SUMMARY
Ethnic differences in breast cancer survival have been a long-standing concern. The objective of this review is to present relevant studies for all major U.S. racial/ethnic groups including African Americans, Hispanics, Native Americans, Japanese, and Native Hawaiians, and to discuss underlying causes of disparity. In comparison to Caucasian women, African American women continue to experience the poorest breast cancer specific survival of all ethnic groups in the US. The prognosis for Latinas, Native Hawaiians, and Native Americans is intermediate, better than for African Americans but not as good as for Caucasians, whereas Japanese women tend to have better outcomes. The following possible contributors to the observed differences are discussed in detail: unfavorable distribution of stage at diagnosis due to low screening rates, limited access to care and treatment, tumor type, comorbidities, socioeconomic status, obesity, and physical activity.
Keywords: Breast carcinoma, Survival, Ethnicity, Risk factors, Trends, SEER registries
INTRODUCTION
According to the 2010 Surveillance, Epidemiology, and End Results (SEER) report [1], the 5-year breast cancer specific survival rate in the U.S. was 89.2%. The strongest determinant of survival is stage at diagnosis: 98.6% for localized disease, 83.4% for regional disease, and 23.4% for metastasized breast cancer [1]. During the last 20 years, improvements in breast cancer survival have been observed in all ethnic groups [2,3], but substantial differences across ethnic groups have been reported repeatedly with worse outcomes among ethnic groups of low socioeconomic status (SES) [4–8]. Several of the predictors proposed as possible explanations for the ethnic-specific survival differences, e.g., early detection, access to care, pre-existing chronic diseases, obesity, poverty, and lifestyle factors, are related to SES [9–11]. This raises the question whether ethnic disparities may be due to SES rather than biologic differences, such as tumor types or genetic susceptibility [12–15]. The objectives of this review are to compare breast cancer survival rates for all major racial/ethnic groups in the U.S. (African American, Hispanic, Asian, Native Hawaiian/Pacific Islander, and Native American) to rates for Caucasian women and to examine underlying causes. Based on our own expertise, there is a special emphasis on Japanese and Native Hawaiian women. Genetic aspects of susceptibility are not presented as part of this review.
METHODS
Relevant publications were identified in PubMed and in the reference sections of published reports. From the large number of investigations that focused on African American women, we selected the most recent ones and included a large meta-analysis to cover the earlier time period. For the other ethnic groups, we present all available studies even if they were small and published during earlier years. Due to the differences in methodology across studies and the large body of literature, we did not attempt our own meta-analysis or systematic review. Instead, we selected studies that compare breast cancer specific and all cause survival across ethnic groups and illustrate particular causes of disparity. The 24 reports summarized in Table 1 all computed hazard ratios (HR) and 95% confidence intervals (CI). Primarily, they utilized data from one or more cancer registries that participate in the SEER program [5–8,16–22] and sometimes overlap in study population and years of diagnosis. In a number of investigations, this information was enhanced with data from questionnaires, medical records, or Medicare files [23–27]. Also, a few analyses were conducted within a single hospital or institution [28–31], network of health care institutions [32], or large clinical studies [33,34]. First, the results of the review are presented separately by ethnic group (Figure 1); then individual factors that may contribute to ethnic survival differences are discussed in detail.
