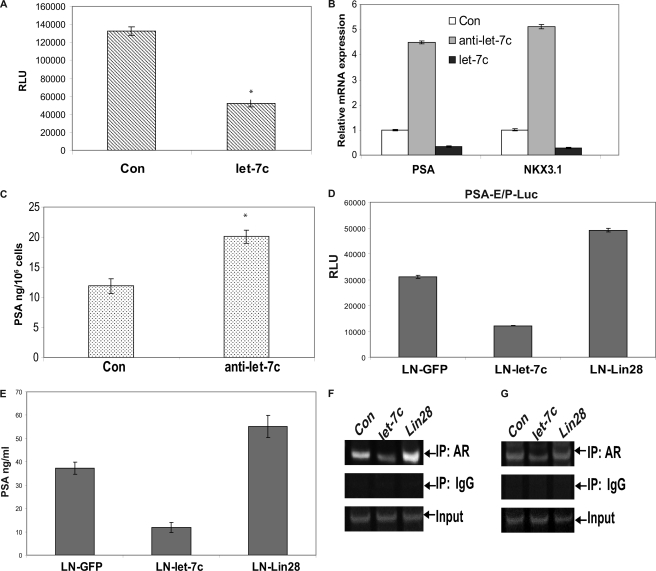
We next examined whether the reduction in expression of AR by let-7c results in inhibition of transcriptional activity of AR. LNCaP cells were cotransfected with the pGL3-PSA6.0-Luc reporter containing the enhancer and promoter regions of the PSA gene and plasmids expressing let-7c. As shown in Fig. 2A, transcriptional activity of the AR in activating reporter gene expression was reduced by ∼60% in the presence of let-7c. These results were confirmed by analysis of PSA mRNA and protein expression by qRT-PCR and ELISA, respectively. LNCaP cells were transfected with let-7c or let-7c antisense oligonucleotides, and PSA mRNA levels were analyzed. Down-regulation of let-7c expression by let-7c antisense oligonucleotides led to a > 4-fold increase in PSA expression, whereas overexpression of let-7c reduced PSA expression by ∼50% (Fig. 2B). In addition to PSA, down-regulation of let-7c expression by let-7c antisense increased, whereas overexpression of let-7c reduced, NKX3.1 (another typical androgen-regulated gene) mRNA expression (Fig. 2B). Similarly, PSA levels in the supernatants of LNCaP cells transfected with let-7c antisense were found to be up-regulated by ∼40% (Fig. 2C). These results were also confirmed in LNCaP and C4-2B cells stably expressing let-7c. Stable expression of let-7c decreased, whereas stable expression of Lin28 increased, transactivation of reporter activity by AR (Fig. 2D) and secretion of PSA by LNCaP cells (E). To determine whether expression of let-7c affects the recruitment of AR to PSA and NKX3.1 promoters, ChIP assays were performed. Overexpression of let-7c in LNCaP cells reduced binding of AR to PSA (Fig. 2F) and NKX3.1 (G) promoters, whereas expression of Lin28, a repressor of let-7c, increased AR binding to these promoters. Collectively, these results demonstrate that the suppression of AR expression by let-7c leads to decrease in the transactivation potential of AR, whereas increased AR expression by let-7c antisense or Lin28 leads to an increase in the transactivation potential of AR and expression of its target genes.
FIGURE 2.
Let-7c inhibits AR activity and transcription of AR target genes. A, LNCaP cells were cotransfected with let-7c and pGL3-PSA6.0-Luc reporter. Data points represent mean ± S.D. of triplicate samples from two independent experiments. Error bars denote mean ± S.D. * denotes p ≤ 0.05. Transactivation of the PSA promoter by AR was reduced with let-7c overexpression. B, LNCaP cells were transfected with control or let-7c or let-7c antisense, and levels of PSA and NKX3.1 mRNAs were analyzed by qRT-PCR. Results are presented as relative fold change compared with expression levels in LNCaP cells transfected with control oligonucleotides. Down-regulation of let-7c enhanced the levels of PSA and NKX3.1 mRNAs, whereas overexpression of let-7c reduced their expression. Con, control. C, PSA secretion in LNCaP cells was enhanced when let-7c expression was down-regulated. * denotes p ≤ 0.05. D, LNCaP cells stably expressing control, let-7c, or Lin28 were transfected with pGL3-PSA6.0-Luc reporter, and luciferase activities were assayed. Transactivation of the reporter by AR was reduced in cells expressing let-7c, whereas it was increased in cells expressing Lin28. RLU, Relative Luciferase Units. E, secretion of PSA by LNCaP cells stably expressing let-7c or Lin28 was analyzed by ELISA. PSA secretion was reduced in cells expressing let-7c and increased in cells expressing Lin28. Recruitment of AR to AREs in PSA (F) and NKX3.1 (G) promoters was analyzed by ChIP assays in LNCaP cells transfected with let-7c or Lin28. Recruitment of AR to the AREs was reduced in cells overexpressing let-7c and was enhanced in cells expressing Lin28. IP, immunoprecipitation.
