Abstract
Background. Vibrio cholerae excreted by cholera patients is “hyperinfectious” (HI), which can be modeled by passage through infant mice. Immunization of adult female mice with V. cholerae outer-membrane vesicles (OMVs) passively protects suckling mice from challenge. Although V. cholerae is unable to colonize protected pups, the bacteria survive passage and have the potential to be transmitted to susceptible individuals. Here, we investigated the impact of OMV immunization and the HI state on V. cholerae transmission.
Methods. Neonatal mice suckled by OMV- or sham-immunized dams were challenged with HI V. cholerae. The infectivity of spatially and temporally separate V. cholerae populations obtained from infected naive or protected pups was tested. Recombination-based in vivo expression technology was used to assess virulence gene expression within these populations.
Results. OMV immunization significantly reduced colonization of neonates challenged with HI V. cholerae. Vibrio cholerae that had colonized the naive host was HI, whereas V. cholerae excreted by neonates born to OMV-immunized dams, although viable, was hypoinfectious and failed to fully induce virulence gene expression.
Conclusions. OMV immunization can significantly reduce the V. cholerae burden upon challenge with HI V. cholerae and can also block transmission from immune mice by reducing the infectivity of shed bacteria.
Vibrio cholerae is the causative agent of cholera and a natural inhabitant of aquatic environments [1, 2]. Pathogenic V. cholerae evolved to multiply in the human small intestine (SI), in part by acquiring genes encoding cholera toxin and the toxin-coregulated pilus [3]. Induction of these genes occurs in the infant mouse model of colonization and is required for pathogenesis in humans [4–6].
Vibrio cholerae excreted in stool are transiently hyperinfectious (HI) [7, 8], which is proposed to help explain the rapid spread of cholera [9]. This state can be modeled by passage through suckling mice [10]. One factor correlated with the HI state is an alteration in chemotaxis [7, 8] that enables V. cholerae to colonize proximal regions of the SI [10, 11].
Outer-membrane vesicles (OMVs) are naturally produced by V. cholerae and, when delivered to adult female mice intranasally or orally, can protect their suckling neonates from challenge with in vitro grown, non-HI V. cholerae [12, 13]. The lipopolysaccharide O-antigen is the major protective OMV antigen, and inhibition of V. cholerae motility may contribute to protection [14]. V. cholerae is unable to colonize the SI of protected pups but passes out of the host without being killed [14].
Because natural infection may occur predominantly by exposure to HI stool-shed V. cholerae, we investigated the ability of the OMV vaccine to block infection by HI V. cholerae and the ability of V. cholerae from challenged immune and nonimmune neonatal mice to participate in disease transmission.
MATERIALS AND METHODS
Bacteriology
The V. cholerae strains that we used are described in the Supplementary methods below. Bacteria were cultured in Luria-Bertani (LB) broth with aeration or on LB agar at 37°C (unless otherwise stated), supplemented with 100 μg/mL streptomycin (Sm), 50 μg/mL rifampicin (Rif), 30 μg/mL ampicillin (Ap), or 3 μg/mL tetracycline (Tc). Competitions using blue/white screening were LB agar supplemented with 80 μg/mL 5-bromo-4-chloro-3-indolyl-d-galactoside (X-gal).
Mice
BALB/c mice (Charles River Laboratories) were used in experiments in Boston, and Swiss Webster mice were used in Dhaka. Mice were housed with food and water ad libitum and monitored in accordance with the rules of the Department of Laboratory Animal Medicine at Tufts Medical Center or the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B).
Immunization
OMVs from V. cholerae strains E7946 and A1552 were prepared as described [12]. Immunization was carried out with 3 doses (days 0, 14, and 28) of 25 μg of OMV, and mice were mated 41 days postimmunization [12]. For immunization of mice in Dhaka, a 1:1 mixture of Ogawa and Inaba OMVs was used; in Boston, Ogawa OMVs were used.
For Swiss Webster mice in Dhaka, overall pregnancy rates were only approximately 45%. Mice that had not given birth were rebred and rice-water-stool challenges were carried out at 143–149 days and final bleeds were carried out at 153 days postimmunization.
Measuring Anti-OMV Antibodies
Enzyme-linked immunosorbent assays (ELISAs) against OMVs were carried out as described [12, 14]. Because BALB/c mice mount an immunoglobulin (Ig) G1–dominant response [12], this isotype is reported. Responses of Swiss Webster mice to OMV immunization had not previously been tested; therefore, IgG1, IgG2, IgA, and IgM were monitored.
Mouse-passaged V. cholerae Challenge and Median Infective Dose
Naive 5- to 6-day-old pups were infected with approximately 105 colony-forming units (CFU) of E7946 in 50 μL by intragastric inoculation and returned to their dams as described [12]. After 24 hours mice were euthanized and SIs were homogenized in 1 mL of saline, pooled (from 2 or 3 mice), filtered (100-μm pores), and diluted 10-, 100-, and 1000-fold. Neonates from BALB/c mice, OMV- or sham-immunized, were challenged intranasally with 50 μL of primary infection homogenate. Twenty-four hours after inoculation, mice were euthanized and SIs were homogenized. Inputs and outputs were diluted and plated for determination of viable counts.