Table 1.
Selected studies comparing breast cancer specific survival by ethnic group
| Author (Year) Population source |
N of subjects % Early stage |
Cases | Deaths | HR (95% CI) | Covariates |
|---|---|---|---|---|---|
| LeMarchand (1984) [5] HTR (1960–1979) |
N=2,956 58% Loc |
W: 1,174 | Ref | Age, stage, SES | |
| J: 973 | 1.0 (0.8–1.3) | ||||
| H: 458 | 1.6 (1.3–2.1) | ||||
| C: 225 | 1.0 (0.7–1.4) | ||||
| F: 127 | 1.6 (1.1–2.4) | ||||
| Sugarman (1994) [7] Seattle SEER, Portland IHS (1974–1989) |
N=25,315 59% 0/Loc |
W: 25,213 | 10,038 | Ref | Age, stage, urban residence, treatment |
| NA: 102 | 49 | 1.4 (1.1–1.9) | |||
| Meng (1997) [12] HTR (1980–1988) |
N=3,345 62% Loc Equal access |
W: 1,191 | 172 | 1.2 (0.9–1.5) | Age, stage, census SES, menopausal status, marital status, geographical residence |
| J: 1,130 | 132 | Ref | |||
| H: 549 | 122 | 1.7 (1.3–2.2) | |||
| C: 242 | 34 | 1.3 (0.9–1.9) | |||
| F: 233 | 46 | 1.6 (1.1–2.3) | |||
| Li (2003) [8] SEER (1992–1998) |
N=124,934 48% I |
W: 97,999 | 14,089 | Ref | Age, stage, ER/PR, surgery, radiation therapy |
| B: 10,560 | 2,393 | 1.5 (1.4–1.6) | |||
| J: 2,420 | 187 | 0.6 (0.5–0.8) | |||
| F: 2,125 | 227 | 0.9 (0.8–1.1) | |||
| C: 1,852 | 177 | 0.8 (0.7–1.0) | |||
| H: 689 | 88 | 1.3 (1.0–1.7) | |||
| L: 7,219 | 1,117 | 1.1 (1.0–1.1) | |||
| NA: 322 | 69 | 1.7 (1.3–2.3) | |||
| Wampler (2005) [13] 3 SEER programs (1973–1996) |
N=2,555 54% Loc |
W: 2,044 | Ref | Age, marital status, stage, tumor size, lymph nodes, therapy | |
| NA/AN: 511 | 1.6 (1.3–2.0) | ||||
| Field (2005) [27] CRN (1993–1998) |
N=21,155 67% Loc Equal access |
W: 18,879 | 3,474 | Ref | Age, histology, enrollment history, health plan |
| B: 2,276 | 596 | 1.3 (1.2–1.5) | |||
| Chlebowski (2005) [29] WHI (1993–1998) |
N=156,570 74% Loc |
W: 3,455 | 191 | Ref | Age, BMI, tumor stage, study component |
| B: 242 | 21 | 1.8 (1.1–3.1) | |||
| Tammemagi (2005) [28] Ford Health System (1985–1990) |
N=906 35% 0/I |
W: 642 | 115 | Ref | Age, tumor stage, ER, therapy, comorbidity |
| B: 264 | 64 | 1.5 (1.1–2.1) | |||
| Braun (2005) [14] HTR (1990–2002) |
N=7,722 54% I |
W: 2,363 | 239 | Ref | Age, stage, ER/PR |
| J: 2,666 | 175 | 0.8 (0.6–0.9) | |||
| H: 1,330 | 173 | 1.5 (1.2–1.9) | |||
| F: 847 | 110 | 1.4 (1.1–1.7) | |||
| C: 516 | 55 | 0.9 (0.7–1.2) | |||
| Newman (2006) [4] 20 studies (1958–2003) |
N=90,124 | W: 76,111 | Ref | Age, stage, SES | |
| B: 14,013 | 1.2 (1.1–1.3) | ||||
| Goggins (2007) [15] SEER (1991–2004) |
N=348,358 47% I |
W: 310,024 | Ref | Tumor size, grade, histology, ER/PR | |
| B: 34,293 | 1.4 (1.4–1.5) | ||||
| H: 1,873 | 1.1 (1.0–1.3) | ||||
| NA: 1,702 | 1.3 (1.1–1.5) | ||||
| S: 187 | 1.6 (1.2–2.1) | ||||
| Du (2008) [23] SEER-Medicare (1992–1999) |
N=35,029 59% I |
W: 30,484 | Ref | Age, marital status, tumor details, ER/PR, SES, comorbidity, treatment | |
| B: 1,971 | 1.2 (1.0–1.5) | ||||
| Curtis (2008) [22] SEER-Medicare (1994–1999) |
N=41,020 56% 0/I |
W: 35,878 | 4,672 | Ref | Age, region, screening, tumor type, ER, grade, treatment, comorbidity, community, SES |
