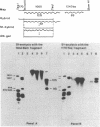
Abstract
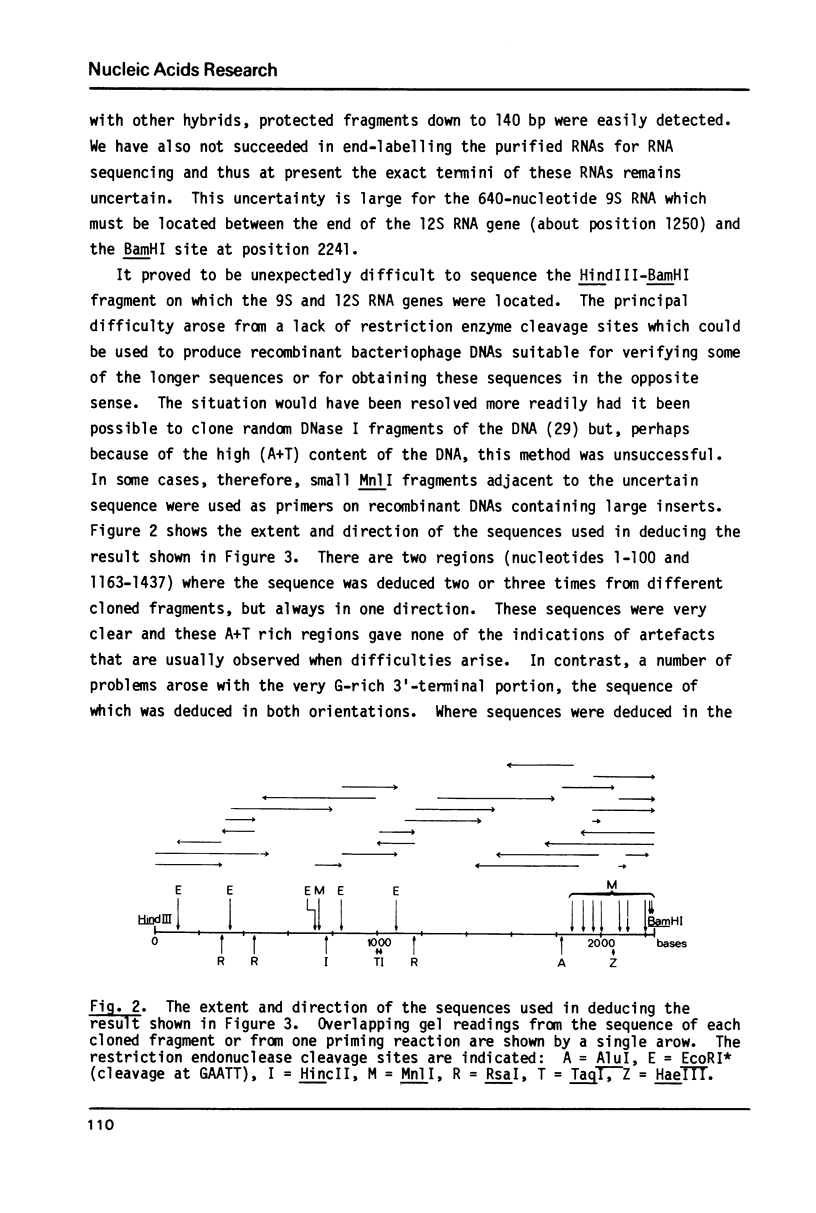
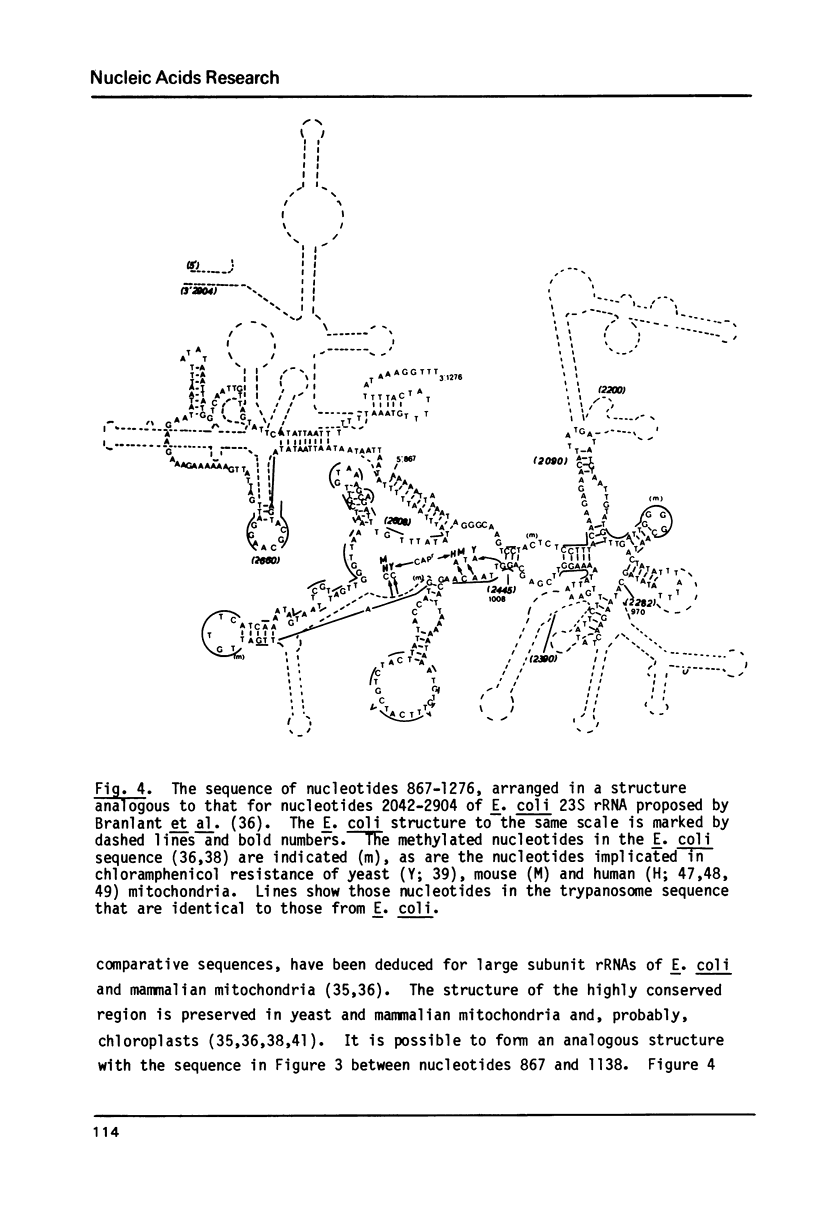
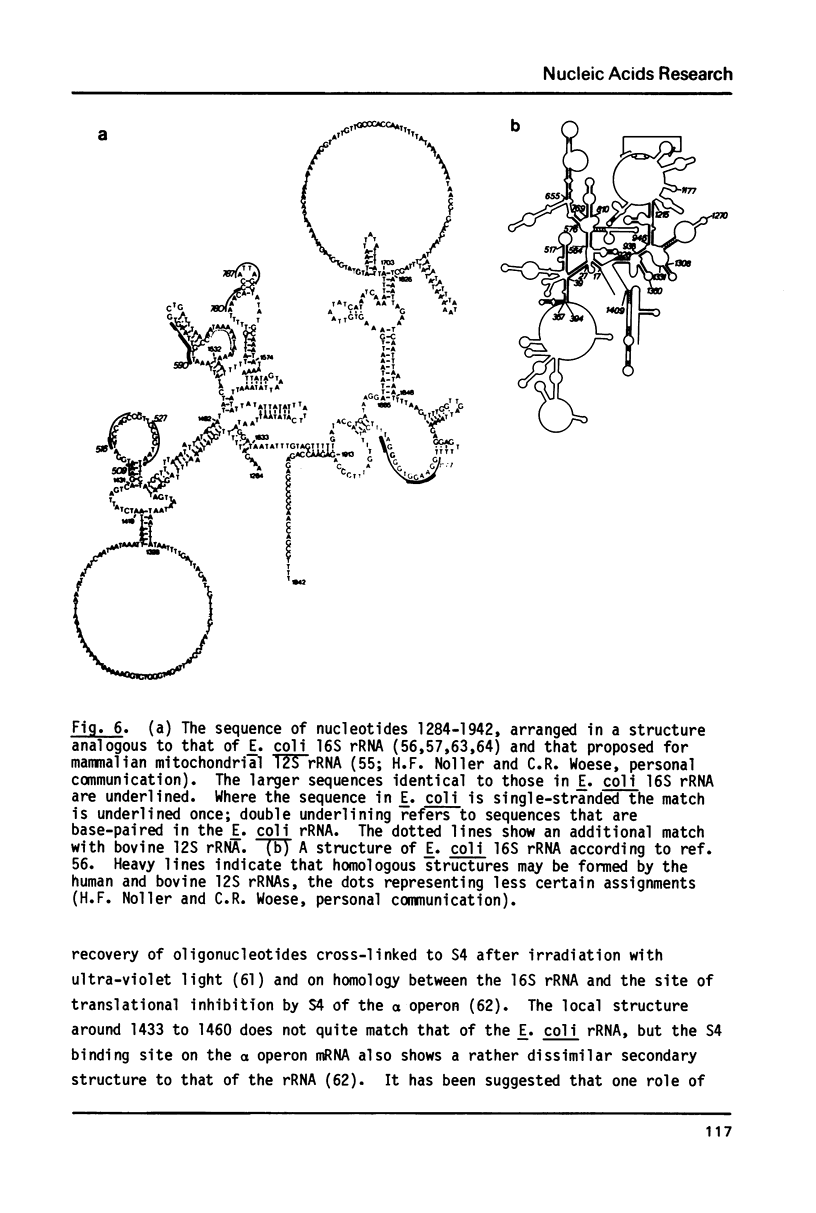
The nucleotide sequence has been determined of a 2.2 kb segment of kinetoplast DNA, which encodes the major mitochondrial transcripts (12S and 9S) of Trypanosoma brucei. The sequence shows that the 12S RNA is a large subunit rRNA, although sufficiently unusual for resistance to chloramphenicol to be predicted. The 9S RNA has little homology with other rRNAs, but a possible secondary structure is not unlike that of the 2.5-fold larger E. coli 16S rRNA. We conclude that the 12S RNA (about 1230 nucleotides) and the 9S RNA (about 640 nucleotides) are the smallest homologues of the E. coli 23S and 16S rRNAs yet observed.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Rochaix J. D. Structure analysis at the ends of the intervening DNA sequences in the chloroplast 23S ribosomal genes of C. reinhardii. Cell. 1979 Sep;18(1):55–60. doi: 10.1016/0092-8674(79)90353-2. [DOI] [PubMed] [Google Scholar]
- Anderson S., Gait M. J., Mayol L., Young I. G. A short primer for sequencing DNA cloned in the single-stranded phage vector M13mp2. Nucleic Acids Res. 1980 Apr 25;8(8):1731–1743. doi: 10.1093/nar/8.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Baer R. J., Dubin D. T. Methylated regions of hamster mitochondrial ribosomal RNA: structural and functional correlates. Nucleic Acids Res. 1981 Jan 24;9(2):323–337. doi: 10.1093/nar/9.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blanc H., Adams C. W., Wallace D. C. Different nucleotide changes in the large rRNA gene of the mitochondrial DNA confer chloramphenicol resistance on two human cell lines. Nucleic Acids Res. 1981 Nov 11;9(21):5785–5795. doi: 10.1093/nar/9.21.