Repression of AR by let-7c Is Mediated by Myc
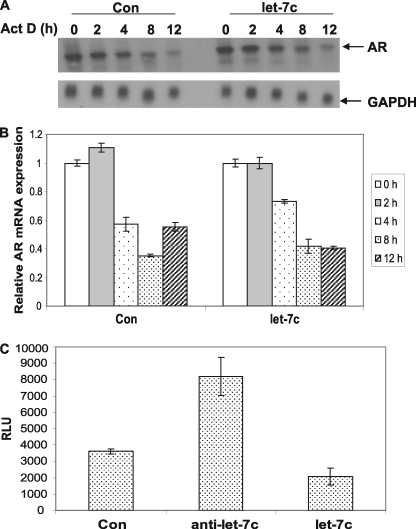
MiRNAs target several genes by binding to consensus binding sites in the 3′ untranslated region of the transcript, leading to degradation of the mRNA via the RISC complex. Therefore, we analyzed whether let-7c binding sites exist in the 3′ untranslated region of the AR mRNA using algorithms from miRBase, TargetScan, PictarVert, and Microcosm. Analysis of the 3′ untranslated region of AR failed to detect the presence of let-7c binding sites. To confirm whether let-7c leads to degradation of the AR mRNA, we analyzed the stability of AR mRNA in LNCaP cells expressing high levels of let-7c. LNCaP cells were transfected with plasmids expressing let-7c, were treated with vehicle or 50 μm actinomycin D (to inhibit de novo RNA synthesis), and total RNAs were isolated. Northern blotting (Fig. 3A) and qRT-PCR (B) were performed with a probe specifically against AR mRNA and primers amplifying AR mRNA, respectively. As shown in Fig. 3, A and B, the half-life of AR mRNA in LNCaP cells was ∼3.5 h in the presence of androgen, which was not altered when let-7c was overexpressed in LNCaP cells. These results suggest that let-7c does not enhance the degradation of AR mRNA, implying that a mechanism other than direct mRNA degradation may be involved in let-7c-mediated AR inhibition. Next, we examined whether let-7c affects transcription of AR. Let-7c or let-7c antisense oligos were cotransfected with a luciferase reporter driven by the full-length (∼6 kb) promoter of the AR gene into LNCaP cells, and luciferase assays were performed. Down-regulation of let-7c expression by let-7c antisense increased, whereas overexpression of let-7 decreased, the activation of the AR promoter (Fig. 3C), suggesting that suppression of AR expression by let-7c may be at the level of transcription.
FIGURE 3.
Let-7c regulates AR expression at the level of transcription. LNCaP cells were transfected with a control (Con) or let-7c plasmid in the presence or absence of actinomycin D, and AR mRNA levels were analyzed by Northern blotting (A) or qRT-PCR (B). Half-life of AR mRNA was not altered in the presence of let-7c. C, LNCaP cells were cotransfected with control or let-7c or with let-7c antisense oligos along with the pGL4-AR-Prom-Luc reporter. Data points represent the mean ± S.D. of triplicate samples from two independent experiments. AR promoter activity was repressed in let-7c-overexpressing cells and was enhanced when let-7c was down-regulated. RLU, Relative Luciferase Units.
