Abstract
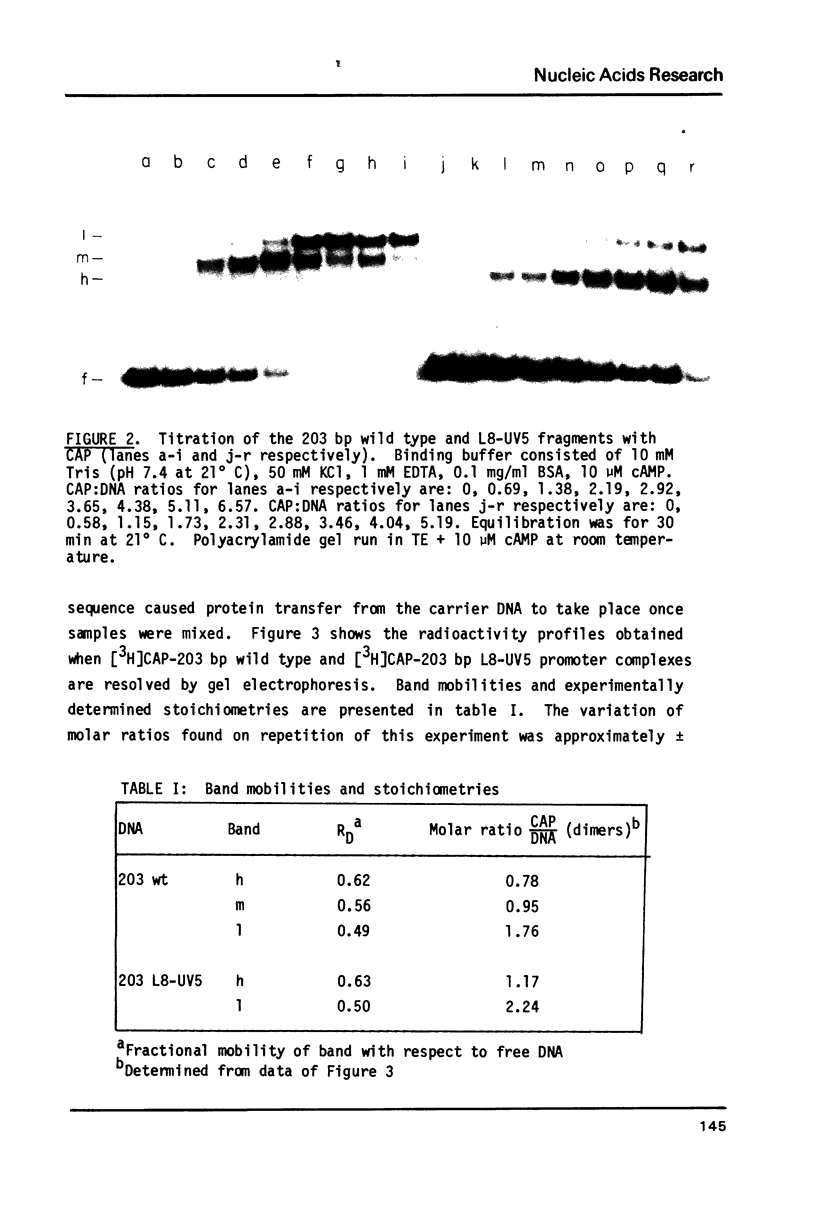
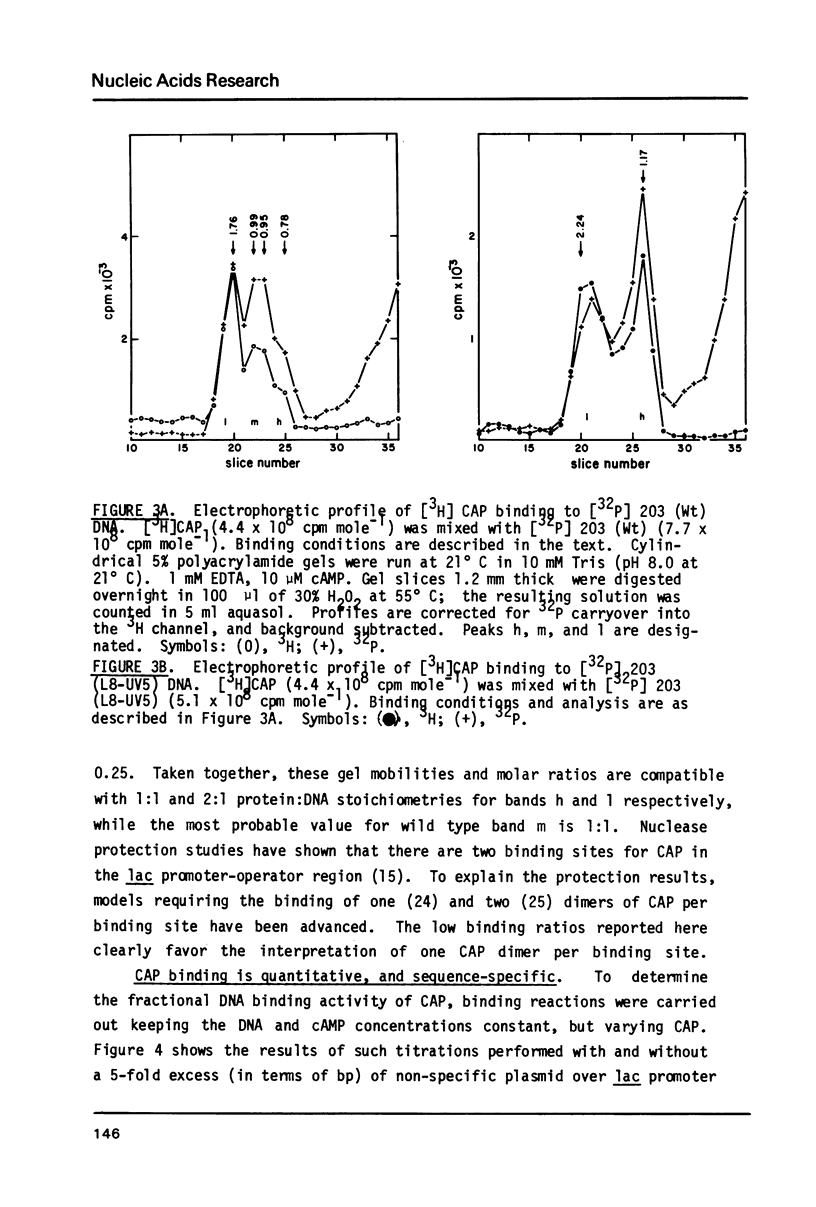
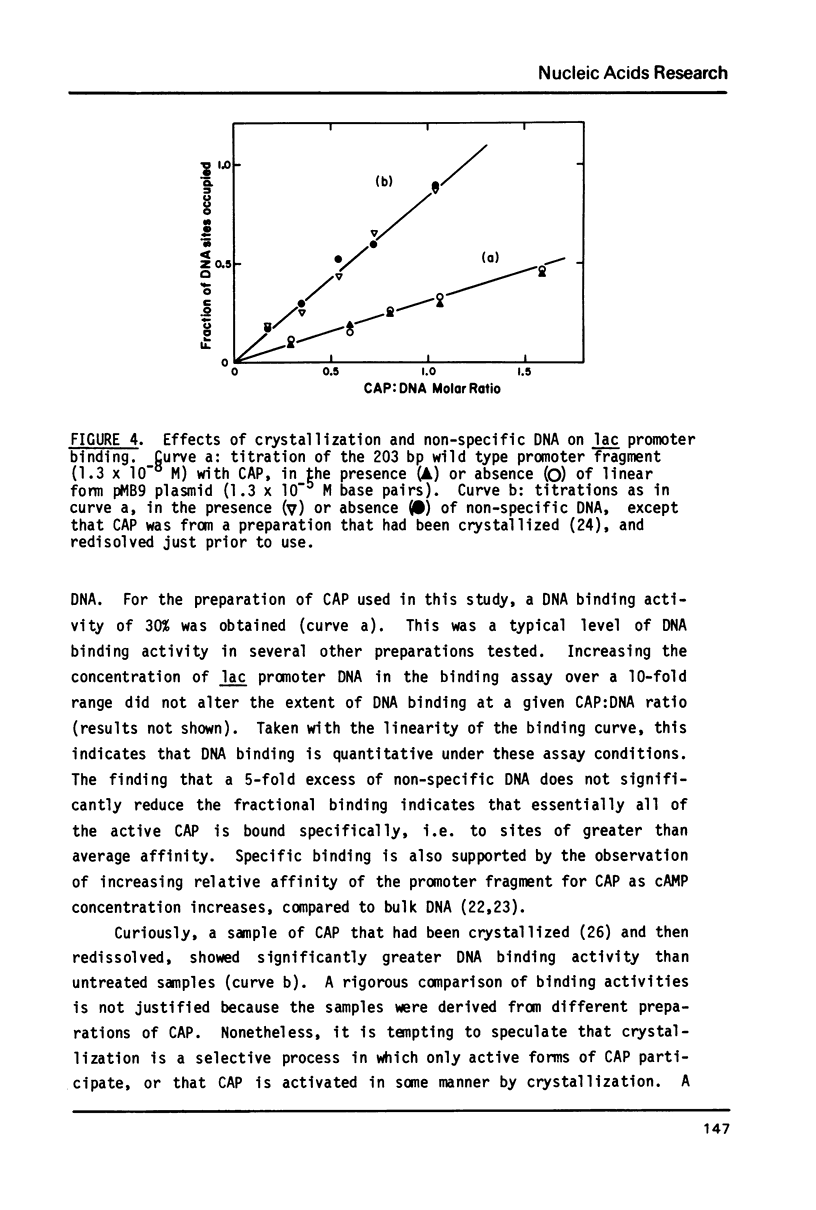
The binding stoichiometries of the complexes formed when the E. coli cyclic AMP receptor protein (CAP) binds to 203 bp lac promoter-operator restriction fragments have been determined. Under quantitative binding conditions, a single dimer of CAP occupies each of two sites in the promoter. Different electrophoretic mobilities are observed for 1:1 complexes formed with L8-UV5 mutant, L305 mutant, and wild type promoter fragments, indicating sequence-specific structural differences between the complexes. The differences in gel mobility between L8-UV5 and wild type complexes disappear when the promoter fragments are cleaved with Hpa II restriction endonuclease. Models in which CAP alters DNA conformation or in which CAP forms a transient intramolecular bridge between two domains of a DNA molecule could account for these observations. The selective binding of RNA polymerase to CAP-promoter complexes is demonstrated: the binding of a single CAP dimer to the promoter is sufficient to stimulate subsequent polymerase binding. Functional CAP molecules are not released from the promoter on polymerase binding.
Full text
PDF







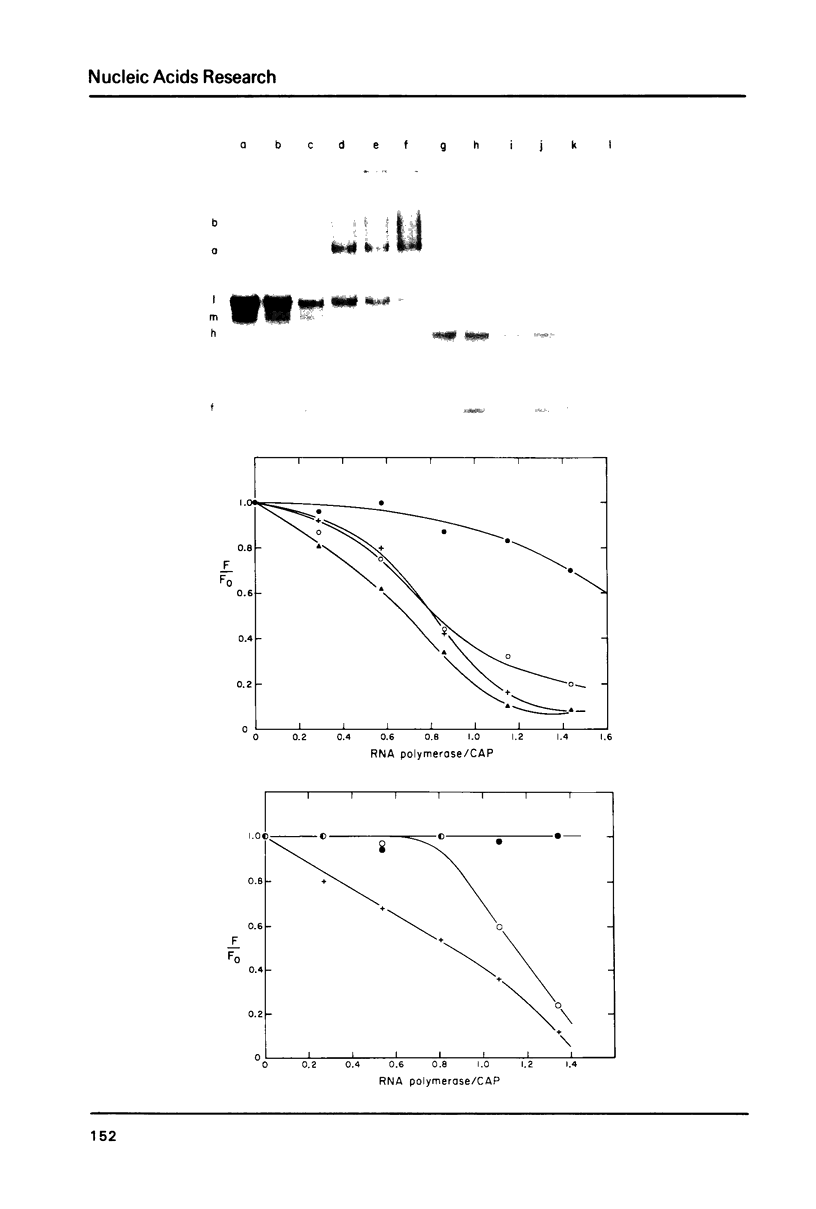






