Abstract
Alveolar macrophages are immunoregulatory effector cells that interact directly with respiratory virus pathogens in vivo. We examined the role of alveolar macrophages in acute infection with pneumonia virus of mice (PVM), a rodent pneumovirus that replicates the clinical sequelae of severe human respiratory syncytial virus disease. We show that PVM replicates in primary mouse macrophage culture, releasing infectious virions and proinflammatory cytokines. Alveolar macrophages isolated from PVM-infected mice express activation markers Clec43 and CD86, cytokines TNFα, IL-1, IL-6, and numerous CC and CXC chemokines. Alveolar macrophage depletion prior to PVM infection results in small but statistically significant increases in virus recovery but paradoxically prolonged survival. In parallel, macrophage depleted PVM-infected mice exhibit enhanced NK cell recruitment and increased production of IFNγ by NK, CD4+ and CD8+ T cells. These results suggest a protective, immunomodulatory role for IFNγ, as overproduction secondary to macrophage depletion may promote survival despite increased virus recovery.
Keywords: inflammation, cytokines, respiratory virus, infection
Introduction
Respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory tract infection in children, and is increasingly recognized as a significant pathogen among the elderly. Although most cases of RSV infection are mild to moderate, severe bronchiolitis, typically in premature infants, in immunocompromised hosts, and even in those with no known risk factors, involves substantial inflammation, with mucus secretion and sloughing of the bronchiolar epithelium, ultimately leading to respiratory compromise (Hall et al., 2009; van Bleek et al., 2011; Krilov, 2011).
Alveolar macrophages are specialized cells of the respiratory tract, and the predominant leukocyte population at homeostasis; they express unique cell surface antigens, pattern-recognition receptors and immunomodulatory mediators, and function to promote immune surveillance against microbial invasion (Gordon & Read, 2002; Gordon & Taylor, 2004). Although virus antigens are detectable in alveolar macrophages isolated from RSV-infected human subjects (Midulla et al., 1993) and virions are capable of inducing the synthesis and release of proinflammatory mediators by both human and mouse macrophages in culture (Tsutsumi et al., 1996; Matsuda et al. 1996; Franke et al., 1994; Franke-Ullmann, et al. 1995; Stadnyck et al., 1997) the precise contributions of alveolar macrophages to the pathogenesis of RSV disease remains uncertain. For example, in a study performed by Pribul and colleagues (2008), RSV-challenged macrophages in BALB/c mice contributed to the early, immediate release of pro-inflammatory cytokines, although macrophage depletion had no impact on RSV-mediated T cell recruitment, weight loss, or lung function. In contrast, Reed and colleagues (2008) found that macrophage depletion prior to RSV challenge resulted in prominent airway occlusion in association with ongoing disease. Of note, although mouse challenge models have been employed extensively to study RSV disease, standard experimental RSV virus strains replicate minimally in commonly-available wild-type experimental mice and ordinarily elicit a limited spectrum of clinical symptoms (Durbin & Durbin, 2004; Bem et al., 2011).
Pneumonia virus of mice (PVM, strain J3666) is a highly virulent natural rodent pathogen of the same family (Paramyxoviridae) and genus (Pneumovirus) as RSV. First characterized by Horsfall and Curnen (1946), mice that are inoculated with fewer than 0.1 TCID50 units (Percopo et al., 2011) respond with robust replication in bronchiolar epithelial cells, and develop severe acute inflammation, which ultimately progresses to respiratory failure and death, with histopathologic findings similar to that of severe RSV infection in human infants (reviewed in Easton et al., 2006; Rosenberg & Domachowske, 2008).
Here we examine PVM infection in primary macrophage culture and we explore the impact of macrophage ablation on the pathogenesis of and survival in response to acute pneumovirus infection in vivo.
Results and Discussion
PVM replicates in mouse macrophages and elicits production of infectious virions and release of proinflammatory cytokines
We previously documented the replication of PVM and production of proinflammatory cytokines in a mouse macrophage cell line (RAW 264.7; Dyer et al. 2007). Here we examined PVM replication in primary cultures of mouse peritoneal macrophages. Similar to findings with RAW 264.7 cells, virus recovery from infected mouse macrophages increased over time [Figs. 1A and 1B]. To assess the production and release of infectious virions, supernatants from PVM-infected mouse macrophage cultures were harvested and used to inoculate RAW 264.7 reporter cultures as previously described (Dyer et al. 2009). Using this method, we detected infectious virions in culture supernatants from PVM-infected peritoneal macrophage cultures on days 5 and 7 after inoculation [Fig. 1C]. Interestingly, little to no RSV replication was reported in primary mouse macrophage culture (Franke et al., 1994; Franke-Ullmann et al. 1995; Stadnyck et al., 1997), although Hegele and colleagues (1998) identified a minor population of high-density guinea pig alveolar macrophages that supported virion assembly. We also determined that PVM infection induced the expression of transcripts encoding proinflammatory mediators CCL2 and CCL3 in peritoneal macrophage cultures [Fig. 2A]; immunoreactive CCL2 and CCL3 were detected in all culture supernatants, but significant expression over background (ie., no virus, heat-inactivated virus inoculations) was detected in response to PVM infection [Fig. 2B]. CCL2 and CCL3 contribute significantly to the pathogenesis of acute PVM infection in mice (Domachowske et al., 2000; Bonville et al. 2006); we have shown previously that blockade of CCL3 signaling results in dramatic improvements in survival (Bonville et al., 2003; Bonville et al., 2004). Likewise, CCL2 and CCL3 are detected prominently in the airway washings of infants with severe RSV disease (Harrison et al., 1999; Garofalo et al., 2001; McNamara et al., 2005); Garofalo and colleagues (2001) have reported an inverse correlation between levels of CCL2 and CCL3 in the airways and oxygen saturation.
Figure 1. PVM replicates in primary mouse macrophages.