The ID50 (the doses at which half of the animals are infected) of mouse-passaged V. cholerae E7946 from 24-hour SI homogenates and from 2-hour large intestinal (LI) homogenates, postinoculation of pups born to sham- or OMV-immunized dams were determined by primary and secondary infection as described above, with primary inputs of 105 CFU for 24-hour or 107 CFU for 2-hour infections. Groups of 4–7 mice were infected with serial dilutions of homogenates in saline. The proportion of mice infected, with cutoff set to >100 CFU/SI, was plotted against input dose. The ID50 required to colonize half of the mice was calculated using Hill regression.
Challenge With Rice-Water-Stool V. cholerae
Stool samples were collected from cholera patients aged ≥15 years. Use of these samples was approved by the institutional review boards at both Massachusetts General Hospital and the ICDDR,B. Dark field–positive, serologically confirmed stool samples containing 1.7–1300 × 106 CFU/mL V. cholerae were utilized for infections. Stool was clarified by 5 minutes’ centrifugation at 380g. The supernatant was diluted 1000-fold (high dose) and 10 000-fold (low dose) in LB. Oral challenge of Swiss Webster neonates was then carried out as described above. Half of each litter was infected with low dose and half with high dose. Twenty-four hours postinoculation, the neonates were euthanized, and SIs were harvested and homogenized in 1 mL LB 15% glycerol. Stomachs were also extracted and frozen for future analysis of stomach contents (milk). Infection inputs and SI outputs were diluted and plated on taurocholate-tellurite-gelatin agar and MacConkey agar plates for enumeration of V. cholerae and Escherichia coli contamination, respectively. Two samples that contained >2% E. coli CFU were excluded. All isolates of E. coli tested were polymerase chain reaction–negative for heat-labile or heat-stable toxins (data not shown), suggesting that V. cholerae, rather than enterotoxigenic E. coli, was the cause of the patients’ rice-water stool.
Competition Experiments
Competition experiments were carried out using wild-type and lacZ mutant V. cholerae. Primary infections with in vitro–grown (from LB agar) V. cholerae were carried out with either lacZ-positive or negative bacteria as described above, with input doses of 105 or 107 CFU for 24-hour and 2-hour infections, respectively. Neonates were euthanized and the whole intestine, SI or LI, was extracted and homogenized in 1 mL of saline. Homogenates diluted 10-fold and/or in vitro–grown bacteria were mixed 1:1 and used for secondary naive neonatal mouse infections with an input dose of approximately 5 × 104 CFU/50 μL. Twenty-four hours after secondary infection, the neonates were euthanized and SIs were homogenized in 1 mL LB 15% glycerol. Dilutions of both inocula and infection outputs were plated on X-gal plates for enumeration of lacZ-positive (blue) and lacZ-negative (white) colonies.
Induction of tcpA In Vivo
Infections were carried out with AC585, which is a derivative of the SmR V. cholerae strain E7946, having a TcR gene flanked by resolvase recognition sequences inserted into the native lacZ gene, and tnpR resolvase under control of the native tcpA promoter via integration of an ApR suicide vector into the tcpA locus. Bacteria were prepared from LB SmApTet agar and diluted in LB to give either 105 CFU (24-hour infection of naive mice) or 107 CFU (2-hour or 24-hour infection of immune mice) in 50 μL. In parallel, the bacteria were diluted 1000-fold into 10 mL of LB or in the tcpA-inducing medium known as “AKI” (0.5% sodium chloride, 0.3% sodium bicarbonate, 0.4% yeast extract, 1.5% Bacto-Peptone) [15]. Cultures were grown shaking (LB) or statically (AKI) for 4 hours, then 1 mL was removed and grown shaking for 4 hours. Twenty-four hours postinoculation, the mice were euthanized and SI or LI were homogenized separately. Homogenates and 8-hour in vitro cultures were diluted and plated on LB SmAp. The resulting colonies were replica plated onto LB SmAp and LB SmApTc to determine the percentage of resolved (TcS) CFUs.
Visualization of V. cholerae In Vivo
Intestinal homogenates were fixed with 3.7% formaldehyde for 30 minutes, vortexed, and mixed with a pipette; 10-μL samples were then spotted on poly-L-lysine slides (Polysciences Inc), dried at 37°C, dipped into ethanol, and washed in phosphate-buffered saline (PBS). Samples were blocked with PBS 5% goat serum 1% bovine serum albumin for 1 hour, then stained for 1 hour with fluorescein isothiocyanate (FITC)–conjugated mouse IgG1 anti–V. cholerae O1 antibody (250-fold dilution; New Horizons), DNA stain (2 μg/mL; Hoescht-33342, Molecular Probes), and a lectin that highlights mucus (2 μg/mL tetramethylrhodamine-5-(and 6)-isothiocyanate–wheat germ agglutinin, Sigma Aldrich). Negative control FITC-mouse IgG1 was used to stain consecutive sections (data not shown).
Statistics
The majority of data were abnormally distributed; therefore, 2 groups were compared using Mann–Whitney U tests and >2 groups were compared using the Kruskal–Wallis and Dunn tests. Normally distributed data were analyzed using 1-sample t test or 1-way analysis of variance and post hoc t tests with Bonferroni adjustment. For ratios and percentages, data were log10-transformed prior to analysis. GraphPad Prism software, version 5.0a, was used for all statistical analysis.
