Abstract
We analyzed more than 1 million small molecules with the goal of finding simple synthetic compounds that potently inhibit cancer cell growth. We identified three such compounds with unknown mechanisms of action. Subsequent studies revealed that all three of these small molecules target microtubules. These three scaffolds can serve as templates for developing new microtubule-targeted agents, overcoming the limits of existing microtubule-inhibiting drugs derived from complex natural products.
Keywords: Small molecules, cell-based screening, microtubule, anticancer drug leads
One potential benefit of using small molecules for studying biological processes is the possibility of obtaining leads for further drug development efforts. Typically, small molecules in large libraries are selected for druglike properties through computational filters. Nonetheless, even with the best filters, many hits from screens lack druglike properties.1 Such compounds may contain reactive groups, have poor water solubility, or have low potency. Compounds with such liabilities may lead to identification of novel target proteins, which may in turn provide a starting point to create more druglike scaffolds acting through the same targets.
In some cases, high potency can compensate for lack of other druglike properties. Natural products such as paclitaxel and vinblastine are clinically used anticancer drugs with high molecular weights and poor water solubility; these drugs require complex formulations to be used clinically, which limits their bioavailability and ultimate clinical efficacy.
In oncology, where the rate of successful drug development is less than 5%, a major reason for drug attrition is lack of clinical efficacy, which has a strong correlation with drug potency.2 The NCI DTP performed a retrospective analysis of 2306 compounds submitted for NCI60 testing and found a striking correlation between potency and subsequent in vivo activity, which indicates that compounds with high potency are expected to result in good efficacy in later stages of drug development.3
In an effort to find small molecule anticancer drug candidates with high potency, we generated and analyzed cell-based screening data using more than 1 million compounds and selected lethal compounds with the highest potency. We then selected only those high potency compounds with simple synthetic structures for further analysis, leading to three novel compounds. We used the NCI60 platform to elucidate the mechanism of action of these three compounds as microtubule inhibitors; they have potential benefits as drug leads for cancer chemotherapy.
We screened compounds for growth inhibitory activity in engineered tumorigenic cell lines, including BJeLR cells.4,5 BJeLR cells are derived from human skin tissue and were engineered to express the catalytic subunit of human telomerase (hTERT), the SV40 large T and small T antigens (LT and ST), and an oncogenic allele of HRAS (HRASG12V). We found that the number of lethal compounds with high potency was small, which led us to conclude that potency of lethal compounds alone can be a stringent selection criterion in cell-based assays.
For example, we were able to identify only 29 compounds out of >1 million compounds tested that had EC50 values less than 100 nM for growth inhibition (see Figure S1 in the Supporting Information). To deduce the molecular mechanism of these highly potent compounds, we grouped them based on their structures and classified them according to their known target proteins (Table 1).
Table 1. Grouping of Highly Potent Lethal Compounds Based on Their Structures and Known Activities.
| known bioactivity | no. of compds |
|---|---|
| microtubule inhibitor | 20 |
| translation inhibitor | 2 |
| kinase inhibitor | 1 |
| transcription inhibitor | 1 |
| nucleotide analog | 1 |
| topoisomerase II inhibitor | 1 |
| unknown | 3 |
| total | 29 |
The structural information on each compound and dose–response curves for viability are presented in Figure S1 in the Supporting Information. Microtubule inhibitors were the most common type of mechanism, representing more than 68% of the total. Translation inhibition, nonspecific kinase inhibition, transcription inhibition, a nucleotide mimicry, and topoisomerase II inhibition were additional mechanisms identified.
