Background: Both p75 and Par3 become localized in a polarized manner in Schwann cells.
Results: BDNF activates Rac1 at the axon-glial interface, regulated by Par3, thereby facilitating proper alignment between the axon and Schwann cells.
Conclusion: Polarized localization of Rac1 activation is critical for myelination.
Significance: Par3 is involved in Rac1 activation by BDNF-p75 at the axon-glial interface, thereby promoting myelination.
Keywords: BDNF, Development, Rac1, Schwann Cells, Signaling, Par3, Myelination, p75
Abstract
Brain-derived neurotrophic factor (BDNF) was shown to play a role in Schwann cell myelination by recruiting Par3 to the axon-glial interface, but the underlying mechanism has remained unclear. Here we report that Par3 regulates Rac1 activation by BDNF but not by NRG1-Type III in Schwann cells, although both ligands activate Rac1 in vivo. During development, active Rac1 signaling is localized to the axon-glial interface in Schwann cells by a Par3-dependent polarization mechanism. Knockdown of p75 and Par3 individually inhibits Rac1 activation, whereas constitutive activation of Rac1 disturbs the polarized activation of Rac1 in vivo. Polarized Rac1 activation is necessary for myelination as Par3 knockdown attenuates myelination in mouse sciatic nerves as well as in zebrafish. Specifically, Par3 knockdown in zebrafish disrupts proper alignment between the axon and Schwann cells without perturbing Schwann cell migration, suggesting that localized Rac1 activation at the axon-glial interface helps identify the initial wrapping sites. We therefore conclude that polarization of Rac1 activation is critical for myelination.
Introduction
During development, cell-to-cell contact plays a critical role in facilitating the exchange of signals between neighboring cells. Myelination by Schwann cells is perhaps one of the best examples, where myelinating glia are in intimate, continuous contact with the axons that they myelinate, forming a reciprocal signaling network between the two cell types. This contact and bidirectional communication begins from the time Schwann cells migrate along the axonal surface and continues until they begin ensheathing the axons. The process of wrapping axons is particularly polarized, being initiated at the axon-glial interface and continuing until mature myelin is formed.
Partitioning-Defective 3 (Par3), a member of the polarization complex that includes Par6 and protein kinase C (1), was recently shown to play a critical role in this process. Par3 was localized at the axon-glial interface, recruiting p75 in response to BDNF3 (2), which is secreted by the axons (3). In particular, Par3 knockdown markedly inhibited myelination in vitro (2). Par3 contains three PDZ domains, rendering it capable of interacting with a large number of proteins aside from Par6 and aPKC in a polarization complex. They include Tiam1 (4), KIF3A (5), Inscrutable (6), Nectins (7), 14-3-3 (8), JAM (9), Ku70 (10), and dynein (11). Considering the ability of Par3 to interact with such a variety of proteins, it is possible that the previously reported effect of Par3 knockdown in myelination cultures (2) could have been due to disruption of its association with a protein critical for myelination.
Here, we report that Par3 is responsible for localized small GTP binding protein, Rac1, activation in response to BDNF but not by NRG1-Type III in Schwann cells. Par3, therefore, plays a critical role in distinguishing two different axonal signals, BDNF and NRG1- Type III, thereby regulating myelination.
EXPERIMENTAL PROCEDURES
Reagents
The Fc recombinant proteins were purchased from R&D Systems and BDNF from Promega. The antibodies used in the study include p-PAK (Cell Signaling Technology, Inc.), actin, and fyn (Santa Cruz Biotechnology, Inc.), Rac1, Par3, and neurofilament (Millipore), p75 (Promega), and myc (Covance). PKI-166 was a gift from Novartis.
Constructs
The Par3 construct was a gift from Dr. Ian Macara. The PDZ 1, 2, and 3 constructs were described in Ref. 2.
RNAi Sequences
The Par3 and its control RNAi sequences were published (2), as were the p75 and its control RNAi sequences (12). The oligonucleotides containing the shRNA of the individual RNAi sequences were placed into pSIREN-RetroQ-ZsGreen1 vector (Clontech) using the BamHI and XbaI sites as directed by the vendor. Retroviruses were generated following transient transfection of the shRNA constructs in PlatE cells (Cell Biolabs), and the viral supernatants were concentrated by centrifugation at 20,000 rpm for 4 h at 15 °C using an SW28 rotor (Beckman). The viral pellet was resuspended in a small volume of media and frozen at −80 °C until used. For infection of Schwann cells in vitro and in vivo, a combined mixture of three different RNAi viruses were used for efficient knockdown.
Primary Schwann Cell Culture
Primary rat Schwann cells were isolated from P0-P1 rats and maintained according to Ref. 13. For RNAi knockdown experiments, Schwann cells were infected with the retroviruses that express the shRNA sequences, and the lysates were harvested 3 days later.
