Abstract
Hemorrhage is a prominent clinical manifestation of systemic anthrax. Therefore, we have examined the effects of anthrax lethal and edema toxins on human platelets. We find that anthrax lethal toxin fails to cleave its target, mitogen-activated protein kinase 1, and anthrax edema toxin fails to increase intracellular cyclic adenosine monophosphate. Surface expression of toxin receptors tumor endothelial marker 8 and capillary morphogenesis gene 2, as well as coreceptor low density lipoprotein receptor-related protein 6 (LRP6), are markedly reduced, preventing toxin binding to platelets. Our studies suggest that the hemorrhagic clinical manifestations of systemic anthrax are unlikely to be caused by the direct binding and entry of anthrax toxins into human platelets.
Inhalation anthrax is a highly fatal, acute disease characterized by severe hemorrhage, pleural effusions, hypotension, and, ultimately, shock-induced death [1]. Anthrax is caused by the gram-positive bacterium Bacillus anthracis, whose virulence is attributed to its poly-D-glutamic acid capsule and the A-B toxins: lethal factor (LF), edema factor (EF), and protective antigen (PA). PA binds the host receptors tumor endothelial marker 8 (TEM8) and capillary morphogenesis gene 2 (CMG2), facilitating entry of EF and/or LF into host cells [2]. LF is a metalloprotease that cleaves the N-terminus of mitogen-activated protein kinase kinase (MEK) 1, 2, 3, 4, 6 and 7 [3]. EF is an adenylate cyclase that increases intracellular cyclic adenosine monophosphate (cAMP) levels [4]. Lethal toxin (LT) refers to the combination of LF and PA, whereas edema toxin (ET) refers to the combination of EF and PA.
Previous studies using anthrax animal models have documented severe hemostasis abnormalities including hemorrhagic lesions, fibrin deposits, thrombocytopenia, increased prothrombin time, elevated activated partial thromboplastin time, vascular permeability, pleural effusions, and decreased fibrinogen levels [5, 6]. In human cases, hemorrhagic lesions—capillary and vascular lesions consisting of fibrin deposits, pleural effusion, blood vessel leakage, and disseminated intravascular coagulopathy—have been documented [7]. These clinical manifestations suggest B. anthracis is capable of inducing severe defects in hemostasis; however, the exact mechanisms underlying these manifestations remain to be fully defined.
Platelets are anuclear fragments of megakaryocytes that aid in hemostasis. Previous studies investigating rabbit platelets have shown that ET suppresses platelet aggregation, induces a rise in cAMP, and activates protein kinase A [8]. Additional investigations have shown anthrax LT inhibits arachidonic acid–induced whole blood aggregation, inhibits platelet P-selectin expression, and increases mortality in mice receiving aspirin and rhodostomin [9]. We have sought to further elucidate the mechanisms of severe hemostasis abnormalities documented in anthrax victims by investigating the direct effects of these toxins on purified human platelets.
Materials and Methods
Human whole blood was collected by venous puncture from healthy volunteers into Aster-Jandl anticoagulant in accordance with US Department of Health and Human Services guidelines and University of Florida Institutional Review Board approval. Human platelet-rich plasma was obtained by centrifugation at 100 g for 10 minutes, and purified platelets were resuspended to 1 × 106 platelets/mL in Tyrode-Hepes buffer. For mouse platelet studies, mice were anesthetized with carbon dioxide followed by cervical displacement. Cardiac puncture was performed, and platelet-rich plasma was obtained using the same methods described for human platelets.
HeLa cells and THP-1 cells (American Type Culture Collection) were grown in complete Dulbecco's Modified Eagle Medium (DMEM)and Roswell Park Memorial Institute 1640 medium (Cellgro), respectively, and used at a concentration of 1 × 106 cells/mL.
Reagents used were thrombin receptor agonist peptide (TRAP) (Sigma–Aldrich), forskolin (Fsk) (Sigma–Aldrich), 3-isobutyl-1-methylxanthine (IBMX) (Sigma–Aldrich), streptavidin-conjugated DyLight 488 (Thermo Scientific), SB203580 (Calbiochem), and CD62P–fluorescein isothiocyanate (FITC) (BD Bioscience). PA, LF, and EF were purified as described elsewhere [10] and incubated with cells for a minimum of 2 hours and for as long as 12 hours.
For Western blot analysis, cells were lysed in sodium dodecyl sulfate and 40-μg total protein was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by transfer to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). Antibodies used were reactive against MEK1 (Upstate), TEM8/CMG2 (Abcam), low density lipoprotein receptor-related protein 6 (LRP6) (Santa Cruz), Alexa Fluor 488 goat anti-rabbit immunoglobulin G (H+L) (Invitrogen), isotype control/VASP antibody (Cell Signaling), total HSP27 (Cell Signaling), phosphorylated serine 82 HSP27 (Cell Signaling), or β-actin (Sigma).