RESULTS
OMV Immunization Protects Against Mouse-Passaged Hyperinfectious Challenge
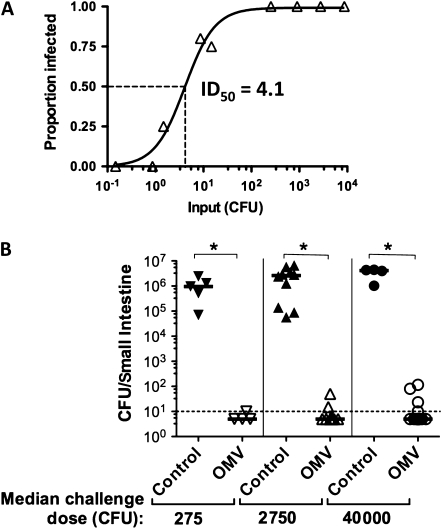
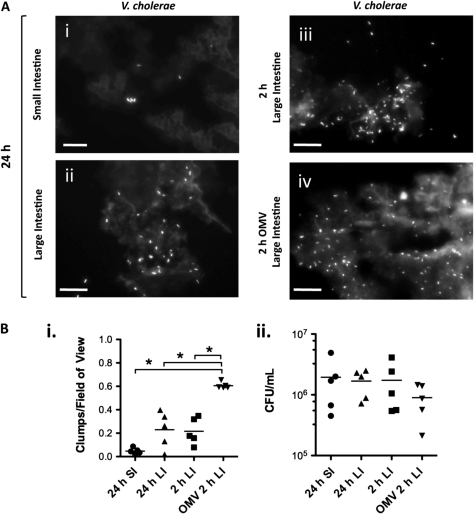
To test whether OMV immunization, which protects from in vitro–grown V. cholerae challenge [12–14], can also protect from host-passaged HI V. cholerae challenge, we first determined the ID50 for the latter in suckling BALB/c mice. The ID50 for strain E7946 was 4.1 CFU (95% confidence interval [CI], 2.2–6.0; R2 = 0.98; Figure 1A), which is 50 times lower than LB-grown E7946 [12]. We considered that clumping could cause input doses to be underestimated. We scored clumps of V. cholerae in formaldehyde-fixed homogenates prepared as if for infection and found very few in 24-hour postinoculation SI homogenates (Figure 2A and 2B), suggesting that a genuine change in bacterial physiology upon host passage accounts for the low ID50.
Figure 1.
Challenge of outer-membrane vesicle (OMV)–immunized mice with hyperinfectious, mouse-passaged Vibrio cholerae. A, ID50 (the dose at which half the animals are infected) for secondary infection using, as inoculae, mouse-passaged V. cholerae E7946 recovered from primary infection small intestinal (SI) homogenates. Infected BALB/c neonates were returned to their dams postinoculation. Primary infection was with 105 E7946 from Luria-Bertani agar plates. Secondary infection was with SI homogenates diluted in saline to between 0.1 and 3 × 104 colony-forming units (CFU)/50 μL challenge dose. Secondary infections were done in groups of 4–10 mice per challenge dose. Both primary and secondary infections were for 24 hours. The proportion of mice infected, with a colonization cutoff of >100 CFU, versus challenge dose is shown, from which the ID50 was calculated to be 4.1 (95% confidence interval, 2.200–5.995; R2 = 0.9814). B, Challenge of neonates born to sham- (control) or OMV-immunized dams with mouse-passaged V. cholerae was carried out with 3 challenge doses (median challenge doses indicated below the graph). Each symbol represents 1 neonate. Neonates from 3 mice are represented in each group. Bars = medians; dotted line = limit of detection. *P < .05 comparing pups born to controls and immunized mice infected with the same challenge dose (Mann–Whitney U tests). The anti-OMV enzyme-linked immunosorbent assay immunoglobulin G1 titers for immunized dams whose pups were used for this experiment ranged from 10.5 to 159 (median, 143) μg/mL.
Figure 2.
Clumping of Vibrio cholerae within infected intestinal homogenates. Clumping of V. cholerae within infected mouse intestinal homogenates was assessed by visualization of formalin-fixed samples stained with fluorescein isothiocyanate–mouse anti-O1 V. cholerae antibody. A, Representative images of clumps within homogenates from small intestine (SI) (i) or large intestine (LI) (ii–iv) harvested 24 hours (i, ii) or 2 hours (iii, iv) postinoculation and stained for V. cholerae. Scale bars = 20 μm. B, (i) Clumps of V. cholerae were scored in at least 20 fields of view with a ×60 objective from 2 samples taken from 5 fixed SI or LI homogenate samples that were harvested 2 hours or 24 hours postinoculation, as indicated. The number of clumps per field of view is shown. *P < .05 (1-way analysis of variance and post hoc t tests with Bonferroni adjustment). (ii) Viable counts (colony-forming units [CFU]/mL) for each homogenate, prepared as if for mouse infection, determined by serial dilution and plating prior to formaldehyde fixing. Each symbol represents 1 homogenate; bars = means. Abbreviation: OMV, outer membrane vesicle.
Pups from OMV- and sham-immunized mice were challenged with mouse-passaged E7946 from infected SI homogenates (median inputs in Figure 1B). Pups born to OMV-immunized mice were significantly protected from colonization by HI mouse-passaged V. cholerae, even at the highest input dose (Figure 1B).