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F., Hoeijmakers J. H., Frasch A. C. Variations in maxi-circle and mini-circle sequences in kinetoplast DNAs from different Trypanosoma brucei strains. Biochim Biophys Acta. 1980 Dec 11;610(2):197–210. doi: 10.1016/0005-2787(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt A., Ebel J. P. The secondary structure of the protein L1 binding region of ribosomal 23S RNA. Homologies with putative secondary structures of the L11 mRNA and of a region of mitochondrial 16S rRNA. Nucleic Acids Res. 1981 Jan 24;9(2):293–307. doi: 10.1093/nar/9.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Sriwidada J., Brimacombe R. RNA sequences associated with proteins L1, L9, and L5, L18, L25, in ribonucleoprotein fragments isolated from the 50-S subunit of Escherichia coli ribosomes. Eur J Biochem. 1976 Nov 15;70(2):483–492. doi: 10.1111/j.1432-1033.1976.tb11039.x. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Davison J., Thi V. H., Merchez M. Cloning and expression of Trypanosoma brucei kinetoplast DNA in Escherichia coli. Gene. 1980 Dec;12(3-4):223–234. doi: 10.1016/0378-1119(80)90104-3. [DOI] [PubMed] [Google Scholar]
- Carbon P., Ebel J. P., Ehresmann C. The sequence of the ribosomal 16S RNA from Proteus vulgaris. Sequence comparison with E. coli 16S RNA and its use in secondary model building. Nucleic Acids Res. 1981 May 25;9(10):2325–2333. doi: 10.1093/nar/9.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Simpson L. Isolation and characterization of kinetoplast DNA and RNA of Phytomonas davidi. Plasmid. 1978 Jun;1(3):297–315. doi: 10.1016/0147-619x(78)90047-1. [DOI] [PubMed] [Google Scholar]
- Cordingley J. S., Turner M. J. 6.5 S RNA; preliminary characterisation of unusual small RNAs in Trypanosoma brucei. Mol Biochem Parasitol. 1980 Apr;1(2):91–96. doi: 10.1016/0166-6851(80)90003-1. [DOI] [PubMed] [Google Scholar]
- Czempiel W., Klose J., Bass R. Mammalian mitochondrial ribosomes:characterization of ribosomal proteins by two-dimensional gel electrophoresis. FEBS Lett. 1976 Mar 1;62(3):259–262. doi: 10.1016/0014-5793(76)80070-1. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Timko K. D., Baer R. J. The 3' terminus of the large ribosomal subunit ("17S") RNA from hamster mitochondria is ragged and oligoadenylated. Cell. 1981 Jan;23(1):271–278. doi: 10.1016/0092-8674(81)90291-9. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Eckerman D. J., Symons R. H. Sequence at the site of attachment of an affinity-label derivative of puromycin on 23-S ribosomal RNA of Escherichia coli ribosomes. Eur J Biochem. 1978 Jan 2;82(1):225–234. doi: 10.1111/j.1432-1033.1978.tb12015.x. [DOI] [PubMed] [Google Scholar]