RacGTP Assays and Western Blotting
RacGTP assays were performed as described (14). For quantification, RacGTP signals were adjusted to total Rac1 to obtain “relative RacGTP levels.” The lysates were prepared and processed as described (14).
Preparation of Neuregulin 1 Type III
Neuregulin 1 type III cDNA was transfected into 293T cells, and the membranes were prepared according to Ref. 15. As a control, the membranes from untransfected 293T cells were prepared in parallel. To add the membranes to Schwann cells, 8 μg of membrane preparations was placed onto Schwann cell monolayers by a 3-min spin in a dish at 3000 rpm as described (15, 16). The cDNA for NRG 1-Type III was a generous gift from Dr. Doug Falls.
Injection of Fc Fusion Proteins and Retroviruses into Neonate Mice
Injection of Fc proteins or retroviruses into P0 mouse sciatic nerves were performed as described (2). Briefly, 2 μl of Fc solutions (1 μg/μl) or 5 μl of concentrated retroviruses was injected using a glass-pulled pipette over the sciatic nerve midway between the knee and hip by penetrating through the outer skin. For every injection, one side was injected with experimental reagents, whereas the other side was injected with appropriate controls in a single mouse. Four days after the injection, the 1-cm sciatic nerve segment surrounding the injection site was isolated for immunohistochemistry and biochemical analyses. For statistical analyses, Student's t test was used.
P75 Knockout and Wild-type Mice
The p75 knockout mice that carried the mutation in exon 3 of the p75 gene (17) and the wild type mice were obtained from heterozygote mating as littermates. The mice were backcrossed to C57/BL6 for 10 generations to make them congenic. Their genotype was determined by PCR analyses of tail DNA according to Bentley and Lee (18). For experiments, both sexes were used.
Myelination Cultures with p75 Knockdown
Rat Schwann cells were transfected with the control-shRNA or p75-shRNA as described (19). Cells were plated in Ultraculture media (BioWhittaker) supplemented with 10% FBS, 2 mm l-glutamine, and 50 ng/ml NGF at a density of 80,000 cells/2.2 cm2 per collagen-coated coverslip. Myelination was induced 5 days later by adding 50 μg/ml of ascorbic acid in the growth media. Growth media and ascorbic acid were replaced every 2 days. Following 10 days of treatment, cells were fixed and immunostained for MBP. For quantification, MBP+ internodes were quantified in a blinded manner. For statistical analysis, a Student's t test was used.
TEM Analyses and Quantification
For TEM of the tissues, the sciatic nerves encompassing the segment from the hip to the knee were dissected (∼0.5 cm) 4 days after retrovirus injections and divided further into three equal parts using a sharp razor blade. The tissues were fixed for 2 h at room temperature with 2% paraformaldehyde/2% glutaraldehyde in 0.1 m Na cacodylate buffer (pH 7.2), rinsed in 0.1 m Na cacodylate buffer, and placed in 1% osmium/0.1 M Na cacodylate for 60–90 min at room temperature. The tissues were stained en bloc for 1 h in 2% uranyl acetate and embedded in Spurr resin following dehydration procedures. Sections were cut on a coronal plane at 80 nm using a Reichert Ultracut E ultramicrotome and collected on 300 mesh grids. Sections were stained in 2% uranyl acetate and Reynolds lead citrate before observation in Field Electron & Ion Source Company Technai G2 Spirit TEM at 60kV (Ohio State University Campus Microscopy and Imaging Facility).
For quantification of myelinated axons, three random images were obtained from each cross-section of the sciatic nerve, from three cross-sections per mouse (the number of images = 36 from 4 mice). The electron photomicrographs were prepared at ×2550 magnification, and the number of myelinated axons, myelin thickness, and g ratio were counted using ImageJ software. For statistical analyses, Student's t test was used.
Morpholinos
pard3 antisense morpholino (MORPH1404, sequence 5′-TCCAACACTCCTTCCCGAATCCAAG-3′) was obtained from Open Biosystems. MO2 (5′-TCAAAGGCTCCCGTGCTCTGGTGTC-3′) and a random sequence control MO3 were obtained from Gene Tool LLC. Both MOs were resuspended in H2O at a concentration of 1.0 mm. Each MO was diluted to a working concentration of 0.25 mm in H2O and phenyl red and injected (1–2 nl) into Tg(sox10(7.2):mRFP);Tg(olig2:EGFP) zebrafish embryos (20, 21) at one cell stage. The embryos were raised at 28.5 °C until analysis at 4 dpf.