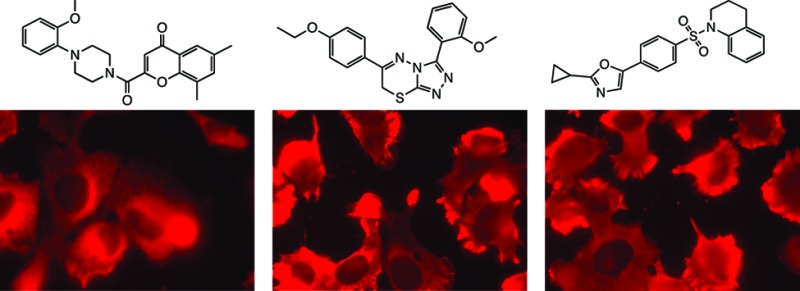
We also discovered three novel compounds whose biological activities had not been studied. We examined the structures of these uncharacterized compounds and found that they represented distinct scaffolds (Figure 1). New batches of compounds were purchased, and the authenticity of each compound was determined using MS and NMR analysis (see Figure S3 in the Supporting Information). The compounds were tested in three different cell lines that were used in the original screening to confirm their activities (Figure 1). The activity of each compound was reproducible in these cell lines, albeit with varying potency. Compound 1 is built around a 4-chromone ring system, compound 2 contains a triazolothiadiazine system, and compound 3 contains oxazole and sulfonamide groups. We were interested in all three compounds because they reproducibly inhibited the growth of cancer cell lines with less than 100 nM EC50 values (Figure 1), and they were all relatively simple synthetic compounds.
Figure 1.
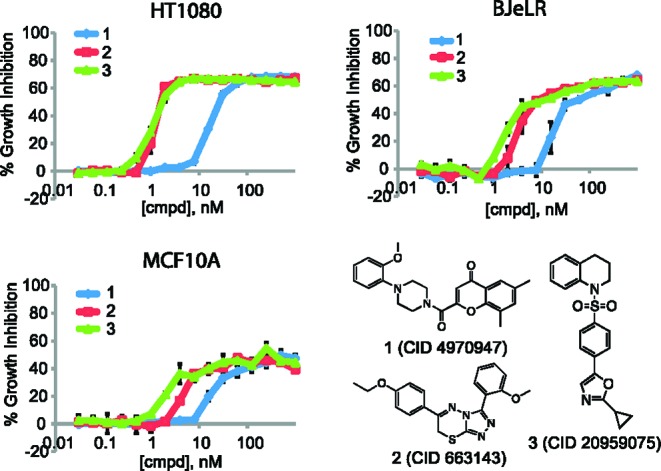
Confirmation of growth inhibitory activity in three different cell lines. HT-1080 and BJeLR cells are derived from human fibroblasts; MCF10A cells are derived from a breast tissue.
We could not deduce a specific mechanism based on their structures, so we decided to submit these compounds to the NCI DTP for NCI60 testing, in which each compound's growth inhibitory activity is examined across 60 different cancer cell lines derived from different tissues of origins.6 A sensitivity profile across the 60 cancer cell lines was obtained, which can be used as a seed to find compounds with similar sensitivity profiles within NCI's database, using the algorithm known as COMPARE.7 If the pattern of sensitivity in the 60 cell lines is similar to any known cytotoxic compound, it is likely that these two compounds have common targets or mechanisms for inducing cell death or growth inhibition. For example, the Yamori group successfully identified the mechanism of a PI3K inhibitor using this approach.8 When we analyzed the sensitivity profile of these compounds with COMPARE, we found microtubule inhibitors were the top-ranked compounds, suggesting that these novel compounds might also be microtubule-targeting compounds (Table 2).
Table 2. Analysis of the NCI60 Database Using the COMPARE Algorithma.
| rank | correlation | compd | biological target |
|---|---|---|---|
| seed: 1 (CID 4970947) | |||
| 1 | 0.636 | paclitaxel (Taxol) | microtubule |
| 2 | 0.62 | maytansine | microtubule |
| 3 | 0.581 | S-trityl-l-cysteine | KIF11(EG5) |
| seed: 2 (CID 663143) | |||
| 1 | 0.713 | maytansine | microtubule |
| 2 | 0.698 | vinblastine sulfate | microtubule |
| 3 | 0.684 | rhizoxin | microtubule |
| seed: 3 (CID 20959075) | |||
| 1 | 0.808 | maytansine | microtubule |
| 2 | 0.754 | vinblastine sulfate | microtubule |
| 3 | 0.682 | rhizoxin | microtubule |
The sensitivity profile of each hit compound was used as a seed for COMPARE analysis. Each table lists the top three ranked compounds that showed the most similar pattern to the sensitivity profile of each hit compound using the NCI's database. KIF11 is a microtubule-binding protein.
To test the hypothesis generated by the COMPARE analysis, we used an antitubulin antibody to stain cellular microtubules and examined changes in microtubule dynamics upon compound treatment. As shown in Figure 2A, a hairlike microtubule network was visualized upon immunofluorescence staining of control HT-1080 cells. However, this microtubule network disappeared when inhibitors of microtubule polymerization (colchicine or vinblastine) were added to HT-1080 cells (Figure 2A).