- Edwards K., Kössel H. The rRNA operon from Zea mays chloroplasts: nucleotide sequence of 23S rDNA and its homology with E.coli 23S rDNA. Nucleic Acids Res. 1981 Jun 25;9(12):2853–2869. doi: 10.1093/nar/9.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann B., Backendorf C., Ehresmann C., Ebel J. P. Characterization of the regions from E. coli 16 S RNA covalently linked to ribosomal proteins S4 and S20 after ultraviolet irradiation. FEBS Lett. 1977 Jun 15;78(2):261–266. doi: 10.1016/0014-5793(77)80319-0. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Faye G., Sor F. Analysis of mitochondrial ribosomal proteins of Saccharomyces cerevisiae by two dimensional polyacrylamide gel electrophoresis. Mol Gen Genet. 1977 Sep 21;155(1):27–34. doi: 10.1007/BF00268557. [DOI] [PubMed] [Google Scholar]
- Glotz C., Brimacombe R. An experimentally-derived model for the secondary structure of the 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1980 Jun 11;8(11):2377–2395. doi: 10.1093/nar/8.11.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., Thurlow D. L., Gerbi S. A., Zimmermann R. A. Specific binding of a prokaryotic ribosomal protein to a eukaryotic ribosomal RNA: implications for evolution and autoregulation. Proc Natl Acad Sci U S A. 1981 May;78(5):2722–2726. doi: 10.1073/pnas.78.5.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Cunningham I., Gardiner P. R., Taylor A. M., Luckins A. G. Cultivation of infective forms of Trypanosoma congolense from trypanosomes in the proboscis of Glossina morsitans. Parasitology. 1981 Feb;82(1):81–95. doi: 10.1017/s0031182000041883. [DOI] [PubMed] [Google Scholar]
