Abstract
Structure–activity relationship translation offers an expeditious means for discovery of new active series. This approach was applied to discover tetrahydroisoquinoline (THIQ)-based steroidomimetic microtubule disruptors. The two A-ring elements of a three-point steroidal pharmacophore were incorporated into a THIQ-based A,B-ring mimic to which an H-bond acceptor was attached as the third motif. Optimization of the representative 6c through conformational biasing delivered a 10-fold gain in activity and a new series of microtubule disruptors (e.g., 9c) with antiproliferative activity in the nanomolar range. The THIQ derivatives match, or surpass, the activities of the steroidal series and exhibit improved physicochemical properties.
Keywords: Tetrahydroisoquinoline, microtubule disruptor, structure−activity relationship, steroidomimetic
Despite great promise, exploration of the anticancer applications of colchicine site binding microtubule disruptors has yet to deliver an approved drug for clinical use. In contrast, compounds binding to the vinca and taxane sites on tubulin have found wide application and are arguably the most successful class of anticancer agents available.1 Colchicine site binders such as the combretastatins, 2-methoxyestradiol, and molecules inspired by, or derived from, these and related compounds do, however, remain in various stages of development.2 Our interest in this class arose from the discovery that sulfamoylation of 2-methoxyestrone yields a compound that, as well as inhibiting steroid sulfatase, a new enzyme target for postmenopausal hormone-dependent breast cancer,3 displays far higher in vitro and in vivo antiproliferative activity against cancer cells than 2-substituted estradiols.4,5 Further studies established that the various anticancer effects of the sulfamoylated 2-substituted estrogen derivatives can be ascribed to their ability to disrupt normal microtubule polymerization6 and that, unlike 2-methoxyestradiol, these sulfamates display high oral bioavailability.7 The steroidal derivatives bind to tubulin in a competitive manner versus colchicine,8 and their activity is independent of estrogen receptor status.4
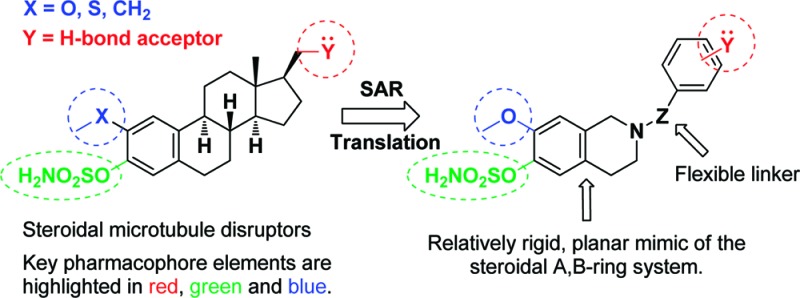
Having established the structure–activity relationship for antiproliferative activity in the sulfamoylated steroidal microtubule disruptor series (Figure 1),5,8−14 we wished to explore the potential to generate new series of anticancer agents by translating the key elements of the steroidal pharmacophore into alternate scaffolds. It seemed reasonable to commence by constructing prototypical systems from appropriately functionalized heterocyclic mimics of the A,B-ring system to which groups with an appropriate hydrogen bond acceptor to address a third key interaction at the colchicine site could be appended. Tetrahydroisoquinoline (THIQ)-based systems appeared ideal for this purpose since they possess a good degree of the rigidity present in the steroidal system, the N-2 nitrogen offers an achiral site of attachment for library generation, and a convergent, flexible entry to screening candidates could be envisaged (Figure 1). Furthermore, precedent for the successful use of tetrahydroisoquinolines in the generation of selective estrogen receptor modulators was available,15 and we have recently applied the same template for the generation of chimeric microtubule disruptors.16 We now describe how this approach was used to discover a new series of microtubule disruptors optimized to surpass, in many respects, the steroidal template that inspired their design.
Figure 1.
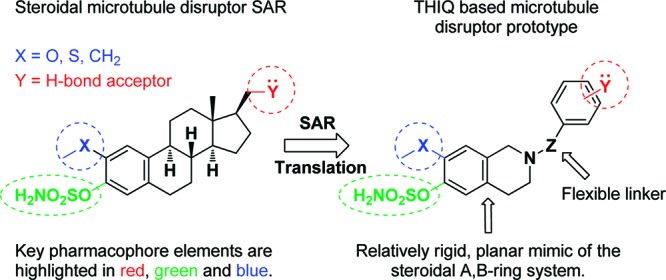
Design of prototypical tetrahydroisoquinoline-based microtubule disruptors by SAR translation.