For cAMP analysis, platelets were treated with cAMP agonists: 10 μmol/L Fsk plus 100 μmol/L IBMX, or varying concentrations of ET. Intracellular cAMP levels were measured using an enzyme-linked immunoassay (Amersham Biosciences and Arbor Assays) following kit protocol.
For P-selectin expression, platelets were fixed in a 1:1 ratio of 3.7% formaldehyde, and the relative amount of FITC-P-selectin was determined using flow cytometry analysis.
For toxin binding and internalization, PA was labeled with biotin (Thermo Scientific) according to the manufacturer's instructions. Platelets or THP-1 cells were incubated with PA-biotin, fixed, and probed with FITC-streptavidin. The relative amount of PA binding and internalization was determined using flow cytometry analysis.
For surface expression of LRP6 and TEM8/CMG2, human platelets or THP-1 cells were fixed, incubated for 1 hour with primary rabbit antibody—either isotype control or specific polyclonal antibody directed against TEM8/CMG2 or LRP6—and followed by the secondary Alex 488 goat anti-rabbit immunoglobulin G antibody. All experiments were performed 3 or more times using a minimum of 3 different platelet donors for each study.
Results
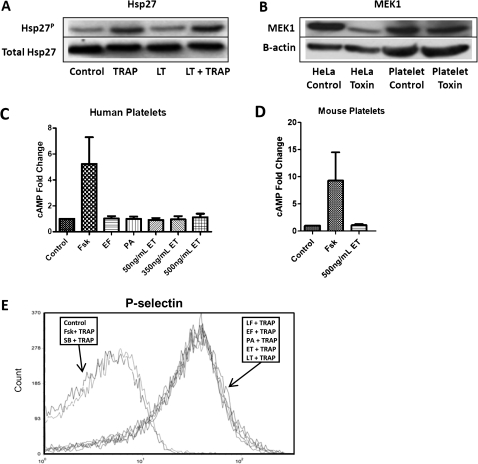
To determine the activity of LT, human platelets or HeLa cells were treated with 500 ng/mL LT. Because LT is known to cleave the N-terminus of MEK and prevent phosphorylation of the downstream substrate Hsp27, a Western blot analysis was performed. LT did not cleave MEK1 or prevent Hsp27 phosphorylation in human platelets (Figure 1A and 1B); however, HeLa cells were susceptible to MEK1 cleavage after LT treatment (Figure1B). We next sought to determine the effects of ET on human and mouse platelets. It is well documented that ET induces intracellular cAMP levels [4]; thus, measurement of cAMP after ET treatment is an additional assay used to measure toxin entry and activity. Treatment of human platelets with increasing concentrations of ET, surprisingly failed to increase intracellular cAMP levels; however, platelets treated with the positive control Fsk/IBMX, demonstrated at least a 5-fold increase in cAMP (Figure 1C). Mouse platelets also failed to show an increase in cAMP levels after a 500 ng/mL treatment of ET; however, the positive control led to an increase in cAMP levels (Figure 1D).
Figure 1.
Effects of lethal toxin (LT) and edema toxin (ET) on human platelets. A, Western blot showing phosphorylated Hsp27 in human platelets treated with 500 ng/mL LT for 4 hours at room temperature and stimulated with 30 μmol/L thrombin receptor agonist peptide (TRAP) for 2 minutes or left unstimulated. Blots were stripped and reprobed with a primary antibody to total Hsp27 to show equal loading. Data are representative of 4 platelet donors. B, Western blot showing mitogen-activated protein kinase kinase (MEK) 1 in human platelets or HeLa cells treated with 500 ng/mL LT for 4 hours at room temperature or left untreated. Blots were stripped and reprobed with a primary antibody to β-actin to show equal loading. Data are representative of 4 platelet donors. C, D, Enzyme-linked immunoassay measuring intracellular cyclic adenosine monophosphate (cAMP) levels in human platelets (C) treated with 50, 350, or 500 ng/mL ET for 2 hours at 37°C. Negative control platelets were treated with 500 ng/mL edema factor (EF) or 500 ng/mL protective antigen (PA) for 2 hours at 37°C. Positive control platelets were treated with forskolin (Fsk (10 μmol/L) and 3-isobutyl-1-methylxanthine (IBMX) (100 μmol/L) for 20 minutes at 37°C. Error bars indicate standard error of the mean (SEM) of 9 experiments using 3 different platelet donors. D, Mouse platelets were treated with 500 ng/mL ET for 2 hours at 37°C. Error bars indicate SEM of 4 experiments using platelets from 4 mice. cAMP levels were significantly higher than control levels only for Fsk/IBMX. E, Flow cytometry measuring P-selectin levels in human platelets after 2 hours of toxin treatment at 37°C with either 1000 ng/mL EF, 1000 ng/mL lethal factor (LF), 1000 ng/mL PA, 1000 ng/mL ET, or 1000 ng/mL LT, or after 20 minutes at 37°C with SB203580 (400 μmol/L) or with forskolin (10 μmol/L) and IBMX (100 μmol/L). Platelets were then stimulated with TRAP (30 μmol/L) for 2 minutes, fixed, probed for P-selectin, and analyzed using FACScan (BD) and FCS Express (DeNovo) software. Data are representative of 6 experiments performed with platelets from 3 donors.
