Abstract
Background. Few studies have addressed the timing of cervical cytologic abnormalities and human papillomavirus (HPV) positivity during the course of an infection. It remains largely unknown how infections detected by HPV and cytology wax and wane relative to each other. The aim of this analysis was to assess the longitudinal relationship of abnormal cytology and HPV positivity in a 7-year prospective study of 2500 women in Guanacaste, Costa Rica.
Methods. At each semiannual or annual visit, cervical specimens were screened using liquid-based cytology and tested for >40 HPV types with use of MY09/MY11 L1 degenerate primer polymerase chain reaction–based methods. On the basis of previous work, we separated prevalent and newly detected infections in younger and older women.
Results. Among newly detected HPV- and/or cytology-positive events, HPV and cytology appeared together ∼60% of the time; when discordant, HPV tended to appear before cytology in younger and older women. Combining newly and prevalently detected events, HPV and cytology disappeared at the same time >70% of the time. When discordant, HPV tended to disappear after cytology in younger and older women.
Conclusions. Detection of HPV DNA and associated cytological abnormalities tend to come and leave together; however, when discordant, detection of HPV DNA tends to precede and/or last longer than associated cytologic abnormalities.
Persistent infection with 1 of 10–15 carcinogenic types of human papillomavirus (HPV) causes virtually all cases of cervical cancer [1–3]. Screening for the cytologic manifestation of HPV (Papanicolaou test) has been the successful mainstay of cervical cancer prevention for a half century. Testing directly for HPV DNA or RNA is more sensitive but less specific than cytology [4]. Because these existing tests are complementary, cervical cancer screening in the future will incorporate both cytology and HPV testing.
In fact, cotesting for women ≥30 years of age (past the peak of acute and benign sexually transmitted HPV infection) is already approved by the US Food and Drug Administration [4]. Moreover, important clinical advisory groups, including the American Cancer Society and the American College of Obstetricians and Gynecologists, have recommended cotesting with cervical cytology and HPV for women >30 years of age [4]. In addition, several randomized controlled trials in the Netherlands, Sweden, and the United Kingdom are under way that evaluate the implementation of high-risk HPV testing in cervical screening, and results indicate health and economic benefit from HPV testing with cytology triage [5–7].
If cervical cancer screening programs are going to rely on 2 tests that measure different aspects of HPV infection, it is important to understand how they relate. We and others have documented in cross-sectional analyses that using conventional methods, a cytologic abnormality is found less than one-third of the time when an HPV infection is positive for a carcinogenic type.
However, what about over time? How do HPV DNA positivity and cytology wax or wane relative to each other? The majority of HPV infections and associated abnormalities clear within 1–2 years. It is currently unknown which test will yield a positive result first and which will revert to a negative result first when a woman is HPV positive and cytologically abnormal at the same visit. There have been few studies of the longitudinal relationship of HPV positivity and cytologic abnormality [8–11], and the scant published literature is inconsistent.
To inform cotesting, the aim of this analysis was to assess the longitudinal relationship of HPV positivity and abnormal cytology in a 7-year population-based prospective study in Guanacaste, Costa Rica.
METHODS
Study Population
From June 1993 through December 1994, a population-based cohort of randomly selected women aged 18–97 years from Guanacaste, Costa Rica, was established to study prospectively the natural history of HPV infection and cervical neoplasia. A total of 10 049 women were enrolled and provided informed consent (93.6% participation rate) [12]. Loss to follow-up was <10% [12]. The study design and methods of the Guanacaste Natural History Study have been described elsewhere [12, 13]. The study protocol was reviewed and reapproved annually by the National Cancer Institute and a Costa Rica Institutional Review Board.
