Abstract
Background. The effect of herpes simplex virus type 2 (HSV-2) suppression on human immunodeficiency virus type 1 (HIV-1) RNA in the context of prevention of mother-to-child transmission (PMTCT) interventions is unknown.
Methods. Between April 2008 and August 2010, we conducted a randomized, double-blind trial of twice daily 500 mg valacyclovir or placebo beginning at 34 weeks gestation in 148 HIV-1/HSV-2 coinfected pregnant Kenyan women ineligible for highly active antiretroviral therapy (CD4 > 250 cells/mm3). Women received zidovudine and single dose nevirapine for PMTCT and were followed until 12 months postpartum.
Results. Mean baseline plasma HIV-1 RNA was 3.88 log10 copies/mL. Mean plasma HIV-1 was lower during pregnancy (−.56 log10 copies/mL; 95% confidence interval [CI], −.77 to −.34) and after 6 weeks postpartum (−.51 log10 copies/mL; 95% CI, −.73 to −.30) in the valacyclovir arm than the placebo arm. Valacyclovir reduced breast milk HIV-1 RNA detection at 6 and 14 weeks postpartum compared with placebo (30% lower, P = .04; 46% lower, P = .01, respectively), but not after 14 weeks. Cervical HIV-1 RNA detection was similar between arms (P = .91).
Conclusions. Valacyclovir significantly decreased early breast milk and plasma HIV-1 RNA among women receiving PMTCT.
Clinical Trials Registration. NCT00530777.
Despite the success of preventing mother-to-child human immunodeficiency virus (HIV) transmission (PMTCT) programs, more than 350000 infants become infected with human immunodeficiency virus type 1 (HIV-1) worldwide each year [1]. In 2010 the World Health Organization (WHO) issued recommendations to use more aggressive PMTCT antiretroviral regimens: highly active antiretroviral therapy (HAART) when CD4 counts are ≤350 cells/mm3 and either HAART until cessation of breastfeeding or a short-course regimen consisting of zidovudine (ZDV), single dose nevirapine (sdNVP), and lamivudine (3TC) when CD4 counts are >350 cells/mm3 [2]. However, access to HAART or short-course regimens is not universal; it is estimated that in 2009 53% of HIV-infected pregnant women received any PMTCT antiretrovirals in low- and middle-income countries [3]. Additional strategies that decrease postnatal HIV-1 transmission, are inexpensive and safe, and can be incorporated into existing PMTCT programs and in conjunction with antiretrovirals are still needed. Herpes simplex virus type 2 (HSV-2) suppression, which fulfills many of these characteristics, might be one such intervention.
HSV-2 infection, the leading cause of genital ulcers, is common among HIV-1–infected women, with HSV-2 seroprevalence ranging from 75% to over 95% in sub-Saharan Africa [4–6]. Clinical and sub-clinical HSV-2 reactivation is associated with higher HIV-1 plasma and genital RNA levels [5, 7–11], demonstrating that HSV-2 increases HIV-1 replication. Although clinical trials have shown that HSV-2 suppressive therapy significantly reduces plasma and cervical HIV-1 RNA levels [12–17], it did not decrease risk of heterosexual HIV-1 transmission [15]. However, the effect of HSV-2 suppression on breast milk HIV-1 RNA levels or on mother-to-child HIV-1 transmission (MTCT) is unknown. It is plausible that a reduction in HIV-1 RNA during pregnancy and breastfeeding would have a substantial effect on reducing MTCT since HIV-1 RNA levels in plasma and breast milk are important predictors of transmission [18]. Furthermore, HSV-2 suppression also reduces genital ulcers, another important risk factor for MTCT [4, 19–21].
For these reasons, we conducted a randomized, double-blind, placebo-controlled trial of valacyclovir 500 mg twice daily to quantify the effect of HSV-2 suppression on reducing plasma, cervical, and breast milk HIV-1 RNA levels among pregnant and postpartum Kenyan women coinfected with HIV-1 and HSV-2. We also evaluated the effect of valacyclovir suppressive therapy on genital HSV DNA shedding during pregnancy.