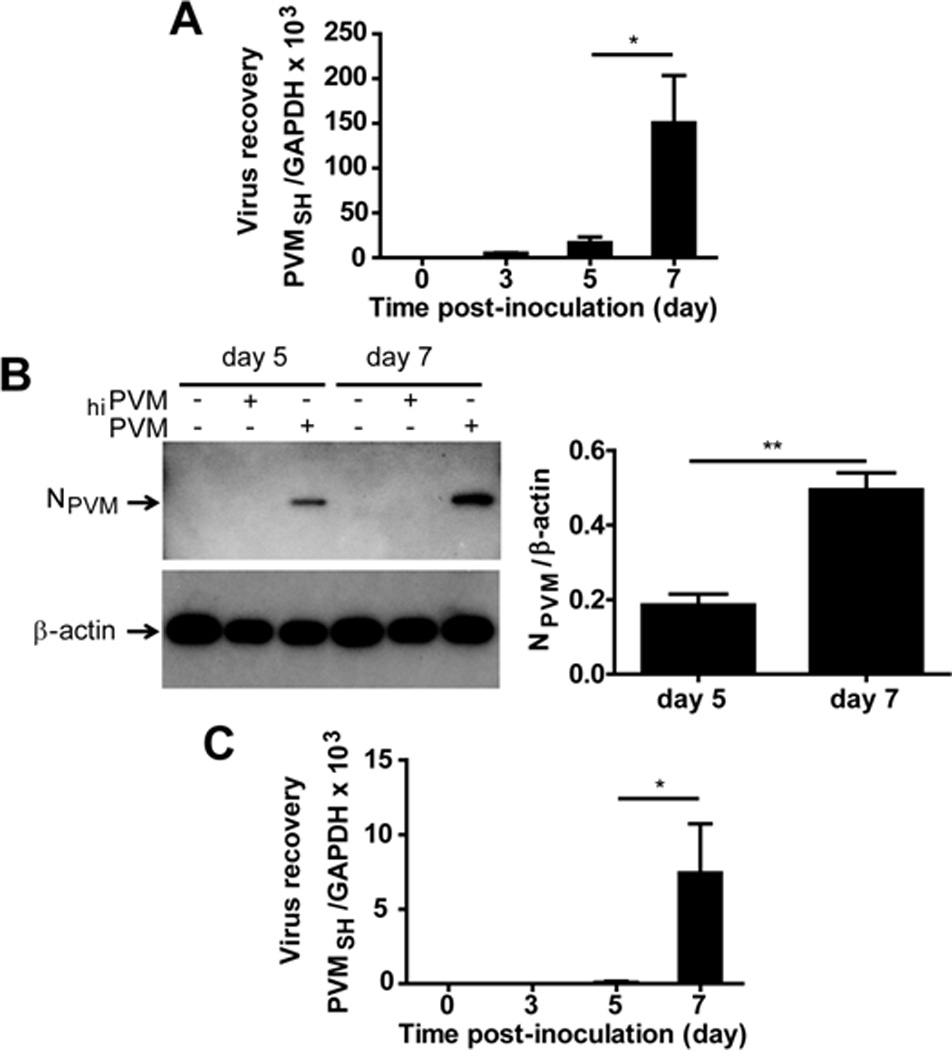
(A) Macrophage cultures were inoculated with PVM at day 0; virus recovery was determined by qRT-PCR as described in Gabryszewski et al. (2011). (B) Total protein extracts from naïve macrophage cultures, and cultures inoculated with heat-inactivated (hi) or replication-competent PVM probed with rabbit polyclonal anti-PVM N protein antibody (NPVM); anti–mouse β-actin antibody was used as a control for protein loading; three immunoblots from PVM-infected cultures shown in (B) were evaluated quantitatively by densitometry. (C) Supernatants from PVM-infected peritoneal macrophage cultures sampled at time points indicated were used to inoculate RAW 264.7 reporter cultures as described in Dyer et al. (2009); virus copy number in the reporter cells was assessed by qRT-PCR at 7 days after inoculation. All data are representative of at least 2 independent experiments performed in quadruplicate; *p < 0.05, ** p < 0.01.
Figure 2. PVM-infection of mouse macrophages elicits production of proinflammatory cytokines.
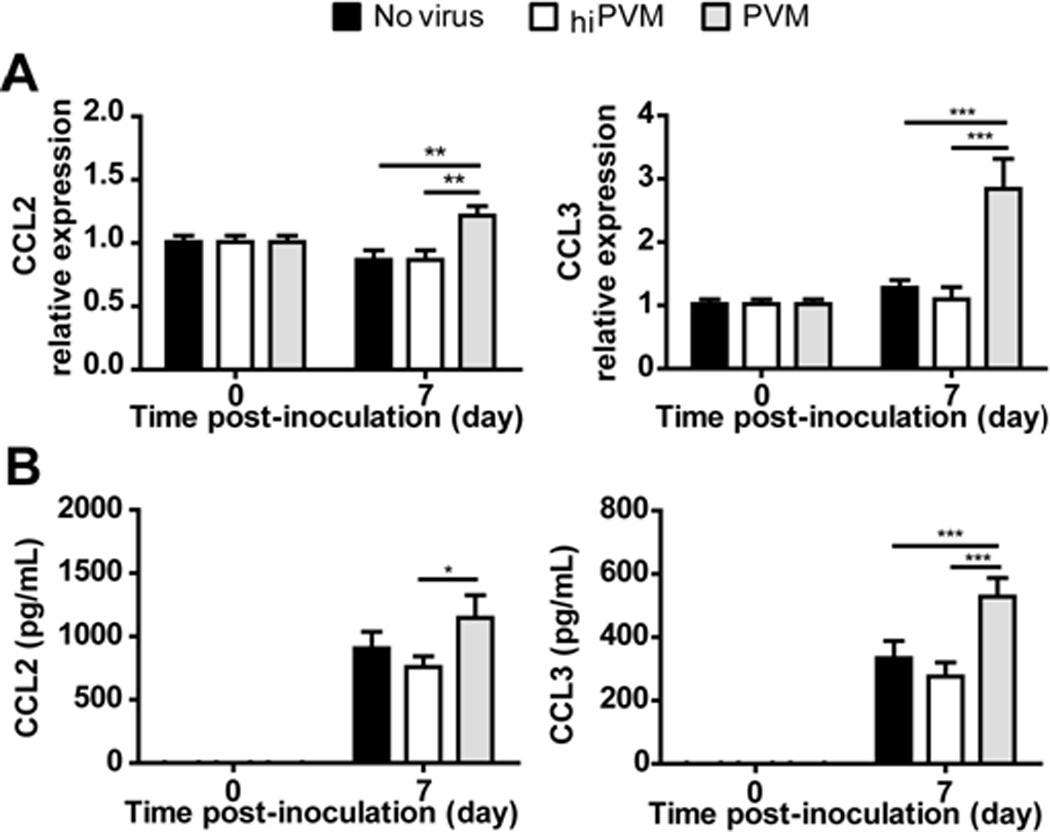
(A) Relative expression of transcripts encoding CCL2 and CCL3 in response to inoculation with PVM (gray bars) or heat-inactivated (hi) PVM (white bars) normalized to expression in naive cells (no virus control; black bars). (B) Detection of immunoreactive CCL2 and CCL3 in supernatants of macrophage cultures described in (A). All data shown are compiled from two separate experiments run independently in quadruplicate; *p < .05, ** p < .01, *** p < .001.
Alveolar macrophages from PVM-infected mice express activation markers and proinflammatory cytokines
Alveolar macrophages display a characteristic antigen profile (F4/80+CD204+CD11chighCD11blow) that has been used successfully in purification strategies (Bedoret et al., 2009; Gough and Gordon, 2000; GeurtsvanKessel et al. 2008) We show here that this approach can also be applied to the isolation of alveolar macrophages from lungs of virusinfected mice, although PVM infection alters the profile of co-purifying leukocytes. Specifically, after anti-CD11c magnetic bead selection, the target CD204+CD11chighCD11blow cells represented 76% of the lung cells from uninfected mice [Fig. 3A], but only 31% of the cells from the infected mice [Fig. 3B]; among the CD11c+ cells from the PVM infected mice were a prominent, heterogeneous population of CD204+ CD11clow CD11bhigh cells, likely dendritic cells and monocytes recruited in response to virus infection. However, the final sorting procedure resulted in 99.3% and 98.5% pure alveolar macrophages from lung cells of uninfected and infected mice, respectively [Fig. 3C and Fig. 3D]; this finding was confirmed by visual inspection [Fig. 3E and Fig. 3F]. RNA was extracted from alveolar macrophages isolated from uninfected and infected mice, and differential expression of activation markers and proinflammatory mediators was assessed. Among the most prominent of these findings, we report 25-fold increased expression of the C-type lectin Clec4e, which is a signature gene of activated macrophages [Fig. 3G]. Clec4e, also known as Mincle (macrophage-inducible C-type lectin) is a type II membrane protein that detects and interacts with a wide variety of carbohydrate-bearing ligands (damaged cells, fungi, mycobacteria), and elicits production of proinflammatory cytokines (Miyake et al., 2010). To the best of our knowledge, this is the first report regarding expression of this molecule in association with pneumovirus infection. Transcripts encoding CD86 were also upregulated, likewise indicative of an activated phenotype. Among the proinflammatory cytokines, we detect augmented expression of transcripts encoding TNF-α, IL-1 and IL-6, and, consistent with our observations from PVM-infected macrophages in culture, we observe prominent expression of Ccl2 and Ccl3 [Fig. 3H]. Expression of Cxcl10 increased dramatically, more than 500 fold; other chemokine genes differentially expressed in alveolar macrophages from PVM-infected mice include Ccl5, Ccl17, Cxcl1, Cxcl2 and Cxcl3. Other transcripts expressed alveolar macrophages from PVM-infected mice include IFN-β1 and CXCL11 (data not shown).