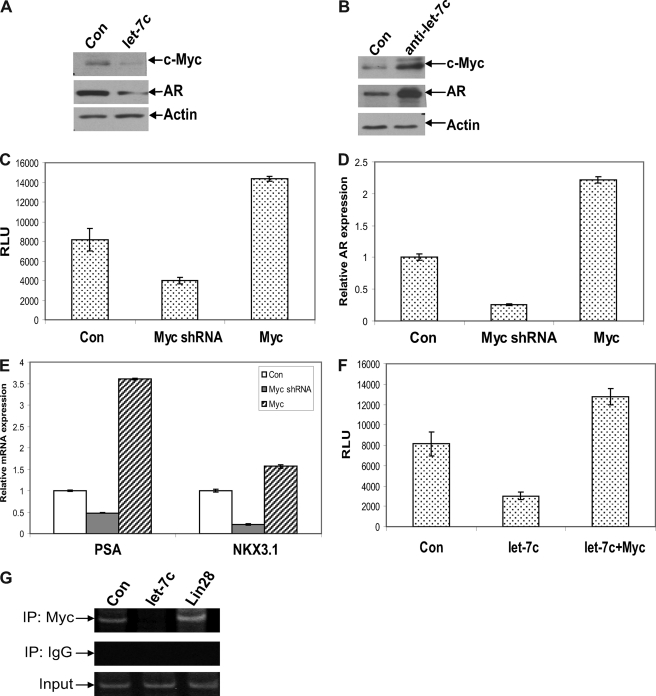
Because our results showed that suppression of AR expression and activity by let-7c is not through typical miRNA-mediated mRNA degradation but at the level of transcription, we hypothesized that a let-7c target gene may function as a transcriptional regulator of AR. We analyzed whether any of the transcription factors binding to the AR promoter was a target of let-7c using MatInspector and miRBase and found that Myc, which activates AR transcription by binding to a consensus element in the AR promoter (12), was one of the targets of let-7c. LNCaP cells overexpressing let-7c were analyzed by Western blotting to confirm whether let-7c reduces Myc expression. Results showed that Myc expression was down-regulated in LNCaP cells expressing let-7c (Fig. 4A). Similarly, we found an increase in Myc expression in LNCaP cells transfected with let-7c antisense oligos (Fig. 4B). In addition, the levels of Myc are correlated with AR expression (Fig. 4B). These results were confirmed in LNCaP and C4-2B cells stably expressing let-7c (supplemental Fig. 2, A and B). Down-regulation of Myc by Myc shRNA reduced AR promoter activity (Fig. 4C) and AR mRNA expression (D), whereas overexpression of Myc enhanced AR promoter activity (C) and AR mRNA expression (D). Down-regulation of Myc also reduced the levels of PSA and NKX3.1 mRNA, whereas overexpression of Myc enhanced the levels of PSA and NKX3.1 mRNA (E).
FIGURE 4.
Down-regulation of AR by let-7c is mediated by down-regulation of Myc. Expression levels of AR and c-Myc were analyzed by Western blotting in LNCaP cells transfected with let-7c (A) or let-7c antisense oligos (B). Protein levels of AR and c-Myc were reduced in let-7c-overexpressing cells and were increased when let-7c was down-regulated. Con, control. C, LNCaP cells were transfected with plasmids expressing Myc or shRNA against Myc along with the pGL4-AR prom-Luc reporter. Luciferase assays showed that down-regulation of Myc reduced transactivation of the AR promoter, whereas overexpression of Myc enhanced reporter activity. RLU, Relative Luciferase Units. D and E, mRNA levels of AR, PSA, and NKX3.1 were measured by qRT-PCR in LNCaP cells transfected with Myc or shRNA against Myc. Expression levels of all three genes were decreased when Myc was down-regulated and were enhanced when Myc was overexpressed. F, LNCaP cells were transfected with let-7c and Myc alone or together along with the pGL4-AR prom-Luc reporter. Overexpression of Myc could overcome the repression of AR promoter activity by let-7c. G, recruitment of Myc to the Myc binding site in the AR promoter was analyzed by ChIP assays. Overexpression of let-7c reduced and expression of Lin28 enhanced the recruitment of Myc to the AR promoter. IP, immunoprecipitation.
To determine whether reduced expression of Myc is responsible for let-7c-mediated AR suppression, we cotransfected let-7c with Myc in LNCaP cells expressing a luciferase reporter driven by the full-length AR promoter. The results showed that let-7c suppressed AR promoter activity, which was reversed by overexpression of Myc (Fig. 4F). ChIP assays were performed using primers spanning the consensus binding site for Myc in the AR promoter to determine whether let-7c affects the recruitment of Myc to the promoter of the AR gene. Overexpression of let-7c reduced, whereas overexpression of Lin28 increased the recruitment of Myc to the AR promoter (Fig. 4G). To confirm that Myc mediates regulation of AR by let-7c, we transfected Myc along with the pGL4-AR prom-Luc reporter into LNCaP and C4-2B cells stably expressing let-7c and performed luciferase assays. AR promoter activity was lower in cells stably expressing let-7c, which was reversed by overexpression of Myc (supplemental Fig. 3, A and B). Previous studies have shown that Myc is a direct target of let-7c (31). Conserved let-7c binding sites exist in the 3′ untranslated region of Myc that are conserved across several vertebrate classes (supplemental Fig. 4, A and B). Collectively, these results demonstrate that AR suppression by let-7c is mediated by direct down-regulation of Myc.