To further explore the functional effects of LT and ET on platelet activation, we measured platelet P-selectin expression after toxin treatment. P-selectin is a glycoprotein that is translocated to the platelet surface from α granules with cell activation and is used as a marker for platelet activation [11]. Platelets were treated with anthrax toxins and then activated with TRAP, a known P-selectin stimulus; P-selectin levels were then determined. Pretreatment with both Fsk/IBMX, which represents a positive control for ET by raising intracellular cAMP levels, and SB203580, which represents a positive control for LT by preventing activation of the p38 pathway, inhibited TRAP-induced P-selectin surface expression, whereas pretreatment with LT or ET failed to block TRAP-induced P-selectin expression on human platelets (Figure 1E). P-selectin surface expression is an important component for aggregate formation of platelets. These results indicate neither LT nor ET directly inhibit TRAP-induced platelet activation.
Figure 2.
Assessment of anthrax toxin binding to human platelet and platelet surface expression of anthrax toxin receptors. A, B, Flow cytometry measuring protective antigen (PA) binding and internalization by human platelets (A) and by THP-1 cells (B) after 2 hours of treatment with 1000 ng/mL PA-biotin at 4°C, 37°C, or incubation in buffer alone. Cells were fixed in a 1:1 ratio of 3.7% formaldehyde, probed with fluorescein isothiocyanate (FITC)–streptavidin, and analyzed using FACScan (BD) and FCS Express (DeNovo) software. Data are representative of 4 experiments from 3 platelet donors and 3 experiments using separate THP-1 cell preparations. C, D, Flow cytometry measuring anthrax receptor surface expression in human platelets (C) and THP-1 cells (D). Cells were fixed, probed for tumor endothelial marker 8 (TEM8)/capillary morphogenesis gene 2 (CMG2), LRP6, or an isotype control, followed by secondary goat anti-rabbit Alexa Fluor 488–conjugated antibody, and analyzed using Accuri C6 (BD Biosciences) and FCS Express (De Novo). Data are representative of 3 experiments using 3 donors. E, Fold changes in peak fluorescent intensity of bound polyclonal antibodies directed against anthrax receptors: TEM8/CMG2 and LRP6 in human platelets and THP-1 cells. Brackets show the standard error of the mean for 3 donors. The fold change in peak fluorescence intensity for each condition was calculated by comparing each sample with the peak fluorescence intensity of an isotype control polyclonal primary rabbit antibody.
To determine whether a lack of cellular responses to anthrax toxin was due to ineffective toxin internalization or binding, a PA-biotin conjugate was used. Purified platelets and THP-1 cells were treated with PA-biotin at 4°C to determine toxin binding or at 37°C to determine toxin internalization. PA-biotin was probed with streptavidin-FITC, which was followed by flow cytometry analysis. THP-1 cells showed an increase in PA binding at 4°C and internalization of the toxin after subsequent warming to 37°C (Figure 2A), whereas purified platelets failed to exhibit PA binding at 4°C or internalization at 37°C (Figure 2B). The failure of PA to bind to the platelet surface indicated a defect in anthrax toxin binding to its cognate receptor.
We next sought to determine whether the anthrax receptors TEM8 CMG2 and the coreceptor LRP6 were present on human platelets. LRP6 is required for anthrax toxin lethality and has been shown to play a role in toxin binding and internalization [12]. Western blot analysis of solubilized human platelets confirmed the presence of these anthrax receptors in human platelets (data not shown). However, flow cytometry revealed a low surface expression of TEM8/CMG2 and LRP6 (Figure 2C). Human platelets demonstrated 2 populations of TEM8/CMG2 surface expression. One population (47%) exhibited low TEM8/CMG2 expression (peak fluorescence intensity 2.16 ± 0.18–fold higher than isotype control, [mean ± standard error of the mean [SEM]; n = 3 donors), and a second population (53%) showed modest expression of TEM8/CMG2 (11.74 ± 0.76–fold higher than isotype control [SEM]; n = 3). LRP6 platelet surface expression was low (2.03 ± 0.20–fold higher than isotype control [SEM]; n = 3) (Figure 2C and 2E). In contrast, the human monocytic THP-1 cell line exhibited a single population with high-level TEM8/CMG2 surface expression (76.67 ± 2.79–fold [SEM]; 3 cell preparations) and moderate surface expression of LRP6 (10.6 ± 2.85–fold [SEM]; n = 3) (Figure 2D and 2E).