Figure 3. Alveolar macrophages isolated from PVM-infected mice express proinflammatory cytokines.
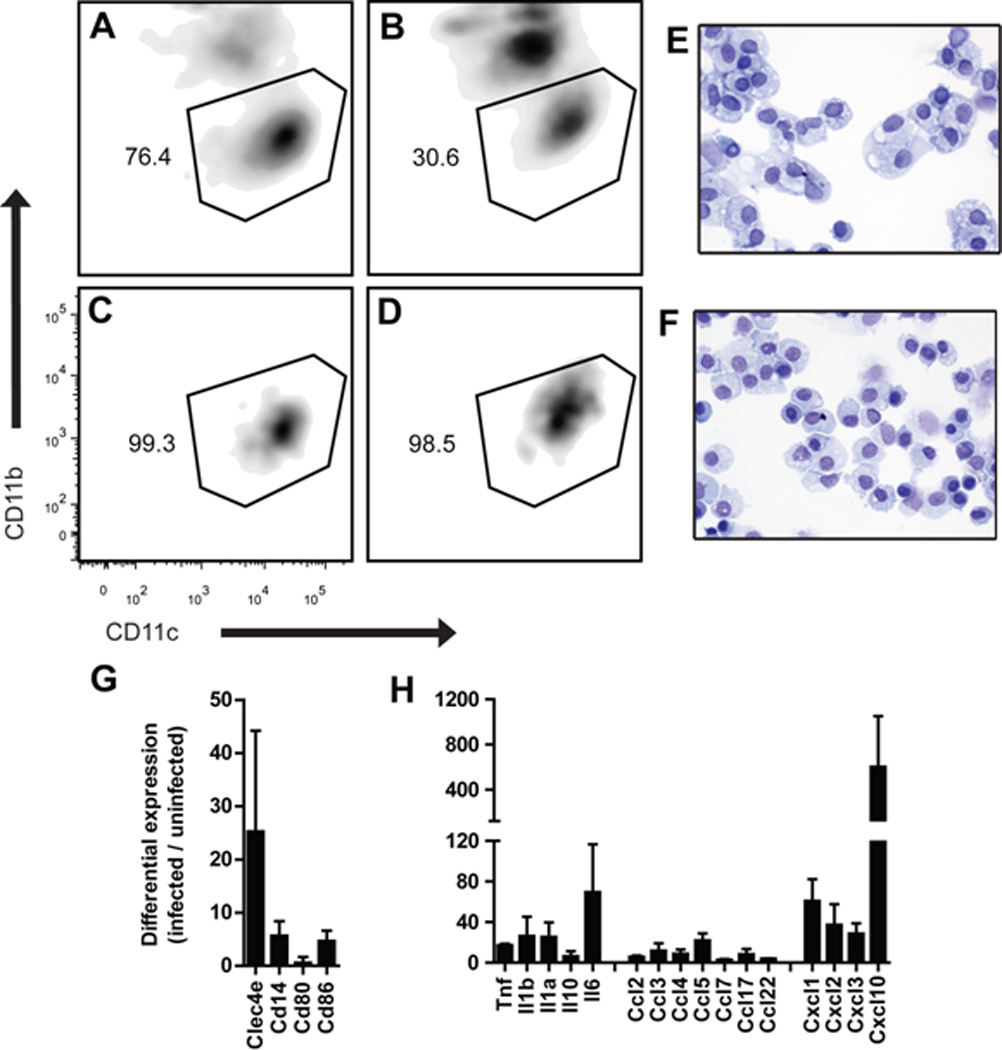
Alveolar macrophages (CD204+ CD11blow CD11chigh) were isolated from naïve and PVM-infected mice by positive selection with anti-CD11c-coupled magnetic beads followed by flow cytometric sorting. (A, B) Profiles of magnetic-bead isolated, CD204+ cells from uninfected and PVM-infected mice, respectively. (C, D) Profiles of pure, sorted alveolar macrophages from A and B, respectively. (E, F) Cytospin preparations of cells in C and D, respectively, stained with modified Giemsa. Flow cytometric profiles and Cytospin preparations are representative of 3 separate experiments run independently. (G, H) Alveolar macrophages were isolated from PVM-infected mice (day 5 after inoculation) and uninfected mice; differential expression was evaluated by RT-PCR array. Data shown are differentially expressed transcripts (infected / uninfected) compiled from three independent experiments (n = 15 mice/experimental group).
Alveolar macrophage depletion results in prolonged survival of PVM-infected mice
PVM-infected mice typically suffer from marked morbidity, weight loss and life-threatening respiratory failure, at least in part in response to the severe, acute inflammatory response (Easton et al., 2006; Rosenberg & Domachowske, 2008). In order to examine the contribution of alveolar macrophages to the pathogenesis of acute pneumovirus infection, these cells were depleted by intratracheal instillation with clodronate-filled liposomes. As shown in Fig. 4A (also Suppl. Fig. 1), intratracheal instillation of clodronate-filled liposomes (Cl2-MDP-liposomes) resulted in a 25-fold depletion of alveolar macrophages (CD204+CD11chighCD11blow cells, as above) from mouse lung tissue within 24 hrs that persisted for up to 5 days. Instillation of PBS alone or an equivalent number of PBS-liposomes had no impact on the recovery of alveolar macrophages. Mice that received PBS-liposomes as control 24 hrs prior to virus inoculation succumbed to the negative sequelae of acute PVM infection within 5 to 11 days after inoculation, with a median survival time of 8 days; Fig 4B). In contrast, macrophage-depleted mice experience prolonged survival, with a median survival time of 9 days (p < 0.001). Interestingly, we observed a small but statistically significant increase in virus recovery from lung tissue of mice subjected to alveolar macrophage depletion [Fig. 5A], similar to results of Pribul and colleagues (2008) who evaluated macrophage-depleted RSV-challenged BALB/c mice. Although our results seem paradoxical in light of the significantly prolonged survival, we have previously reported that changes in peak virus recovery do not necessarily predict survival (Gabryszewski et al., 2011). Furthermore, we observed that depletion of alveolar macrophages had no impact on the recruitment of CD4+ or CD8+ T cells, or on the recruitment of neutrophils or eosinophils [Figs. 5B – 5C]. A small but statistically significant increase in NK cell recruitment was detected in mice depleted of macrophages [Fig. 5C]. Although the precise role played by NK cells in host defense against pneumovirus infection remains uncertain, relative NK cell deficiency was reported as a feature of severe RSV disease in human infants (DeWeerd et al. 1998); recently, Kaiko and colleagues (2010) demonstrated that NK cell depletion of RSVchallenged BALB/c mice resulted in suppression of IFN-γ production and the development Th2 cytokine responses and subsequent allergic lung disease
Figure 4. Alveolar macrophage depletion results in prolonged survival of PVM-infected mice.