| B: 2,479 | 477 | 1.1 (1.0–1.2) | |||
| L: 1,172 | 171 | 0.9 (0.8–1.0) | |||
| API: 1,086 | 82 | 0.6 (0.5–0.8) | |||
| Dawood (2008) [16] SEER (1988–2003) |
N=15,438 All stage IV |
W: 11,049 | Ref | Age, grade, ER/PR surgery, marital status, | |
| B: 2,219 | 0.9 (0.7–1.2) | ||||
| Chu (2009) [25] Louisiana State University (1998–2008) |
N=786 35% 0/I Equal access |
W: 318 | 46 | Ref | Age, stage, therapy, grade, income, SES |
| B: 468 | 86 | All deaths 0.9 (0.6–1.3) | |||
| Albain (2009) [30] SWOG Phase III trials |
N=6.676 Equal access |
W: 6,014 | Ref | Age, tumor size, number of lymph nodes | |
| B: 662 | 1.4 (1.1–1.8) | ||||
| Holmes (2010) [17] SEER (1992–1998) |
N=6,951 56% 0/I |
W: 5,981 | 1.5 (1.1–1.9) | Age, marital status, stage, surgery, radiation | |
| B: 506 | 2.6 (1.9–3.6) | ||||
| A: 359 | Ref | ||||
| Gomez (2010) [18] CCR, SEER (1988–2005) |
N=20,747 13% I |
J (US): 2,130 | 239 | Ref | Age, immigrant status, marital status, stage, grade, histology, ER, surgery/treatment, neighborhood SES |
| J (foreign): 1,456 | 159 | 1.1 (0.9–1.4) | |||
| C (US): 1,217 | 114 | 1.5 (1.3–1.7) | |||
| C (foreign): 4,437 | 647 | 1.5 (1.3–1.7) | |||
| F (US): 614 | 62 | 1.2 (0.9–1.5) | |||
| F (foreign): 6,814 | 1076 | 1.5 (1.3–1.7) | |||
| Hill (2010) [19] SEER, Mammography Project (1995–2004) |
N=3,891 49% I |
W: 2,881 | Ref | Age, detection, income, education, rural residence, tumor size, stage, grade | |
| L: 1,010 | 1.2 (1.0–1.5) | ||||
| Komenaka (2010) [24] Wishard Memorial Hospital, IN (1997–2006) |
N=574 53% 0/I Equal access |
W: 259 | 30 | Ref | Age, stage, ER/PR, employment, comorbidity |
| B: 315 | 55 | 1.3 (0.8–2.0) | |||
| Barcenas (2010) [26] Medical College, GA (1990–2005) |
N=1,159 33% 0/I |
W: 670 | 225 | Ref | Age, stage, therapy, surgery |
| B: 489 | 217 | 1.4 (1.1–1.6) | |||
| Ooi (2011) [6] SEER (2000–2006) |
N=229,594 48% I |
W: 176,094 | 10,823 | Ref | Age, SEER registry, stage, ER/PR, treatment, poverty, education |
| B: 20,486 | 2,808 | 1.5 (1.4–1.6) | |||
| L: 14,951 | 1,188 | 1.1 (1.0–1.2) | |||
| J: 2,658 | 105 | 0.8 (0.6–1.0) | |||
| H: 885 | 68 | 1.5 (1.1–2.0) | |||
| NA: 1,004 | 79 | 1.1 (0.8–1.4) | |||
| Maskarinec (2011) [20] HTR (1995–1996) Plus interview |
N=382 65% 0/I Equal access |
J: 137 | 8 | Ref | Age, menopausal status, BMI, stage, ER/PR, treatment, toxicity, comorbidity, health insurance |
| W: 93 | 7 | 0.4 (0.1–1.2) | |||
| H: 49 | 16 | 0.6 (0.2–1.9) | |||
| C: 45 | 20 | 2.3 (0.9–6.0) | |||
| F: 27 | 19 | 1.7 (0.5–5.7) | |||
| Conroy (2011) [21] MEC, CA county cancer surveillance program, CCR, HTR (1993–2007) |
N=3,842 71% Loc |
J: 1,141 | 65 | 0.8 (0.6–1.1) | Age, ER/PR, treatment, comorbidity |
| W: 991 | 93 | Ref | |||
| B: 748 | 115 | 1.4 (1.1–2.0) | |||
| L: 623 | 72 | 1.2 (0.9–1.7) | |||
| H: 339 | 31 | 1.0 (0.7–1.6) |
Abbreviations: SEER=Surveillance Epidemiology End Results, HTR=Hawaii Tumor Registry (part of SEER), IHS=Indian Health Service, CRN=Cancer Research Network, CCR=California Cancer Registry, MEC=Multiethnic Cohort Study, SWOG=South West Oncology Group, SES=Socioeconomic Status, BMI=Body Mass Index
Stages: 0=in situ, Loc=localized, I=stage I; ER/PR=estrogen and progesterone receptor
Ethnic groups: A=Asian , AN=Alaska Native, B=African American, C=Chinese American, F=Filipino, H=Native Hawaiian, J=Japanese American, L=Latino, NA=Native American, PI=Pacific Islander, S=Samoan, W=Caucasian
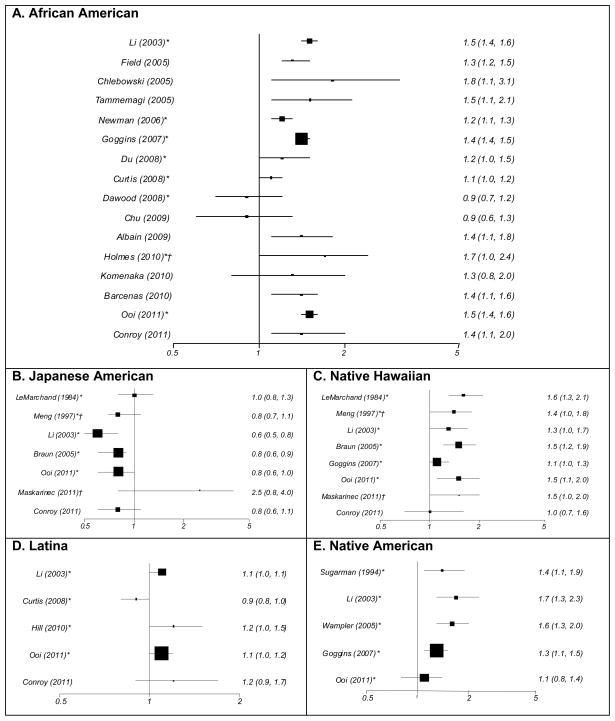
Figure 1.
