Abstract
(See the editorial commentary by Johnson, on pages 353–4.)
Background. Clostridium difficile infection (CDI) can cause a wide range of disease, from mild diarrhea to fulminant systemic disease. The incidence of systemic CDI with fatal consequence has increased rapidly in recent years.
Methods. Using an ultrasensitive cytotoxicity assay, we measured C. difficile toxin A (TcdA) and C. difficile toxin B (TcdB) in sera and body fluids of piglets and mice exposed to C. difficile to investigate the relationship between the presence of toxins in body fluids and systemic manifestations of CDI.
Results. We found that both TcdA and TcdB disseminate systemically, with toxins present in the sera and body fluids of infected animals, and toxemia is significantly correlated with the development of systemic CDI. The systemic administration of neutralizing antibodies against both toxins blocked the development of systemic disease in mice. We measured cytokine concentrations in the sera of mice and piglets with systemic and nonsystemic CDI and found that proinflammatory mediators were considerably elevated in animals with systemic CDI.
Conclusion. Our study demonstrates the existence of a strong correlation between toxemia and the occurrence of systemic disease, supporting the hypothesis that systemic CDI is most likely due to the toxicity of TcdA and TcdB and the induction of proinflammatory cytokines by the toxins.
Clostridium difficile is an important nosocomial pathogen and the most commonly diagnosed cause of antibiotic-associated diarrhea and colitis [1]. C. difficile infection (CDI) can cause a wide range of disease, from mild diarrhea to fulminant systemic disease. Since 2000, outbreaks of CDI with greater morbidity and mortality have occurred in many developed countries [2]. CDI is thought to be caused by 2 glucosylating toxins secreted by the bacterium C. difficile toxin A (TcdA) and toxin B (TcdB) because strains lacking the toxins are avirulent. Recent studies show that both toxins can cause disease in hamsters [3], but their relative roles in the disease are unclear. C. difficile infection commonly results in severe gastrointestinal pathology, including pseudomembranous colitis and toxic megacolon, but may also result in a variety of observed systemic complications. Reported systemic complications in patients with CDI include ascites [4, 5], pleural effusion [6, 7], cardiopulmonary arrest [8, 9], hepatic abscess [10], abdominal compartment syndrome [11], acute respiratory distress syndrome [12], multiple organ dysfunction syndrome [13], and renal failure [14]. Although bacteremia has been identified as a cause of systemic manifestations of CDI in some cases, it is not always present, and other mechanisms by which C. difficile causes these systemic effects are not well understood. The 2 glucosylating toxins produced by the bacteria are likely involved, and a greater understanding of the systemic effects of C. difficile infection and why they occur in some patients, but not others, is important because these effects are often life-threatening in nature.
Animals infected by C. difficile often experience systemic manifestations of disease, in addition to typical gastrointestinal signs. Naturally infected piglets sometimes develop respiratory distress and hydrothorax, as well as ascites [15, 16]. Administration of TcdB to zebrafish causes cardiac damage [17], a finding that places more importance on understanding the systemic role of these toxins in mammals. An obstacle to investigations of the relationship between systemic disease and toxemia in CDI has been the lack of a sensitive detection assay for toxins in blood or tissue fluid. We recently reported the development of an ultrasensitive immunocytoxicity (ICT) assay, which can detect toxin concentrations as low as 1 pg/mL in samples of the serum and body fluids of piglets with CDI [18, 19]. Using this assay, we investigated the relationship between the occurrence of systemic disease and toxemia in gnotobiotic piglet and mouse models of CDI.
MATERIALS AND METHODS
Animals and Inoculation
Gnotobiotic piglets were delivered via cesarean section into a sterile isolator and maintained in groups of 2–4 inside isolators for the duration of the study, as previously described [18]. We orally inoculated a total of 79 piglets, ranging in age from 2 days to 7 weeks, with 105–109 C. difficile spores using the NAP1/027/BI strain UK6 [20]. For 1 group of 8 piglets, we collected blood daily after inoculation, and for all piglets, we collected blood at the time of euthanasia. We performed a necropsy on each piglet following euthanasia or death, assessed gross gastrointestinal and systemic lesions, and collected body fluids, including pleural and abdominal effusion, if present.
We maintained 6- to 9-week-old C57BL/6 mice (Jackson Laboratory) in pathogen-free facilities in groups of 5. We orally inoculated mice with 106 C. difficile spores using the NAP1/027/BI strain UK1 [20] after a series of antibiotic treatments, as previously described by Chen et al [21]. We collected blood daily after inoculation to assess the progression to toxemia.