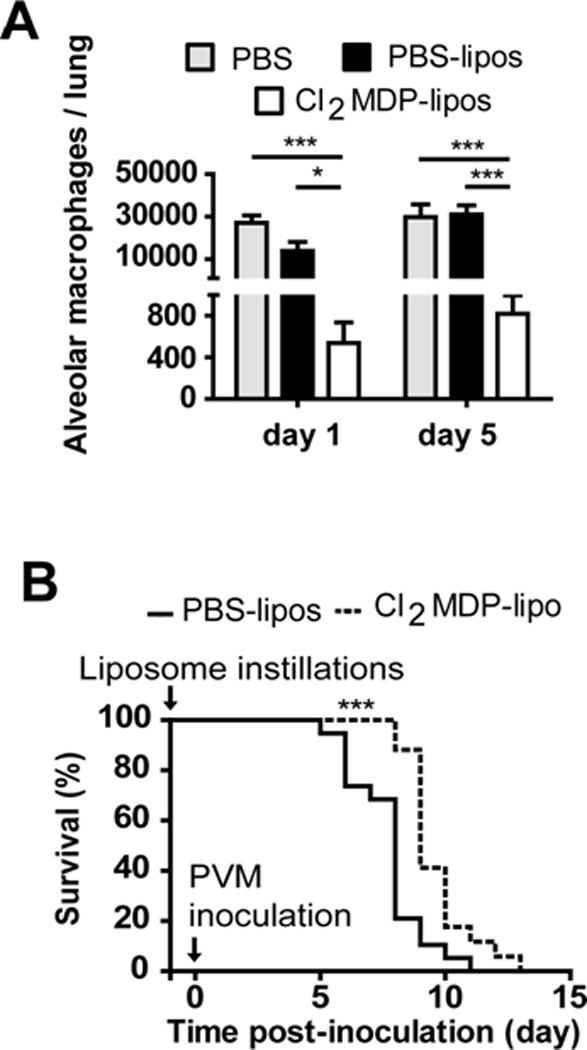
(A) Detection of alveolar macrophages (CD204+ CD11chigh CD11blow) by flow cytometry on days 1 and 5 after intratracheal instillation of PBS (grey bars), PBS liposomes (black bars) or clodronate (Cl2MDP) liposomes (white bars); data shown are representative of 2 independent experiments (n = 5 mice/experimental group); *p < 0.05, *** p < 0.001; representative flow profile is shown in Supplemental Figure 1. (B) Twenty-four hours before PVM inoculation, mice underwent intratracheal instillation of Cl2MDP-liposomes (dotted line) or PBS-liposomes (filled line). Data shown are compiled from two separate experiments run independently (n = 15 mice/experimental group); *** p < 0.001.
Figure 5. Impact of macrophage depletion on virus recovery and cell recruitment in response to PVM infection.
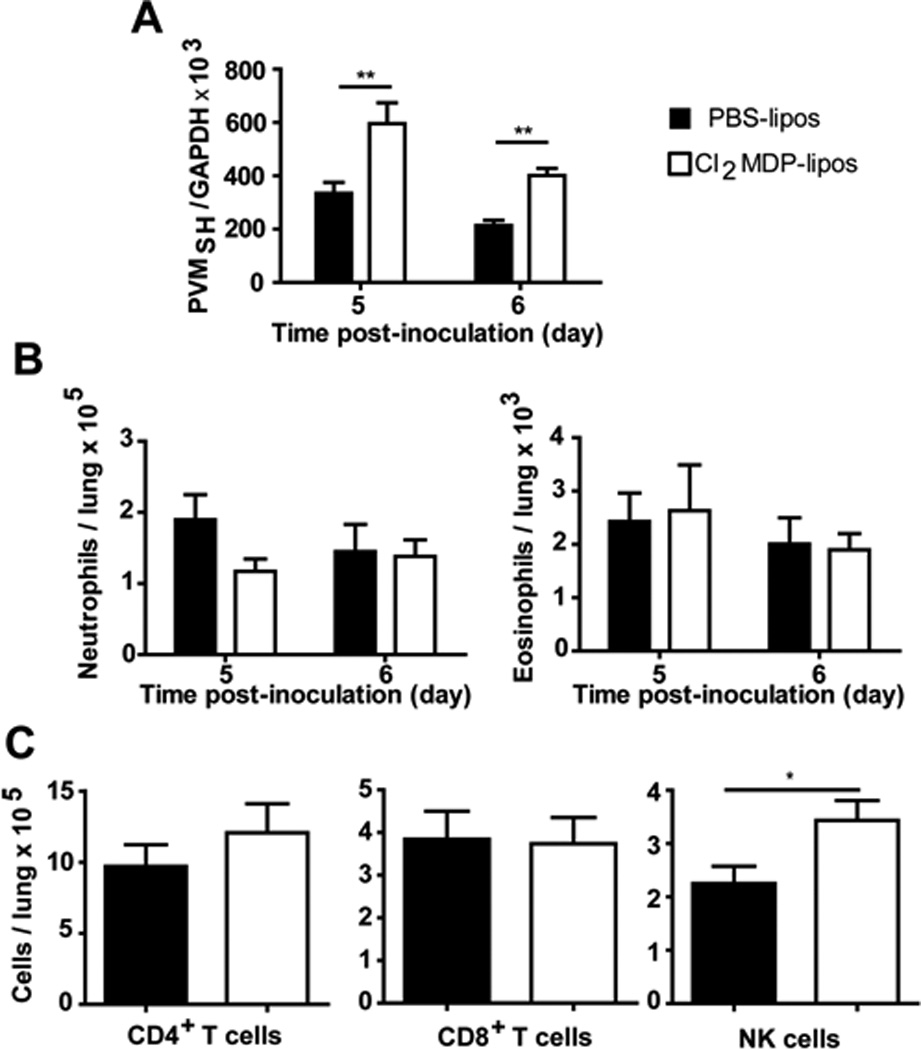
(A) Virus recovery from macrophage-depleted PVM-infected mice; PVM genome copies were detected by dual standard curve qRT-PCR. Data shown are representative of 2 independent experiments (n = 5 - 15 mice / experimental group); ** p < 0.01. Cells detected in lung suspensions of macrophage-depleted (white bars, Cl2MDP liposomes) and control (black bars, PBS liposomes), PVM-infected mice, including (B) neutrophils (GR-1high) and eosinophils (SiglecF+ CD11c−); (C) CD4+ T cells (CD3+CD4+CD8−), CD8+ T cells (CD3+CD4−CD8+) and NK cells (CD3−DX5+) at day 6 after inoculation; * p < 0.05.