OMV Immunization Protects Against Rice-Water-Stool V. cholerae Challenge
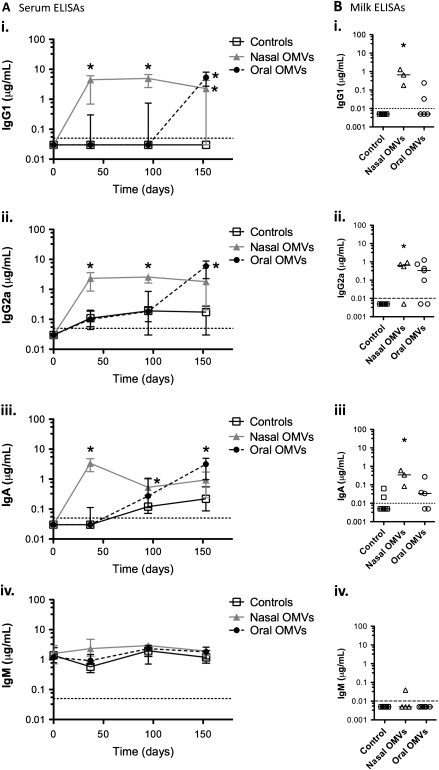
Mouse-passaged V. cholerae competes 1:1 with rice-water-stool V. cholerae in the mouse infection model [10]. However, it is not clear whether mouse passage elicits the same HI phenotype as human passage. To test whether pups from immunized mice are protected from human-passaged HI V. cholerae, we performed challenge studies with rice-water stool at the ICDDR,B using Swiss Webster mice. We first characterized the immune response of Swiss Webster mice after OMV immunization. Anti-OMV antibody titers in unimmunized control mice remained below the limit of detection, except for a slight increase above baseline for IgG2a and IgA over time (Figure 3). Anti-OMV IgG1, IgG2a, and IgA titers were above control levels for intranasally OMV-immunized mice 38–95 days (153 for IgG1) postimmunization (Figure 3A) but were lower than those seen with BALB/c mice [12, 14]. Oral immunization induced less robust immune responses than intranasal immunization (Figure 3A), as previously observed [14]. A delayed increase in anti-OMV IgG1, IgG2a, and IgA in the orally OMV-immunized group, reaching levels higher than those of the controls at days 95–153 (Figure 3A), could be due to environmental contamination boosting the response; a small rise in anti-OMV IgG2a and IgA was observed in unimmunized controls over time (Figure 3A). Anti-OMV IgM was detectable in OMV-immunized mouse serum but was at similar levels in preimmune and control sera (Figure 3A) and thus probably represents innate anticarbohydrate antibodies [16]. Similar ELISA results were seen with O1 Inaba (data not shown) and O1 Ogawa (Figure 3) OMVs as the ELISA substrate.
Figure 3.
Immune responses of outer-membrane vesicle (OMV)–immunized Swiss Webster mice. Adult female Swiss Webster mice were immunized with three 25-μg doses of a 1:1 mixture of O1 Ogawa and O1 Inaba OMVs either intranasally (nasal, 10 mice) or by oral gavage (oral, 9 mice) at days 0, 14, and 28. Controls (19 mice) were cohoused with the immunized mice. Enzyme-linked immunosorbent assays (ELISAs) for serum and milk samples from these mice were carried out against O1 Ogawa OMVs. A, Serum levels of anti-O1 Ogawa OMV immunoglobulin (Ig) G1 μg/mL (i), IgG2a μg/mL (ii), IgA μg/mL (iii), and IgM μg/mL (iv) assessed by ELISA for preimmune serum (day 0) and serum samples taken at various days postimmunization, as indicated on the x-axis of each graph: day 37, before mating; day 95, after initial neonatal rice-water-stool challenges; day 153, after second round of challenge experiments. Sera from at least 6 mice per group were analyzed at each time point, except for day 153 when only 4 mice remained in the orally immunized group. Symbols = medians; error bars = interquartile range. *P < .05 compared with controls at the same time point (Kruskal–Wallis and Dunn tests). B, Levels of anti-O1 Ogawa OMV IgG1 μg/mL (i), IgG2a μg/mL (ii), IgA μg/mL (iii), and IgM μg/mL (iv) in milk extracted from the stomachs of pups born to OMV-immunized (nasally or orally delivered as indicated) or control mice. Each symbol represents milk pooled from the stomachs of at least 3 pups suckled by 1 dam; bars = medians. *P < .05 compared with controls (Kruskal–Wallis and Dunn tests).