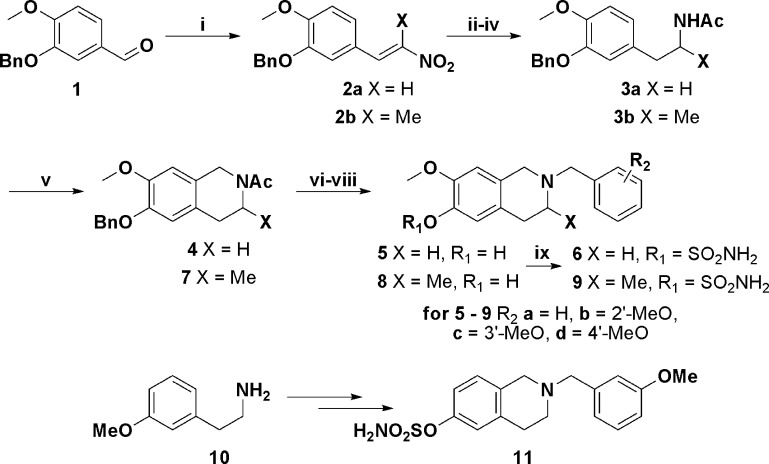
We required an efficient synthesis of 6-hydroxy-7-methoxy tetrahydroisoquinoline and selected an approach based on the Pictet–Spengler reaction (Scheme 1).17,18 Benzyl isovanillin 1 was subjected to a Henry aldol reaction with nitromethane, and the resultant nitro alkene 2a was reduced in two steps to yield the corresponding phenethylamine. To achieve good yields from the key Pictet–Spengler cyclization, it proved expeditious to acylate the phenethylamine to give 3a, since purification of the cyclized product 4 was greatly simplified and higher isolated yields were accordingly obtained. With the tetrahydroisoquinoline core 4 in hand, conversion to the prototypical screening set of N-benzyl and 2′-, 3′-, and 4′-methoxybenzyl derivatives was carried out by cleaving the N-acetyl group and then benzylating at N-2 with the appropriate benzyl halide. Deprotection of the 6-O-benzyl group gave the phenols 5, followed by sulfamoylation with sulfamoyl chloride in DMA to deliver the desired putative steroidomimetics 6.
Scheme 1. Synthesis of THIQ-Based Microtubule Disruptors.
Reagents and conditions: (i) For 2a, MeNO2, HOAc, for 2b EtNO2; NH4OAc, reflux (ii) for 3a Dioxane/DMF, for 3b EtOH; NaBH4, 0 °C (iii) Raney Ni, NH2NH2·H2O, MeOH, 50 °C (iv) Ac2O, TEA, DCM, 0 °C to rt (v) (CH2O)n, p-TsOH, PhMe, 120 °C (vi) KOH, EtOH/water, reflux (vii) ArCH2Cl,* TEA, EtOH, 130 °C, microwave (viii) H2, Pd/C, EtOH/THF (ix) H2NSO2Cl, DMA, 0 °C to rt: *R2a = H, b = 2′-MeO, c = 3′-MeO, d = 4′-MeO.
The prototypical library of phenols 5a–d and sulfamates 6a–d was then screened for antiproliferative activity against DU-145 prostate cancer cells.14 The results obtained are presented in Table 1. Phenol derivatives 5a–d show no significant activity. In contrast, the sulfamates 6a–d display promising activity, with the 2′- and 3′-methoxybenzyl derivatives 6b and 6c causing 50% growth inhibition of DU-145 cells at concentrations of 7.8 and 2.1 μM, respectively. The unsubstituted benzyl 6a and 4′-methoxybenzyl 6d compounds are inactive, and it would appear that the 2′- and 3′-methoxy substituents best map to the appropriate pharmacophore vector for antiproliferative effect. To further evaluate this hypothesis, we synthesized the analogous sulfamate derivative 11 lacking the 7-methoxy group. THIQ 11 was synthesized in a similar manner to that outlined for the conversion of 3–6 from commercially available 3-methoxyphenethylamine 10 with variation only in the deprotection chemistry applied.19 Screening of 11 for antiproliferative activity revealed that, like 5a–d and 6a, the compound is devoid of significant activity, and thus, when any of the three pharmacophore elements of the design template is deleted from prototypical lead 6c, so is any antiproliferative effect. These results validate our hypothesis that translating the key pharmacophoric elements of the steroidal series into a nonsteroidal motif could deliver new series of active agents.