We collected tissues, including small intestine, large intestine, mesenteric lymph nodes, liver, kidney, spleen, lung, and heart, from piglets and mice at the time of necropsy for histopathologic examination. We handled and cared for all animals according to Institutional Animal Care and Use Committee guidelines.
All piglets and mice were monitored for the development of clinical signs of CDI several times daily after inoculation with C. difficile. Based on the assessment of signs, we classified animals as developing either nonsystemic CDI or systemic CDI. For both piglets and mice, nonsystemic CDI refers to the presence of localized gastrointestinal illness only, evidenced by development of diarrhea, without any systemic clinical signs. Systemic CDI refers to the development of systemic signs of illness in addition to diarrhea. In piglets, clinically observable systemic signs include lethargy, depression, weakness, and dehydration, progressing to complete anorexia and dyspnea. In mice, systemic CDI is evidenced by depression, unkempt haircoat, hunched posture, weakness, and dehydration, progressing to anorexia and abdominal distension. Both piglets and mice were euthanized either when systemic clinical signs were progressively worse over the course of several daily checkpoints or when the animal was found moribund or dyspneic. Animals that never developed any systemic signs of illness were euthanized at a predetermined experimental endpoint, generally 7–10 days after inoculation.
Immunocytotoxicity Assay
To determine the presence of toxins in serum samples, we used the ultrasensitive ICT assay using both the real-time cell electronic sensoring system (RT-CES, Roche Applied Science) as previously described by our laboratory [18], as well as traditional methods of observing cell rounding. In brief, mRG1-1 cells, which express the FCγRI-α chain, were added to wells of microelectrode-imbedded microplates (E plates) or standard 96-well plates. We added the A1H3 mouse anti-TcdA monoclonal antibody generated by our laboratory [22] to wells with cells. A1H3 increases the sensitivity of cells to TcdA, allowing detection of low concentrations of TcdA in samples to the 1–10 pg/mL range [18]. TcdB is more cytotoxic than TcdA and can be detected ∼ 10 pg/mL without an enhancing antibody. Mouse and piglet serum samples were added to cells at a 1:10 final dilution. We used goat anti-TcdA and anti-TcdB serum (TechLab) in the assays to neutralize the activity of both toxins. Rabbit anti-TcdA and alpaca anti-TcdB sera were used for neutralizing TcdA and TcdB, respectively. Recombinant TcdA and TcdB [23] were used as positive controls and standards. After addition of samples, toxins, and antibodies, we connected E plates to the RT-CES and collected cytotoxicity data that was measured via cell index for 12–24 hours. We used a phase-contrast microscope to assess the percentage of cell rounding on standard plates hourly for ≤8 hours and then after overnight incubation.
Rac1 Glucosylation
We evaluated piglet and mouse serum samples, which were toxin positive in the ICT assay, for glucosyltransferase activity by assessing glucosylation of the Rho GTPase Rac as described previously [23]. In brief, mRG1-1 cells were seeded in 24-well plates and incubated until the cells reached confluence. Cells and A1H3 only were used as negative controls, and 10 ng/mL of TcdA with A1H3 was used as a positive control. We used the polysera described previously to neutralize either both toxins or each toxin separately. We harvested cells when toxin control wells reached 100% cell rounding, and we performed immunoblotting. We used antibodies that specifically recognize the nonglucosylated form of Rac1 (clone 102, BD Bioscience) and anti-β-actin (clone AC-40, Sigma) as the primary antibodies and horseradish peroxidase–conjugated anti-mouse immunoglobulin G (Amersham Biosciences) as the second antibody.
In Vivo Neutralizing Antibody Treatment
We orally inoculated C57BL/6 mice with 106 C. difficile UK1 spores after antibiotic treatment, as previously described by Chen et al [21]. We treated mice 4 hours after inoculation with C. difficile, with alpaca polyclonal antisera against TcdA and TcdB. The antiserum for each toxin were generated separately, and mice were dosed based on the neutralizing activity of the sera with a single intraperitoneal injection of the mixed anti-TcdA and anti-TcdB sera. We injected control mice with an equal amount of nonimmune sera. All mice were monitored for development of clinical signs of CDI and progression to systemic CDI and mice that became moribund were euthanized.