- Gray M. W. Unusual pattern of ribonucleic acid components in the ribosome of Crithidia fasciculata, a trypanosomatid protozoan. Mol Cell Biol. 1981 Apr;1(4):347–357. doi: 10.1128/mcb.1.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell P., Harris R. J., Symons R. H. Affinity labelling of 23-S ribosomal RNA in the active centre of Escherichia coli peptidyl transferase. Eur J Biochem. 1974 Dec 2;49(3):539–544. doi: 10.1111/j.1432-1033.1974.tb03858.x. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Hahn U., Lazarus C. M., Lünsdorf H., Küntzel H. Split gene for mitochondrial 24S ribosomal RNA of Neurospora crassa. Cell. 1979 May;17(1):191–200. doi: 10.1016/0092-8674(79)90307-6. [DOI] [PubMed] [Google Scholar]
- Hanas J., Linden G., Stuart K. Mitochondrial and cytoplasmic ribosomes and their activity in blood and culture form Trypanosoma brucei. J Cell Biol. 1975 Apr;65(1):103–111. doi: 10.1083/jcb.65.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., RajBhandary U. L. Organization of tRNA and rRNA genes in N. crassa mitochondria: intervening sequence in the large rRNA gene and strand distribution of the RNA genes. Cell. 1979 Jul;17(3):583–595. doi: 10.1016/0092-8674(79)90266-6. [DOI] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Herr W., Noller H. F. Protection of specific sites in 23 S and 5 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1979 Jun 5;130(4):421–432. doi: 10.1016/0022-2836(79)90432-7. [DOI] [PubMed] [Google Scholar]
- Heyting C., Talen J. L., Weijers P. J., Borst P. Fine structure of the 21S ribosomal RNA region on yeast mitochondrial DNA. II. The organization of sequences in petite mitochondrial DNAs carrying genetic markers from the 21S region. Mol Gen Genet. 1979 Jan 11;168(3):251–277. doi: 10.1007/BF00271497. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Huynh-Van-Tan, Gavrilovic M., Schapira G. Eukaryotic ribosomal proteins: the number of proteins in the subunits and their isoelectric points. FEBS Lett. 1974 Sep 1;45(1):299–303. doi: 10.1016/0014-5793(74)80866-5. [DOI] [PubMed] [Google Scholar]
- Kazemie M. Binding of aminoacyl-tRNA to reconstituted subparticles of Escherichia coli large ribosomal subunits. Eur J Biochem. 1976 Aug 16;67(2):373–378. doi: 10.1111/j.1432-1033.1976.tb10701.x. [DOI] [PubMed] [Google Scholar]