METHODS
Study Design and Population
Between April 2008 and June 2009, HIV-1–infected pregnant women seeking antenatal care at, or referred to, the Mathare North City Council Clinic in Nairobi, Kenya, were screened for study participation. Women were eligible if they were ≥18 years of age, HIV-1 seropositive, HSV-2 seropositive, and at 28–32 weeks gestation, had a CD4 count >250 cells/mm3, and planned on delivering and residing in Nairobi for 12 months postpartum. Exclusion criteria included hypersensitivity to acyclovir or valacyclovir and clinical indication for HAART (WHO stage 3 or 4). Participants were offered counseling and PMTCT antiretrovirals according to Kenyan guidelines: twice daily oral ZDV 300 mg beginning at 28 weeks gestation, oral ZDV 300 mg at the onset of labor and every 3 hours until delivery, and sdNVP 200 mg at the onset of labor for the mother and after delivery for the infant. In June 2009, maternal 3TC and twice daily ZDV for 1 week postpartum were also offered for prophylaxis [22]. Women were referred for treatment if they became eligible for HAART, but they continued study participation. Women received multivitamins during pregnancy and counseling on infant feeding [22]. Study procedures were approved by ethical review committees at the University of Nairobi and the University of Washington, and all participants provided written informed consent. This trial is registered at http://clinicaltrials.gov (NCT00530777).
Randomization
Study clinicians enrolled women and randomized them to twice daily 500 mg valacyclovir or placebo at 34 weeks through 12 months postpartum. Enrollment occurred at 34 weeks gestation in an effort to include the majority of antenatal care attendees in the study (most Kenyan women initiate care in late pregnancy) and to allow for ample time to determine eligibility. An off-site, independent researcher randomly generated sequentially numbered study identifiers using a 1:1 allocation scheme with block sizes of 20, and participants were sequentially enrolled.
Clinical Follow-up
At screening, a questionnaire and exam were administered to ascertain eligibility; blood was drawn for CD4 count and HSV-2 and HIV-1 serology. Antenatal visits occurred at 34 and 38 weeks gestation and postpartum follow-up visits occurred at 2, 6, 10, and 14 weeks and 6, 9, and 12 months postpartum. At all study visits women completed questionnaires, were examined, and underwent pill counts and counseling for adherence. Adherence by pill count was calculated as [(number of pills prescribed − number of pills counted)/number of pills prescribed].
At enrollment, demographic characteristics were ascertained and blood was collected for syphilis serostatus. Blood and cervical swabs were collected for HIV-1 RNA, and genital swabs were collected for HSV DNA at 34 and 38 weeks gestation [23]. Genital specimens were obtained by swabbing the cervicovaginal, vulvar, and perianal areas as described previously [24]. Antenatal care was provided semimonthly. Women were encouraged to deliver at a health care facility and referred to the local tertiary facility for complications. Delivery information was obtained from the hospital chart and study participants. Blood and breast milk samples were collected for HIV-1 RNA assays at 2, 6, and 14 weeks and 6 and 12 months postpartum. Infant blood was collected on filter papers within 48 hours of birth and at all postpartum visits to determine timing of HIV-1 infection.