Because of the longitudinal nature of this analysis of the natural history of HPV infection (measured by HPV DNA testing and cytology), only nonvirginal women who were actively followed up (n = 2655) could contribute events; women who, on the basis of their screening results, were judged to be at low risk of cervical intraepithelial neoplasia (CIN) grade 3+ were followed up passively and excluded. Abnormal cytology of ASCUS or greater led to inclusion; HPV positivity alone did not. We further excluded the small minority of women with prevalent and/or incident CIN2+ by pathology review and women with cytology high-grade squamous intraepithelial lesion (HSIL) (even if histologic CIN2+ was not found; n = 150). Our final analysis cohort included 2505 women.
The visit frequency varied by cytologic interpretation, according to safety guidelines existent at that time. Women in this actively followed cohort were scheduled to be screened every 6 months after a cytologic interpretation of low-grade squamous intraepithelial lesion (LSIL) or annually if they had only atypical squamous cells of undetermined significance (ASCUS).
Specimen Collection and Testing
Specimen collection
At enrollment and all follow-up visits, study clinicians collected exfoliated cervical cells from sexually active women with use of a Cervex broom-type brush (Unimar) to conduct conventional and liquid-based cytology (ThinPrep; Hologic Corporation). Exfoliated cells were also collected using a Dacron swab for HPV DNA detection and genotyping. The cells were stored first in ViraPap DNA transport medium (Digene Corporation, now Qiagen) and, later in the study, in specimen transport medium (STM; Digene) and were kept long-term in a −70°C mechanical or liquid nitrogen freezer until tested.
Cytology
Masked to HPV DNA test results, conventional and liquid-based cytology slides were read and classified using the Bethesda System [14]. Conventional smears were prepared and read in Costa Rica. Liquid-based ThinPreps were read by a single reader (M. L. H.). For this analysis, we present the results from ThinPrep. Reassuringly, we found similar patterns of HPV clearance and cytological regression associated with conventionally detected abnormal cytology as those found with ThinPrep (data not shown). Women with HSIL had already been excluded from the study population; we classified cytology as abnormal (positive), if interpretation was equivocal (ASCUS) or mildly abnormal (LSIL, including koilocytotic atypia and CIN1).
HPV DNA
DNA extracted from a 100 μL aliquot of the STM specimen was amplified using the MY09/MY11 L1 degenerate primer polymerase chain reaction (PCR) system with AmpliTaq Gold polymerase (TaqGold; Perkin-Elmer-Cetus) as described elsewhere [15]. After amplification, dot-blot hybridization of PCR products was conducted using type-specific oligonucleotide probes [16]. Concordant with the latest International Agency for Research on Cancer (IARC) classification [1, 17], we considered carcinogenic HPV types to include HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68. This PCR method of detecting HPV DNA is not ultrasensitive and has similar sensitivity to Hybrid Capture 2.
We do not discuss the common noncarcinogenic HPV types in this report. In the context of cervical screening, they represent noise. Although they may explain some of the abnormalities, 85%–90% of the time, individual infections with these types do not cause cytologic abnormalities. With the exception of some cross-reactivity, HPV testing by commercial kits does not detect them. Thus, we focus on carcinogenic HPV types. The pattern for noncarcinogenic types mirrored the pattern for carcinogenic types (data not shown).
Viral load
Three experienced HPV investigators interpreted type-specific dot-blot results to determine HPV PCR positivity. Two investigators then interpreted the strength of the hybridization signal with use of a semiquantitative index scale of 1 to 5 (weakest = 1; strongest = 5) by observing the density and diameter of the PCR product on the autoradiogram. This measure can be used as a semiquantitative measurement of HPV load and has reasonable agreement with quantitative Taqman PCR in women from this population infected with a single type [18].
Statistical Analysis
At enrollment and all follow-up visits, there were 4 possible HPV and cytology combinations in women without missing data: (1) HPV and cytology negative, (2) HPV negative and cytology positive, (3) HPV positive and cytology negative, and (4) HPV and cytology positive. This analysis explored the longitudinal fate of the joint HPV- and cytology-positive events detected at enrollment (prevalent) and newly detected during follow-up (incident). Although we fully recognize that the term “incident” may be misleading because some newly detected infections may be reappearing from an infection earlier in the woman’s life, for simplicity, we refer to infections newly detected during follow-up as incident.