In Situ RNA Hybridization in Zebrafish
Zebrafish larvae were fixed in 4% paraformaldehyde for 24 h and stored in 100% methanol at −20 °C. The mbp RNA probe was synthesized using a digoxigenin labeling kit (Roche) and T7 RNA polymerase (NEB). After in situ RNA hybridization and staining, embryos were dissected from the yolk and mounted in 75% glycerol on bridged microscope slides. Images were obtained using Volocity software (PerkinElmer Life Sciences) and a Zeiss AxioObserver inverted microscope equipped with differential interference contrast optics and a Retiga Exi color digital camera. Images were exported to Photoshop (Adobe). Image manipulations were limited to cropping, contrast, brightness, and color matching settings.
In Vivo Imaging of Zebrafish
For imaging, larvae were lightly anesthetized using ethyl 3-aminobenzoate methanesulfonic acid (Tricaine), immersed in 0.8% low-melting temperature agarose, and mounted on their sides in glass-bottomed 35-mm dishes (World Precision Instruments). Images were captured using a ×40 oil immersion objective mounted on a motorized Zeiss AxioObserver microscope equipped with a PerkinElmer Life Sciences UltraVIEW VoX spinning disk confocal system. Z image stacks were collected and compiled using Volocity software and exported to Photoshop. Image manipulations were limited to brightness settings and cropping.
RESULTS
BDNF and NRG1-Type III Activate Rac1 during Sciatic Nerve Development
During development in rat sciatic nerves, RacGTP levels peaked before the onset of myelination at embryonic age 19.5 (E19.5) and then gradually declined until reaching the adult level (Fig. 1, A and B). Because Schwann cells are in constant contact with axons in addition to forming a signaling junction with the extracellular matrix, we located the sites of Rac1 activation by phospho-PAK (p-PAK) immunostaining. Although PAK is known to undergo autophosphorylation when bound to Rac1- and Cdc42-GTP (22), p-PAK staining represents mostly Rac1 activation at the premyelinating stage because Cdc42 is not activated until myelination begins (data not shown). In dorsal root ganglion (DRG) neuron Schwann cell cocultures in which myelination was not initiated, p-PAK immunoreactivity was detected within the Schwann cell on the side that was in contact with DRG fibers (Fig. 1C, left panel). Also at postnatal day 3 (P3) sciatic nerves, p-PAK immunoreactivity was asymmetrically localized toward the axon (Fig. 1C, right panel and inset). These results suggest that at least at the premyelinating stage, signals from the axon induce Rac1 activation in Schwann cells. In further support, we failed to detect any change in RacGTP levels in 2- to 3-week-old pure DRG cultures after treating them with BDNF, NRG1-Type III, and laminin (data not shown).
FIGURE 1.
BDNF and NRG1-Type III activate Rac1 in sciatic nerves. A, RacGTP levels are their highest at E19.5 in rats and gradually decline during development. B, quantification of RacGTP levels in A. n = 6–8. C, asymmetric localization of phospho-PAK immunoreactivity in premyelinating Schwann cells in culture and in vivo. Note that phospho-PAK staining is along the side of Schwann cells that were in contact with DRG axons in culture. NF, neurofilament. Scale bar = 5 μm (left panels). Similarly, p-PAK immunoreactivity is found predominantly adjacent to axons in P3 sciatic nerves (right panel). Scale bar = 12 μm). The inset shows a high-magnification view. D, injection of TrkB-Fc and not control Fc into the sciatic nerves of P0 mice resulted in inhibition of Rac1 activation and P0 protein levels. Quantification is shown next to the figure. E, injection of ErbB3-Fc, and not control Fc, into the sciatic nerves of P0 mice resulted in inhibition of Rac1 activation and P0 protein levels. Quantification is shown next to the figure. F, BDNF activates Rac1 in primary Schwann cells. BDNF was added at 50 ng/ml for 1 h. Quantification is shown below the figures. G, NRG1-Type III activates Rac1 in primary Schwann cells. Membrane fractions (8 μg) from 293 cells expressing a vector or NRG1-Type III cDNA were added onto Schwann cells for 1 h. Quantification is shown below the figures. Relative RacGTP levels represent the RacGTP levels adjusted to total Rac1 level in each sample. For statistical analyses, Student's t test was used.
We hypothesized that BDNF and/or neuregulin (NRG) 1, the two well known axonal signals (3, 15, 23), are responsible for activating Rac1 in vivo. To test the hypothesis, TrkB-Fc or ErbB3-Fc was injected into sciatic nerves of P0 mice, whereas the control-Fc was injected into sciatic nerves on the contralateral side. Although the RacGTP levels were readily detected from the control-Fc-injected sciatic nerves at P4, they were reduced by 80% with TrkB-Fc injections (Fig. 1D), and 70% with ErbB3-Fc injections (E). These results suggest that both BDNF and NRG1 are involved in activating Rac1 at P0-P4. It should be noted that myelin protein P0 levels were also reduced with TrkB-Fc and ErbB3-Fc, suggesting that the mechanisms by which NRG1 and BDNF promote myelination are likely to include Rac1 activation. In line with the in vivo data, both BDNF and NRG1-Type III increased RacGTP levels in Schwann cell cultures (Fig. 1, F and G).