Alveolar macrophage depletion in PVM-infected mice results in augmented expression of interferon-gamma
As a further exploration of the mechanism of differential survival, the impact of alveolar macrophage depletion on the production of inflammatory mediators was evaluated. The most prominent and consistent response was augmented expression of IFNγ in PVM-infected macrophage depleted mice [Fig 6A]. Consistent with this findings, we detected significantly more (and significantly greater fractions of) IFNγ+CD4+ T cells, IFNγ+CD8+ T cells and IFNγ+ NK cells in lung cell suspensions of PVM-infected, macrophage depleted mice than from their PBS-liposome treated, PVM-infected counterparts [Figs. 6B - 6D], although we did not determine the fraction of cells that were virus-specific. There are currently two papers in the literature that address the role of virus-specific CD8+T cells in PVM infection. Both Claassen and colleagues (2005) and Frey and colleagues (2008) note that PVM infection is associated with acute influx of total CD8+T cells to the lung tissue and airways, respectively. Frey and colleagues reported that a large fraction of the CD8+T cells isolated from airways of PVM-infected mice were virus-specific, specifically, airway CD8+T cells expressed IFNγ+ upon exposure to PVM-infected mouse RAW 264.7 cells ex vivo. In contrast, Claassen and colleagues examined the responses of CD8+T cells to three dominant PVM-specific epitopes, and found that the virus-specific cells were relatively inactive (IFNγlo or IFNγ−) during acute infection and for prolonged periods thereafter. As such, it is not at all clear how to evaluate or how to interpret findings regarding virus-specific IFNγ+CD8+T cells in acute PVM infection.
Figure 6. Macrophage depletion elicits augmented interferon-gamma response to PVM infection.
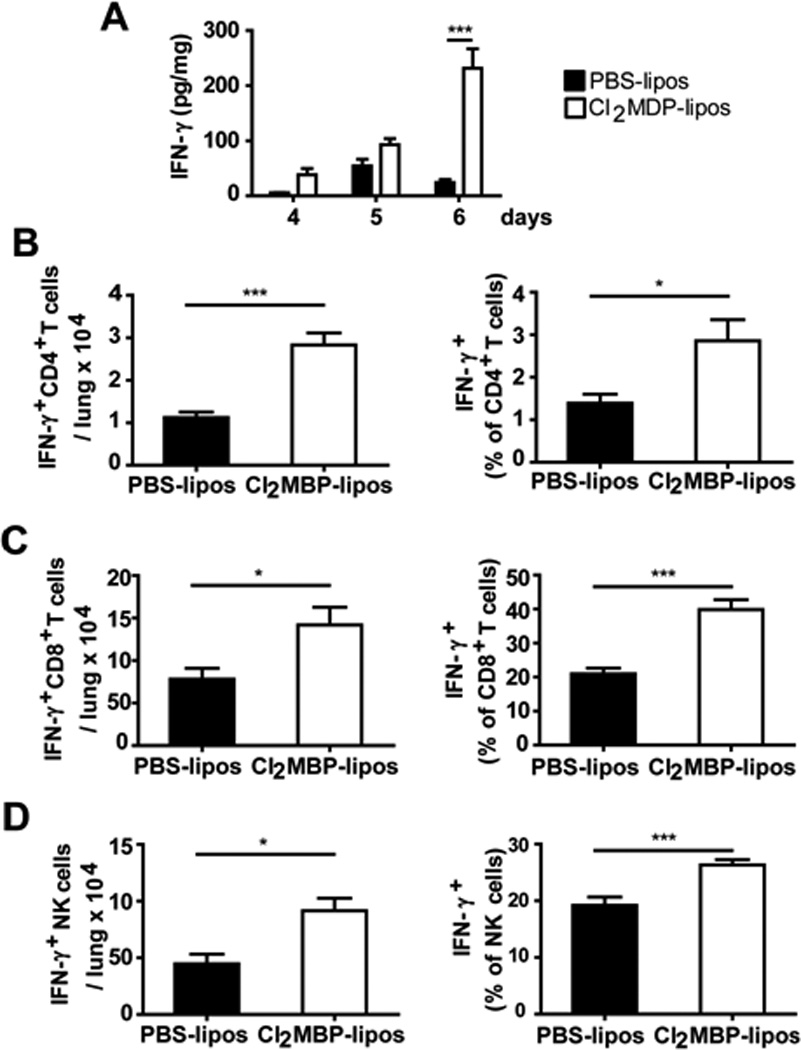
(A) Interferon gamma (IFNγ; pg/mg lung tissue) detected in macrophage-depleted PVM-infected mice. (B – D) Total and relative abundance of IFN-γ+ CD4+ and CD8+ T cells, and IFN-γ+ NK cells in lung tissue of macrophage-depleted (white bars, Cl2MDP liposomes) and control (black bars, PBS liposomes), day 6 after inoculation with PVM. Data shown are representative of 3 independent experiments (n = 6 mice/experimental group); *p < 0.05, ** p < 0.01, *** p < 0.001.
Likewise, although numerous and prominent antiviral activities of IFNγ have been documented vis-à-vis other virus infections (Maher et al., 2007; Schroeder et al., 2004), the primary role of this cytokine in pneumovirus infection remains unclear (Durbin & Durbin, 2004). Our findings are consistent with previously published clinical reports, (Eisenhut 2008; Semple et al., 2007; Aberle et al. 2004; Garcia et al., 2011) which suggest a correlation between decreased production of IFNγ and increasing severity of RSV disease. Among studies that have addressed the mechanistic relationship between IFNγ and various outcomes of acute pneumovirus infection, Harker and colleagues (2010) determined that delivery of IFNγ via a recombinant bicistronic RSV vector results in attenuated virus replication, while Culley and colleagues (2002) suggested that viral replication and IFNγ-mediated responses may not be directly linked; in this latter study, virus replication in neonatal mice was the same as in older mice, despite reduced and delayed IFNγ responses in the former group. In studies focused on secondary responses, Dakhama and colleagues (2008) documented a role for IFNγ signaling in protecting against asthmatic-type responses in mice upon re-challenge with RSV, as did Hussell and Openshaw (2000), who noted that IFNγ from IL-12-activated NK cells was a crucial factor limiting RSV vaccine-mediated immunopathology.
While our results suggest a protective, immunomodulatory role for IFNγ, Frey and colleagues (2008) examined PVM infection in IFNγR gene-deleted mice, and determined that receptor-mediated signaling had no impact on virus recovery or infection-associated weight loss. The aforementioned study was carried out in C57BL/6 mice, in contrast to our findings, which were generated in BALB/c mice. Ahn and colleagues (2006) found that BALB/c mice are substantially more susceptible to PVM infection than C57BL/6 mice. It is possible that IFNγR-mediated signaling contributes to this differential susceptibility; these hypotheses will need to be addressed with further experiments. We have shown previously that IFNγR-mediated signaling is not required to promote seroconversion to PVM antigens, although the actions of this cytokine are crucial for CCL3-mediated neutrophil recruitment (Bonville et al., 2006; Ellis et al., 2007, Bonville et al., 2009). The results featured here suggest that IFNγ may have a protective, immunomodulatory role in PVM infection at least in BALB/c mice, as increased expression, observed in the setting of macrophage depletion, results in prolonged survival despite augmented virus recovery.