We considered each type-specific HPV infection and/or abnormal cytology as a separate event. If a woman was infected with 2 carcinogenic types and the cytology was concurrently abnormal, both types contributed independently to the analysis. Therefore, 1 woman could contribute >1 event. In addition, if there was a carcinogenic and noncarcinogenic HPV type positive at the same time as the abnormal cytology, the event was attributed to the carcinogenic HPV type. We justified this decision based on the previous finding that carcinogenic HPV types are more likely to produce cytologic abnormalities than are noncarcinogenic types [19]. The conclusions of our analysis were not altered by restricting to single carcinogenic infections, although numbers of events and statistical precision were reduced substantially.
The frequency and percentage of abnormal cytology by HPV type was computed separately for younger women (age, <30 years; median age, 25 years), mid-adult women (age, 30 to <42 years; median, 35 years), and older women (age, ≥42 years; median, 52 years). We stratified by age, because prevalent (but not incident) HPV infections, having already lasted a substantial time, tend to persist longer in older women [20].
Similar to previous analyses within the Guanacaste Natural History Study, to standardize time among women, each visit was assigned to 1 of 16 time bins (6-month bins), with time of first detection of an HPV- and cytology-positive event (index event) as time 0. If there was >1 visit in a bin, the worst cytology and worst HPV result were used. Time of clearance of HPV and time to regression of cytology were defined as the bin with the first negative result; for cytology, negative was defined as returning to a normal cytology result. These assumptions lead to slight overestimates of time to clearance/regression but do not require assumptions of when in the preceding interval the infection disappeared. They are clinically relevant assumptions, because very frequent repeat testing is rarely performed.
If there was an intervening negative or normal between 2 positives, it was assumed to be false and a positive result was imputed. In the main analysis, for relevance to the clinical setting, we stratified age by younger (<30 years) and older (≥30 years), thus performing the analysis independently for 4 groups: younger women prevalent, younger women incident, older women prevalent, and older women incident.
For each incident event (HPV positivity and cytologic abnormality occurring together, following bins in which at least 1 was negative), we could assess which measure became positive first. We could also assess which became negative first. For prevalent events, we could only assess return to negativity (clearance for HPV detection or regression for cytology).
Starting from the index bin for each event (when HPV positivity and cytologic abnormality were first found together), we counted backward or forward to tabulate the relative appearance or disappearance of HPV and cytology, compared with each other. Then, we averaged over the entire study population.
Rather than present the results in mean number bins in which HPV or cytology lasted longer, we estimated the mean number of months as follows: (sum total number of discordant bins/total number of events) × 6 months. Of importance, we converted bins to months, and thus, time is estimated from discrete (time-censored) values.
For statistical testing, we used nonparametric statistics for paired and unpaired data, as follows: difference in the mean time between HPV positivity and cytologic abnormality was assessed within each of the 4 groups using a nonparametric Wilcoxon signed-rank test. Differences in overall mean time between the 4 groups were assessed using nonparametric Kruskal-Wallis test. Difference in the mean viral load between discordant events was assessed using Wilcoxon signed-rank test. All P values were 2-sided, and statistical significance was set at P < .05. Analyses were done using SAS version 9.1 (SAS Institute).
RESULTS
HPV-Positive, Cytologically Abnormal Visits
The 2505 women contributing to the analysis, after exclusions mentioned in the “Methods” section, contributed 17 440 visits. The visits were spaced at a mean of 12.5 months (median, 12 months). The mean length of follow-up was 74.8 months (median, 84 months).
Of the 2505 women in this subcohort at enrollment and throughout follow-up, 1013 (40%) were only ever carcinogenic HPV negative and cytology negative, 626 (25%) were only ever carcinogenic HPV negative and cytology positive, and 369 (15%) were only ever carcinogenic HPV positive and cytology negative. The relative proportions of these cytology/HPV combinations reflected which women were actively followed in the Guanacaste Natural History Study; i.e., ASCUS or worse cytology led to inclusion while HPV positivity with normal cytology did not. One hundred forty-four women (6%) were ever carcinogenic HPV and cytology positive, but not at the same time; 353 (14%) were ever carcinogenic HPV and cytology positive at the same time at least once.