Figure 2.
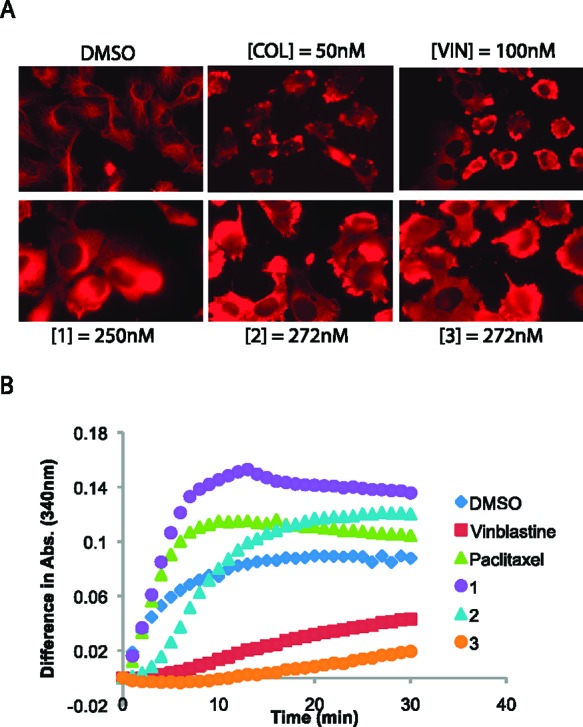
Microtubule network is the target for 1–3. (A) Immunocytochemistry confirmed alteration of microtubule dynamics after compound treatment. (B) An in vitro microtubule polymerization assay revealed that 3 is a microtubule-destabilizing agent, whereas 1 is a microtubule-stabilizing agent. It is likely that 2 targets a microtubule-regulating protein, not microtubules themselves. Compounds were tested at 5 μM.
Cells treated with 2 and 3 also lost their microtubule network in a manner similar to vinblastine-treated cells, confirming the microtubule-targeting activity of these two compounds (Figure 2A). In contrast, cells treated with 1 retained their microtubule network, albeit with a shortened length. This pattern was similar to what was reported in paclitaxel-treated cells.9
The effects of these compounds on tubulin polymerization were further examined in an in vitro assay using purified porcine brain tubulin.10 In this assay, tubulin monomer was self-polymerized to microtubules under buffered conditions, increasing light scattering at 340 nm. Microtubule-depolymerizing drugs, such as vinblastine and colchicine, are known to inhibit self-polymerization activity of tubulin in this assay. When we added vinblastine to the reaction mixture, it inhibited self-polymerization, as expected (Figure 2B). Compound 3 also inhibited self-polymerization of tubulin monomers, implying that it directly binds to tubulin and induces depolymerization of microtubule network within cells (Figure 2B).
On the other hand, paclitaxel, a microtubule-stabilizing agent, accelerated polymerization, showing faster kinetics than untreated controls (Figure 2B). Compound 1 displayed similar, faster kinetics in the microtubule reconstitution experiment, indicating that it binds and stabilizes the microtubule network, as paclitaxel does. The kinetics of 2-treated samples were complex; they did not show a clear stabilization or destabilization effect on microtubule formation, although immunocytochemistry clearly demonstrated a microtubule-disrupting effect of 2 in cells (Figure 2A,B). It is likely that 2 targets microtubule-associated proteins, not tubulin itself, to inhibit the polymerization process within cells, as exemplified by S-trityl-l-cysteine, a KIF11 targeting compound (Table 2).
We then investigated the cell death pathways that were activated upon compound treatment. It has been reported that microtubule-disrupting agents activate the cellular apoptotic machinery to kill cancer cells.11,12 Indeed, we observed dose- and time-dependent activation of cellular caspases after treatment with each of these three compounds (Figure S2A in the Supporting Information).