Laboratory Procedures
Maternal HIV-1 serostatus was confirmed using Vironostika HIV Uni-Form II enzyme-linked immunosorbent assay (ELISA) (bioMerieux, France) and HSV-2 serostatus was determined using HerpeSelect ELISA (Focus Technologies, Cypress, CA) [25]. Optical densities ≥3.5 were considered positive for HSV-2. Flow cytometry (FACSCaliber or FACSCount, Becton Dickinson, Franklin Lakes, NJ) was used for CD4 counts. Syphilis serostatus was determined using rapid plasma reagin (Becton Dickinson, Franklin Lakes, NJ). Plasma, cervical, and breast milk specimens were cryopreserved and shipped to Seattle for testing. The Fred Hutchinson Cancer Research Center conducted HIV-1 RNA assays using the Gen-Probe assay (Gen-Probe Inc, San Diego, CA), a transcription-mediated amplification method sensitive for HIV-1 subtypes common in Kenya and validated for samples from plasma and cervical swabs [26, 27]. HIV-1 RNA levels below the lower limit of detection, 150 copies/mL for plasma and 100 copies/mL for cervical swabs and breast milk, were re-coded as half the value of the lower limit of detection. Genital swabs were analyzed for HSV DNA using a polymerase chain reaction (PCR) assay described previously and reported as positive if ≥150 copies/mL were detected [28, 29]. HIV-1 and HSV shedding were defined as having HIV-1 RNA or HSV DNA copies/mL above the lower limit of detection of the assay, respectively. As part of the existing PMTCT program in Kenya, infant blood collected on filter paper specimens collected at 6 weeks of age were tested using the Amplicor HIV-1 Test, version 1.5 (Roche Molecular Systems, Inc, Branchburg, NJ). Gag and pol PCR methods previously described [30, 31] were used to confirm positive HIV-1 DNA 6 week results and to test samples collected within 48 hours of birth, and at 2 weeks and 6 and 12 months of age. Positive samples were retested to confirm the result, and samples collected prior to the positive result were tested in order to determine the timing of infection.
Statistical Analysis
We estimated that with 148 women we would have 80% power to detect a 0.5 log10 copies/mL difference between the mean plasma HIV-1 RNA levels in the two arms, assuming a standard deviation of 1.0 with 15% attrition and a 5% 2-sided type I error rate. An independent data safety and monitoring board reviewed the study to evaluate safety concerns periodically during the trial; aside from safety, no criteria for stopping the trial were provided. We used a modified intention-to-treat analysis for our primary outcome, change in plasma HIV-1 RNA levels, excluding women who were lost to follow-up after enrollment.
Wilcoxon rank-sum and χ2 or Fisher’s Exact tests were used to compare continuous and categorical variables, respectively. T tests were used to determine the difference between the change in plasma HIV-1 RNA levels in the valacyclovir and placebo arms during pregnancy. Linear regression was used to evaluate the effect of treatment on plasma HIV-1 RNA levels between 2 consecutive study visits at 2 and 6 weeks postpartum, corresponding to the period of HIV-1 rebound after PMTCT effects wane. A linear mixed effects model with random intercepts, random slopes, and an unstructured correlation matrix was constructed to evaluate the effect of treatment on plasma HIV-1 RNA levels at multiple study visits between 6 weeks and 12 months postpartum. Changes in cervical shedding of HIV-1 and genital shedding of HSV were assigned a value on an ordinal scale, and Wilcoxon rank-sum tests were used to compare differences in shedding between the treatment arms. Cox proportional hazards regression models were used to evaluate the effect of treatment on HIV-1 transmission and infant mortality. Statistical analyses were performed using Stata version 11 (StataCorp LP, College Station, TX).
RESULTS
Population
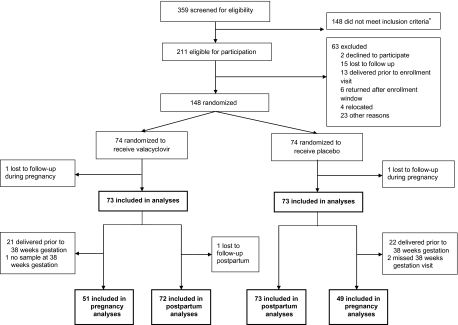
At the Mathare North Clinic, 5599 women sought antenatal care and 620 (11%) were HIV-1 seropositive. A total of 359 women came for a screening visit—223 from the study clinic and 136 from referring clinics. Among 211 eligible women, 148 were enrolled, with 74 randomized to each arm; 146 women had at least one follow-up visit and were included in our analyses (Figure 1). A total of 100 women (49 placebo, 51 valacyclovir) were included in the pregnancy analyses and 145 in the postpartum analyses (73 placebo, 72 valacyclovir); 140 women (71 placebo, 69 valacyclovir) who were able to express breast milk were included in the breast milk analysis.