At enrollment, 719 (29%) of the 2505 women were younger, 838 (33%) were mid-adult, and 948 (38%) were older. Ninety-seven (13%) of 719 younger women, 63 (8%) of 838 mid-adult women, and 44 (5%) of 948 older women had at least 1 carcinogenic HPV-positive and abnormal cytology event at enrollment; 88 (12%) of 719, 75 (9%) of 838, and 50 (5%) of 948 younger, mid-adult, and older women, respectively, had at least 1 carcinogenic HPV-positive and abnormal cytology event during follow-up.
Thus, as expected from previous reports of HPV positivity and cytologic abnormality in the Guanacaste Natural History Study, the percentage of women with a HPV-positive and abnormal cytology event decreased with increasing age. This trend reflected the combination of 2 subtrends: HPV DNA detection decreased with age (data not shown [21]), and when HPV DNA was found, the proportion of abnormal cytology decreased with age. However, we found in the present analysis that, within age group, the number of HPV-positive women showing cytologic abnormality was similar between prevalent and incident events.
Within each group defined by age and prevalence/incidence, carcinogenic infections were associated with LSIL more than with ASCUS; the ratio was ∼3:2, and there was not a significant difference in the proportion of LSIL and ASCUS caused by carcinogenic infections across the 6 groups (χ2 test, P = .74). Among events that were ASCUS positive and carcinogenic HPV positive, mean viral load was 3.73; mean viral load was 4.14 for events LSIL positive and carcinogenic HPV positive. Although based on very small numbers, analyses by individual α-species groups were not informative in terms of ASCUS versus LSIL patterns; mean viral loads were similar for the various alpha species (μα-5 = 4.08; μα-6 = 4.38; μα-7 = 3.72; μα-9 = 4.00). For relevance to the clinical setting where screening of women ≥30 years is being considered, we present the remaining analyses combining mid-adult and older women.
Main Longitudinal Analysis
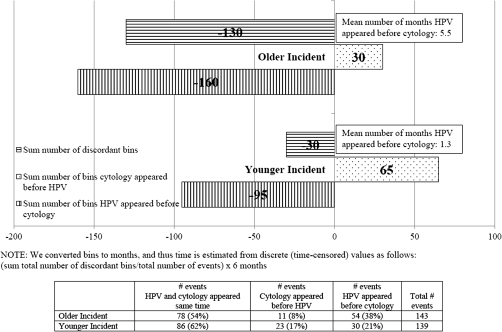
We could examine the relative appearance of HPV DNA and abnormal cytology among incident events only. HPV and cytology appeared at the same visit in 62% of cases among younger women and 54% of cases among older women; however, when discordant, HPV was detected a mean of 1.3 months (P = .36) and 5.5 months (P < .0001) earlier than abnormal cytology in younger and older women, respectively (Figure 1). There was a statistically significant difference in mean time HPV appeared before cytology between younger and older women (5.5 vs. 1.3, P = .01). Younger women contributed a mean of 7.6 visits per woman; older women contributed 8.6 visits per woman.
Figure 1.
Timing of appearance of incident carcinogenic human papillomavirus (HPV) infection/abnormal cytology (before index bin). Bins were converted to months, and thus, time is estimated from discrete (time-censored) values as follows: (sum total number of discordant bins/total number of events) × 6 months.