However, we found that caspase activation only partially contributed to the cell death, as two different caspase inhibitors, z-VAD-fmk and Boc-D-fmk, showed small inhibitory effects on the compounds' efficacy but no effect on potency (Figure S2B in the Supporting Information). This observation led us to explore additional cell death modulators to find key mechanisms regulating cell death induced by microtubule inhibitors. Accordingly, we tested 32 chemical and genetic perturbations to cell death for their effects on the sensitivity of tumor cells to these microtubule inhibitors (Table S1 in the Supporting Information). These cell death modulators were selected based on previous studies on validated suppressors and sensitizers of various forms of cell death. We confirmed the cell death modulating activity of the chemicals and genetic materials in our previous report.13 Vinblastine and colchicine were included in the analysis in parallel to see if there are cell death modulators common to microtubule-targeting agents. This analysis revealed several cell death modulators that showed stronger effects than caspase inhibitors in this experimental setting (Figure 3).
Figure 3.
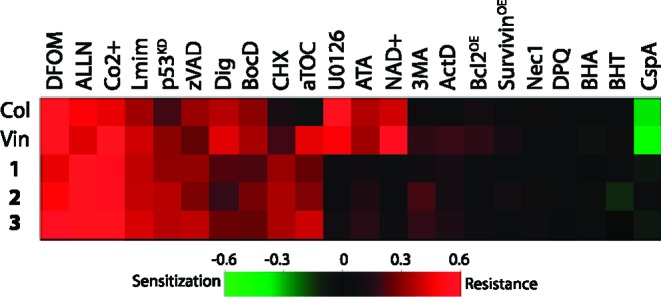
Suppressor screening of MTI-induced cell death. The heat map shows changes in the sensitivity of HT-1080 cells to MTI-induced cell death in the presence of known cell death modulators. See the Supporting Information for methods in detail.
These modulators are an iron chelator (deferoxamine), a calpain inhibitor (ALLN), and a calcium channel blocker (Co2+). The interaction between paclitaxel and calpain inhibitors was reported by Wang et al.14 In the paper, the authors concluded that a calpain inhibitor could ameliorate paclitaxel-induced sensory neuropathy in a mouse model, which is consistent with the data that we have obtained. On the other hand, several calcium channel blockers have been used as chemosensitizers for MTIs,15−17 which is contradictory to our observation. The mechanism of chemosensitization is known to be an off-target effect of some calcium channel blockers on competitive inhibition of the multiple drug resistance (MDR) gene product. It is possible that, unlike other calcium channel blockers, Co2+ does not inhibit MDR, leading to the protective effect in our assay. The effect of iron chelation was particularly striking. Further efforts to improve the efficacy of microtubule inhibitors could be directed toward these mechanisms (Figure 3).
The results of this modulator screening using small molecules and genetic reagents are significant, because they raise questions about the mechanism of cell death induced by microtubule-targeting agents, which may illuminate mechanisms of drug resistance to such agents. Three main causes of drug resistance are known for microtubule-targeting agents: drug efflux by ATP binding cassette (ABC) family membrane transporters,18 quantitative or qualitative alterations in microtubules,19 and deficiency in apoptotic processes.20 Our modulator screening results suggest that calcium channels, calpain, and iron metabolism are additional factors related to MTI drug resistance.
There are eight MTIs currently in clinical use; vinblastine, docetaxel, vinorelbine tartrate, ixabepilone, vincristine sulfate, halaven, colchicine, navelbine, and cabazitaxel. All of these compounds are natural product derivatives violating Lipinski's rule of 5, with the exception of colchicine. Although these drugs are in the mainstream in chemotherapy regimens, their poor solubility, synthetic tractability, and serious side effects are still problematic. Antitumor agents derived from synthetic small molecules, such as the compounds reported here, may overcome the liabilities of current MTIs.
Acknowledgments
We are grateful to the NIH MLPCN and GNF for assistance in generating growth inhibitory screening data.
Supporting Information Available
Supplementary table, figures, and methods. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
All authors contributed to the writing of the manuscript.