Cytokine Measurement
In piglet serum samples, cytokine concentration was determined for interleukin 1β (IL-1β), interleukin 4 (IL-4), interleukin 6 (IL-6), interleukin 8, interleukin 10 (IL-10), interleukin 12 (IL-12), tumor necrosis factor α (TNF-α), transforming growth factor β, and interferon γ (IFN-γ) using porcine cytokine quantification kits (Invitrogen and R&D). In total, 69 piglet serum samples were analyzed, including 29 from animals with systemic CDI and 40 from animals with nonsystemic CDI. For mice, a total of 19 serum samples from 69 mice were analyzed, including 7 from animals with systemic CDI, 7 from animals with nonsystemic CDI, and 5 from animals treated with alpaca polyclonal antisera against TcdA and TcdB described previously. The cytokine concentration for IFN-γ, IL-12, IL-10, TNF-α, IL-6, KC (CXCL1), and IL-1β was determined, following the manufacturer’s directions, with murine cytokine quantification kits (Invitrogen and R&D).
Statistical Analysis
We compared categorical variables, such as presence of toxemia and systemic CDI, for each group using the Fisher exact test. We used the t test to analyze continuous variables, such as cytokine measurements. All analyses were performed using SPSS statistical software version 16.0.
RESULTS
Both TcdA and TcdB Are Liberated Into Serum and Body Fluids
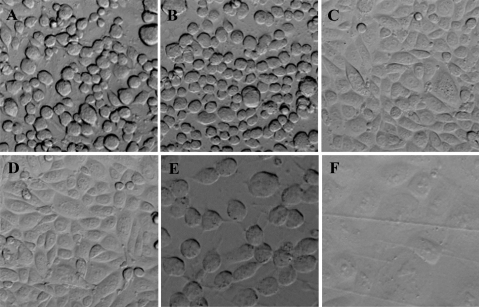
Using the ICT assay [18], we found that 12 of 43 piglet serum samples with CDI (27.9%) and 23 of 69 mouse serum samples with CDI (33.3%) were positive for toxin. Additionally, 6 of 13 pleural fluid samples (46.2%) and 11 of 14 ascites samples (78.6%) collected from piglets and 5 of 5 pleural fluid samples (100%) and 2 of 2 ascites samples (100%) collected from mice were positive for toxin (Table 1). The concentrations of TcdA or TcdB detected in the sera and fluids ranged from 1 pg/mL to 10 ng/mL, and were determined either by the cell index curve or percentage of cell rounding. Figure 1 depicts the appearance of cells in the ICT assay using a standard 96-well plate. Serum samples collected from a piglet and mouse with systemic CDI caused typical cell rounding (Figure 1B and 1E), similar to that of TcdA as a control (Figure 1A). Preinoculation serum or the toxemic samples mixed with neutralizing anti-TcdA and TcdB antibodies had no ability to cause cell rounding (Figure 1C, D, F), indicating that the cell rounding is indeed caused by the toxins.
Table 1.
Toxin in Body Fluid Samples and Association With Systemic Clostridium difficile Infection in Piglets and Mice With C. difficile Infection
| Seruma |
Pleural Fluid |
Ascites |
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Piglets (total) | 12 | 31 | 6 | 7 | 11 | 3 |
| Systemic CDI (18) | 12 | 6 | 6 | 7 | 11 | 2 |
| Nonsystemic CDI (25) | 0 | 25 | 0 | 0 | 0 | 1 |
| Mice (total) | 23 | 46 | 5 | 0 | 2 | 0 |
| Systemic CDI (27) | 23 | 4 | 5 | 0 | 2 | 0 |
| Nonsystemic CDI (34) | 0 | 42 | 0 | 0 | 0 | 0 |
Serum and body fluid samples were collected from piglets and mice at the time of euthanasia and necropsy.
Abbreviation: CDI, C. difficile infection.
P < .0001 for association of toxemia with systemic CDI in piglets and mice using the Fisher exact test.
Figure 1.
Cell rounding caused by piglet and mouse serum samples. A, 10 ng/mL of Clostridium difficile toxin A (TcdA); B, Serum from a piglet with severe C. difficile infection (CDI); C, Piglet serum in (B) plus anti-TcdA and C. difficile toxin B (TcdB) polysera; D, Piglet preinoculation serum; E, Serum from a mouse with severe CDI; F, Mouse serum in (E) plus anti-TcdA and anti-TcdB polysera.