- Kazemie M. The importance of Escherichia coli ribosomal proteins L1, L11 and L16 for the association of ribosomal subunits and the formation of the 70-S initiation complex. Eur J Biochem. 1975 Oct 15;58(2):501–510. doi: 10.1111/j.1432-1033.1975.tb02398.x. [DOI] [PubMed] [Google Scholar]
- Kearsey S. E., Craig I. W. Altered ribosomal RNA genes in mitochondria from mammalian cells with chloramphenicol resistance. Nature. 1981 Apr 16;290(5807):607–608. doi: 10.1038/290607a0. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Borst P. Are 50% of all cellular proteins synthesized on mitochondrial ribosomes in Crithidia luciliae? Biochim Biophys Acta. 1975 Apr 16;390(1):78–81. doi: 10.1016/0005-2787(75)90010-6. [DOI] [PubMed] [Google Scholar]
- Leister D. E., Dawid I. B. Physical properties and protein constituents fo cytoplasmic and mitochondrial ribosomes of Xenopus laevis. J Biol Chem. 1974 Aug 25;249(16):5108–5118. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W., Miller F., Michel R., Hallermayer G. Mitochondrial ribosomes of Neurospora crassa: isolation, analysis, and use. Methods Enzymol. 1979;56:79–92. doi: 10.1016/0076-6879(79)56011-x. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Woese C. R. Secondary structure of 16S ribosomal RNA. Science. 1981 Apr 24;212(4493):403–411. doi: 10.1126/science.6163215. [DOI] [PubMed] [Google Scholar]
- Nomiyama H., Sakaki Y., Takagi Y. Nucleotide sequence of a ribosomal RNA gene intron from slime mold Physarum polycephalum. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1376–1380. doi: 10.1073/pnas.78.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Evers R. F., Van der Laan J. C., Tabak H. F. A putative precursor for the small ribosomal RNA from mitochondria of Saccharomyces cerevisiae. Nucleic Acids Res. 1981 Mar 25;9(6):1351–1364. doi: 10.1093/nar/9.