Discussion
Hemostatic abnormalities have been well documented during anthrax infections, but the precise underlying mechanisms have yet to be identified. Pathologic findings, including hemorrhagic lesions, fibrin deposits, and prolonged clotting times, suggest a defect in platelet function [6, 7]. Our extensive analysis of the interaction of anthrax toxins with human platelets strongly suggests that neither anthrax LT nor ET has a direct effect on human platelet function, and our findings contradict those of previous platelet studies in mice and rabbits [8, 9]. We are at a loss to explain these differences. The same highly purified preparations of LT and ET used in our platelet studies have respectively been shown to impair human neutrophil Hsp27 phosphorylation [13] and induce a marked rise in neutrophil cAMP [14], and both toxins effectively impair the actin-based motility of human neutrophils [14, 15]. Our findings for human platelets were unexpected and encouraged us to examine multiple human platelet donors and to repeat our experiments multiple times to assure their validity.
The inability of PA to bind to and enter platelets is likely to be the consequence of low surface expression of PA receptors TEM8 and CMG2 and coreceptor LRP6 on platelets, and these findings can explain the inability of LT and ET to directly impair platelet function. Our findings should guide future investigators to focus on alternative mechanisms to explain defective hemostasis in systemic anthrax.
Notes
Acknowledgments.
The authors thank Dr Conrad Quinn of the Centers for Disease Control and Prevention for providing his expertise knowledge on platelets and for providing purified LF and PA, Dr Wei-Jen Tan of the University of Chicago for providing purified EF, Dr Minoru Satoh of the University of Florida for helpful advice on the design and analysis of our flow cytometry experiments, and Dr Bradley Fletcher of the University of Florida for providing his knowledge on the analysis of the anthrax receptors.
Financial support.
This work was supported by the National Institutes of Health (grant RO1AI064891).
Potential conflicts of Interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Borio L, Frank D, Mani V, et al. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA. 2001;286:2554–9. doi: 10.1001/jama.286.20.2554. [DOI] [PubMed] [Google Scholar]
- 2.Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- 3.Duesbery NS, Webb CP, Leppla SH, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–7. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 4.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–6. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culley NC, Pinson DM, Chakrabarty A, Mayo MS, Levine SM. Pathophysiological manifestations in mice exposed to anthrax lethal toxin. Infect Immun. 2005;73:7006–10. doi: 10.1128/IAI.73.10.7006-7010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twenhafel NA, Leffel E, Pitt ML. Pathology of inhalational anthrax infection in the African green monkey. Vet Pathol. 2007;44:716–21. doi: 10.1354/vp.44-5-716. [DOI] [PubMed] [Google Scholar]
- 7.Guarner J, Jernigan JA, Shieh WJ, et al. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am J Pathol. 2003;163:701–9. doi: 10.1016/S0002-9440(10)63697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam S, Gupta M, Bhatnagar R. Inhibition of platelet aggregation by anthrax edema toxin. Biochem Biophys Res Commun. 2006;339:107–14. doi: 10.1016/j.bbrc.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Kau JH, Sun DS, Tsai WJ, et al. Antiplatelet activities of anthrax lethal toxin are associated with suppressed p42/44 and p38 mitogen-activated protein kinase pathways in the platelets. J Infect Dis. 2005;192:1465–74. doi: 10.1086/491477. [DOI] [PubMed] [Google Scholar]
- 10.Quinn CP, Shone CC, Turnbull PC, Melling J. Purification of anthrax-toxin components by high-performance anion-exchange, gel-filtration and hydrophobic-interaction chromatography. Biochem J. 1988;252:753–8. doi: 10.1042/bj2520753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shebuski RJ, Kilgore KS. Role of inflammatory mediators in thrombogenesis. J Pharmacol Exp Ther. 2002;300:729–35. doi: 10.1124/jpet.300.3.729. [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Lu Q, Chaudry GJ, Leppla SH, Cohen SN. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell. 2006;124:1141–54. doi: 10.1016/j.cell.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 13.During RL, Gibson BG, Li W, et al. Anthrax lethal toxin paralyzes actin-based motility by blocking Hsp27 phosphorylation. EMBO J. 2007;26:2240–50. doi: 10.1038/sj.emboj.7601687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szarowicz SE, During RL, Li W, Quinn CP, Tang WJ, Southwick FS. Bacillus anthracis edema toxin impairs neutrophil actin-based motility. Infect Immun. 2009;77:2455–64. doi: 10.1128/IAI.00839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.During RL, Li W, Hao B, et al. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J Infect Dis. 2005;192:837–45. doi: 10.1086/432516. [DOI] [PubMed] [Google Scholar]