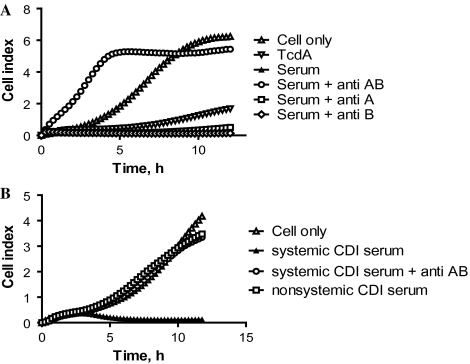
To determine which of the 2 toxins were present in the samples, neutralizing antibodies against individual toxins were added to sample wells in the ICT assay. Figure 2A illustrates representative data from the assay with serum from 1 piglet with systemic CDI. Cells in the control well displayed normal attachment and growth (Figure 2A) with a rapid rise in the cell index, and cells with 10 ng/mL of TcdA displayed retarded growth with a minimal increase in cell index (Figure 2A). The cell index of the well containing the piglet serum remained at baseline levels throughout the 24-hour period (Figure 2A). Addition of anti-TcdA and anti-TcdB antibodies to the serum sample fully neutralized the toxicity, indicating that it is the presence of the toxins in the serum causing cytotoxicity (Figure 2A). Addition of only anti-TcdB antibody did not improve the cell index (Figure 2A), and addition of only anti-TcdA antibody resulted in a marginal increase in the cell index (Figure 2A), indicating that both toxins were present in the serum in cytotoxic concentrations. Similarly, in Figure 2B, sera from 1 representative mouse with systemic CDI and 1 mouse with nonsystemic CDI were evaluated for the presence of toxin using the ICT assay. Only serum from the mouse with systemic CDI caused a retarded growth curve (Figure 2B), and addition of antibodies against TcdA and TcdB fully neutralized the toxic effect of the serum (Figure 2B).
Figure 2.
Toxins in piglet and mouse sera. A, Serum from 1 representative piglet with systemic Clostridium difficile infection (CDI) was assessed for the presence of toxemia, using antibodies against C. difficile toxin A (TcdA) and C. difficile toxin B (TcdB) separately to demonstrate the presence of each toxin. B, Sera from 1 representative mouse with systemic CDI and 1 mouse with nonsystemic CDI were assessed for the presence of toxemia. Control = cells only; anti AB = goat anti-TcdA and anti-TcdB polysera; anti A = alpaca anti-TcdA polysera; anti B = alpaca anti-TcdB polysera.
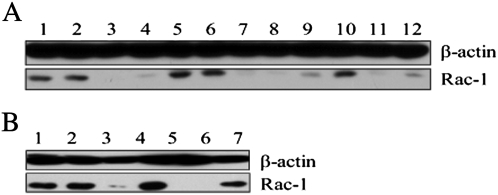
To further verify the presence of clostridial glucosylating toxins, we evaluated the glucosylation of Rac1 in cells in the presence of the serum samples. When TcdA or TcdB is present in a sample, the Rac1 in mRG1-1 cells incubated in wells with the sample will be glucosylated by the toxin and thus will be undetectable by the anti-Rac1 antibody, which recognizes only the nonglucosylated protein [23]. In control wells containing only cells or cells plus neutralizing antibodies alone, Rac1 was readily detectable (Figure 3A , lane 1, 2), but when toxin was incubated with the cells as a positive control, Rac1 was not detected (Figure 3A, lane 3, 4). Mouse presera and sera collected 1 day postinoculation were negative for toxin based on the presence of Rac1, but samples collected from mice 2 days postinoculation, with development of systemic CDI, were positive (Figure 3A, lanes 5–9). When anti-TcdA and TcdB antibodies were added to the samples and incubated with cells, toxin activity was completely neutralized, and Rac1 levels were similar to that of controls (Figure 3A, lane 10). When only anti-TcdA or only anti-TcdB antibodies were added to the sample, toxin activity could not be fully neutralized, indicating the presence of both toxins in the serum (Figure 3A, lanes 11, 12). The Rac1 glucosylation assay was also performed with piglet serum samples obtained from the same animal as in Figure 2. Serum from this piglet was negative at day 5 postinoculation, but positive on day 7, when systemic disease was noted, and again, the glucosylation was completely abrogated by addition of anti-TcdA and anti-TcdB antibodies to the cells with samples (Figure 3B).
Figure 3.
Glucosyltransferase activity in mouse and pig sera. The mRG1-1 cells were exposed to mouse (A) or pig (B) sera before being harvested for detection of Rac1 glucosylation by immunoblotting as described in Materials and Methods. A, Lane 1: cells only; lane 2: A1H3 antibody only; lane 3: 10 ng/mL Clostridium difficile toxin A (TcdA) + A1H3; lane 4: 1 ng/mL TcdA + A1H3; lane 5: mouse preinoculation serum (1:10) + A1H3; lane 6: mouse serum (1:20) 1 d postinoculation (PI) + A1H3; lane 7: mouse serum (1:20) 2 d PI + A1H3; lane 8: mouse serum (1:50) 2 d PI + A1H3; lane 9: mouse serum (1:200) 2 d PI + A1H3; lane 10: mouse serum (1:20) 2 d PI + A1H3 + anti-TcdA and anti-TcdB polysera (1:1000); lane 11: mouse serum (1:20) 2 d PI + A1H3 + anti-TcdA polysera (1:1000); lane 12: mouse serum (1:20) + A1H3 + anti-TcdB polysera (1:1000); B, Lane 1: cells-only control; lane 2: A1H3 antibody control; lane 3: 10 ng/mL TcdA + A1H3; lane 4: piglet serum (1:10) 5 d PI + A1H3; lane 5: piglet serum (1:50) 7 d PI + A1H3; lane 6: piglet serum (1:50) + A1H3 + anti-TcdA and anti-TcdB polysera.