Conclusions
We have shown that the natural rodent pneumovirus pathogen, PVM, replicates in primary mouse macrophage culture and induces production and release of infectious virions and proinflammatory cytokines CCL2 and CCL3. Alveolar macrophages isolated from PVM-infected mice express activation markers Clec43 and CD86 as well as numerous proinflammatory cytokines and chemokines. Depletion of alveolar macrophages by intratracheal instillation with clodronate-filled liposomes results in small but statistically significant increases in virus recovery, but, paradoxically, prolonged survival in response to PVM infection. Alveolar macrophage depletion resulted in an increase in NK cell recruitment, but had no impact on the recruitment of granulocytes, CD4+ or CD8+T cells. We observe a profound increase in virus-induced expression of IFNγ, in association with significantly more (and significantly greater fractions of) IFNγ+ CD4+ T cells, IFNγ+ CD8+ T cells and IFNγ+ NK cells present in lung tissue. Taken together, these results suggest that macrophage-depletion results in augmented production of IFNγ from multiple sources, uncovering a potential protective, immunomodulatory functions of this pleiotropic cytokine in primary pneumovirus infection.
Materials and methods
Mouse and Virus stocks
6 – 8 week old BALB/c mice were purchased from Division of Cancer Therapeutics (DCT, National Cancer Institute, National Institutes of Health). All procedures were reviewed and approved according to Animal Study Protocol LAD8E, approved by the National Institute of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee. Mouse-passaged PVM strain J3666 was used in all experiments. Virus copy number in stocks and inocula were evaluated by qRT-PCR targeting the unique SH gene as previously described (Gabryszewski et al., 2011). Prior to inoculation of cells in culture, clarified lung homogenates were dialyzed at 4°C overnight against phosphate buffered saline (50 kDa MW cut off dialysis tubing, Spectrum Laboratories).
Cell culture
Isolated alveolar and peritoneal macrophages were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin and 100 IU/mL M-CSF (R&D Systems). RAW 264.7 cells (ATCC TIB-71; ATCC) were maintained in IMDM supplemented with 10% FBS, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin.
Isolation of peritoneal macrophages and challenge with PVM
Peritoneal cavities of naïve mice were lavaged with RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin and 25 μM 2-mercaptoethanol. Peritoneal fluids were pooled and centrifuged (400 × g, 5 min). Pelleted cells were suspended in DMEM/F12 supplemented with 10% FBS, 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin and 100 IU/mL M-CSF and seeded in culture plates. Cells were permitted to adhere to the plates for 40 min prior to washing with PBS. Routinely, more than 90% of the cells obtained by this procedure displayed a macrophage phenotype as assessed by visual inspection and by anti-F4/80 staining. Peritoneal macrophages were challenged 24 hours later with PVM, heat-inactivated PVM (hiPVM; PVM virions heated to 95°C and then flash frozen in a dry ice–methanol bath, repeated 3 times) at a multiplicity of infection (MOI) of 100 or were sham treated with an equal volume of IMDM. The cells and virus were co-incubated for 4 hours at 37°C in a humidified 5% CO2 incubator, after which the cells were washed twice with PBS and placed in fresh complete medium. At the time points indicated, aliquots of culture media were collected for analysis of virus recovery by qRT-PCR, cytokine release by ELISA (R&D Systems), or Western blot probed with anti-PVM N antibody (Percopo et al., 2011) and anti-beta actin to determine relative loading (Cell Signaling, Millipore Corporation).
Determination of virus recovery
Mouse lung tissues were stored in RNAlater (Ambion) overnight at 4°C prior to RNA extraction. RNA was isolated from lungs with RNAzol Bee reagent (Tel-Test) according to manufacturer’s instructions. Virus titer was determined by quantitative RT-PCR with two standard curves (PVM SH gene and cellular GAPDH) as previously described (Gabryszewski et al., 2011). Western blot was carried out by standard procedures. PVM anti-N antibody was characterized as described in Percopo et al. (2011).
Release of infectious virions released by cultured macrophages
Supernatants from infected macrophage cultures were collected at the time points indicated and stored at -80°C until use. Aliquots of these culture supernatants (100 μL) were used to inoculate RAW 264.7 reporter cultures seeded in 900 μL of medium. After 7 days, total cellular RNA was isolated, and virus recovery was determined by qRT-PCR.
Isolation and identification of leukocytes from lungs of PVM-infected mice
Mice were anesthetized by a brief inoculation of 20% (v/v) halothane (Ayerst Laboratories) diluted in mineral oil. Anesthetized mice were inoculated intranasally with 5 × 105 copies of PVM strain J3666 in IMDM or IMDM as control. Five or six days after inoculation, mice were sacrificed. A PBS solution supplemented with 10 mM EDTA was injected into the heart to perfuse lung tissues. Collected lungs were finely chopped with a razor blade and digested in RPMI containing 5% FBS, 2 mg/mL collagenase D (Roche Diagnostics) and 20 μg /mL grade II DNase I (Roche Diagnostics). After 45 min incubation at 37°C under stirring, 1 volume of fresh digestion medium was added; 10 mM EDTA was added after a total of 90 min digestion time and the lung digest was placed on ice for 5 min. The cell suspension was strained onto a 70 μm nylon mesh and centrifuged (400 × g, 5 min, 4°C). Red cells were lysed with ACK buffer and remaining cells were washed in PBS containing 2% FBS and 20 μg /mL grade II DNase I. Lung cells were stained with LIVE/DEAD Fixable Near IR stain (Invitrogen) in PBS (30 min, 4°C). To assess neutrophils and eosinophil recruitment to the lung, cells were then probed with anti-mouse GR-1 V450 (BD Biosciences), CD11c Alexa 488 (eBioscience), Siglec-F PE (BD Biosciences), CD45 PE-Cy5 (BD Biosciences) in HBSS containing 10 mM EDTA, 10% FBS in the presence of blocking anti-mouse CD16/CD32 (eBioscience) for 20 min at 4°C. To assess T cell and NK cell recruitment, fluorochrome-coupled antibodies included anti-mouse CD3 FITC (BD Biosciences), CD8 PE (BD Biosciences), CD4 PerCp-Cy5.5 (eBioscience) and DX5 PE-Cy7 (BD Biosciences). To enumerate alveolar macrophages, cells were probed with anti-mouse CD11c Alexa 488 (eBioscience), CD11b PE (BD Biosciences) and CD204 Alexa 647 (AbD Serotec). Parallel samples were stained with isotype-matched controls replacing the aforementioned specific mAbs. Antibody-bound cells were washed twice in HBSS supplemented with 10 mM EDTA and 10% FBS and analyzed by flow cytometry (LSRII, BD Biosciences).
Purification of alveolar macrophages
Lung cell suspensions were prepared as described above; CD11c+ lung cells were purified from isolated lung cells with a murine CD11c+ cells isolation kit (Miltenyi Biotec) in accordance to manufacturer’s instructions. Live CD11c+ cells were identified with LIVE/DEAD Fixable Near IR stain (Invitrogen) in PBS (30 min, 4°C) followed by anti-mouse CD11c Alexa 488 (eBioscience), CD11b PE (BD Biosciences), CD204 Alexa 647 (AbD Serotec) in HBSS containing 10 mM EDTA, 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin in the presence of blocking anti-mouse CD16/CD32 (eBioscience) for 20 min at 4°C. Antibody-bound cells were washed twice in HBSS supplemented with 10 mM EDTA, 10% FBS, 100 units /mL penicillin and 100 μg/mL streptomycin. CD204+ CD11chigh CD11blow LIVE/DEAD stainlow (viable alveolar macrophages) were sorted by flow cytometry (FACSAria, BD Biosciences). The purity of sorted cells was > 95 %. A fraction of isolated cells was subjected to cytospin, methanol-fixation and staining with modified Giemsa (DiffQuik, Fisher Scientific) to confirm purity and identification as macrophages.