Par3 Is Necessary for BDNF-mediated but not NRG1-Type III-mediated Rac1 Activation
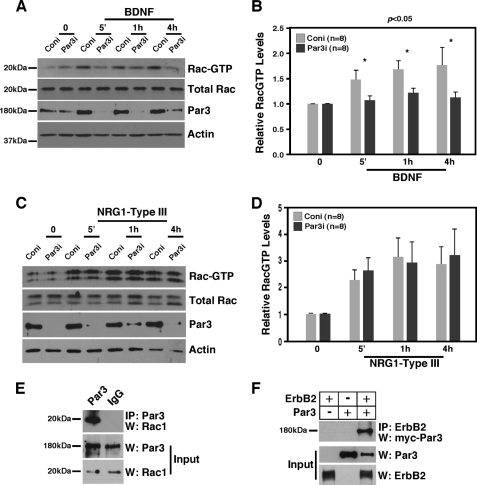
Par3, partitioning-defective protein 3, was shown to be asymmetrically localized on the axon-glial interface during the premyelination stage, recruiting p75 upon BDNF stimulation (2). Par3 can also bind RacGEF and Tiam1, thereby regulating Rac1 activation in epithelial cells (4). Because the p-PAK expression pattern mimics that of Par3 (2) and Par3 interacted with Rac1 in P3 sciatic nerves (Fig. 2E), we tested whether Par3 plays a role in BDNF- and/or NRG1-Type III-dependent Rac1 activation in Schwann cells. Par3 was knocked down in Schwann cells using retroviruses carrying Par3 functional RNAi or control nonfunctional Par3 RNAi (2), and the changes in RacGTP levels were measured at various times after treatment with BDNF or NRG1-Type III. Par3 knockdown reduced RacGTP levels to the basal level with BDNF (Fig. 2, A and B) but not with NRG1-Type III (C and D). The reason for the difference was not clear because ErbB2 can bind Par3 (Fig. 2F). These results nonetheless suggest that the mechanism by which BDNF activates Rac1 is distinct from that of NRG1-Type III in Schwann cells. More importantly, these data indicate that Par3 plays a role in distinguishing the two different axonal signals.
FIGURE 2.
Par3 is necessary for BDNF-mediated but not for NRG1-Type III-mediated Rac1 activation in Schwann cell cultures. A, Par3 is necessary for BDNF-mediated Rac1 activation in Schwann cell cultures. Following infection with the retrovirus carrying Par3 RNAi (Par3i) or control RNAi (Coni), Schwann cells were treated with 50 ng/ml BDNF for the indicated period of time. Control Par3 Western blot analysis demonstrates the extent of Par3 knockdown. B, quantification of A. Relative RacGTP levels represent the RacGTP levels adjusted to total Rac1 level in each sample. For statistical analyses, Student's t test was used. C, knocking down Par3 in Schwann cells failed to inhibit Rac1 activation by NRG1-Type III. Following infection with the retrovirus carrying Par3 RNAi, Schwann cells were treated with 8 μg/ml NRG1-Type III for the indicated period of time. D, quantification of C. E, Par3 interacts with Rac1 in P3 sciatic nerves. Par3 was immunoprecipitated and blotted for Rac1. F, Par3 binds ErbB2 in 293T cells. Full-length Par3 and ErbB2 were transfected to 293T cells, and the resulting lysates were subjected to immunoprecipitation with ErbB2 and Western blot analysis with Par3. Control inputs are shown (5% of the lysates used for immunoprecipitation).
Selective Knockdown of Par3 in Schwann Cells in Vivo Inhibits Polarized Rac1 Activation and Myelination
P75 is expressed both in DRG neurons and in Schwann cells, tending to obscure whether p75 plays a positive role in myelination from the neuronal side or the Schwann cell side. Our discovery of Par3-mediated selectivity for BDNF over NRG1-Type III signaling presents an opportunity for testing whether it is the p75 in Schwann cells that is responsible for its effect in myelination. Accordingly, we knocked down Par3 only in Schwann cells by injecting the retrovirus for Par3-RNAi and its control RNAi to the P0 sciatic nerve and examined whether polarized activation of Rac1 as well as myelination are affected. The effect of Par3 knockdown on Rac1 activation was assessed via p-PAK immunoreactivity. The extent of infection is shown in Fig. 3A.
FIGURE 3.