Toxemia Is Associated With the Development of Systemic Manifestations of CDI
In both the mouse and piglet models of CDI, the severity of disease ranged from mild, localized gastrointestinal illness to severe, systemic, and often fatal disease. To examine whether the presence of toxin in the systemic circulation is associated with systemic manifestations of CDI, we divided the animals into 2 groups: systemic CDI and nonsystemic CDI. Of the 43 piglet serum samples tested, 18 were from animals with systemic CDI and 25 were from animals with nonsystemic CDI (Table 1). Twelve of 18 (66.67%) serum samples from piglets with systemic CDI had detectable levels of toxin. In piglets with nonsystemic CDI, however, toxin was never present in the serum at detectable levels (Table 1). The association of toxemia in piglets with the occurrence of systemic CDI is highly significant (P < .0001). Similarly, in mice, 27 of 61 serum samples tested were from animals with systemic CDI and 34 were from animals with nonsystemic CDI. Of 27 animals with systemic CDI, 23 (85.2%) had detectable levels of toxin, but none of the animals with nonsystemic CDI had detectable toxin levels (Table 1). The association of toxemia with systemic CDI in mice is also highly significant (P < .001). We found no evidence of bacteremia or bacteria in the lungs of piglets or mice with systemic CDI ([19] and data not shown). As evidenced by the results from a representative piglet and mice (Figure 3), toxemia coincides with the development of systemic CDI, and neither TcdA nor TcdB was detectable in the sera of animals before the onset of systemic clinical signs of CDI.
Systemic Manifestations of CDI Occur in Piglets and Mice With Toxemia
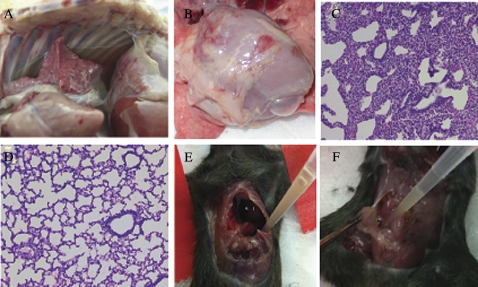
All piglets inoculated with C. difficile developed diarrhea within 48 hours postinoculation, and progression to systemic CDI occurred within 3–6 days postinoculation. On necropsy, typical gross systemic manifestations in pigs with systemic CDI included cranial-ventral lung consolidation or petechiae in 79.5%, pleural effusion in 74.4% (Figure 4A), and ascites in 79.5%, with the rare lesion of pancreatitis in 2.6%. Ascites was the only systemic manifestation ever found to occur in piglets that did not exhibit clinical signs of systemic illness, occurring in 10.9%. The association of ascites, pleural effusion, and pulmonary lesions with systemic CDI is highly significant (P < .0001).
Figure 4.
Necropsy and histopathologic images from piglets and mice with Clostridium difficile infection (CDI). A, Necropsy image of the thorax of a piglet with systemic CDI showing pleural effusion. B, Necropsy image of the thorax of a mouse with systemic CDI showing pleural effusion; C, Necropsy image of the abdomen of a mouse with systemic CDI showing ascites; D, Section of lung from a normal piglet for comparison; E, Section of lung from a piglet with systemic CDI showing regional atelectasis and interstitial thickening with no bacteria or neutrophilic inflammation present.
In C. difficile–infected piglets, histopathology of the large intestine showed submucosal and mesenteric edema, neutrophilic inflammation, and mucosal ulcerations and erosions, as previously described [19]. Systemic lesions were most notable in the lungs, and interlobular edema, alveolitis, interstitial thickening, and regional atelectasis were observed, but neither bacteria nor neutrophils were present (Figure 4E). Similarly, in mice, systemic manifestations, including pleural effusion (Figure 4B) and ascites (Figure 4C), were also observed.