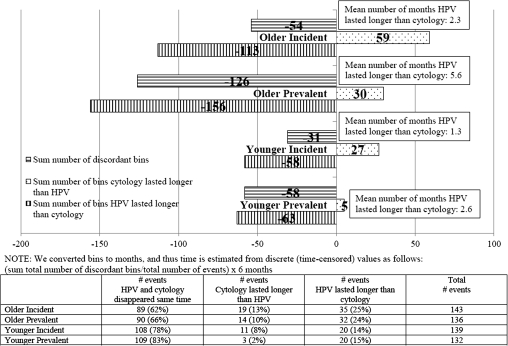
We could examine disappearance of HPV and cytologic abnormalities in both incident and prevalent HPV- and cytology-positive events. Among both combined, HPV and cytology cleared at the same time before the next visit in >70% of the events; however, when discordant, abnormal cytology tended to clear before HPV (Figure 2). HPV cleared a mean of 1.3 months after cytology (P = .10) in younger women with an incident HPV- and cytology-positive event, and 2.6 months after cytology (P < .0001) in younger women with a prevalent HPV- and cytology-positive event (Figure 2). Among older women, HPV cleared a mean of 2.3 months after cytology (P = .09) and 5.6 months after cytology (P = .0004) for incident and prevalent HPV- and cytology-positive events, respectively. There were no statistically significant differences in the means (mean time HPV lasted longer than cytology) between the groups (2.3 vs. 5.6 vs. 1.3 vs. 2.6, P = .58).
Figure 2.
Timing of disappearance of carcinogenic human papillomavirus (HPV) infection/abnormal cytology (after index event). Bins were converted to months, and thus, time is estimated from discrete (time-censored) values as follows: (sum total number of discordant bins/total number of events) × 6 months.
We explored whether mean semiquantitative carcinogenic viral load, as measured by hybridization signal strength, differed in the discordant events. In each of the 4 groups, the mean HPV load was higher but not statistically different in events in which cytology cleared first, compared with events in which HPV cleared first (Wilcoxon, P = .26, P = .66, P = .09, and P = .54 for younger prevalent, younger incident, older prevalent, and older incident, respectively). When we combined the 4 groups, mean HPV load remained nonstatistically different between discordant events (P = .37). Thus, although higher HPV DNA copy number has been shown in HPV-positive women with abnormal rather than normal cytology [22] and we found mean viral load was higher with LSIL than with ASCUS cytology, viral load did not appear to substantially influence whether HPV or cytologic abnormality cleared/regressed first.
When we removed those events in which neither carcinogenic HPV nor cytology ever cleared (ie, were both still positive at the final visit), we found the same pattern remained and the mean time difference did not change meaningfully (data not shown). Similarly, when we removed those events in which either HPV or cytology never cleared, the same pattern remained. However, because most of the events removed were events in which HPV lasted longer than cytology, the mean time that HPV lasted longer than cytology decreased in all 4 groups (data not shown).
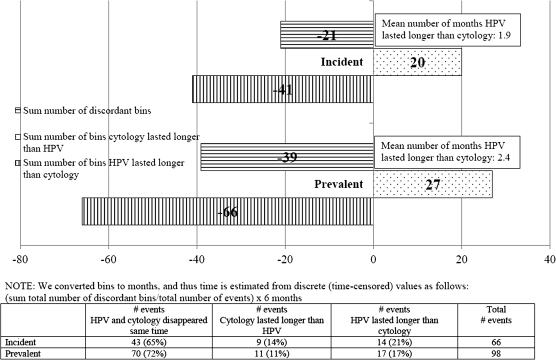
Finally, when we combined older and younger women and restricted the analysis to HPV and cytology events among women with a single carcinogenic infection ever detected during the cohort study, the same patterns remained (Figure 3), despite much reduced statistical power.
Figure 3.
Timing of disappearance of carcinogenic human papillomavirus (HPV) infection/abnormal cytology (after index event) among women infected with a single carcinogenic HPV type ever. Bins were converted to months, and thus, time is estimated from discrete (time-censored) values as follows: (sum total number of discordant bins/total number of events) × 6 months.