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of tRNALys and tRNA2Glu to 16 S RNA at the P site of Escherichia coli ribosomes. J Biol Chem. 1979 Jun 10;254(11):4745–4749. [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Zakharyev V. M., Krayev A. S., Skryabin K. G., Bayev A. A. The structure of the yeast ribosomal RNA genes. I. The complete nucleotide sequence of the 18S ribosomal RNA gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Dec 11;8(23):5779–5794. doi: 10.1093/nar/8.23.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M., Maden B. E. Nucleotide sequence of Xenopus laevis 18S ribosomal RNA inferred from gene sequence. Nature. 1981 May 21;291(5812):205–208. doi: 10.1038/291205a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Simpson A. G. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978 May;14(1):169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Séquence nucléotidique du gène de l'ARN ribosomique 15S mitochondrial de la levure. C R Seances Acad Sci D. 1980 Dec 8;291(12):933–936. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spithill T. W., Shimer S. P., Hill G. C. Inhibitory effects of chloramphenicol isomers and other antibiotics on protein synthesis and respiration in procyclic Trypanosoma brucei brucei. Mol Biochem Parasitol. 1981 Feb;2(3-4):235–255. doi: 10.1016/0166-6851(81)90103-1. [DOI] [PubMed] [Google Scholar]
- Staden R. A computer program to search for tRNA genes. Nucleic Acids Res. 1980 Feb 25;8(4):817–825. [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Veldman G. M., Klootwijk J., de Regt V. C., Planta R. J., Branlant C., Krol A., Ebel J. P. The primary and secondary structure of yeast 26S rRNA. Nucleic Acids Res. 1981 Dec 21;9(24):6935–6952. doi: 10.1093/nar/9.24.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Glotz C., Brimacombe R. Secondary structure comparisons between small subunit ribosomal RNA molecules from six different species. Nucleic Acids Res. 1981 Aug 11;9(15):3621–3640. doi: 10.1093/nar/9.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H., de Jonge J. C., Bakker H., Meurs H., Kroon A. Laboratory of Physiological Chemistry, State University Groningen, Netherlands. Nucleic Acids Res. 1979;6(5):1791–1803. doi: 10.1093/nar/6.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]