Summary of breast cancer specific survival by ethnic group^
^Shown are authors, year of publication, and hazard ratios with 95% confidence intervals multivariately adjusted as presented in the original publication (Table 1); the size of symbol reflects the number of study subjects
*Based on SEER data
†Japanese Americans were used as reference group in the original report
RESULTS
African Americans
The literature on ethnic-specific survival differences is most abundant for African Americans. The higher breast cancer mortality rates have been a concern for many years, in particular in light of the incidence rates below those for Caucasians [1,3]. Despite some exceptions, the majority of publications show an elevated risk of dying for African Americans compared to Caucasian breast cancer cases with HRs ranging between 1.2 and 2.6 (Figure 1A). A meta-analysis summarizing 20 studies up to 2005 [4] found a higher risk of breast cancer specific mortality for African American women (HR = 1.3; 95% CI 1.2–1.4). Even after controlling for an area-wide measure of SES the association remained significant; the risk estimate was attenuated to 1.2 (95% CI 1.1–1.3). Two earlier investigations that did not have SES information reported risk estimates of 1.5 [8] and 1.3 [32]; the latter study included women with equal access to health care. Since the meta-analysis, a number of conflicting reports have been published. No ethnic differences in outcome were detected in a SEER-Medicare analysis that controlled for SES and comorbidities [26] and in a population of women with low SES who were treated at a single academic center [29]. In a review of medical records within one medical center, no difference in overall survival was observed after adjustment for sociodemographic factors, but the risk for recurrence was non-significantly elevated among African Americans (HR = 1.3; p = 0.11) [28]. Although a study limited to metastasized breast cancers found no ethnic difference in survival, an interaction term between ethnicity and year of diagnosis was statistically significant, indicating that the gap for African American relative to Caucasian patients, despite being small, increased over time [20]. A range of elevated risks for breast cancer-specific mortality were described in more recent studies ranging from 1.2 [27] and 1.4 [19,25,30] to 1.5 [6,31], and 2.6 [21]. Although a wide range of covariates were included in the models, the predictors varied across reports, e.g., education, SES, and region [6]; obesity, stage, treatment, comorbidities, and hormone receptor status [25]; or access to care [30]. Therefore, the results in Figure 1 reflect the remaining ethnic differences after accounting for different sets of explanatory factors in each study and are difficult to compare. The ideal method to control for differences in treatment is clinical trials; however, an analysis of phase III clinical trials conducted by the Southwest Oncology Group (SWOG) showed that African American ethnicity was associated with a 40% higher mortality in patients with early-stage premenopausal breast cancer and a 50% higher mortality among early-stage postmenopausal breast cancer cases, despite treatment on the same protocols and adjustment for multiple prognostic factors [34]. Many of the comparative reports noted the presence of worse predictors of outcome, such as advanced stage, negative ER/PR status, and triple negative tumors, among African American women [11,25,28,29,32].
Asian Americans
Due the large differences in SES across Asian subgroups, mortality differs considerably by subgroup and also by immigrant status. For example, in a California study, foreign born Chinese and Filipino women had poorer outcomes than those born in the US [22]. For women of Japanese and Chinese ancestry, generally more favorable outcomes have been reported than for Caucasians, whereas Filipina, Korean, South Asian, and Vietnamese immigrants tend to experience poorer outcomes [8,16,22]. When risk estimates for Asians as a group were presented, outcomes were better than for Caucasians [21,26]. Given the limited data on most Asian subgroups, we only summarize studies with Japanese Americans in Figure 1B. Whereas the risk of dying from breast cancer was similar as for Caucasians in four investigations [5,16,24,25], two reports described survival advantages in the range of 20% [18] to 40% [8] without adjusting for BMI or other lifestyle factors.
Native Hawaiians/Pacific Islanders
Similar to African Americans, health disparities in Native Hawaiians and other Pacific Islanders due to low SES and lack of access to care have been an area of great concern and active research [35]. Because only one report presented separate results for Samoans [19], all investigations shown in Figure 1C refer to Native Hawaiians. Out of eight studies, all but three [19,24,25] observed statistically significant poorer outcomes. The risk estimates ranged from 1.3 [8] to 1.5 [6,18] and 1.6 [5] when compared to Caucasians; in comparison to Japanese the HR was 1.7 in one report [16]. The higher risk estimates tended to be in SEER-based analyses without additional information on treatment and comorbidity [6,8,18], whereas studies performed in recent years and controlling for more detailed information on lifestyle factors tended to observe less disparity in survival [24,25]. A study linking insurance claims with Hawaii SEER data, thus controlling for comorbidities and adequacy of treatment, also showed little ethnic differences [36]. This indicates that obesity and comorbidities may explain part of the poorer survival observed among Native Hawaiians who suffer from high rates of obesity, hypertension, and diabetes [37,38]. The poor survival of Samoan women as reported by a SEER-based investigation was most likely due to late diagnosis and lack of access to appropriate screening and treatment [6].
Latinas
Of the five studies that included Latinas (Figure 1D), three described poorer breast cancer survival outcomes for Latina women who had immigrated from different countries [6,8,23] and two observed no differences [25,26]. However, in comparison with the risk estimates for African Americans, the HRs for Latinas were relatively small. In a SEER-based report, the risk of dying from breast cancer was 10% higher for Latinas than Caucasians while controlling for stage, age, treatment, ER/PR status, and access to health care [8]. Later research by Ooi [6] and Hill [23], also based on SEER data for Latinas, found an excess risk of 10% in one study after controlling for stage, age, income, education, residence, ER/PR status, and treatment [6]; and 23% in the other study after adjustment for method of detection [23]. Two later studies that detected no ethnic differences controlled for additional variables, i.e., equal insurance status (Medicare), detection method, comorbidities, SES, surrounding community, and region [26] as well as BMI [25].