DISCUSSION
In this analysis of >2500 actively followed-up women from the Guanacaste Natural History Study, we investigated longitudinally the concordance of 2 measures of HPV infection, namely, HPV DNA detection and minor cytologic abnormality. Our analysis showed that, at the level of discrimination provided by common clinical screening intervals, HPV DNA and associated cytological abnormalities tend to come and leave together; however, when discordant, HPV DNA precedes and/or lasts longer than associated cytologic abnormalities.
The findings were constant, regardless of age or prevalence/incidence of finding HPV DNA and abnormal cytology together. In each group, >70% of the HPV- and cytology-positive events reverted to negative DNA/normal cytology a mean of 12 months after initial concordant detection, therefore providing evidence for short duration of dual HPV infection and mildly abnormal cytology. Of note, the appearance and disappearance of HPV and cytology is part of a continuous infection process in both concordants and discordants and that, within the confines of our screening tools, we can only see this process through sequential snapshots.
It was unsurprising that HPV tended to appear before abnormal cytology when discordant, based on ample epidemiologic data that HPV often precedes and predicts abnormal cytology. There are several cross-sectional analyses showing that the majority of women positive for high-risk HPV have no evidence of abnormal cytology [23, 24]. The data in the present study are complementary, showing that HPV positivity precedes and lasts longer than cytologic abnormality. However, it is unclear to what degree the broader and longer base of HPV infection, detected molecularly, is a biological or methodological phenomenon. Although more women were HPV negative and cytology positive than HPV positive and cytology negative, this mainly reflects which women were actively followed in the Guanacaste Natural History Study. Moreover, >80% of cytology positive, HPV negative women had ASCUS. This finding could also be the result of nonspecificity of the equivocal finding of ThinPrep ASCUS as interpreted in this study [25]. Thus, it is important to consider the choice of HPV assay and cytological detection method in a study of this kind. In addition, HPV testing may be more sensitive and less specific than cytology at any time point, rather than a reflection of a true absence of rare abnormal cells at the beginning and/or end of an acute infection. Of note, we did use a sensitive microscopic method of detecting cytological abnormalities (ThinPrep read with a relaxed threshold for ASCUS), and our method of HPV detection (PCR) has similar sensitivity to the method used in the clinical setting (HC2 HPV DNA Test). In addition, a confirmatory analysis based on conventional cytology showed the same pattern. Thus, we feel that the conclusions are generalizable.
The literature on timing of HPV clearance and cytological regression is sparse and conflicting. Among women in the ALTS trial, we reported that HPV cleared a mean 2.5 months after cytological regression [8]. However, 3 other studies reported HPV cleared a mean of 1–4 months before cytological regression and concluded that, in women with cytological abnormalities, HPV clearance predicts cytological regression [9–11]. In this analysis, we tried to replicate these previous findings by requiring 2 successive type-specific HPV negatives and 2 successive normal cytologies, limiting the analysis to only those women who had cleared both HPV and cytology and limiting to those women who cleared either HPV or cytology during the observation period. We were unable to replicate the previous studies and found that regardless of the restrictions used, HPV tended to clear after cytological regression when events were discordant.
We cannot convincingly explain the differences among the findings but note that the studies are not comparable in several ways. In the previous studies, HPV detection, although carcinogenic, was non–type-specific, and HPV clearance was defined as negative HPV at the next visit, whereas cytological regression required normal cytology in 2 consecutive cervical smears. Differences between the studies could also be attributed to smaller samples sizes, different statistical analyses, different restriction criteria used to define cytological regression and HPV clearance, and/or international variation in interpretations of abnormal cytology. Regardless of differing results, a common finding from all studies is that, when HPV and cytology are cotested positive for the first time, on average, they will both be negative by the next clinical visit (ie, HPV and cytology will typically clear prior to 12 months). In addition, our data show that, if cytology regresses before HPV after a woman is cotested positive, HPV will clear, on average, within 6 months following cytological regression. Furthermore, data from our same cohort show that, in general, <10% of carcinogenic HPV infections persist past 6 years; however, risk of CIN3+ increases as infections continue to persist [17].