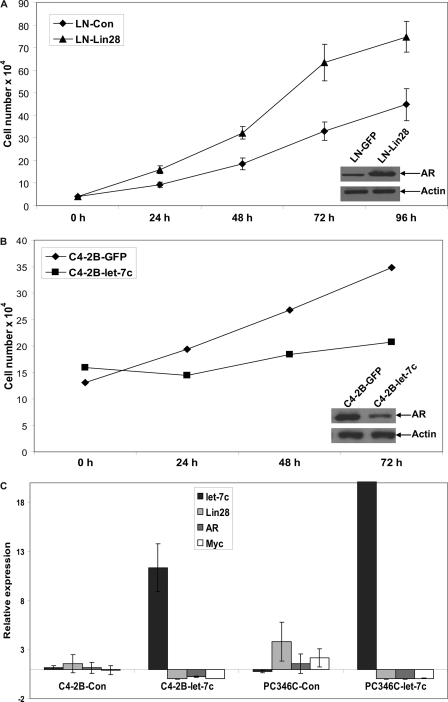
To test whether reduction of AR expression by let-7c affects proliferation of PCa cells in vitro, we analyzed cell growth of LNCaP and C4-2B cells stably expressing Lin28 or let-7c, respectively. As shown in Fig. 5, A and B, expression of Lin28 enhanced growth of LNCaP cells, indicating that increased expression of AR due to Lin28 leads to increased growth of PCa cells. Similarly, C4-2B cells stably expressing let-7c exhibited a lower rate of growth compared with controls, indicating that suppression of AR expression by let-7c leads to reduced survival of PCa cells. Expression levels of AR in these cell lines were confirmed by Western blotting (Fig. 5, A and B, insets).
FIGURE 5.
Let-7c reduces proliferation of PCa cells. A, cell growth of LNCaP cells stably expressing Lin28 was determined by cell counting up to 96 h. Expression of Lin28 enhanced the growth rate of LNCaP cells. The inset (Western blot analysis) shows the increase in expression of AR with increased expression of Lin28. B, cell growth of C4-2B cells stably expressing let-7c was analyzed up to 72 h. Expression of let-7c decreased the growth rate of C4-2B cells. The inset (Western blot analysis) shows the reduction in AR protein levels with expression of let-7c. C, relative expression levels of let-7c, AR, Lin28, and Myc were analyzed by qRT-PCR in PCa xenografts injected intratumorally with let-7c expressing lentiviruses. Enhanced expression of let-7c suppressed expression of AR, Lin28, and Myc. Data points represent mean ± S.D. of triplicate samples from two independent experiments. Error bars denote mean ± S.D. p ≤ 0.05.
Expression of let-7c Suppresses AR, Lin28, and Myc in PCa Xenografts
To determine whether let-7c suppresses AR expression in xenografts of PCa cells in vivo, we generated xenografts of AR-positive C4-2B and PC346C human PCa cells in nude mice. After the tumors reached 0.5 cm3, the control mice were injected with control lentiviruses, whereas experimental mice were injected intratumorally with lentiviruses expressing let-7c. At the end of 3 weeks, the tumors were excised, RNAs prepared, and levels of let-7c, AR, Lin28, and Myc were analyzed by qRT-PCR. As shown in Fig. 5C, expression of let-7c suppressed AR, Lin28, and Myc levels in the xenograft tissues.
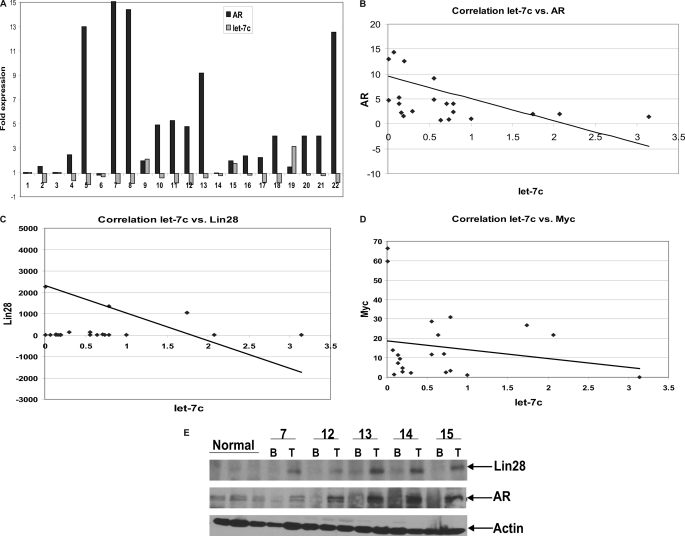
Let-7c and AR Are Negatively Correlated in Human PCa
We have demonstrated that let-7c represses AR expression and that the levels of let-7c are inversely correlated with AR in cell culture and xenografts of PCa mouse models. To determine whether a correlation exists between expression levels of let-7c and AR in clinical PCa, we analyzed RNAs from 22 human PCa specimens by quantitative RT-PCR. RNAs were isolated from human tissues, reverse-transcribed, and subjected to qRT-PCR using LNA-conjugated let-7c primers (Exiqon). This was followed by measurement of expression levels of AR using primers specifically amplifying AR mRNA. The levels of let-7c and AR were negatively correlated, with a correlation coefficient of −0.52 using a two tailed Student's t test in Microsoft Excel Tools (Fig. 6, A and B). Expression levels of Lin28 and Myc were also examined in these specimens and were correlated negatively with expression levels of let-7c with correlation coefficients of −0.1765 and −0.3354, respectively, using a two-tailed Student's t test in Microsoft Excel Tools (Fig. 6, C and D). The correlation coefficients do not show a perfect negative correlation between expression levels of the respective genes but demonstrate a trend toward negative correlation, which should be validated with larger numbers of samples.