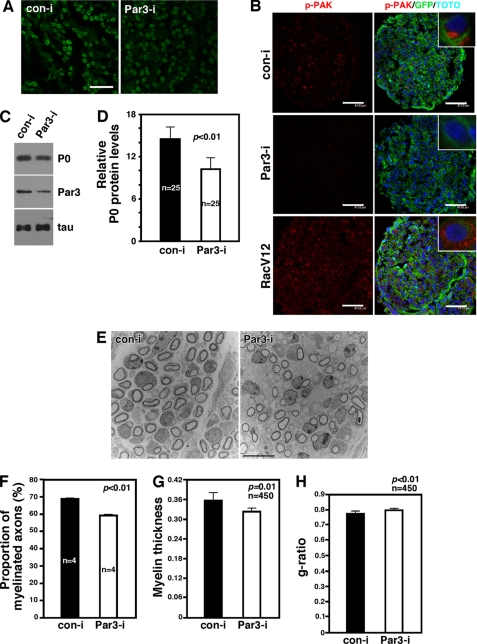
Selective knockdown of Par3 among Schwann cells in vivo results in disruption of polarized Rac1 activation and attenuation in myelination. A, representative images of the retrovirus-infected sciatic nerves. GFP signals indicate the circular myelin sheath formed by the Schwann cells that were infected with the retroviruses. The retroviruses carry GFP, so infected myelinating Schwann cells appear green in doughnut shapes. Scale bar = 15 μm. B, knockdown of Par3 in vivo inhibits polarized Rac1 activation in Schwann cells. Note that p-PAK immunoreactivity is polarized within Schwann cells (inset). Although p-PAK immunoreactivity is detected throughout the cross-section of the sciatic nerve from the control virus-infected mice, it is significantly reduced in the nerves that were infected with shRNA-carrying retroviruses of Par3. In contrast, RacV12 infection increased p-PAK immunoreactivity throughout the sciatic nerve and disrupted its polarization. Scale bars = 45–47 μm. The inset shows a high-magnification view. C, knockdown of Par3 in vivo resulted in reduction in P0 protein levels. Par3i, Par3 RNAi; Coni, control RNAi. Also shown is the control Western blot analysis, Par3, and tau. D, quantification of P0 proteins from C. For statistical analyses, Student's t test was used. E, representative EM images of the retrovirus-infected sciatic nerves at P4. Scale bar = 10 μm. F, quantification of the proportion of the axons that were myelinated. Note that there were four different mice that had been injected with the virus. For statistical analyses, Student's t test was used. G, myelin thickness was reduced with Par3 knockdown. H, the g ratio was increased with Par3 knockdown.
In the control virus-infected Schwann cells, red p-PAK immunoreactivity was polarized to one side of GFP+ Schwann cells (Fig. 3B, inset). Par3 knockdown resulted in a significant reduction in Rac1 activation (Fig. 3B), as it did with p75 knockdown (Fig. 5C). These results provide additional evidence that p75 and Par3 regulate Rac1 activation in Schwann cells. When we activated Rac1 constitutively using a RacV12 retrovirus, however, Rac1 activity was no longer polarized but distributed throughout Schwann cells (Fig. 3B, inset). We found that loss of polarized Rac1 activation by Par3 knockdown impacts myelination. P0 protein levels were reduced by 30% in Par3 knockdown nerves compared with that in the control nerves. The extent of Par3 knockdown was 57% (Fig. 3, C and D). The effect on myelination is also supported by EM analyses of the infected sciatic nerves. Not only was the proportion of the axons that were myelinated reduced by 15% by Par3 knockdown compared with the control (Fig. 3, E–G), but the myelin thickness was also reduced by 9.2%. In line with thinner myelin, the g ratio was increased by 2.9% by Par3 knockdown (Fig. 3H). It should be noted that the observed extent of changes in the myelin profile is likely to have been an underestimate because not all Schwann cells were infected with the virus. We therefore interpret these data as suggesting that the p75-ErbB2-Par3 complex regulates polarized activation of Rac1, thereby regulating certain aspects of myelination in vivo.
FIGURE 5.
P75 signals from Schwann cells activate Rac1 and promote myelination. A, knockdown of p75 in rat Schwann cells inhibited the extent of myelination in culture. Also shown are the control Westerns for the p75 knockdown with RNAi. For statistical analyses, Student's t test was used. B, representative images of MBP staining of the cocultures. Scale bar = 10 μm. C, knockdown of p75 among Schwann cells reduced the extent of p-PAK and MBP immunoreactivity. Note that we are using the same image from Fig. 3B for the control shRNA-infected nerves. Scale bars = 45–47 μm. D, P75 is necessary for Rac1 activation in vivo. RacGTP levels are reduced in p75−/− mice compared with those in p75+/+ at E18.5. As controls, the protein levels of p75, tau, and P0 are also shown. Note that there is a reduction in tau protein levels, reflecting a reduction in DRG fibers. E, quantification of RacGTP from D.