This research was supported by grants from the U.S. National Institutes of Health (R01CA097061, R01GM085081, and R01CA161061), the Arnold and Mabel Beckman Foundation, and NYSTAR. B.R.S. is an Early Career Scientist of the Howard Hughes Medical Institute.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Oprea T. I.; Bologa C. G.; Boyer S.; Curpan R. F.; Glen R. C.; Hopkins A. L.; Lipinski C. A.; Marshall G. R.; Martin Y. C.; Ostopovici-Halip L.; Rishton G.; Ursu O.; Vaz R. J.; Waller C.; Waldmann H.; Sklar L. A. A crowdsourcing evaluation of the NIH chemical probes. Nat. Chem. Biol. 2009, 57441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I.; Landis J. Can the pharmaceutical industry reduce attrition rates?. Nature Rev. 2004, 38711–715. [DOI] [PubMed] [Google Scholar]
- Johnson J. I.; Decker S.; Zaharevitz D.; Rubinstein L. V.; Venditti J. M.; Schepartz S.; Kalyandrug S.; Christian M.; Arbuck S.; Hollingshead M.; Sausville E. A. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer 2001, 84101424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S.; Lessnick S. L.; Hahn W. C.; Stockwell B. R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 33285–296. [DOI] [PubMed] [Google Scholar]
- Yang W. S.; Stockwell B. R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 153234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R. H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 610813–823. [DOI] [PubMed] [Google Scholar]
- Paull K. D.; Shoemaker R. H.; Hodes L.; Monks A.; Scudiero D. A.; Rubinstein L.; Plowman J.; Boyd M. R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81141088–1092. [DOI] [PubMed] [Google Scholar]
- Yaguchi S.; Fukui Y.; Koshimizu I.; Yoshimi H.; Matsuno T.; Gouda H.; Hirono S.; Yamazaki K.; Yamori T. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J. Natl. Cancer Inst. 2006, 988545–556. [DOI] [PubMed] [Google Scholar]
- Li P. K.; Pandit B.; Sackett D. L.; Hu Z.; Zink J.; Zhi J.; Freeman D.; Robey R. W.; Werbovetz K.; Lewis A.; Li C. A thalidomide analogue with in vitro antiproliferative, antimitotic, and microtubule-stabilizing activities. Mol. Cancer Ther. 2006, 52450–456. [DOI] [PubMed] [Google Scholar]
- Jiang J. D.; Davis A. S.; Middleton K.; Ling Y. H.; Perez-Soler R.; Holland J. F.; Bekesi J. G. 3-(Iodoacetamido)-benzoylurea: A novel cancericidal tubulin ligand that inhibits microtubule polymerization, phosphorylates bcl-2, and induces apoptosis in tumor cells. Cancer Res. 1998, 58235389–5395. [PubMed] [Google Scholar]
- von Haefen C.; Wieder T.; Essmann F.; Schulze-Osthoff K.; Dorken B.; Daniel P. T. Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene 2003, 22152236–2247. [DOI] [PubMed] [Google Scholar]
- Park S. J.; Wu C. H.; Gordon J. D.; Zhong X.; Emami A.; Safa A. R. Taxol induces caspase-10-dependent apoptosis. J. Biol. Chem. 2004, 2794951057–51067. [DOI] [PubMed] [Google Scholar]
- Wolpaw A. J.; Shimada K.; Skouta R.; Welsch M. E.; Akavia U. D.; Pe'er D.; Shaik F.; Bulinski J. C.; Stockwell B. R. Modulatory profiling identifies mechanisms of small molecule-induced cell death. Proc. Natl. Acad. Sci. U.S.A. 2011, 10839E771–E780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. S.; Davis A. A.; Culver D. G.; Wang Q.; Powers J. C.; Glass J. D. Calpain inhibition protects against Taxol-induced sensory neuropathy. Brain 2004, 127Part 3671–679. [DOI] [PubMed] [Google Scholar]
- Safa A. R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc. Natl. Acad. Sci. U.S.A. 1988, 85197187–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. M.; Hait W. N. Pharmacologic circumvention of multidrug resistance. Cytotechnology 1993, 121–3171–212. [DOI] [PubMed] [Google Scholar]
- Horio M.; Lovelace E.; Pastan I.; Gottesman M. M. Agents which reverse multidrug-resistance are inhibitors of [3H]vinblastine transport by isolated vesicles. Biochim. Biophys. Acta 1991, 10611106–110. [DOI] [PubMed] [Google Scholar]
- Fojo A. T.; Menefee M. Microtubule targeting agents: Basic mechanisms of multidrug resistance (MDR). Semin. Oncol. 2005, 326 Suppl. 7S3–S8. [DOI] [PubMed] [Google Scholar]
- Dumontet C.; Jordan M. A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nature Rev. 2010, 910790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinedo F.; Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 2003, 85413–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