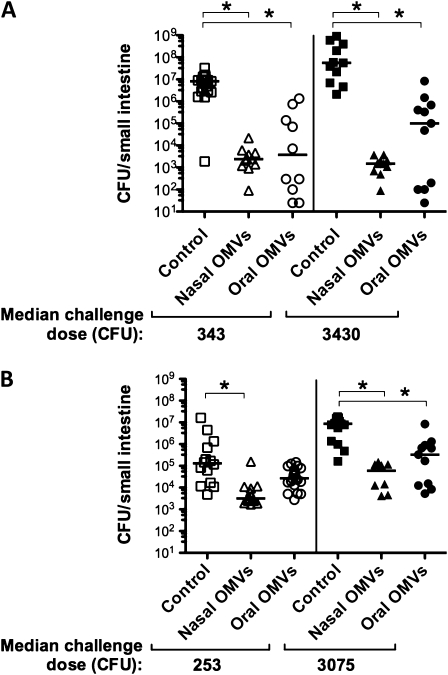
To determine if pups from OMV-immunized mice are protected from challenge with rice-water-stool V. cholerae, stools were collected from patients in the ICDDR,B. During this period (April–May 2009), all V. cholerae–positive stool samples analyzed contained O1 serotype Ogawa. Two dilutions of rice-water stool were used for infections. Despite relatively low antibody responses in the dams (Figure 3), their pups showed significantly lower intestinal burdens than cohoused unimmunized controls challenged 62–76 days postimmunization (Figure 4A). In the second challenge experiment, 143–149 days postimmunization, protection did not reach statistical significance for the oral group with low input dose due to poor colonization of controls, but intestinal burdens were significantly reduced in all other immunization groups (Figure 4B). These data indicate that OMV immunization of dams provides passive protection to pups challenged with naturally transmitted rice-water-stool V. cholerae.
Figure 4.
Challenge of outer-membrane vesicle (OMV)–immunized Swiss Webster mice with rice-water-stool Vibrio cholerae. Neonates born to intranasally or orally O1 OMV-immunized or cohoused (control) dams were challenged with cholera patient rice-water-stool samples diluted to give the median challenge doses indicated. Challenges were carried out either (A) 62–76 days or (B) 143–149 days postimmunization of the dam. Each symbol represents viable counts in the small intestine of 1 challenged neonate; bars = medians. *P < .05 significantly lower viable counts compared with controls with the same challenge dose (Kruskal–Wallis and Dunn tests). Abbreviation: CFU, colony-forming units.
Suckling from an immunized dam provides the majority of neonatal protection [13]. IgG1, IgG2a, and IgA, but not IgM, responses were detectable in OMV-immunized mouse milk samples and were significantly higher than those of controls after intranasal immunization (Figure 3B).
V. cholerae Excreted by Challenged Protected Mice Is Hypoinfectious
We previously observed that challenged pups suckled by OMV-immunized dams contain live V. cholerae in the LI 2–4 hours postinoculation, similar to challenged pups born to sham-immunized mice [14]. We were interested in determining whether bacteria excreted by protected neonates were infectious.
First, we determined that 107 CFU challenge of pups from immune mice provides enough V. cholerae in the LI 2 hours postinoculation to be used for reinfection (data not shown). Again, we were concerned that clumping could confound our interpretation of input doses. More clumps (Figure 2B), but similar viable counts (Figure 2B), were seen in 2-hour postinoculation homogenates from challenged pups born to OMV-immunized rather than to sham-immunized mice. This suggests that upon challenge of secondary mice with the former inoculum, we are introducing more clumped bacteria than with the latter. However, the clumps appeared to be debris to which multiple individual bacteria were sparsely attached, rather than bacteria intimately associated with each other (Figure 2A). We also confirmed, by staining of V. cholerae in infected intestinal samples and scoring of bacterial localization, that bacteria in the LI are in transit within the lumen as opposed to colonizing (Supplementary Figure 1).
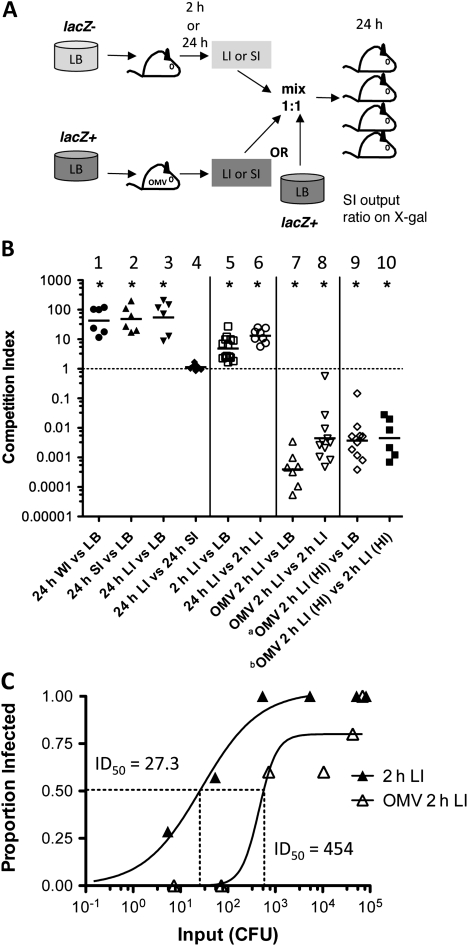
With these controls in place, we carried out competition experiments with differentially marked, equally virulent (lacZ plus or minus) strains of V. cholerae to determine the infectivity of different host-passaged bacterial populations, as depicted in Figure 5A. First, we compared infected whole-intestine, SI, and LI homogenates harvested 24 hours postinoculation in competition with in vitro LB-grown V. cholerae. Regardless of whether the bacteria were exiting the host via the LI or were in the SI, they were all HI (Figure 5B, competitions 1–3). Competition between SI and LI homogenates 24 hours postinoculation confirmed that they compete approximately 1:1 (Figure 5B, competition 4). LI homogenates 2 hours postinoculation contain V. cholerae that failed to colonize the SI and were in transit out of the host. This population from naive mice only slightly, but significantly, outcompeted LB-grown bacteria (Figure 5B, competition 5). Competition of 2-hour LI homogenates with 24-hour LI homogenates showed an in-between phenotype (Figure 5B, competition 6). These data together show that passive passage of V. cholerae through the host (2-hour LI) provides a small advantage over in vitro–grown bacteria, but this advantage is an order of magnitude less than bacteria that have colonized the SI 24 hours postinfection.