Systemic Neutralizing Antibodies Block Toxemia and Systemic Disease
Because both TcdA and TcdB are liberated into circulation in animals with toxemia, we examined whether neutralizing the 2 toxins would eliminate systemic manifestations of disease and thus reduce severity. Mice treated with systemic neutralizing antibodies against TcdA and TcdB (antitoxins) were completely protected from the development of toxemia, systemic CDI, and death. Of the nonimmune serum-treated control mice, 40% developed fatal systemic CDI (Figure 5A) with systemic clinical signs of CDI such as weight loss, anorexia, dehydration, lethargy, dyspnea, and poor body condition. The antitoxin-treated mice, on average, lost <6% of their body weight and quickly recovered body weight compared with control mice (Figure 5B).
Figure 5.
Survival and body-weight changes in mice treated with systemic polyclonal anti–Clostridium difficile toxin A and anti–C. difficile toxin B antibodies. A, Kaplan-Meier survival curves of antitoxin antibody-treated mice compared with those of mice treated with nonimmune sera (CRT) (P = .007); B, Relative body-weight change in mice after inoculation with C. difficile. The data shown are pooled from 2 independent experiments (n = 15). Significant differences were determined using the t test. *P < .001.
Proinflammatory Cytokines Are Elevated in Animals With Systemic CDI
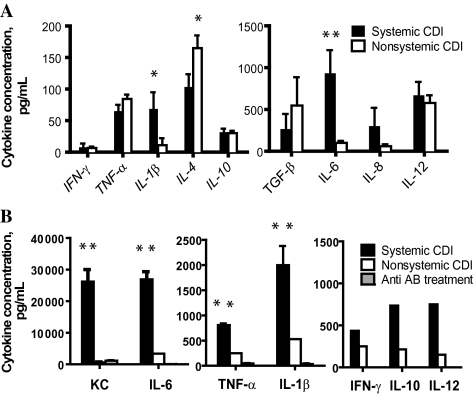
Both TcdA and TcdB possess inflammatory properties [24–28]; therefore, we hypothesized that toxemia would induce systemic cytokine production. For piglet sera, the we divided samples into systemic-CDI (n = 29) or nonsystemic-CDI (n = 40) groups, and the mean cytokine concentrations were compared for significant differences between groups with the t test for each cytokine. In piglet sera, IL-1β, IL-4, and IL-6 concentrations were significantly different between groups, with P values of .03, .04, and .001, respectively (Figure 6A). Piglets developing systemic CDI had significantly greater levels of the inflammatory cytokines IL-1β and IL-6, but significantly lower levels of IL-4, an anti-inflammatory cytokine (Figure 6A). Likewise, mice with systemic CDI also had elevations in the concentrations of several cytokines measured. CXCL1 (KC) and IL-6 were most elevated in mice with systemic CDI, with a trend for elevations also noted for IFN-γ,IL-1β, IL-10, and IL-12 (Figure 6B).
Figure 6.
Piglet and mouse serum cytokine concentrations. A, Serum from piglets (n = 69) was grouped according to disease severity, as systemic (black bar, n = 29) or nonsystemic (open bar, n = 40) Clostridium difficile infection (CDI); B, Serum samples from 7 mice with systemic CDI (black bar), 7 mice with nonsystemic CDI (open bar), or 5 mice treated with anti–Clostridium difficile toxin A and anti–C. difficile toxin B polysera (anti AB, gray bar) were run separately in cytokine assays. Columns represent the mean cytokine concentration with standard error bars for each cytokine. Statistical differences were determined by the t test. *P < .05; ** P < .01. Abbreviations: IFN-γ, interferon γ; IL, interleukin; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
DISCUSSION
C. difficile is an important nosocomial pathogen in developed countries [29]. The increased morbidity and mortality associated with CDI, which began in the early 2000s with the emergence of NAP1/027/BI strains, has reached epidemic proportions [29]. In the United States, some 500 000 cases of CDI, with approximately 15 000–20 000 deaths, are reported annually [29, 30]. Systemic complications of CDI are often life threatening, and although large-scale studies on systemic CDI in humans are unavailable, there exists a wealth of case reports describing a variety of extracolonic complications [31–33]. Although suspected, a linkage between systemic CDI and toxemia has not yet been confirmed with direct evidence in humans. We conducted experiments in mice and piglets, aiming to define the features that distinguish systemic CDI from nonsystemic CDI.
We have previously reported the detection of toxins in sera and body fluids of systemically ill animals with CDI [18, 19], which suggested a potential association with systemic involvement. In contrast, in piglets and mice with CDI that exhibit diarrheal symptoms with no apparent systemic manifestations, toxins are undetectable in the sera or body fluids. Recent reports describing the relative importance of TcdA and TcdB in animal models of CDI have suggested that TcdB was likely linked with systemic disease [17, 34]. Our findings show that both toxins are present in the serum at cytotoxic concentrations, observations consistent with a recent report that TcdA and TcdB are implicated in the pathogenesis of systemic CDI [3].