Table 1. Activity of THIQ Derivatives against the Proliferation of DU-145 Human Prostate Cancer Cellsa.
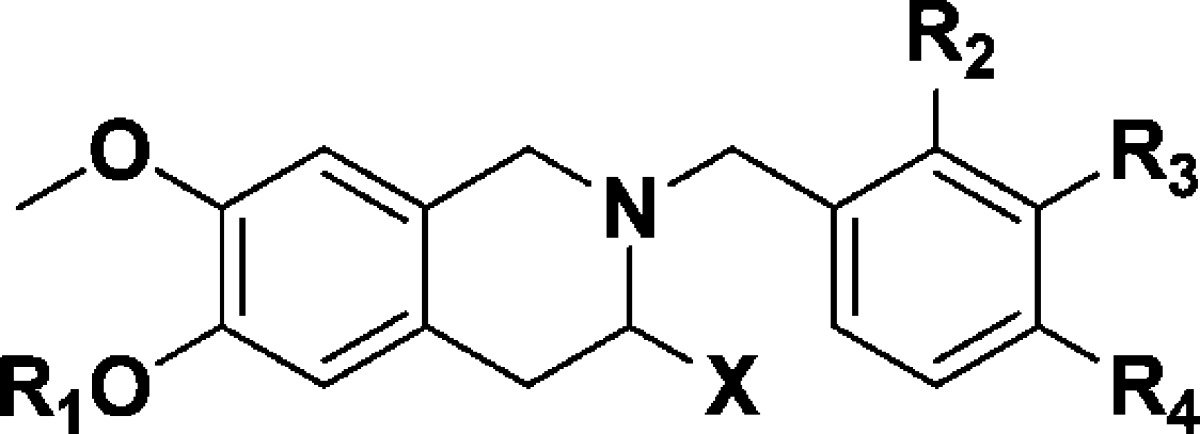
| R1 | X | R2 | R3 | R4 | DU-145 GI50 (μM) | |
|---|---|---|---|---|---|---|
| 5a | H | H | H | H | H | >100 |
| 6a | SO2NH2 | H | H | H | H | 80.5 |
| 5b | H | H | OMe | H | H | >100 |
| 6b | SO2NH2 | H | OMe | H | H | 7.8 |
| 5c | H | H | H | OMe | H | >100 |
| 6c | SO2NH2 | H | H | OMe | H | 2.1 |
| 5d | H | H | H | H | OMe | >100 |
| 6d | SO2NH2 | H | H | H | OMe | 57.2 |
| 8a | H | Me | H | H | H | >100 |
| 9a | SO2NH2 | Me | H | H | H | 2.2 |
| 8b | H | Me | OMe | H | H | >100 |
| 9b | SO2NH2 | Me | OMe | H | H | 3.4 |
| 8c | H | Me | H | OMe | H | 3.73 |
| 9c | SO2NH2 | Me | H | OMe | H | 0.222 |
| 8d | H | Me | H | H | OMe | >100 |
| 9d | SO2NH2 | Me | H | H | OMe | 1.1 |
| 11b | >100 |
SE ≤ 7%, n = 3.
For the structure of 11, see Scheme 1.