FIGURE 6.
Expression levels of let-7c and AR are negatively correlated in human PCa. A, relative expression levels of let-7c and AR were measured by qRT-PCR in total RNAs extracted from 22 human prostate cancer samples. Levels of let-7c and AR were correlated negatively with each other. p = 0.015 by two-tailed Student's t test. B, plot showing the negative correlation between levels of let-7c and AR mRNAs in the above samples. The correlation coefficient (R) was found to be −0.5135. C and D, plots showing negative correlation between relative expression levels of let-7c and Lin28 and let-7c and Myc, respectively, in the above samples. r = −0.1765 (Lin28) and r = −0.3354 (Myc). E, representative Western blot analysis showing protein levels of Lin28 and AR in extracts from normal, benign (B), and adjacent tumor (T) containing human prostate samples. Actin is shown as a loading control. The levels of Lin28 and AR are positively correlated with each other.
As Lin28 is a key regulator of let-7c expression, we examined Lin28 expression in 42 archival matched pairs of benign and cancerous human prostate samples and 20 samples of normal prostate by Western blotting using an antibody specifically against Lin28 (Abcam). The levels of Lin28 protein expression were higher in most of the tumors compared with the matched benign prostates (Fig. 6E). We found that 86% of tumor tissues were positive for Lin28, whereas only 47% of benign and 40% of normal tissues exhibited Lin28 expression. We also analyzed expression levels of AR in these samples by Western blotting, and the results show that AR levels correlate with Lin28 levels (Fig. 6E). Collectively, these data suggest that expression levels of let-7c and AR are negatively correlated with each other and that Lin28 is overexpressed in prostate cancer versus benign prostate and is correlated positively with AR.
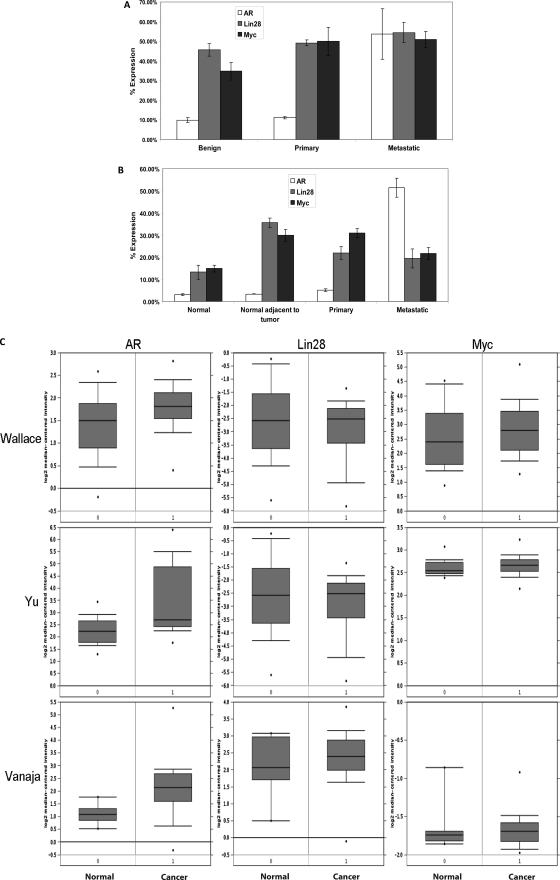
Expression Levels of AR, Lin28, and Myc Are Increased in Human PCa
To validate our above results from clinical PCa specimens, gene expression analysis was performed using public domain data sets deposited in the Gene Expression Omnibus of the National Center for Biotechnology Information and in Oncomine. The former utilized data sets generated by Affymetrix human genome microarrays of well described human tissues, whereas the latter utilized the Oncomine cancer transcriptome data. Analysis of Affymetrix human gene arrays demonstrated that expression levels of AR, Lin28, and Myc were enhanced (p ≤ 0.05) in primary and metastatic prostate cancer specimens compared with benign prostate specimens (Fig. 7, A and B). Similarly, significant (p ≤ 0.001) increases were observed in expression levels of AR, Lin28, and Myc in prostate cancer specimens compared with their normal counterparts and were positively correlated with each other (Fig. 7C). These results imply that Lin28 and Myc play important roles in the regulation of AR expression in human PCa. Down-regulation of expression of let-7c in human PCa may lead to higher levels of expression of Lin28 and Myc, which in turn enhance the expression of AR, which is an important survival factor for PCa cells.