Figure 5.
Infectivity of neonatal mouse host-passaged Vibrio cholerae 2 hours or 24 hours postinoculation and the impact of outer-membrane vesicle (OMV) immunization. A, Scheme for competitions between V. cholerae passaged through immunized (OMV) or unimmunized mice for 2 hours or 24 hours, or V. cholerae E7946 grown on Luria-Bertani (LB) plates. Secondary infection of naive mice was with mixes of mouse-passaged whole intestine (WI), small intestine (SI), or large intestine (LI) homogenates or LB-grown (LB) bacteria mixed ∼1:1. After 24 h, SIs were harvested from secondary infections and plated for viable counts. Strains were differentially marked by the presence or absence of lacZ, allowing them to be differentiated in competition outputs by the ratio of blue to white bacteria on 5-bromo-4-chloro-3-indolyl-D-galactoside (X-gal) plates. B, Competition index = ratio of SI output/ratio of input. Input ratios were <10:1 and >0.1:1 unless otherwise stated. Competitions were carried out between (1) 24 h WI homogenate V. cholerae (24 h WI) vs LB-grown V. cholerae E7946 (LB); (2) 24 h SI homogenate (24 h SI) vs LB; (3) 24 h LI homogenate (24 h LI) vs LB; (4) 24 h SI vs 24 h LI; (5) 2 h LI homogenate (2 h LI) vs LB; (6) 24 h LI vs 2 h LI; (7) 2 h LI homogenate from neonates born to OMV-immunized dams (OMV 2 h LI) vs LB; (8) OMV 2 h LI vs 2 h LI. Where primary infections were carried out with mouse-passaged hyperinfectious (HI) 24-h SI homogenate, rather than with LB-grown V. cholerae, is indicated by (HI). Competitions were carried out between (9) OMV 2 h LI (HI) vs LB; (10) OMV 2 h LI (HI) vs 2 h LI (HI). Each symbol represents the competitive index from 1 SI homogenate from a naive mouse infected with the indicated competition mix; bars = geometric means (GeoMean). *P < .05 significantly different from 1 (single sample t tests). In each case, a lacZ mutant and lacZ wild-type swap was performed. The pooled data from at least 2 experiments, including the lacZ swap, with at least 6 neonates per group are shown. A ∼1:1 input ratio LB vs LB control competition had a GeoMean competitive index of 1.75 (data not shown). a Competition 9 input ratios were 1:32 and 1:45; to clarify the impact of this deviation from a 1:1 input ratio, an additional control LB vs LB competition was carried out, with inputs of 1:26 and 1:33, which resulted in a GeoMean competitive index of 0.18 (data not shown); thus, for competition 9 the GeoMean competitive index of 0.001148 cannot be attributed only to the lack of a 1:1 input ratio. b Outlier identified and excluded with the Grubb test (P < .01). Where OMV-immunized mice were infected (competitions 7–10), the anti-O1 Ogawa OMV immunoglobulin (Ig) G1 titers by enzyme-linked immunosorbent assay (ELISA) (terminal bleeds) ranged from 23.4 to 112.8 (median, 36.7) μg/mL. C, ID50(the dose at which half of animals are infected) for 2-hour postinoculation LI homogenates from neonates born to unimmunized (2-h LI) or intranasally OMV-immunized (OMV 2-h LI) mice used to infect naive neonates with doses from 1 to 105 colony-forming units (CFU). The anti-O1 Ogawa OMV ELISA IgG1 titers (terminal bleeds) for 4 immunized dams whose pups were used for the ID50 experiment ranged from 18.6 to 114 (median, 73.2) μg/mL.
To shed light on whether the OMV vaccine has the potential to reduce transmission, we competed 2-hour LI homogenates from OMV-immunized mice with in vitro–grown or 2-hour passively passaged (without colonization) bacteria. The bacteria from immune pups were severely outcompeted in each case (Figure 5B, competitions 7 and 8), indicating that the former bacteria are hypoinfectious. Vibrio cholerae that started off HI, then passed through an immune host, were also unable to compete against LB-grown V. cholerae (Figure 5B, competition 9) or V. cholerae exiting naive mice 2 hours after challenge with HI bacteria (Figure 5B, competition 10).
We also determined the ID50 of LB-grown V. cholerae that had passed transiently (2 hours) through naive or immune hosts. The ID50 for bacteria passively transiting through the LI of naive hosts was 27.3 CFU (95% CI, 1–57; R2 = 0.97; Figure 5C). This is 7-fold higher than 24-hour SI homogenates (Figure 1A) and 10-fold lower than LB-grown bacteria (ID50 = 200 CFU [12]). In contrast, V. cholerae from immune hosts were slightly reduced in their capacity to colonize a new host compared with LB-grown bacteria, with an ID50 of 454. However, there was a great deal of variability in the data (95% CI, 1–2411; R2 = 0.91; Figure 5C).