Relative expression of proinflammatory cytokines in alveolar macrophages from PVMinfected mice
RNA was isolated from sorted alveolar macrophages from both control and PVM-infected mice using the RT2 qPCR-grade RNA Isolation Kit (SA Biosciences, Frederick, MD). First-strand cDNA was generated using the RT2 First-strand Kit (SA Biosciences) and, in conjunction with the RT2 SYBR Green/ROX qPCR Master Mix (SABiosciences), analyzed for differential gene expression using the RT2 Profiler Toll-Like Receptor Signaling Pathway PCR Array and the Inflammatory Response and Autoimmunity PCR Array (SA Biosciences). Alternatively, sorted alveolar macrophages were seeded in macrophage complete medium (see above). Alveolar macrophages supernatants were aliquoted at specified time points and tested for cytokine production by multiplex bead array (BioRad) or ELISA (R&D Systems).
Depletion of alveolar macrophages
Clodronate (dichloromethylene diphosphonate, Cl2MDP) or PBS encapsulated liposomes (Encapsula Nanosciences) were injected intratracheally. (60 μL, 1:2 dilution in PBS) to each anesthetized naïve recipient. Anesthesia was achieved by injecting 80 μL of 2 mg/mL xylazine, 12.5 mg/mL ketamine in PBS via the intraperitoneal route. Twenty-four hrs later, mice were infected with 1 × 105 copies of PVM strain J3666 after inhalation anesthesia with 20% (v/v) halothane (Ayerst Laboratories) in mineral oil. Mouse survival was evaluated every 24 h. Whole lung tissue was stored in RNAlater (Ambion) for determination of virus recovery, or blade-homogenized in ice-cold PBS-BSA 0.01% (w/v), followed by centrifugation (2000 × g, 15 min, 4°C) for preparation of clarified tissue homogenates. Clarified supernatants were stored in aliquots at -80 °C until use. Cytokine concentration was determined by ELISA (R&D Systems). Datapoints were normalized to total protein content, which was measured by micro BCA protein essay (Pierce Biotech).
Intracellular cytokine staining
Intracellular cytokine staining was performed according to previously published methods (Foster et al. 2007). Cells were washed twice and resuspended in RPMI 1640 medium supplemented with 5% FBS, 100 U/ml penicillin, 100 μg/mL streptomycin and 1 mM L-glutamine. Cells were sham-stimulated or stimulated with 20 ng/mL phorbol myristate acetate and 1 μM ionomycin in the presence of 10 μg/mL brefeldin A. Stimulation was performed in 16-mm × 125-mm culture tubes (Corning, 4 × 106 cells, 2 mL, 37°C) with gentle stirring. After 4 hr, the samples were washed twice in cold PBS, labeled with LIVE/DEAD Fixable Violet Dead Cell Stain (Invitrogen) according to the manufacturer's instructions, washed in cold PBS, and stained with mAb diluted in PBS containing 10 mM EDTA, 10% FBS in the presence of blocking anti-mouse CD16/CD32 (eBioscience) for 20 min at 4°C. The following 5-color panel was used: Violet LIVE/DEAD in combination with anti-mouse CD3 FITC (BD Biosciences), CD8 PE (BD Biosciences), CD4 PerCp-Cy5.5 (eBioscience) and DX5 PE-Cy7 (BD Biosciences). Cells were washed twice in cold PBS, fixed in 4% paraformaldehyde (Sigma), washed in PBS and stored 16 hr at 4°C. For intracellular staining, cells were blocked in PBS with 0.1% saponin and 5% nonfat dried milk for 60 minutes and stained thereafter with anti-mouse IFNγ APC diluted in PBS/saponin/nonfat dried milk for 30 minutes. Stained cells were washed twice with PBS, and resuspended in PBS with 0.1% BSA (w/v) for analysis. Parallel samples were stained with isotype-matched controls replacing the anti- mAbs.
Statistical analysis
All data points indicate the average of samples ± SEM. All analyses were performed using GraphPad Prism 5 (GraphPad). Log-rank test was used in survival studies. In other cases, statistical significance was determined by an ANOVA test, followed by a Bonferroni test for comparison between groups. Significant outliers were excluded by using Grubbs test. All experiments were repeated at least 2 times to confirm results.
Supplementary Material
Acknowledgements
The authors are grateful to Ms. Caroline M. Percopo for her expert training in intratracheal inoculation techniques, to Dr. Alfonso Gozalo and the staff of the 14BS animal facility for the care of the mice used in these experiments, and to Mr. Stanislaw Gabryszewski, Dr. Tolga Barker, and Dr. Kimberly Dyer for critical reading of the manuscript. The studies carried out in our laboratory are supported by funding from the NIAID Division of Intramural Research, #AI000943.
Abbreviations
- PVM
pneumonia virus of mice
- hi
heat inactivated
- Cl2MDP
clodronate
- IFNγ
interferon gamma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle JH, Aberle SW, Rebhandl W, Pracher E, Kundi M, Popow-Kraupp T. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin Exp Immunol. 2004;137:146–150. doi: 10.1111/j.1365-2249.2004.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn DB, Faisca P, Desmecht DJ. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am J Physiol Lung Cell Mol Physiol. 2006;291:L426–L435. doi: 10.1152/ajplung.00483.2005. [DOI] [PubMed] [Google Scholar]
- Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, de Leval L, Van Rooijen N, Le Moine A, Vanderplasschen A, Cataldo D, Drion PV, Moser M, Lekeux P, Bureau F. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301:L148–L156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonville CA, Easton AJ, Rosenberg HF, Domachowske JB. Altered pathogenesis of severe pneumovirus infection in response to combined antiviral and specific immunomodulatory agents. J. Virol. 2003;77:1237–1244. doi: 10.1128/JVI.77.2.1237-1244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonville CA, Lau VK, DeLeon JM, Gao JL, Easton AJ, Rosenberg HF, Domachowske JB. Functional antagonism of chemokine receptor CCR1 reduces mortality in acute pneumovirus infection in vivo. J. Virol. 2004;78:7984–7989. doi: 10.1128/JVI.78.15.7984-7989.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonville CA, Bennett NJ, Koehnlein M, Haines DM, Ellis JA, DelVecchio AM, Rosenberg HF, Domachowske JB. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349:87–95. doi: 10.1016/j.virol.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Bonville CA, Percopo CM, Dyer KD, Gao J, Prussin C, Foster B, Rosenberg HF, Domachowske JB. Interferon-gamma coordinates CCL3-mediated neutrophil recruitment in vivo. BMC Immunol. 2009;10:14. doi: 10.1186/1471-2172-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen EA, van der Kant PA, Rychnavska ZS, van Bleek GM, Easton AJ, van der Most RG. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. J. Immunol. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakhama A, Kaan PM, Hegele RG. Permissiveness of guinea pig alveolar macrophage subpopulations to acute respiratory syncytial virus infection in vitro. Chest. 1998;114:1681–1688. doi: 10.1378/chest.114.6.1681. [DOI] [PubMed] [Google Scholar]
- De Weerd W, Twilhaar WN, Kimpen JL. T cell subset analysis in peripheral blood of children with RSV bronchiolitis. Scand. J. Infect. Dis. 1998;30:77–80. doi: 10.1080/003655498750002349. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J Immunol. 2000;165:2677–2682. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Durbin RK. Respiratory syncytial virus-induced immunoprotection and immunopathology. Viral Immunol. 2004;17:370–380. doi: 10.1089/vim.2004.17.370. [DOI] [PubMed] [Google Scholar]