FIGURE 7.
Expression levels of AR, Lin28 and Myc are correlated with each other in human PCa. A, comparison of AR, Lin28, and Myc expression in the dataset GDS1439 (23). Benign, n = 6; primary prostate cancer, n = 7; and metastatic prostate cancer, n = 6. B, comparison of AR, Lin28, and Myc levels in GDS2547 (24). Normal prostate tissue, n = 18; normal tissue adjacent to tumor, n = 16; primary prostate cancer, n = 65; and metastatic prostate cancer, n = 25. Data are expressed as mean ±S.E. (in percentages) of the maximum single channel count determined in each data set. C, gene expression analysis using the Oncomine database showing the relative expression levels of AR, Lin28, and Myc in three datasets comparing normal prostate tissue and prostate cancer. Wallace_prostate: normal, n = 20; cancer, n = 69. Yu_prostate: normal, n = 23; cancer, n = 64. Vanaja_prostate: normal, n = 8; cancer, n = 32. Data are presented as mean ± S.E. of normalized expression units according to Oncomine output.
DISCUSSION
In this study, we found that let-7c suppresses AR expression via a transcriptional mechanism involving Myc. Down-regulation of let-7c by antisense oligonucleotides increased AR expression, thereby conferring a survival advantage on PCa cells. We also found that down-regulation of Lin28 in PCa cells led to an up-regulation of let-7c expression and inhibited cell growth. These results suggest that this novel pathway by which miRNA let-7c regulates AR expression and PCa cell proliferation may be exploited for therapeutic applications.
A tumor suppressor role has been attributed to the let-7 family of miRNAs in several human cancers. Here, we demonstrate that let-7c functions as a tumor suppressor that inhibits prostate tumor growth by regulating AR expression. The most important and well studied targets of let-7 are the oncogenes: HMGA2, RAS, and Myc. Each of these proteins is an important transcription factor, and it is conceivable that by repressing their expression directly, let-7 may influence cancer cell proliferation in a cell type- and target-specific manner. In our study, we show that by targeting Myc, which is a pivotal transcription factor regulating the expression of AR in PCa cells, let-7c suppresses AR expression and plays an important tumor suppressor role in PCa. Our results also indicate that, if feasible strategies to reconstitute let-7c in prostate tumors can be developed, reconstitution of let-7c may become a potential therapeutic agent for prostate cancer treatment. The down-regulation of potential oncogenic and survival factors by let-7c may represent an attractive therapeutic opportunity in human PCa.
It is well documented that Lin28 plays a major role in regulation of let-7c expression (14, 32). Overexpression of Lin28 inhibited let-7c expression in LNCaP cells, whereas knockdown of Lin28 expression increased let-7c in C4-2B cells. In addition, we showed that expression levels of let-7c and Lin28 are correlated inversely with each other in the tumors of prostate cancer xenograft models as well as in prostate cancer clinical specimens, indicating that a negative feedback loop exists between Lin28 and let-7c. Since overexpression of Lin28 enhanced AR expression and increased cell growth in prostate cancer cells, it is possible that overexpression of let-7c suppresses AR expression on one hand while negatively regulating Lin28 expression on the other hand, leading to further inhibition of prostate tumor growth.
The AR is an important survival factor in prostate cells and tumors. Up-regulation of AR expression has been recognized as a major determinant in the pathogenesis of PCa. Up-regulation of AR expression has been shown to be sufficient for conversion of a castration-sensitive phenotype to a castration-resistant phenotype (33). CRPC tissues expressing higher levels of the AR require 80% lower concentrations of androgens to maintain androgen signaling (34). High levels of AR expression also convert AR antagonists like bicalutamide and flutamide into agonists and confer promiscuity to ligands like estrogens (33). Recent data also indicate that elevated expression of the AR detected in biopsy specimen predicts response or resistance to therapy (35). Silencing AR signaling by down-regulating its expression has been the focus of research efforts in the recent past. Use of synthetic siRNA, AR antisense, geldanamycin analogs, and selective AR modulators (SARMs) has demonstrated that down-regulation of AR expression is sufficient to slow prostate tumor growth and induce apoptosis (36–39). In view of the above studies and the importance of the AR in PCa pathogenesis, our study, which shows a novel way of antagonizing AR expression and thereby CRPC progression by let-7c, may have important translational implications.