Virulence Gene Induction Is Blocked in Immune Mice and During Passive Transit in Naive Mice
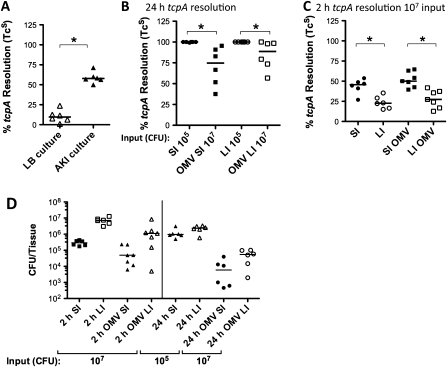
Upon host entry, V. cholerae induces virulence genes including tcpA encoding the pilin subunit for the toxin-coregulated pilus. We monitored tcpA induction using a strain with a resolvase transcriptional fusion to tcpA and a resolvable cassette encoding TcR, as described previously [6]. For El Tor biotype V. cholerae, the tcpA operon can be induced in vitro in AKI broth [15]. As a control we showed that AKI results in 60% resolution of the reporter strain (Figure 6A). Infection of mice with 105 V. cholerae induced the fusion, resulting in complete resolution after 24 hours in the SI (Figure 6B). Using a high (107) input dose, 1000–100 000 V. cholerae overcame inhibition of colonization in immune mice and managed to reside in the host for 24 hours (Figure 6D). V. cholerae populations in both the SI and LI, however, showed significantly less tcpA induction than those that had colonized an unimmunized host (Figure 6B). This suggests that the niche in which the bacteria managed to reside in an immune host is not conducive to tcpA activation.
Figure 6.
Expression of tcpA by Vibrio cholerae upon passage through immunized or unimmunized mice monitored using resolution. Resolution under the control of the tcpA promoter was monitored through the proportion of resolved, tetracycline-sensitive (TcS) V. cholerae AC585. Percentage of tcpA resolution = [(Total CFU – TcR CFU)/Total CFU) × 100/% resolution of the input]. A, tcpA expression in control conditions of Luria-Bertani (LB)–grown (noninducing condition) or what is known as “AKI” culture (inducing condition). Each symbol represents 1 culture. B, tcpA expression 24 hours postinoculation in V. cholerae from homogenates of small intestine (SI) or large intestine (LI) from neonates born to unimmunized mice infected with 105 CFU or outer-membrane vesicle (OMV)–immunized mice challenged with 107 colony-forming units (CFU). C, tcpA expression 2 hours postinoculation in SI and LI upon infection of neonates born to unimmunized or OMV-immunized (OMV) mice with a V. cholerae challenge dose of 107 CFU. Each symbol represents the percentage of resolution for1 infected neonate; bars = medians. *P < .05 (Mann–Whitney U test). Where pups suckled by OMV-immunized mice were infected, anti-OMV immunoglobulin (Ig) G1 antibody titers (terminal bleeds) for the dams were between 23.4 and 25.7 (median, 25.3) μg/mL. D, Total tissue viable counts for V. cholerae infections for which resolution data are shown in (B) and (C). The input doses are indicated. Each symbol represents viable counts for the SI or LI from 1 infected unimmunized or OMV-immunized neonate either 2 hours or 24 hours postinoculation, as indicated. Bars = medians.
Transient passage through a host for 2 hours induced tcpA to a low level, which was significantly higher in the SI (where colonization occurs) than in the LI, regardless of whether the host was suckling from an OMV- or sham-immunized dam (Figure 6C). This suggests that signals for tcpA induction are more active for bacteria in the early stages of SI colonization than for V. cholerae being excreted through the LI without colonization. However, full induction does not occur in either environment early after infection.
DISCUSSION
The physiological state of stool-borne, HI V. cholerae is different from that of laboratory-grown bacteria [7, 8, 17]. Here we show that OMV immunization of mouse dams provides significant protection to their suckling pups challenged with HI V. cholerae, using both infant mouse-passaged and human rice-water-stool HI V. cholerae. This was true for inbred mice mounting a strong antibody response (BALB/c) and for outbred mice (Swiss Webster) mounting a less robust response. Thus, OMV immunization can significantly reduce the intestinal burden upon challenge with HI V. cholerae, as would occur during a cholera outbreak.
The question of whether immunity to cholera after natural infection or immunization leads to killing of bacteria or just prevents their colonization, but without sterilizing immunity, is important with respect to blocking of cholera transmission by infection- or vaccination-mediated immunity. In the suckling mouse passive-protection model with OMV immunization, although anti-V. cholerae antibodies prevent V. cholerae from colonizing the SI, and vibriocial antibodies are present in serum from immunized mice, the bacteria are not killed (by viable counts 1 hour postinfection) and can be followed into the colon 2–4 hours postinfection [14]. Using competitions and ID50 assays, we show that V. cholerae that has passed through an immune host is compromised in its ability to colonize a new host. We hypothesize that V. cholerae is bound by antibodies that reduce its ability to infect new hosts through a reduction of motility and/or clumping. Thus, in the mouse passive-protection model, OMV immunization inhibits both primary infection and transmission to secondary hosts despite a lack of sterilizing immunity.