Knockdown of pard3 in Zebrafish Larvae Inhibits MBP Expression by Disrupting Normal Wrapping of Axons
Although the EM data suggest that a smaller number of axons is myelinated with Par3 knockdown in mouse sciatic nerves, it is not clear at which step Par3 regulates myelination. To determine the precise step at which Par3 plays its role during myelination, we utilized fluorescently marked transgenic zebrafish in which Par3 expression was knocked down by injecting into single-cell embryos one of two different antisense morpholino oligonucleotides (MO1 and MO2) designed to block translation of pard3 mRNA. At 4 dpf, larvae injected with pard3 MO had a significant reduction in endogenous RacGTP levels as well as in Pard3 protein (Fig. 4A). This result indicates that Par3 regulates Rac1 activation in zebrafish as it does in rodent Schwann cells. We next investigated whether pard3 knockdown also attenuates the extent of myelination by performing in situ RNA hybridization using mbp as a marker. In all the larvae that received no MO (n = 31) or a control MO (n = 19), mbp RNA was readily detected at motor nerves (Fig. 4B) and the posterior lateral line nerve (Fig. 4C). In larvae that were injected with either MO1 (n = 20, Fig. 4, D and E) or MO2 (n = 65, Fig. 4, F and G), mbp RNA levels were significantly reduced along both motor nerves and the posterior lateral line nerves. These results suggest that Par3 regulates Schwann cell myelination both in zebrafish and rodents.
FIGURE 4.
Knockdown of pard3 in zebrafish larvae resulted in abnormal Schwann cell wrapping and a significant reduction in mbp expression. A, pard3 MO1 reduces Pard3 protein levels and inhibits RacGTP levels in zebrafish larvae. Embryos at 4 dpf were deyolked, and proteins were extracted for RacGTP assays and control Western blot analyses. B–G, lateral views at the level of the trunk of 4 dpf larvae hybridized for mbp. In control larvae (B and C), mbp is expressed in stripes associated with ventral motor roots (B, asterisks) and along the posterior lateral line nerve (C, arrow). In pard3 MO1- (D and E) and MO2 (F and G)-injected larvae, mbp expression is lost at the ventral roots (asterisks) and the posterior lateral line nerve (arrows), whereas it is still evident in the spinal cord (sc, brackets). H and I, lateral views at the level of the trunk of 4 dpf Tg(olig2:EGFP);Tg(sox10:mRFP) larvae. Motor axons are green, and Schwann cells are red. In a control larva (H), Schwann cells wrap motor axons (arrows), whereas in a MO1 injected larva (I), Schwann cells failed to align along the axon, forming a loose association with the axon. The MO-injected larva also has a deficit of axon wrapping by oligodendrocytes, which are marked by RFP expression, in the spinal cord.
To investigate the steps at which pard3 regulates Schwann cell myelination, we injected MO into embryos at the one-cell stage in Tg(sox10(7.2):mRFP);Tg(olig2:EGFP) embryos, grew them until 4 dpf, and performed live cell imaging. This transgenic combination marks motor axons with enhanced green fluorescent protein driven by olig2 regulatory DNA (24) and Schwann cells with membrane-tethered RFP driven by sox10 regulatory DNA (20). In control larvae, Schwann cell wrapping of motor axons is initiated by 4 dpf (Fig. 4H, arrows). In comparably staged pard3-injected larvae, Schwann cells migrated to the motor roots but did not appear to wrap axons tightly (Fig. 4I, arrows). These results are consistent with the possibility that in the absence of Par3 signaling, Schwann cells lose polarity and fail to form a tight interaction with the axon that they should myelinate. We therefore conclude that Par3-mediated signaling in Schwann cells is critical for proper myelination by helping to establish and maintain the axonal contact at the time of initial wrapping.
P75 Signals from Schwann Cells to Activate Rac1 and Promote Myelination
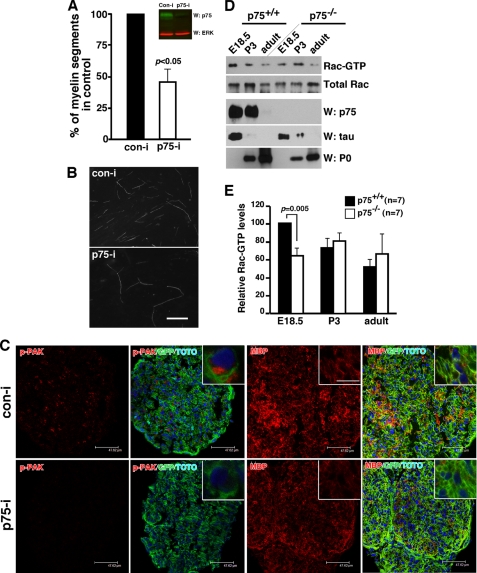
Although our Par3 knockdown data suggest that BDNF acts on Schwann cells most likely through p75, we cannot rule out the possibility that Par3 has an unknown target that plays a role in myelination. We therefore tested whether knocking down p75 itself in Schwann cells affects myelination in vivo and in vitro. First, in an in vitro study, we utilized DRG-Schwann cell cocultures where p75 expression was knocked down by transfecting Schwann cells with the p75-RNAi prior to seeding them onto pure DRG neurons as described (19). When the extent of myelination was assessed by staining for MBP on days 10–14 day following ascorbic acid treatment, the number of MBP+ fibers was reduced by 62% in the p75 knockdown Schwann cells compared with the control cultures (Fig. 5, A and B). These results suggest that it is the p75 in Schwann cells that plays a signaling role in myelination in vitro.