Native Americans
We identified five studies (Figure 1E) among Native American women. A 1974–1989 investigation from Washington state [7] reported poorer breast cancer survival among Native Americans than Caucasians in the same area (HR=1.4) after controlling for age, stage, residence, and treatment. Three later reports also showed 30–70% higher risks of dying from breast cancer for Native American women [8,17,19] after adjustment for different predictors. The most recent study that controlled for stage, age, ER/PR status, and treatment in addition to access to health care, poverty, education, and geographic location detected no statistical difference between Native Americans and Caucasians [6].
DISCUSSION
While there is little doubt that mortality from breast cancer has decreased for all ethnic groups during the last 20 years [1,2], it is clear that survival differences between ethnic groups have not been eradicated. Based on many reports confirming disproportionate numbers of late stage disease, lack of screening and early detection due to limited access to health care remain important contributors to this situation [9,23]. Nevertheless, the role of lifestyle factors, such as obesity and physical activity, has become better understood and gained more attention [39]. The differences and time trends across populations are not easily disentangled since the studies do not control for a common set of covariates known to influence survival. As described in an excellent review [10], many of these factors are related to SES and affect prognosis in combination and through multiple pathways. For example, poverty may directly be responsible for lack of screening but also indirectly affect tumor biology because obesity, smoking, and poor nutrition may promote the development of tumors with adverse characteristics. We will consider the major factors that have been identified as contributors to the observed differences and explain the inconsistencies in the reports discussed above.
Early Detection and Access to Care
Given the importance of stage at diagnosis for prognosis [1], screening participation and early detection are the most important predictors of survival. There are many examples of this in the literature, such as the analysis of 229,594 cases from the SEER registries that shows respective rates of 50% and 57% stage I cases among Caucasians and Japanese, compared to 35%, 38%, 42%, and 45% in African Americans, Latinas, Pacific Islanders, and Native Americans [6]. In a study among Latinas, the screen-detected proportion of cancers was only 52% compared to 61% in Caucasians, and adjustment for type of detection reduced the ethnic difference [23]. As described in a large trend analysis for 1987–2005 [2], absolute ethnic disparities declined for mammography screening, stage at diagnosis, and 5-year cause-specific probability of death during this time period; however, relative ethnic disparities in 5-year cause-specific probability of death persisted.
Lack of early detection is further complicated by delay in treatment initiation [9]. Another important issue is compliance with consensus recommendations to treat breast cancer, which have been associated with improved survival [40]. Many reports suggest less surgery, radiation therapy, and hormone treatment and 20–50% rates of inappropriate treatment among African Americans as compared to Caucasians [8,32]. When detailed information from medical charts was used to assess breast cancer treatment [41] or comorbidity and treatment patterns from insurance claims were included as covariates [36], survival times were relatively similar across ethnic groups in Hawaii with a rather unique health care environment. Because cancer registries do not typically collect information on treatment beyond 6 months, many of the published reports are missing detailed treatment information after initial treatment. However, access to care and health insurance rates [42] are probably not the only reason for these delays; geographic distance from good treatment centers and cultural factors probably also contribute as suggested by the disparate outcome in the SWOG trials [34].
Tumor Type
Advances in molecular classification and characterization of breast tumors into distinct subtypes by using DNA microarrays independent of disease stage has allowed stratification of cases by prognosis [43,44]. Whereas the respective proportions for the major subtypes appear to be similar in Japanese and Chinese Americans as in Caucasians [45], the distribution in African Americans is skewed toward the subtypes with poorer outcomes [46,47]. African American women are more likely to be diagnosed with higher tumor grades as well as ER/PR negative and triple negative tumors than Caucasians, partly as a result of later stage at diagnosis [9,11,33,48]. The same observation was made for Latina women, although to a lesser degree [6]. Interestingly, a study among a socioeconomically deprived population with high obesity rates showed more tumors with poor prognostic features, i.e., late stage, triple negative, and lymph node metastases [49]. This suggests that a low SES regardless of ethnic background is associated with breast cancer tumors that have an unfavorable prognosis [10].
Comorbidity
Closely related to the question of appropriate treatment is the issue of pre-existing conditions. Given the high rates of obesity, diabetes, and hypertension among African American and Hispanic [42] as well as Pacific Islander women [37,38], the presence of these conditions may be responsible for worse breast cancer outcomes, possibly due to less tolerance to cancer treatments. Women with comorbidities may receive less aggressive treatment, as described for diabetic women, due to disease-related complications [50]. The association of comorbidity with lower survival rates among breast cancer patients has been shown repeatedly [36,51,52]; however, comorbidity contributes more to higher overall mortality in breast cancer patients than to breast cancer specific mortality [31]. On the other hand, controlling for comorbidity does not eliminate all ethnic differences as can be seen in several studies within the meta-analysis [4].
Socioeconomic Status
As for many conditions, outcomes tend to be worse for individuals with lower education and income than for those with higher SES [53]. In a recent report on health disparities [42], the persisting ethnic differences in education, health insurance coverage, and poverty status are well documented. Although many survival analyses included SES indicators, the registry-based studies usually only had neighborhood-level SES indicators with well documented limitations available since more accurate individual-level SES data are difficult to collect [54]. Nevertheless, in the meta-analysis of studies among African American women, the area-wide measure of SES narrowed the survival difference considerably [4]. Interestingly, it appears from a limited number of studies within Caucasian populations with low SES that poverty is associated with similar cancer disparities as observed across ethnic groups [2,10,49]. Adjustment for SES probably remains insufficient because a person’s economic and social situation is more complex than just assessing income and education; housing, environmental, dietary, cultural, behavioral, and access to health care issues are additional contributors to health effects and are not easily captured by traditional demographic data [4,53,55]. Since SES is not a biologic risk factor itself and rather a marker for other factors, such as access to screening and health care, it is also challenging to interpret studies that controlled for SES and true mediating variables, such as obesity, at the same time.