In humans, the extent of killing of V. cholerae in immune individuals varies. A human volunteer challenge study showed that after rechallenge following primary infection, only 1 of 16 volunteers excreted culturable V. cholerae [18], suggesting that after natural infection, adult immunity is sterilizing. However, when volunteers immunized with oral live-attenuated or whole-cell killed cholera vaccines were challenged with V. cholerae, shedding of the challenge strain was observed in the majority of individuals who were protected from symptomatic cholera [19–21]. Therefore, it may be that bacterial killing is more effectively induced by natural infection than by immunization. Our data in mice suggest that upon exiting an immunized host, stool-shed V. cholerae are hypoinfectious, making them less likely to be transmitted. This may be a contributing factor to the herd immunity reported following immunization with the whole-cell killed oral vaccine Dukoral [22, 23].
In making observations about transmission in mouse models, we also uncovered further information about the nature of the HI state of host-passaged V. cholerae. We found that passive transit through the intestinal lumen of naive mice does not confer hyperinfectivity, but instead, SI colonization is required. This suggests that the SI lumen and epithelium represent distinct environments with respect to the signals needed for inducing hyperinfectivity. Consistent with this, we found that full induction of tcpA expression only occurs within the colonized population, as previously observed [6], whereas passive transit through the SI failed to fully induce this gene. Whether other genes induced during infection also require colonization, rather than transient passage, for their induction remains to be determined.
We observed that a fraction of a high-dose V. cholerae inoculum can overcome the vaccine protection and is retained in the SI for up to 24 hours. However, these bacteria fail to fully induce tcpA, suggesting that they are in a microenvironment that is different from that of bacteria that colonize a naive host. It may be that these bacteria colonize the mucus layer in the SI of immune mice but fail to penetrate further to the SI epithelium, perhaps being held up by antibody binding.
In summary, we have shown that OMV immunization can reduce SI colonization in a passive-protection model even when the challenge bacteria are in the natural rice-water-stool HI state. We show that V. cholerae exiting an immune host, having failed to colonize, occurs on large clumps and is hypoinfectious. And, finally, we show that colonization is required for full virulence gene expression and to induce the HI state.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank Lauren Richey and Derek Papalegis in the Animal Histology Core of the Research Animal Health and Pathology Support Laboratory, Division of Laboratory Animal Medicine, at Tufts University for their expert services of paraffin embedding and tissue sectioning.
Financial support.
This work was supported by the US National Institutes of Health (NIH) grant AI055058 awarded to A. C. and NIH grant AI058935 to S. B. C. A. C. is a Howard Hughes Medical Institute investigator.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–33. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 2.Pruzzo C, Vezzulli L, Colwell RR. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol. 2008;10:1400–10. doi: 10.1111/j.1462-2920.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 3.Childers BM, Klose KE. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2007;2:335–44. doi: 10.2217/17460913.2.3.335. [DOI] [PubMed] [Google Scholar]
- 4.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–92. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klose KE. The suckling mouse model of cholera. Trends Microbiol. 2000;8:189–91. doi: 10.1016/s0966-842x(00)01721-2. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Hava DL, Waldor MK, Camilli A. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell. 1999;99:625–34. doi: 10.1016/s0092-8674(00)81551-2. [DOI] [PubMed] [Google Scholar]
- 7.Merrell DS, Butler SM, Qadri F, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–5. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler SM, Nelson EJ, Chowdhury N, Faruque SM, Calderwood SB, Camilli A. Cholera stool bacteria repress chemotaxis to increase infectivity. Mol Microbiol. 2006;60:417–26. doi: 10.1111/j.1365-2958.2006.05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley DM, Morris JG, Jr, Smith DL. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006;3:e7. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam A, Larocque RC, Harris JB, et al. Hyperinfectivity of human-passaged Vibrio cholerae can be modeled by growth in the infant mouse. Infect Immun. 2005;73:6674–9. doi: 10.1128/IAI.73.10.6674-6679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelichio MJ, Spector J, Waldor MK, Camilli A. Vibrio cholerae intestinal population dynamics in the suckling mouse model of infection. Infect Immun. 1999;67:3733–9. doi: 10.1128/iai.67.8.3733-3739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008;76:4554–63. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77:472–84. doi: 10.1128/IAI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop AL, Schild S, Patimalla B, Klein B, Camilli A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect Immun. 2010;78:4402–20. doi: 10.1128/IAI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30:1075–83. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 16.Hansen GH, Niels-Christiansen LL, Immerdal L, Danielsen EM. Antibodies in the small intestine: mucosal synthesis and deposition of anti-glycosyl IgA, IgM, and IgG in the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol. 2006;291:G82–90. doi: 10.1152/ajpgi.00021.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bina J, Zhu J, Dziejman M, Faruque S, Calderwood S, Mekalanos J. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc Natl Acad Sci U S A. 2003;100:2801–6. doi: 10.1073/pnas.2628026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine MM, Nalin DR, Craig JP, et al. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73:3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- 19.Black RE, Levine MM, Clements ML, Young CR, Svennerholm AM, Holmgren J. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987;55:1116–20. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine MM, Kaper JB, Herrington D, et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;2:467–70. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 21.Tacket CO, Cohen MB, Wasserman SS, et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect Immun. 1999;67:6341–5. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali M, Emch M, Von Seidlein L, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366:44–9. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 23.Longini IM, Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.