To study the effect of p75 knockdown in vivo, we opted to inject a retrovirus that carries the same p75 shRNA into mouse sciatic nerves. As with Par3, knockdown of p75 resulted in a significant reduction in p-PAK immunoreactivity as well as MBP staining (Fig. 5C). In correspondence with the p-PAK results, RacGTP levels in p75−/− mice were reduced by 40% compared with that of the wild-type mice at E18.5 (Fig. 5, D and E). The RacGTP levels at P3 and adult stages did not differ between the genotypes. We therefore conclude that p75 promotes myelination from the Schwann cell side, in part by regulating Rac1 activation.
DISCUSSION
Here, we identified two signals that can activate Rac1 in Schwann cells: BDNF and NRG1-Type III, in addition to β1 integrin (25). Of these three different inputs to activate Rac1 in Schwann cells, it is the BDNF-dependent Rac-GTP signal that is located at the axon-glial interface where myelination is likely to occur. It is our contention that the location where Rac1 is activated inside the cell is likely to affect which downstream signaling pathways are to be activated, thereby impacting a particular step that is involved in myelination.
On the basis of Par3 knockdown results from zebrafish, we propose that Par3-dependent Rac1 activation regulates where axon wrapping should commence on the axon surface, although it has little effect on Schwann cell migration along the axon. These data are in agreement with a report in which BDNF was shown to inhibit Schwann cell migration in rat Schwann cells (26). Although it remains to be tested whether Par3 in zebrafish is also linked to BDNF and p75 signaling as it is in rodents, we surmise that BDNF signaling in rodents is likely to play a role in establishing the initial wrapping points along the axon.
A recent study by Xiao et al. (27) demonstrated that BDNF promoted myelination through p75 from the neuron side and that knocking down the receptor in Schwann cells had no effect. This is in direct contrast to our results presented here. The reason for the difference is not clear, but it should be noted that the age of the DRG neurons used in the two studies differ. In our study, DRG neurons were isolated at E15 and cultured for a week in the presence of NGF before Schwann cells were added. This procedure approximates the neuronal stage when myelination occurs during normal development. On the other hand, Xiao et al. isolated DRG neurons at P2 and cultured them for 2–3 weeks until they became independent of neurotrophins before adding Schwann cells. The DRG neurons would essentially be adult neurons. Hence, it is possible that p75 plays a critical role in Schwann cells during myelin formation in development but that in the adult when myelin forms, such as after an injury, the receptor has a more critical role in the neurons. A conditional knockout strategy will be important to better clarify the role of p75 in Schwann cells pertaining to myelination in the future.
This work was supported, in whole or in part, by National Institute of Health grants NS039472 (to S. O. Y.), P30NS045758 (to the Ohio State Neuroscience Center Core), NS062796 (to J. R. C.), NS048249 (to B. D. C.), and NS062717 (to B. A.). This work was also supported by Muscular Distrophy Association Grant MDA115761 (to A. S. L.).
- BDNF
- brain-derived neurotrophic factor
- MBP
- myelin basic protein
- MO
- morpholino
- dpf
- days post-fertilization
- E19.5
- embryonic day 19.5
- DRG
- dorsal root ganglion
- P3
- postnatal day 3
- NRG
- neuregulin
- PKC
- protein kinase C
- PDZ
- postsynaptic density protein
- Drosophila
- disc large tumor suppressor and Zonula Occuludens-1 protein
- JAM
- junctional adhesion molecule
- TEM
- transmission electron microscopy
- PAK
- p-21 activated kinase.