Our work clearly shows that TcdA and TcdB reach systemic circulation following colonization of the intestinal tract by the bacteria, and when they do, systemic disease ensues. Systemic abnormalities in these animals included pleural effusion, ascites, and cardiopulmonary lesions, all observations that have been described in humans with CDI [31]. The mechanisms of systemic toxin uptake and the relative contribution of each toxin to these systemic abnormalities remain unclear. Both toxins are highly cytotoxic to cultured cells, and thus may have a direct effect on various organ tissues, which could explain the cardiac damage seen in the intoxicated zebrafish model [17]. Significant tissue abnormalities, presumably due to intoxication, were found, which included cranial-ventral consolidation of the lung lobes, pleural effusion, and diffuse petechiation of the lung lobes, which may be due to a direct toxic effect.
Because TcdA and TcdB are both proinflammatory [35], we believe that toxemia may have induced a profound inflammatory reaction that contributed to the severity of disease. To investigate one aspect of the inflammatory response, we examined pig and mouse serum samples for the presence of various pro- and anti-inflammatory cytokines. We found that in both pigs and mice, proinflammatory cytokine levels were elevated in animals with systemic CDI compared with those in animals with nonsystemic CDI. In both species, significant elevations were found for levels of IL-1β and IL-6, both of which are proinflammatory mediators. Interestingly, a significant elevation in the anti-inflammatory cytokine IL-4 was observed in piglets with no apparent systemic manifestations of disease. These differences between the 2 groups warrant further investigation, as the proinflammatory response in CDI may contribute to the development of systemic disease. Our findings in both animal-infection models of CDI support the importance of inflammatory mediators in the C. difficile immune response and the potential implications for inflammation-induced tissue damage systemically.
Our findings have implications for understanding the pathogenesis of CDI, the development of novel vaccines, and immunotherapy. Knowing that the toxins can be liberated into circulation and subsequently can cause systemic inflammation and tissue damage illustrates the need to not only target the bacteria with effective antibiotics, but also to prevent or reduce the impact of toxin present in circulation in severe cases. Development of vaccines and monoclonal and polyclonal antibodies directed against the 2 toxins will help prevent the development of systemic CDI. Our observations warrant a close examination of toxemia in humans with systemic CDI, in addition to treating the infection within the gastrointestinal tract.
Notes
Acknowledgments.
We give special thanks to Patricia Boucher and Rachel Nieminen for all of their help with the care of piglets in these studies.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases (NO1-30050, R01AI088748, F32AI081497) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK084509 and K01DK076549) at the National Institutes of Health.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Elliott B, Chang BJ, Golledge CL, Riley TV. Clostridium difficile-associated diarrhoea. Intern Med J. 2007;37:561–8. doi: 10.1111/j.1445-5994.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 2.Blossom DB, McDonald LC. The challenges posed by reemerging Clostridium difficile infection. Clin Infect Dis. 2007;45:222–7. doi: 10.1086/518874. [DOI] [PubMed] [Google Scholar]
- 3.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–13. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 4.Jafri SF, Marshall JB. Ascites associated with antibiotic-associated pseudomembranous colitis. South Med J. 1996;89:1014–7. doi: 10.1097/00007611-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Tsourous GI, Raftopoulos LG, Kafe EE, Manoleris EK, Makaritsis KP, Pinis SG. A case of pseudomembranous colitis presenting with massive ascites. Eur J Intern Med. 2007;18:328–30. doi: 10.1016/j.ejim.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Boaz A, Dan M, Charuzi I, Landau O, Aloni Y, Kyzer S. Pseudomembranous colitis: report of a severe case with unusual clinical signs in a young nurse. Dis Colon Rectum. 2000;43:264–6. doi: 10.1007/BF02236993. [DOI] [PubMed] [Google Scholar]
- 7.Zwiener RJ, Belknap WM, Quan R. Severe pseudomembranous enterocolitis in a child: case report and literature review. Pediatr Infect Dis J. 1989;8:876–82. doi: 10.1097/00006454-198912000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Kent SA, O'Leary KJ, et al. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann Intern Med. 2001;135:434–8. doi: 10.7326/0003-4819-135-6-200109180-00012. [DOI] [PubMed] [Google Scholar]