Clearly, however, the activity of 6c is still an order of magnitude below that of the parent steroidal compounds.11 A number of factors could possibly explain this, including the nonoptimal nature of the methoxy group as an H-bond acceptor, the methylene group as a linker between the THIQ core and our D-ring mimic, or indeed a possible intrinsic reduction of activity due to the presence of the basic amine in the THIQ core. One potential factor that we were keen to explore first is the effect of free precession of the N-benzyl group. This rotational freedom should logically result in only a small population of conformations that project the H-bond acceptor at the appropriate vector to mimic the steroidal compound (Figure 2). We thus examined whether methylation of the THIQ core at C-3 could favor the desired “steroid-like” conformational populations and thus deliver enhanced activity. A second generation of THIQs 9a–d was synthesized to test this hypothesis (Scheme 1). Replacement of nitromethane with nitroethane in the first step of the sequence gave nitroalkene 2b, which was transformed into C-3 methyl THIQ derivatives 8 and 9 in analogy to the synthesis of 5 and 6. THIQs 8a–d and 9a–d were then evaluated as antiproliferative agents and these data are presented in Table 1.
Figure 2.
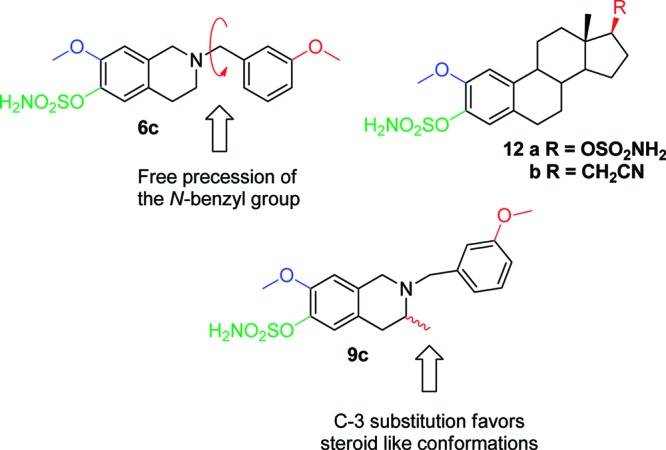
Introduction of C-3 substitution as a means to favor a “steroid-like” conformation.
Comparison of the C-3 methyl derivatives 8a–d and 9a–d with their unsubstituted analogues 5a–d and 6a–d reveals a profound difference in antiproliferative activity. As can be seen 8c, in contrast to the C-3 unsubstituted phenols 5a–d, exhibits activity (3.8 μM) that approaches that of sulfamate 6c (2.1 μM) and 2-methoxyestradiol (1.2 μM). The activities of the sulfamoylated compounds are even more encouraging, with a GI50 of 220 nM obtained for the 3′-methoxybenzyl derivative 9c. A 10-fold gain in activity is thus realized by incorporating a C-3 methyl group as a conformational bias (cf. 9c and 6c). Notable too are the activities of 9a and 9d, whose antiproliferative activities are in the low micromolar range, whereas the corresponding C-3 unsubstituted derivatives 6a and 6d show no significant activities at 100 μM. The activities of 9a (2.2 μM), which lacks the H-bond acceptor, and 9d (1.1 μM), which bears the H-bond acceptor, but apparently not at the vector to the A-ring pharmacophore elements to confer antiproliferative activity, suggests that the benzyl group is picking up positive lipophilic interactions associated with the steroidal D ring and thus serves to some extent as a D-ring mimic. Interestingly, in support of this observation, the relative activities of 9a and 9c are analogous to those displayed by 2-methoxy-17-deoxyestrone-3-O-sulfamate and 2-methoxyestrone-3-O-sulfamate in the steroidal series (GI50 values of 2.2 μM and 300 nM in MCF-7 breast cancer cells),5,10 indicating the complementary nature of the SAR in steroidal and THIQ-based agents. It seems reasonable to conclude that these THIQ derivatives are good steroidomimetics and the strategy could equally be applied to other targets interacting with steroidal ligands.