The main strength of this study is that the use of a large number of events studied with an intensive and long follow-up period. In addition, our use of ThinPrep cytological screening methods, corroboration of patterns using conventional cytology, and inclusion of only the carcinogenic types increase applicability to cotesting conducted in the clinical setting. We predict that, when cotesting is used, clinicians will find that HPV infections and associated equivocal or mildly abnormal cytology (ASCUS or LSIL) tend to appear and disappear at the same time; however, when discordant, HPV will tend to appear and last longer than associated abnormal cytology.
Notes
Financial support.
This work was supported in part by the National Institutes of Health research training grant (NIH, R25 CA098566 to S.C.M.). The Guanacaste Project was supported by National Cancer Institute, NIH, Department of Health and Human Services (NO1-CP-21081, NO1-CP-33061, NO1-CP-40542, and NO1-CP-506535).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bouvard V, Baan R, Straif K, et al. WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 2.Ho GY, Burk RD, Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 4.Waxman AG. Cervical cancer screening in the early post vaccine era. Obstet Gynecol Clin North Am. 2008;35:537–48. doi: 10.1016/j.ogc.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–72. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 6.Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–97. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 7.Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672–82. doi: 10.1016/S1470-2045(09)70156-1. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman M, Wheeler CM, Castle PE. Human papillomavirus DNA remains detectable longer than related cervical cytologic abnormalities. J Infect Dis. 2002;186:1169–72. doi: 10.1086/343816. [DOI] [PubMed] [Google Scholar]
- 9.Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, et al. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet. 2001;358:1782–3. doi: 10.1016/S0140-6736(01)06809-X. [DOI] [PubMed] [Google Scholar]
- 10.Denise Zielinski G, Snijders PJ, Rozendaal L, et al. High-risk HPV testing in women with borderline and mild dyskaryosis: long-term follow-up data and clinical relevance. J Pathol. 2001;195:300–6. doi: 10.1002/path.981. [DOI] [PubMed] [Google Scholar]
- 11.Syrjanen S, Shabalova IP, Petrovichev N, et al. Clearance of high-risk human papillomavirus (HPV) DNA and PAP smear abnormalities in a cohort of women subjected to HPV screening in the New Independent States of the former Soviet Union (the NIS cohort study) Eur J Obstet Gynecol Reprod Biol. 2005;119:219–27. doi: 10.1016/j.ejogrb.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Herrero R, Schiffman MH, Bratti C, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salub Publica. 1997;1:362–74. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 13.Bratti MC, Rodriguez AC, Schiffman M, et al. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10 000 women in Guanacaste, Costa Rica. Rev Panam Salub Publica. 2004;15:75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 14.The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses. National Cancer Institute Workshop. JAMA. 1989;262:931–4. [PubMed] [Google Scholar]
- 15.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 16.Qu W, Jiang G, Cruz Y, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35:1304–10. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravitt PE, Kovacic MB, Herrero R, et al. High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int J Cancer. 2007;121:2787–93. doi: 10.1002/ijc.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacic MB, Castle PE, Herrero R, et al. Relationships of human papillomavirus type, qualitative viral load, and age with cytologic abnormality. Cancer Res. 2006;66:10112–9. doi: 10.1158/0008-5472.CAN-06-1812. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:1–10. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle PE, Fetterman B, Thomas Cox J, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol. 2010;116:76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 22.Schiffman M, Herrero R, Hildesheim A, et al. HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica. JAMA. 2000;283:87–93. doi: 10.1001/jama.283.1.87. [DOI] [PubMed] [Google Scholar]
- 23.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 24.Watt A, Garwood D, Jackson M, et al. High-risk and multiple human papillomavirus (HPV) infections in cancer-free Jamaican women. Infect Agent Cancer. 2009;4(Suppl 1):S11. doi: 10.1186/1750-9378-4-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson ML, Zahniser DJ, Sherman ME, et al. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer. 1999;87:48–55. doi: 10.1002/(sici)1097-0142(19990425)87:2<48::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]