- 9.Siarakas S, Damas E, Murrell WG. Is cardiorespiratory failure induced by bacterial toxins the cause of sudden infant death syndrome? Studies with an animal model (the rabbit) Toxicon. 1995;33:635–49. doi: 10.1016/0041-0101(95)00003-5. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai T, Hajiro K, Takakuwa H, Nishi A, Aihara M, Chiba T. Liver abscess caused by Clostridium difficile. Scand J Infect Dis. 2001;33:69–70. doi: 10.1080/003655401750064112. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh N, Kettern MA, Hanssens Y, Elshafie SS, Louon A. A rare and unsuspected complication of Clostridium difficile infection. Intensive Care Med. 2008;34:963–6. doi: 10.1007/s00134-007-0922-6. [DOI] [PubMed] [Google Scholar]
- 12.Jacob SS, Sebastian JC, Hiorns D, Jacob S, Mukerjee PK. Clostridium difficile and acute respiratory distress syndrome. Heart Lung. 2004;33:265–8. doi: 10.1016/j.hrtlng.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Dobson G, Hickey C, Trinder J. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 2003;29:1030. doi: 10.1007/s00134-003-1754-7. [DOI] [PubMed] [Google Scholar]
- 14.Cunney RJ, Magee C, McNamara E, Smyth EG, Walshe J. Clostridium difficile colitis associated with chronic renal failure. Nephrol Dial Transplant. 1998;13:2842–6. doi: 10.1093/ndt/13.11.2842. [DOI] [PubMed] [Google Scholar]
- 15.Songer JG, Post KW, Larson DJ, Jost BH, Glock RD. Infection of neonatal swine with Clostridium difficile. Swine Health Prod. 2000;8:185–9. [Google Scholar]
- 16.Songer JG, Uzal FA. Clostridial enteric infections in pigs. J Vet Diagn Invest. 2005;17:528–36. doi: 10.1177/104063870501700602. [DOI] [PubMed] [Google Scholar]
- 17.Hamm EE, Voth DE, Ballard JD. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc Natl Acad Sci U S A. 2006;103:14176–81. doi: 10.1073/pnas.0604725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Wang J, Steele J, et al. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J Microbiol Methods. 2009;78:97–100. doi: 10.1016/j.mimet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele J, Feng H, Parry N, Tzipori S. Piglet models of acute or chronic Clostridium difficile illness. J Infect Dis. 2010;201:428–34. doi: 10.1086/649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killgore G, Thompson A, Johnson S, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol. 2008;46:431–7. doi: 10.1128/JCM.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–92. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.He X, Sun X, Wang J, et al. Antibody-enhanced, Fc gamma receptor–mediated endocytosis of Clostridium difficile toxin A. Infect Immun. 2009;77:2294–303. doi: 10.1128/IAI.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, Zhou B, Wang J, et al. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 2008;8:192. doi: 10.1186/1471-2180-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savidge TC, Pan WH, Newman P, O’Brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–20. doi: 10.1016/s0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Kim JS, Jun HC, Oh YK, Song IS, Kim CY. Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in response to Clostridium difficile toxin A. Microbiol Immunol. 2002;46:333–42. doi: 10.1111/j.1348-0421.2002.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 26.Ng EK, Panesar N, Longo WE, et al. Human intestinal epithelial and smooth muscle cells are potent producers of IL-6. Mediators Inflamm. 2003;12:3–8. doi: 10.1080/0962935031000096917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linevsky JK, Pothoulakis C, Keates S, et al. IL-8 release and neutrophil activation by Clostridium difficile toxin–exposed human monocytes. Am J Physiol. 1997;273:G1333–40. doi: 10.1152/ajpgi.1997.273.6.G1333. [DOI] [PubMed] [Google Scholar]
- 28.Flegel WA, Müller F, Däubener W, Fischer HG, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659–66. doi: 10.1128/iai.59.10.3659-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerding DN. Global epidemiology of Clostridium difficile infection in 2010. Infect Control Hosp Epidemiol. 2010;31:S32–4. doi: 10.1086/655998. [DOI] [PubMed] [Google Scholar]
- 30.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs A, Barnard K, Fishel R, Gradon JD. Extracolonic manifestations of Clostridium difficile infections. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2001;80:88–101. doi: 10.1097/00005792-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Lee NY, Huang YT, Hsueh PR, Ko WC. Clostridium difficile bacteremia, Taiwan. Emerg Infect Dis. 2010;16:1204–10. doi: 10.3201/eid1608.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf LE, Gorbach SL, Granowitz EV. Extraintestinal Clostridium difficile: 10 years’ experience at a tertiary-care hospital. Mayo Clin Proc. 1998;73:943–7. doi: 10.4065/73.10.943. [DOI] [PubMed] [Google Scholar]
- 34.Lyras D, O’Connor JR, Howarth PM, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in Clostridium difficile-associated disease: past and present perspectives. Gut Microbes. 2010;1:58–64. doi: 10.4161/gmic.1.1.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]