With these excellent in vitro data in hand, we wished to establish the microtubule disruptor activity of 9c, which was compared with the potent microtubule disruptor combretastatin A-4 (CA-4) and a representative steroid derivative 12a (Table 2). As can be seen, 9c disrupts the polymerization of tubulin with an IC50 of 15 ± 1 μM. Interestingly, this is less active than the steroidal derivatives with comparable antiproliferative activity [e.g., 12a, GI50 (DU-145) 340 nM, IC50 with tubulin 2.5 μM].8 Ultimately, the concentration required in tubulin-based assays far exceeds the antiproliferative dose and, most likely, it suffices to disrupt microtubule dynamics to arrest the cell cycle rather than cause a catastrophic depolymerization event. It should also be recalled that the nominal concentration in antiproliferative assays is that added to the culture medium, rather than the actual concentration of the agent within the cells. We also determined that 9c inhibits colchicine binding to tubulin, and its effect is similar to that of the steroidal derivative 12a, with both agents significantly less active than CA-4. It thus appears reasonable to suggest that the interaction of the novel THIQ derivative 9c can at least partially be ascribed to its ability to disrupt the normal dynamic polymerization of tubulin by interaction at, or around, the colchicine site. Combined with our determination that the pharmacophore elements requisite for activity in the steroidal series are required for optimal antiproliferative activity in THIQs both with, and without, a substituent at C-3 these results serve to validate our design hypothesis.
Table 2. Activity of THIQ Derivative 9c as an Inhibitor of Tubulin Polymerization and Colchicine Binding to Tubulina.
| inhibition of colchicine binding |
|||
|---|---|---|---|
| inhibition of tubulin assembly | % inhibition
± SD |
||
| compd | IC50 (μM) ± SD | 5 μM | 50 μM |
| CA-4 | 1.3 ± 0.1 | 98 ± 0.6 | |
| 12a | 2.5 ± 0.1 | 24.5 ± 5 | 74 ± 4 |
| 12b | 1.3 ± 0.1 | 78 ± 0.9 | |
| 9c | 15 ± 1 | 13 ± 2 | 59 ± 2 |
Data for 12b are taken from ref (8).
To shed more light on the mechanism of action of the novel THIQ derivatives, 6c, 8c, and 9c were evaluated as inhibitors of the proliferation of human CA46 Burkitt lymphoma cells, along with CA-4 and the steroidal compound 12b. Because antitubulin agents classically cause cells to accumulate in mitosis, the mitotic index with each agent at 10 times the IC50 was also determined. In untreated cells, the mitotic index in CA46 cells is always 2–4%.20 These data are presented in Table 3. With all three compounds, as well as CA-4 and 12b, a marked increase in the mitotic index occurred, a strong indication that at the cellular level the antiproliferative mechanism involved an interaction with tubulin.
Table 3. Activity of Compounds against the Proliferation of Human CA46 Burkitt Lymphoma Cells.
| compd | IC50 (nM) ± SD | % mitotic cells at 10 × IC50 |
|---|---|---|
| CA-4 | 6 ± 1 | 68 ± 8 |
| 12b | 350 ± 100 | 60 ± 10 |
| 6c | 2300 ± 300 | 66 ± 6 |
| 8c | 2500 ± 700 | 45 ± 10 |
| 9c | 230 ± 100 | 71 ± 7 |
The effects of C-3 methylation on the energy states of conformers of a model system, 3-methyl-N-benzyl-1,2,3,4-tetrahydroisoquinoline, arising from rotation of the N-benzyl group, and precession of the phenyl ring of this group around its axis, were assessed using computational energy calculations.21 These calculations indicate, as we had postulated, that in the minimal energy conformation the N-benzyl group is projected into the area of space close to that occupied by the steroidal D ring in the estratriene series. This is illustrated in Figure 3 where the minimal energy state of the (R)-enantiomer is overlaid with the energy-minimized estradiol core (see the Supporting Information for further detail). In contrast, in the maximum energy conformation the N-benzyl group eclipses the C-3 methyl group and is thus far away from the area of space occupied by the steroidal D ring.21 The energy difference between these states for either C-3 enantiomer is more than 120 kJ/mol. These calculations support our postulate that C-3 methylation favors adoption of a “steroid-like” conformation, and thus, it seems reasonable to propose that the positive effects of C-3 methylation on activity can, to some degree, be ascribed to this conformational biasing.
Figure 3.