In summary, we show that the miRNA let-7c plays an important role in regulation of androgen signaling by down-regulating AR expression and thereby inhibits PCa cell proliferation. Our results suggest that re-expression of the miRNA let-7c may help target enhanced and hypersensitive AR in advanced PCa.
Supplementary Material
Acknowledgments
We thank Drs. Leland W. K. Chung and Wen-Chin Huang (Cedars-Sinai Medical Center, Los Angeles, CA) and Dr. Donald Tindall (Mayo Clinic, Rochester, MN) for the pGL4-AR-Prom-Luc plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grants CA140468, CA118887, CA109441, and DOD PC080538.

This article contains supplemental Figs. 1–4 and experimental procedures.
- PCa
- prostate cancer
- CRPC
- castration-resistant prostate cancer
- AR
- androgen receptor
- PSA
- prostate-specific antigen
- miRNA
- microRNA
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Chen Y., Sawyers C. L., Scher H. I. (2008) Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol. 8, 440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamont K. R., Tindall D. J. (2010) Androgen regulation of gene expression. Adv. Cancer Res. 107, 137–162 [DOI] [PubMed] [Google Scholar]
- 3. Coppola V., de Maria R., Bonci D. (2010) Endocr. Relat. Cancer 17, F1–F17 [DOI] [PubMed] [Google Scholar]
- 4. Shi X. B., Tepper C. G., deVere White R. W. (2008) Cancerous miRNAs and their regulation. Cell Cycle 7, 1529–1538 [DOI] [PubMed] [Google Scholar]
- 5. Gandellini P., Folini M., Zaffaroni N. (2009) Towards the definition of prostate cancer-related microRNAs. Where are we now? Trends Mol. Med. 15, 381–390 [DOI] [PubMed] [Google Scholar]
- 6. Calin G. A., Sevignani C., Dumitru C. D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., Croce C. M. (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. U.S.A. 101, 2999–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ozen M., Creighton C. J., Ozdemir M., Ittmann M. (2008) Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 27, 1788–1793 [DOI] [PubMed] [Google Scholar]
- 8. Jiang J., Lee E. J., Gusev Y., Schmittgen T. D. (2005) Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 33, 5394–5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyerinas B., Park S. M., Hau A., Murmann A. E., Peter M. E. (2010) The role of let-7 in cell differentiation and cancer. Endocr. Relat. Cancer 17, F19–36 [DOI] [PubMed] [Google Scholar]
- 10. Lee Y. S., Dutta A. (2007) The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 21, 1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. (2005) RAS is regulated by the let-7 microRNA family. Cell 120, 635–647 [DOI] [PubMed] [Google Scholar]
- 12. Kumar M. S., Lu J., Mercer K. L., Golub T. R., Jacks T. (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 39, 673–677 [DOI] [PubMed] [Google Scholar]
- 13. Viswanathan S. R., Daley G. Q., Gregory R. I. (2008) Selective blockade of microRNA processing by Lin28. Science 320, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viswanathan S. R., Daley G. Q. (2010) Lin28. A microRNA regulator with a macro role. Cell 140, 445–449 [DOI] [PubMed] [Google Scholar]
- 15. Chang T. C., Zeitels L. R., Hwang H. W., Chivukula R. R., Wentzel E. A., Dews M., Jung J., Gao P., Dang C. V., Beer M. A., Thomas-Tikhonenko A., Mendell J. T. (2009) Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc. Natl. Acad. Sci. U.S.A. 106, 3384–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dangi-Garimella S., Yun J., Eves E. M., Newman M., Erkeland S. J., Hammond S. M., Minn A. J., Rosner M. R. (2009) Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 28, 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu J., Getz G., Miska E. A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B. L., Mak R. H., Ferrando A. A., Downing J. R., Jacks T., Horvitz H. R., Golub T. R. (2005) MicroRNA expression profiles classify human cancers. Nature 435, 834–838 [DOI] [PubMed] [Google Scholar]
- 18. Nadiminty N., Lou W., Lee S. O., Lin X., Trump D. L., Gao A. C. (2006) Stat3 activation of NF-κB p100 processing involves CBP/p300-mediated acetylation. Proc. Natl. Acad. Sci. U.S.A. 103, 7264–7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nadiminty N., Dutt S., Tepper C., Gao A. C. (2010) Microarray analysis reveals potential target genes of NF-κB2/p52 in LNCaP prostate cancer cells. Prostate 70, 276–287 [DOI] [PubMed] [Google Scholar]