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresloff J. L., Crothers D. M. DNA-ethidium reaction kinetics: demonstration of direct ligand transfer between DNA binding sites. J Mol Biol. 1975 Jun 15;95(1):103–123. doi: 10.1016/0022-2836(75)90339-3. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Lac repressor - lac operator interaction. Circular dichroism study. Nucleic Acids Res. 1981 Oct 10;9(19):5175–5184. doi: 10.1093/nar/9.19.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Blumberg W. E. Binding constants from zone transport of interacting molecules. Biochemistry. 1973 Sep 11;12(19):3648–3662. doi: 10.1021/bi00743a013. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transmission of allosteric effects in DNA. Nature. 1979 Apr 5;278(5704):521–524. doi: 10.1038/278521a0. [DOI] [PubMed] [Google Scholar]
- Hopkins J. D. A new class of promoter mutations in the lactose operon of Escherichia coli. J Mol Biol. 1974 Aug 25;87(4):715–724. doi: 10.1016/0022-2836(74)90080-1. [DOI] [PubMed] [Google Scholar]
- Ippen K., Miller J. H., Scaife J., Beckwith J. New controlling element in the Lac operon of E. coli. Nature. 1968 Mar 2;217(5131):825–827. doi: 10.1038/217825a0. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Binding of RNA polymerase and the catabolite gene activator protein within the cat promoter in Escherichia coli. J Mol Biol. 1981 Aug 5;150(2):185–196. doi: 10.1016/0022-2836(81)90448-4. [DOI] [PubMed] [Google Scholar]