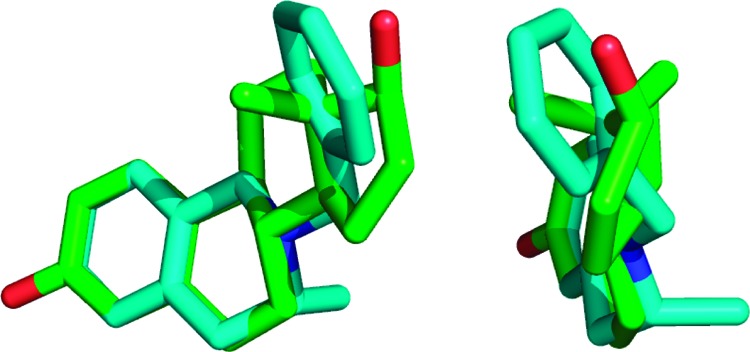
Overlay of the minimum energy conformation of 3(R)-methyl-2-benzyl-1,2,3,4-tetrahydroisoquinoline (cyan) with estradiol (green) viewed from two perspectives.
We have thus demonstrated how translation of pharmacophore elements can be successfully used to “series hop” and deliver new series of microtubule disruptors with excellent antiproliferative activity. These novel THIQ derivatives were optimized by biasing the conformational population through introduction of a steric buttress at C-3. In addition to an activity profile that matches the steroidal compounds from which their design was inspired, the new THIQ derivatives have low molecular weight and an excellent solubility profile. They form water-soluble salts and can be formulated as solutions in mild aqueous acid. We are actively exploring further optimization and preclinical development of this series and the application of THIQ-based steroidomimetics to other therapeutic targets.
Supporting Information Available
Experimental procedures and analytical data for compounds 8c and 9c and details of computational energy calculations. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by Sterix Ltd., a member of the Ipsen group, by VIP awards and a Programme Grant (082837) from the Wellcome Trust.
Supplementary Material
References
- Jordan M. A.; Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [DOI] [PubMed] [Google Scholar]
- Hadfield J. A.; Ducki S.; Hirst N.; McGown A. T.; Meijer L.; Jezequel A.; Roberge M. Progress in Cell Cycle Research. Prog. Cell Cycle Res. 2003, 309–325. [PubMed] [Google Scholar]
- Reed M. J.; Purohit A.; Newman S. P.; Potter B. V. L. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr. Rev. 2005, 26, 171–202. [DOI] [PubMed] [Google Scholar]
- Purohit A.; Hejaz H. A. M.; Walden L.; MacCarthy-Morrogh L.; Packham G.; Potter B. V. L; Reed M. J. The effect of 2-methoxyoestrone-3-O-sulphamate on the growth of breast cancer cells and induced mammary tumours. Int. J. Cancer 2000, 85, 584–589. [PubMed] [Google Scholar]
- Leese M. P.; Hejaz H. A. M.; Mahon M. F.; Newman S. P.; Purohit A.; Reed M. J.; Potter B. V. L. A-ring-substituted estrogen-3-O-sulfamates: potent multitargeted anticancer agents. J. Med. Chem. 2005, 48, 5243–5256. [DOI] [PubMed] [Google Scholar]
- MacCarthy-Morrogh L.; Townsend P. A.; Purohit A.; Hejaz H. A. M.; Potter B. V. L; Reed M. J.; Packham G. Differential effects of estrone and estrone-3-O-sulfamate derivatives on mitotic arrest, apoptosis, and microtubule assembly in human breast cancer cells. Cancer Res. 2000, 60, 5441–5450. [PubMed] [Google Scholar]
- Ireson C. R.; Chander S. K.; Purohit A.; Perera S.; Newman S. P.; Parish D.; Leese M. P.; Smith A. C.; Potter B. V. L; Reed R. J. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br. J. Cancer 2004, 90, 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan F.; Leese M. P.; Dohle W.; Hamel E.; Ferrandis E.; Newman S. P.; Purohit A.; Reed M. J.; Potter B. V. L. Synthesis, Antitubulin, and Antiproliferative SAR of Analogues of 2-Methoxyestradiol-3,17-O,O-bis-sulfamate. J. Med. Chem. 2010, 53, 2942–2951. [DOI] [PubMed] [Google Scholar]
- Leese M. P.; Newman S. P.; Purohit A.; Reed M. J.; Potter B. V. L. 2-Alkylsulfanyl estrogen derivatives: synthesis of a novel class of multi-targeted anti-tumour agents. Bioorg. Med. Chem. Lett. 2004, 14, 3135–3138. [DOI] [PubMed] [Google Scholar]