Obesity and Physical Activity
The role of obesity at diagnosis and later has become much clearer over recent years and is important in light of the higher obesity rates in non-Caucasian women [37,42]. There is increasing evidence that obese women with breast cancer experience worse breast cancer survival, as much as 30% higher mortality, than survivors with normal weight [39,56]. This difference appears to be due to estradiol formation in adipose tissue, which stimulates neoplastic cell proliferation in obese women [57] contributing to more biologically aggressive tumors [58]. Estrogen-independent pathways, in particular adipokine production, e.g., adiponectin and leptin, may also contribute to an aggressive breast cancer phenotype [59,60]. Furthermore, obese women may be given lower doses of chemotherapy because the ideal body surface area rather than true body surface area is used to estimate the dose of chemotherapy [61]. Epidemiologic data for other modifiable factors such as physical activity and healthful diets are not as convincing [39], but a growing number of large observational studies have demonstrated that participation in moderate intensity recreational physical activity after diagnosis may improve survival in women with breast cancer [62,63].
Methodological Issues
In the studies included in this review (Table 1), variations in study populations and research methodology appear to be major contributors to the conflicting findings. In general, it appears that smaller ethnic differences in survival are observed when more predictors were included [24] or when patients come from a similar background and are treated in the same institution [28,29]. However, each study controlled for a different combination of covariates, which makes it difficult to determine which factors explain the ethnic disparity across populations. Sample sizes and especially the number of cause-specific deaths were very small in several investigations. Although the studies were based on different populations across the country, the majority relied on data collected by SEER registries. While the SEER data provide information for a large number of cases and follow a standardized protocol for data collection, they lack information on personal and lifestyle factors, such as BMI, SES, access to healthcare, and comorbidities. Another limitation is that the majority of studies with Asians and Pacific Islanders relied on similar datasets or geographic locations because the number of breast cancer patients with Asian and Pacific Islander ancestry in the U.S. is limited. Thus, the multiple SEER analyses are not independent from each other. For example, some of the cases in smaller SEER studies may also be included in the larger or national SEER studies and other reports are updates of earlier investigations with partial overlap. An ideal study would present the association between ethnicity and survival among women with breast cancer in age-adjusted models and compare this to models that control for other predictors of breast cancer survival separately and in combination. These predictors should include stage at diagnosis, hormone receptor status, tumor characteristics, treatment received, comorbidities, insurance status, SES, obesity, and preferably also smoking, physical activity, and other lifestyle factors. This would answer the question what proportion of ethnic differences in survival are explained by individual factors and their combinations and would suggest the most likely interventions to reduce the disparity in breast cancer survival.
CONCLUSIONS
From the existing evidence, it appears that African American women continue to experience the poorest breast cancer specific survival of all ethnic groups in the US. The prognosis for Latinas, Native Hawaiians, and Native Americans is intermediate, better than for African Americans but not as good as for Caucasians and Japanese. One possible explanation of this evidence is that genetic susceptibility or lifestyle factors predispose some ethnic groups to tumors with more adverse behavior [12–15]. Future genetic investigations may also detect ethnic related genetic polymorphisms of chemotherapy metabolizing enzymes [64]. However, an alternative interpretation is that, regardless of ethnic background, women with low SES and unhealthy lifestyles are more likely to experience late stage disease and to develop more aggressive tumors than more affluent women. This hypothesis is supported by the following pieces of evidence. One, research among Caucasian women with low SES indicates similar tumor types and poor survival as observed in African American populations [49]. Second, improvements in outcomes have occurred over time across ethnic groups [2]. Finally, inclusion of more treatment, comorbidity, SES, and lifestyle information in the statistical analysis appears to diminish the observed ethnic differences (Figure 1).
FUTURE PERSPECTIVE
For the future, Newman et al. [4] proposed two research areas that need to be strengthened in addition to improved early detection and access to care: Methods to measure effects of sociobehavioral issues and poverty, e.g., environmental, economic, cultural, and lifestyle factors, on breast cancer risk and an exploration of associations between ethnicity and variation in primary breast tumor biology. As to efforts in reducing the unequal mortality to breast cancer, improvements in SES, health insurance, and access to care will still achieve great improvements although we do not know at this time whether a part of the observed ethnic differences is due to genetic factors that cannot be modified.
EXECUTIVE SUMMARY.
Background
Although breast cancer survival has improved for all ethnic groups over the last 20 years, poor survival rates in some ethnic groups remain an important concern.
Results
In comparison to other ethnic groups in the US, African Americans have worse breast cancer specific survival rates.
Breast cancer related mortality among Hispanic, Native Hawaiian, and Native American women is intermediate between African Americans and Caucasians, whereas Japanese and some other Asian groups show better survival rates than Caucasians.