REFERENCES
- 1. Goldstein B., Macara I. G. (2007) The PAR proteins. Fundamental players in animal cell polarization. Dev. Cell 13, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan J. R., Jolicoeur C., Yamauchi J., Elliott J., Fawcett J. P., Ng B. K., Cayouette M. (2006) The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science 314, 832–836 [DOI] [PubMed] [Google Scholar]
- 3. Ng B. K., Chen L., Mandemakers W., Cosgaya J. M., Chan J. R. (2007) Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. J. Neurosci. 27, 7597–7603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X., Macara I. G. (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol. 7, 262–269 [DOI] [PubMed] [Google Scholar]
- 5. Nishimura T., Kato K., Yamaguchi T., Fukata Y., Ohno S., Kaibuchi K. (2004) Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 [DOI] [PubMed] [Google Scholar]
- 6. Schober M., Schaefer M., Knoblich J. A. (1999) Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature 402, 548–551 [DOI] [PubMed] [Google Scholar]
- 7. Takekuni K., Ikeda W., Fujito T., Morimoto K., Takeuchi M., Monden M., Takai Y. (2003) Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J. Biol. Chem. 278, 5497–5500 [DOI] [PubMed] [Google Scholar]
- 8. Hurd T. W., Fan S., Liu C. J., Kweon H. K., Hakansson K., Margolis B. (2003) Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr. Biol. 13, 2082–2090 [DOI] [PubMed] [Google Scholar]
- 9. Ebnet K., Suzuki A., Horikoshi Y., Hirose T., Meyer Zu Brickwedde M. K., Ohno S., Vestweber D. (2001) The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J. 20, 3738–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fang L., Wang Y., Du D., Yang G., Tak Kwok T., Kai Kong S., Chen B., Chen D. J., Chen Z. (2007) Cell polarity protein Par3 complexes with DNA-PK via Ku70 and regulates DNA double-strand break repair. Cell Res. 17, 100–116 [DOI] [PubMed] [Google Scholar]
- 11. Schmoranzer J., Fawcett J. P., Segura M., Tan S., Vallee R. B., Pawson T., Gundersen G. G. (2009) Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 19, 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilar M., Charalampopoulos I., Kenchappa R. S., Simi A., Karaca E., Reversi A., Choi S., Bothwell M., Mingarro I., Friedman W. J., Schiavo G., Bastiaens P. I., Verveer P. J., Carter B. D., Ibáñez C. F. (2009) Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron 62, 72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan J. R., Watkins T. A., Cosgaya J. M., Zhang C., Chen L., Reichardt L. F., Shooter E. M., Barres B. A. (2004) NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 43, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrington A. W., Kim J. Y., Yoon S. O. (2002) Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J. Neurosci. 22, 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taveggia C., Zanazzi G., Petrylak A., Yano H., Rosenbluth J., Einheber S., Xu X., Esper R. M., Loeb J. A., Shrager P., Chao M. V., Falls D. L., Role L., Salzer J. L. (2005) Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurel P., Salzer J. L. (2000) Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI3-kinase activity. J. Neurosci. 20, 4635–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee K. F., Li E., Huber L. J., Landis S. C., Sharpe A. H., Chao M. V., Jaenisch R. (1992) Targeted mutation of the gene encoding the low-affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69, 737–749 [DOI] [PubMed] [Google Scholar]
- 18. Bentley C. A., Lee K. F. (2000) p75 is important for axon growth and Schwann cell migration during development. J. Neurosci. 20, 7706–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon C., Korade Z., Carter B. D. (2008) Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor κB promotes Schwann cell differentiation into a myelinating phenotype. J. Neurosci. 28, 3738–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kucenas S., Takada N., Park H. C., Woodruff E., Broadie K., Appel B. (2008) CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 11, 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flanagan-Steet H., Fox M. A., Meyer D., Sanes J. R. (2005) Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 132, 4471–4481 [DOI] [PubMed] [Google Scholar]
- 22. Manser E., Huang H. Y., Loo T. H., Chen X. Q., Dong J. M., Leung T., Lim L. (1997) Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell Biol. 17, 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michailov G. V., Sereda M. W., Brinkmann B. G., Fischer T. M., Haug B., Birchmeier C., Role L., Lai C., Schwab M. H., Nave K. A. (2004) Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 [DOI] [PubMed] [Google Scholar]
- 24. Shin J., Park H. C., Topczewska J. M., Mawdsley D. J., Appel B. (2003) Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 25, 7–14 [DOI] [PubMed] [Google Scholar]
- 25. Nodari A., Zambroni D., Quattrini A., Court F. A., D'Urso A., Recchia A., Tybulewicz V. L., Wrabetz L., Feltri M. L. (2007) β1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J. Cell Biol. 177, 1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamauchi J., Chan J. R., Shooter E. M. (2004) Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc. Natl. Acad. Sci. U.S.A. 101, 8774–8779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao J., Wong A. W., Willingham M. M., Kaasinen S. K., Hendry I. A., Howitt J., Putz U., Barrett G. L., Kilpatrick T. J., Murray S. S. (2009) BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J. Neurosci. 29, 4016–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]