- Majerfeld I. H., Miller D., Spitz E., Rickenberg H. V. Regulation of the synthesis of adenylate cyclase in Escherichia coli by the cAMP -- cAMP receptor protein complex. Mol Gen Genet. 1981;181(4):470–475. doi: 10.1007/BF00428738. [DOI] [PubMed] [Google Scholar]
- Majors J. Specific binding of CAP factor to lac promoter DNA. Nature. 1975 Aug 21;256(5519):672–674. doi: 10.1038/256672a0. [DOI] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKay D. B., Fried M. G. Crystallization and preliminary X-ray diffraction data for the cyclic AMP receptor protein of Escherichia coli. J Mol Biol. 1980 May 5;139(1):95–96. doi: 10.1016/0022-2836(80)90118-7. [DOI] [PubMed] [Google Scholar]
- McKay D. B., Steitz T. A. Structure of catabolite gene activator protein at 2.9 A resolution suggests binding to left-handed B-DNA. Nature. 1981 Apr 30;290(5809):744–749. doi: 10.1038/290744a0. [DOI] [PubMed] [Google Scholar]
- O'Gorman R. B., Dunaway M., Matthews K. S. DNA binding characteristics of lactose repressor and the trypsin-resistant core repressor. J Biol Chem. 1980 Nov 10;255(21):10100–10106. [PubMed] [Google Scholar]
- O'Neill M. C., Amass K., de Crombrugghe B. Molecuar model of the DNA interaction site for the cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2213–2217. doi: 10.1073/pnas.78.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden S., Haggerty D., Stoner C. M., Kolodrubetz D., Schleif R. The Escherichia coli L-arabinose operon: binding sites of the regulatory proteins and a mechanism of positive and negative regulation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3346–3350. doi: 10.1073/pnas.77.6.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saxe S. A., Revzin A. Cooperative binding to DNA of catabolite activator protein of Escherichia coli. Biochemistry. 1979 Jan 23;18(2):255–263. doi: 10.1021/bi00569a003. [DOI] [PubMed] [Google Scholar]
- Schmitz A. Cyclic AMP receptor proteins interacts with lactose operator DNA. Nucleic Acids Res. 1981 Jan 24;9(2):277–292. doi: 10.1093/nar/9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Gilbert S. G., Jain S. C., Sakore T. D. Organization of DNA in chromatin. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3068–3072. doi: 10.1073/pnas.73.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A. Non-specific interactions of CRP from E. coli with native and denatured DNAs: control of binding by cAMP and cGMP and by cation concentration. Nucleic Acids Res. 1979 Nov 24;7(6):1699–1712. doi: 10.1093/nar/7.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Crothers D. M. Solution structural studies of the A and Z forms of DNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6808–6811. doi: 10.1073/pnas.78.11.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]