Adjustment for prognostic predictors reduces ethnic differences in breast cancer survival.
Lack of early detection, late stage at diagnosis, and limited access to health care are the most important predictors of poor breast cancer survival.
Low socioeconomic status appears to be associated with tumors showing unfavorable characteristics and higher breast cancer related mortality independent of ethnic background.
Comorbidities, obesity, and lifestyle factors have emerged as additional significant determinants of prognosis.
Future perspectives
Improvements in early detection of breast cancer and access to health care will lead to further reductions in breast cancer related mortality.
Better methods of measuring the effects of sociobehavioral factors and poverty on tumor biology are needed to understand these associations.
Explorations of tumor biology and genetic susceptibility may be able to identify additional determinants of ethnic differences.
Acknowledgments
One of the authors (SMC) was supported by a postdoctoral fellowship on grant R25 CA 90956.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
REFERENCES (*of interest)
- 1*.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD, USA: 2011. Provides recent breast cancer incidence, mortality, and survival data for cancer registries in the US. [Google Scholar]
- 2.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005) Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–131. doi: 10.1158/1055-9965.EPI-08-0679. [DOI] [PubMed] [Google Scholar]
- 3.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56(3):168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 4*.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342–1349. doi: 10.1200/JCO.2005.03.3472. Large analyses of 20 different studies that included African American women and adjusted for SES. [DOI] [PubMed] [Google Scholar]
- 5.LeMarchand L, Kolonel LN, Nomura AM. Relationship of ethnicity and other prognostic factors to breast cancer survival patterns in Hawaii. J Natl Cancer Inst. 1984;73(6):1259–1265. [PubMed] [Google Scholar]
- 6.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127(3):729–738. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugarman JR, Dennis LK, White E. Cancer survival among American Indians in western Washington State (United States) Cancer Causes Control. 1994;5(5):440–448. doi: 10.1007/BF01694758. [DOI] [PubMed] [Google Scholar]
- 8.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 10*.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 2009;18(6):883–893. doi: 10.1089/jwh.2008.1127. Presents an insightful analysis how poverty affects the biology and the prognosis of breast tumors. [DOI] [PubMed] [Google Scholar]
- 11.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham JE, Butler WM. Racial disparities in female breast cancer in South Carolina: clinical evidence for a biological basis. Breast Cancer Res Treat. 2004;88(2):161–176. doi: 10.1007/s10549-004-0592-9. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14(8):761–766. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 14.Mountain JL, Risch N. Assessing genetic contributions to phenotypic differences among 'racial' and 'ethnic' groups. Nat Genet. 2004;36(11 Suppl):S48–S53. doi: 10.1038/ng1456. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JE, Montero AJ, Garrett-Mayer E, Berkel HJ, Ely B. Racial differences in the incidence of breast cancer subtypes defined by combined histologic grade and hormone receptor status. Cancer Causes Control. 2010;21(3):399–409. doi: 10.1007/s10552-009-9472-2. [DOI] [PubMed] [Google Scholar]
- 16.Meng L, Maskarinec G, Wilkens L. Ethnic differences and factors related to breast cancer survival in Hawaii. Int J Epidemiol. 1997;26(6):1151–1158. doi: 10.1093/ije/26.6.1151. [DOI] [PubMed] [Google Scholar]
- 17.Wampler NS, Lash TL, Silliman RA, Heeren TC. Breast cancer survival of American Indian/Alaska Native women, 1973–1996. Soz Praventivmed. 2005;50(4):230–237. doi: 10.1007/s00038-004-4020-6. [DOI] [PubMed] [Google Scholar]
- 18.Braun KL, Fong M, Gotay C, Pagano IS, Chong C. Ethnicity and breast cancer in Hawaii: increased survival but continued disparity. Ethn Dis. 2005;15(3):453–460. [PubMed] [Google Scholar]
- 19.Goggins WB, Wong GK. Poor survival for US Pacific Islander cancer patients: evidence from the Surveillance, Epidemiology, and End Results database: 1991 to 2004. J Clin Oncol. 2007;25(36):5738–5741. doi: 10.1200/JCO.2007.13.8271. [DOI] [PubMed] [Google Scholar]
- 20.Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26(30):4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes L., Jr Opara F, Hossain J A five-year breast cancer-specific survival disadvantage of African American women. Afr J Reprod Health. 2010;14(3):195–200. [PubMed] [Google Scholar]
- 22.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. 2010;100(5):861–869. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill DA, Nibbe A, Royce ME, et al. Method of detection and breast cancer survival disparities in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2453–2460. doi: 10.1158/1055-9965.EPI-10-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maskarinec G, Pagano I, Lurie G, Bantum E, Gotay CC, Issell BF. Factors affecting survival among women with breast cancer in Hawaii. J Womens Health (Larchmt) 2011;20(2):231–237. doi: 10.1089/jwh.2010.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112(1):171–180. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du XL, Fang S, Meyer TE. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol. 2008;31(2):125–132. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- 28.Komenaka IK, Martinez ME, Pennington RE, Jr, et al. Race and ethnicity and breast cancer outcomes in an underinsured population. J Natl Cancer Inst. 2010;102(15):1178–1187. doi: 10.1093/jnci/djq215. [DOI] [PubMed] [Google Scholar]