- Leese M. P.; Leblond B.; Newman S. P.; Purohit A.; Reed M. J.; Potter B. V. L. Anti-cancer activities of novel D-ring modified 2-substituted estrogen-3-O-sulfamates. J. Steroid Biochem. Mol. Biol. 2005, 94, 239–251. [DOI] [PubMed] [Google Scholar]
- Leese M. P.; Leblond B.; Smith A.; Newman S. P.; Di Fiore A.; De Simone G.; Supuran C. T.; Purohit A.; Reed M. J.; Potter B. V. L. 2-Substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J. Med. Chem. 2006, 49, 7683–7696. [DOI] [PubMed] [Google Scholar]
- Leese M. P.; Jourdan F. L.; Gaukroger K.; Mahon M. F.; Newman S. P.; Foster P. A.; Stengel C.; Regis-Lydi S.; Ferrandis E.; Di Fiore A.; De Simone G.; Supuran C. T.; Purohit A.; Reed M. J.; Potter B. V. L. Structure–activity relationships of C-17 cyano-substituted estratrienes as anticancer agents.. J. Med. Chem. 2008, 51, 1295–1308. [DOI] [PubMed] [Google Scholar]
- Jourdan F.; Bubert C.; Leese M. P.; Smith A.; Ferrandis E.; Regis-Lydi S.; Newman S. P.; Purohit A.; Reed M. J.; Potter B. V. L. Effects of C-17 heterocyclic substituents on the anticancer activity of 2-ethylestra-1,3,5(10)-triene-3-O-sulfamates: synthesis, in vitro evaluation and computational modelling. Org. Biomol. Chem. 2008, 6, 4108–4119. [DOI] [PubMed] [Google Scholar]
- Bubert C.; Leese M. P.; Mahon M. F.; Ferrandis E.; Regis-Lydi S.; Kasprzyk P. G.; Newman S. P.; Ho Y. T.; Purohit A.; Reed M. J.; Potter B. V. L. 3,17-Disubstituted 2-alkylestra-1,3,5(10)-trien-3-ol derivatives: synthesis, in vitro and in vivo anticancer activity. J. Med. Chem. 2007, 50, 4431–4443. [DOI] [PubMed] [Google Scholar]
- Chesworth R.; Zawistoski M. P.; Lefker B. A.; Cameron K. O.; Day R. F.; Mangano M.; Rosati R. L.; Colella S.; Petersen D. N.; Brault A.; Lu B. H.; Pan L. C.; Perry P.; Ng O.; Castleberry T. A.; Owen T. A.; Brown T. A.; Thompson D. D.; DaSilva-Jardine P. Tetrahydroisoquinolines as subtype selective estrogen agonists/antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 2729–2733. [DOI] [PubMed] [Google Scholar]
- Leese M. P.; Jourdan F.; Kimberley M. R.; Cozier G. E.; Thiyagarajan N.; Stengel C.; Regis-Lydi S.; Foster P. A.; Newman S. P.; Acharya K. R.; Ferrandis E.; Purohit A.; Reed M. J.; Potter B. V. L. Chimeric microtubule disruptors. Chem. Comm. 2010, 46, 2907–2909. [DOI] [PubMed] [Google Scholar]
- Cox E. D.; Cook J. M. The Pictet–Spengler condensation: a new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar]
- Schlosser M.; Simig G.; Geneste H. Three complementary methods offering access to 5-substituted 1,2,3,4-tetrahydroisoquinolines. Tetrahedron 1998, 54, 9023–9032. [Google Scholar]
- Zhong H. M.; Villani F. J.; Marzouq R. Improved and Practical Synthesis of 6-Methoxy-1,2,3,4-tetrahydroisoquinoline Hydrochloride. Org. Process Res. Dev. 2007, 11, 463–465. [Google Scholar]
- Cruz-Monserrate Z.; Vervoort H. C.; Bai R. L.; Newman D. J.; Howell S. B.; Los G.; Mullaney J. T.; Williams M. D.; Pettit G. R.; Fenical W.; Hamel E. Diazonamide A and a Synthetic Structural Analog: Disruptive Effects on Mitosis and Cellular Microtubules and Analysis of Their Interactions with Tubulin. Mol. Pharmacol. 2003, 63, 1273–1280. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information for full details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.