- 20. Nadiminty N., Lou W., Sun M., Chen J., Yue J., Kung H. J., Evans C. P., Zhou Q., Gao A. C. (2010) Aberrant activation of the androgen receptor by NF-κB2/p52 in prostate cancer cells. Cancer Res. 70, 3309–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dhir R., Ni Z., Lou W., DeMiguel F., Grandis J. R., Gao A. C. (2002) Stat3 activation in prostatic carcinomas. Prostate 51, 241–246 [DOI] [PubMed] [Google Scholar]
- 22. Ni Z., Lou W., Lee S. O., Dhir R., DeMiguel F., Grandis J. R., Gao A. C. (2002) Selective activation of members of the signal transducers and activators of transcription family in prostate carcinoma. J. Urol. 167, 1859–1862 [PubMed] [Google Scholar]
- 23. Varambally S., Yu J., Laxman B., Rhodes D. R., Mehra R., Tomlins S. A., Shah R. B., Chandran U., Monzon F. A., Becich M. J., Wei J. T., Pienta K. J., Ghosh D., Rubin M. A., Chinnaiyan A. M. (2005) Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 8, 393–406 [DOI] [PubMed] [Google Scholar]
- 24. Chandran U. R., Ma C., Dhir R., Bisceglia M., Lyons-Weiler M., Liang W., Michalopoulos G., Becich M., Monzon F. A. (2007) Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prasad P. D., Stanton J. A., Assinder S. J. (2010) Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res. 339, 337–347 [DOI] [PubMed] [Google Scholar]
- 26. Wallace T. A., Prueitt R. L., Yi M., Howe T. M., Gillespie J. W., Yfantis H. G., Stephens R. M., Caporaso N. E., Loffredo C. A., Ambs S. (2008) Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 68, 927–936 [DOI] [PubMed] [Google Scholar]
- 27. Yu Y. P., Landsittel D., Jing L., Nelson J., Ren B., Liu L., McDonald C., Thomas R., Dhir R., Finkelstein S., Michalopoulos G., Becich M., Luo J. H. (2004) Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J. Clin. Oncology 22, 2790–2799 [DOI] [PubMed] [Google Scholar]
- 28. Vanaja D. K., Cheville J. C., Iturria S. J., Young C. Y. (2003) Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 63, 3877–3882 [PubMed] [Google Scholar]
- 29. Lee S. O., Lou W., Hou M., de Miguel F., Gerber L., Gao A. C. (2003) Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin. Cancer Res. 9, 370–376 [PubMed] [Google Scholar]
- 30. Lee S. O., Chun J. Y., Nadiminty N., Lou W., Gao A. C. (2007) Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate 67, 764–773 [DOI] [PubMed] [Google Scholar]
- 31. Koscianska E., Baev V., Skreka K., Oikonomaki K., Rusinov V., Tabler M., Kalantidis K. (2007) Prediction and preliminary validation of oncogene regulation by miRNAs. BMC Mol. Biol. 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iliopoulos D., Hirsch H. A., Struhl K. (2009) An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 34. Bonkhoff H., Berges R. (2010) From pathogenesis to prevention of castration-resistant prostate cancer. Prostate 70, 100–112 [DOI] [PubMed] [Google Scholar]
- 35. Donovan M. J., Khan F. M., Fernandez G., Mesa-Tejada R., Sapir M., Zubek V. B., Powell D., Fogarasi S., Vengrenyuk Y., Teverovskiy M., Segal M. R., Karnes R. J., Gaffey T. A., Busch C., Haggman M., Hlavcak P., Freedland S. J., Vollmer R. T., Albertsen P., Costa J., Cordon-Cardo C. (2009) Personalized prediction of tumor response and cancer progression on prostate needle biopsy. J. Urol. 182, 125–132 [DOI] [PubMed] [Google Scholar]
- 36. Heinlein C. A., Chang C. (2004) Androgen receptor in prostate cancer. Endocr. Rev. 25, 276–308 [DOI] [PubMed] [Google Scholar]
- 37. Singh P., Uzgare A., Litvinov I., Denmeade S. R., Isaacs J. T. (2006) Combinatorial androgen receptor-targeted therapy for prostate cancer. Endocr. Relat. Cancer 13, 653–666 [DOI] [PubMed] [Google Scholar]
- 38. Pienta K. J., Bradley D. (2006) Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 12, 1665–1671 [DOI] [PubMed] [Google Scholar]
- 39. Tran C., Ouk S., Clegg N. J., Chen Y., Watson P. A., Arora V., Wongvipat J., Smith-Jones P. M., Yoo D., Kwon A., Wasielewska T., Welsbie D., Chen C. D., Higano C. S., Beer T. M., Hung D. T., Scher H. I., Jung M. E., Sawyers C. L. (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.