- 29.Chu QD, Smith MH, Williams M, et al. Race/Ethnicity has no effect on outcome for breast cancer patients treated at an academic center with a public hospital. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2157–2161. doi: 10.1158/1055-9965.EPI-09-0232. [DOI] [PubMed] [Google Scholar]
- 30.Barcenas CH, Wells J, Chong D, French J, Looney SW, Samuel TA. Race as an independent risk factor for breast cancer survival: breast cancer outcomes from the medical college of georgia tumor registry. Clin Breast Cancer. 2010;10(1):59–63. doi: 10.3816/CBC.2010.n.008. [DOI] [PubMed] [Google Scholar]
- 31.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 32.Field TS, Buist DS, Doubeni C, et al. Disparities and survival among breast cancer patients. J Natl Cancer Inst Monogr. 2005;(35):88–95. doi: 10.1093/jncimonographs/lgi044. [DOI] [PubMed] [Google Scholar]
- 33.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 34*.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992. doi: 10.1093/jnci/djp175. Survival analysis within a large clinical trial that treated all women on the same protocol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaisdell RK. The health status of the Kanaka Maoli (Indigenous Hawaiians) Asian, American, and Pacific Islander Journal of Health. 1993;1:116–160. [PubMed] [Google Scholar]
- 36.Maskarinec G, Pagano I, Yamashiro G, Issell BF. Influences of Ethnicity, Treatment, and Comorbidity on Breast Cancer Survival. J Clin Epidemiol. 2003;56(7):678–685. doi: 10.1016/s0895-4356(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 37.Davis J, Busch J, Hammatt Z, et al. The relationship between ethnicity and obesity in Asian and Pacific Islander populations: a literature review. Ethn Dis. 2004;14(1):111–118. [PubMed] [Google Scholar]
- 38.Salvail F, Nguyen H, Liang S. 2010 State of Hawaii Behavioral Risk Factor Surveillance System. Hawaii Department of Health; Honolulu, HI, USA: 2011. [Google Scholar]
- 39*.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. Summarizes studies that examined the effect of obesity and physical activity on breast cancer survival. [DOI] [PubMed] [Google Scholar]
- 40.Hebert-Croteau N, Brisson J, Latreille J, Rivard M, Abdelaziz N, Martin G. Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. J Clin Oncol. 2004;22(18):3685–3693. doi: 10.1200/JCO.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Issell BF, Maskarinec G, Pagano I, Gotay CC. Breast cancer treatment among women of different ethnicity in Hawaii. Cancer Invest. 2005;23(6):497–504. doi: 10.1080/07357900500201442. [DOI] [PubMed] [Google Scholar]
- 42.Frieden TR. Forward: CDC Health Disparities and Inequalities Report - United States, 2011. MMWR Surveill Summ. 2011;60 (Suppl):1–2. [PubMed] [Google Scholar]
- 43.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorlie T. Molecular classification of breast tumors: toward improved diagnostics and treatments. Methods Mol Biol. 2007;360:91–114. doi: 10.1385/1-59745-165-7:91. [DOI] [PubMed] [Google Scholar]
- 45.Telli ML, Chang ET, Kurian AW, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127(2):471–478. doi: 10.1007/s10549-010-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 47.Ihemelandu CU, Naab TJ, Mezghebe HM, et al. Treatment and survival outcome for molecular breast cancer subtypes in black women. Ann Surg. 2008;247(3):463–469. doi: 10.1097/SLA.0b013e31815d744a. [DOI] [PubMed] [Google Scholar]
- 48.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 49*.Vona-Davis L, Rose DP, Hazard H, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3319–3324. doi: 10.1158/1055-9965.EPI-08-0544. Shows that Caucasian women with low socoioconomic status experience poor breast cancer survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27(13):2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 52.West DW, Satariano WA, Ragland DR, Hiatt RA. Comorbidity and breast cancer survival: a comparison between black and white women. Ann Epidemiol. 1996;6(5):413–419. doi: 10.1016/s1047-2797(96)00096-8. [DOI] [PubMed] [Google Scholar]
- 53.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don't. Ann NY Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 54.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annual Reviews of Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 56.Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev. 2006;7(4):333–340. doi: 10.1111/j.1467-789X.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 57.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 58.Lonning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol. 2009;117(1–3):31–41. doi: 10.1016/j.jsbmb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Rose DP, Gilhooly EM, Nixon DW. Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review) Int J Oncol. 2002;21(6):1285–1292. [PubMed] [Google Scholar]
- 60.Brakenhielm E, Veitonmaki N, Cao R, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101(8):2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165(11):1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 62.Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic review. Physiother Can. 2010;62(1):25–34. doi: 10.3138/physio.62.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmichael AR, Daley AJ, Rea DW, Bowden SJ. Physical activity and breast cancer outcome: a brief review of evidence, current practice and future direction. Eur J Surg Oncol. 2010;36(12):1139–1148. doi: 10.1016/j.ejso.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Neber DW, Roe AL. Ethnic and genetic differences in metabolism genes and risk of toxicity and cancer. Sci Total Environ. 2001;274(1–3):93–102. doi: 10.1016/s0048-9697(01)00732-x. [DOI] [PubMed] [Google Scholar]