- Dyer KD, Schellens IM, Bonville CA, Martin BV, Domachowske JB, Rosenberg HF. Efficient replication of pneumonia virus of mice (PVM) in a mouse macrophage cell line. Virol. J. 2007;4:48. doi: 10.1186/1743-422X-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AJ, Domachowske JB, Rosenberg HF. Pneumonia virus of mice. In: Cane P, editor. Perspectives in Medical Virology. vol. 12. 2006. pp. 299–319. [Google Scholar]
- Eisenhut M. Determinants of reduced interferon gamma expression in severe respiratory syncytial virus disease. Exp Biol. Med. 2008;233:493–494. [PubMed] [Google Scholar]
- Ellis JA, Martin BV, Waldner C, Dyer KD, Domachowske JB, Rosenberg HF. Mucosal inoculation with an attenuated mouse pneumovirus strain protects against virulent challenge in wild type and interferon-gamma receptor deficient mice. Vaccine. 2007;25:1085–1095. doi: 10.1016/j.vaccine.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im0624s78. Chapter 6: Unit 6-24. [DOI] [PubMed] [Google Scholar]
- Franke G, Freihorst J, Steinmüller C, Verhagen W, Hockertz S, Lohmann-Matthes ML. Interaction of alveolar macrophages and respiratory syncytial virus. J Immunol. Methods. 1994;174:173–184. doi: 10.1016/0022-1759(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Franke-Ullmann G, Pförtner C, Walter P, Steinmüller C, Lohmann-Matthes ML, Kobzik L, Freihorst J. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J. Immunol. 1995;154:268–280. [PubMed] [Google Scholar]
- Frey S, Krempl CD, Schmitt-Graff A, Ehl S. Role of T cells in virus control and disease after infection with pneumonia virus of mice. J. Virol. 2008;82:11619–11627. doi: 10.1128/JVI.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, Rosenberg HF. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol. 2011;186:1151–1161. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Soriano-Fallas A, Lozano J, Leos N, Gomez AM, Ramilo O, Mejias A. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhino virus bronchiolitis. Pediatr. Infect. Dis. J. 2011 doi: 10.1097/INF.0b013e31822dc8c1. in press. [DOI] [PubMed] [Google Scholar]
- Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Brit. Med. Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature Rev. Immunol. 2004;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gough PJ, Gordon S. The role of scavenger receptors in the innate immune system. Microbes Infect. 2000;2:305–311. doi: 10.1016/s1286-4579(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. New Engl. J. Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker JA, Lee DC, Yamaguchi Y, Wang B, Bukreyev A, Collins PL, Tregoning JS, Openshaw PJ. Delivery of cytokines by recombinant virusin early life alters the immune response to adult lung infection. J. Virol. 2010;84:5294–5302. doi: 10.1128/JVI.02503-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am. J. Respir. Crit. Care Med. 1999;159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- Horsfall FL, Curnen EC. Studies on pneumonia virus of mice (PVM): I. The precision measurements in vivo of the virus and antibodies against it. J. Exp. Med. 1946;83:25–42. [PubMed] [Google Scholar]
- Hussell T, Openshaw PJ. IL-12 activated NK cells reduce lung eosinophilia to the attachment protein of respiratory syncytial virus but do not enhance the severity of illness in CD8 T cell-immunodeficient conditions. J. Immunol. 2000;165:7109–7115. doi: 10.4049/jimmunol.165.12.7109. [DOI] [PubMed] [Google Scholar]
- Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185:4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- Krilov LR. Respiratory syncytial virus disease: update on treatment and prevention. Expert Rev. Anti Infect. Ther. 2011;9:27–32. doi: 10.1586/eri.10.140. [DOI] [PubMed] [Google Scholar]
- Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, Joetham A, Gelfand EW, Dakhama A. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2008;177:208–218. doi: 10.1164/rccm.200612-1890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: cellular executioner or white knight? Curr. Med. Chem. 2007;14:1279–1289. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Tsutsumi H, Sone S, Yoto Y, Oya K, Okamoto Y, Ogra PL, Chiba S. Characteristics of IL-6 and TNF-alpha production by respiratory syncytial virus-infected macrophages in the neonate. J. Med. Virol. 1996;48:199–203. doi: 10.1002/(SICI)1096-9071(199602)48:2<199::AID-JMV13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- McNamara PS, Flanagan BF, Hart CA, Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2005;191:1225–1232. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- Midulla F, Villani A, Panuska JR, Dab I, Kolls JK, Merolla R, Ronchetti R. Respiratory syncytial virus lung infection in infants: immunoregulatory role of infected alveolar macrophages. J. Infect. Dis. 1993;168:1515–1519. doi: 10.1093/infdis/168.6.1515. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Ishikawa E, Ishikawa T, Yamasaki S. Self and nonself recognition through C-type lectin receptor, Mincle. Self Nonself. 2010;1:310–313. doi: 10.4161/self.1.4.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percopo CM, Dubovi EJ, Renshaw RW, Dyer KD, Domachowske JB, Rosenberg HF. Canine pneumovirus replicates in mouse lung tissue and elicits inflammatory pathology. Virology. 2011;416:26–31. doi: 10.1016/j.virol.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribul PK, Harker J, Wang B, Wang H, Tregoning JS, Schwarze J, Openshaw PJ. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 2008;82:4441–4448. doi: 10.1128/JVI.02541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Brewah YA, Delaney T, Welliver T, Burwell T, Benjamin E, Kuta E, Kozhich A, McKinney L, Suzich J, Kiener PA, Avendano L, Velozo L, Humbles A, Welliver RC, Sr, Coyle AJ. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2008;198:1783–1793. doi: 10.1086/593173. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF, Domachowske JB. Pneumonia virus of mice: severe respiratory virus infection in a natural host. Immunol. Lett. 2008;118:6–12. doi: 10.1016/j.imlet.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Semple MG, Dankert HM, Ebrahimi B, Correia JB, Booth JA, Stewart JP, Smyth RL, Hart CA. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PloS ONE. 2007;2:e1038. doi: 10.1371/journal.pone.0001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnyk AW, Gillan TL, Anderson R. Respiratory syncytial virus triggers synthesis of IL-6 in BALB/c mouse alveolar macrophages in the absence of virus replication. Cell Immunol. 1997;176:122–126. doi: 10.1006/cimm.1996.1075. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H, Matsuda K, Sone S, Takeuchi R, Chiba S. Respiratory syncytial virus-induced cytokine production by neonatal macrophages. Clin. Exp. Immunol. 1996;106:442–446. doi: 10.1046/j.1365-2249.1996.d01-874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bleek GM, Osterhaus AD, de Swart RL. RSV 2010: Recent advances in research on respiratory syncytial virus and other pneumoviruses. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.07.114. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