Figure 1.
Screening, enrollment, and follow-up of study participants. Women from the Mathare North City Council Clinic and women referred from 5 neighboring clinics were screened for eligibility between 28 and 32 weeks gestation. Analyses were separated by the pregnancy and postpartum periods due to several women delivering prior to 38 weeks gestation and not having follow-up visits during pregnancy. *Reasons women did not meet inclusion criteria (not mutually exclusive) included: age <18 years (n = 1), HSV-2 seronegative (n = 85), CD4 <250 cells/mm3 (n = 67), eligible for HAART (n = 70), planned to deliver/reside outside of Nairobi (n = 24).
The median age of the 146 participants was 25 years (interquartile range [IQR], 22–29). All women received antenatal ZDV prophylaxis for PMTCT; 97% started at or prior to enrollment with a median gestational age at ZDV initiation of 29 weeks (IQR, 28–31). The median CD4 count was 459 cells/mm3, and mean plasma HIV-1 RNA level was 3.88 log10 copies/mL. There were no significant differences in baseline demographic or clinical characteristics between the valacyclovir and placebo arms (Table 1); similar distributions of baseline characteristics were observed for women included in the pregnancy and postpartum analyses. Medication adherence did not differ between arms; median adherence was 86% in both arms (IQR, 80%–92%).
Table 1.
Demographic and Clinical Characteristics of Study Participants at Baseline and Delivery, by Treatment Arm
| Median (IQR) or n (%) |
||
| Baseline characteristics | Valacyclovir (n = 73) | Placebo (n = 73) |
| Age (years) | 25 (22–30) | 25 (22–29) |
| Married | 57 (78) | 58 (79) |
| Education (years) | 8 (7–12) | 8 (7–10) |
| Employed | 22 (30) | 18 (25) |
| Monthly rent ($/month)a,b | 24 (19–33) | 21 (13–33) |
| Lifetime number of sex partners | 3 (2–4) | 3 (2–4) |
| History of commercial sex work | 2 (3) | 3 (4) |
| History of sexually transmitted diseases | 20 (27) | 18 (25) |
| History of genital ulcer disease | 10 (14) | 13 (18) |
| Syphilis seropositivec | 0 (0) | 0 (0) |
| Informed partner of HIV-1 statusd | 37 (57) | 35 (50) |
| CD4 count (cells/mm3)b | 452 (351–560) | 481 (340–598) |
| WHO stagee | ||
| 1 | 68 (93) | 62 (85) |
| 2 | 5 (7) | 11 (15) |
| On ZDV by enrollment | 73 (100) | 68 (93) |
| Plasma HIV-1 RNA (log10 copies/mL)f | 3.89 (3.66–4.11) | 3.87 (3.67–4.06) |
| Genital HSV DNA detected | 16 (22) | 15 (21) |
| Genital HSV DNA (log10 copies/mL)g | 3.88 (2.63–5.93) | 4.06 (2.37–6.70) |
| Cervical HIV-1 RNA (log10 copies/mL) | 2.03 (1.70–3.28) | 2.00 (1.70–2.99) |
| Maternal delivery characteristics | ||
| Gestational age (weeks) | 39 (38–40) | 39 (38–41) |
| Preterm (<37 weeks) | 14 (19) | 9 (12) |
| Cesarean delivery | 5 (7) | 9 (12) |
| Delivery location | ||
| Medical facility | 64 (88) | 63 (86) |
| Home | 4 (6) | 6 (8) |
| Traditional birth attendant | 4 (6) | 2 (3) |
| In transit | 1 (1) | 2 (3) |
| Live birth | 72 (99) | 71 (97) |
| Delivery/postpartum antiretroviralsh,i | 71 (99) | 69 (97) |
| sdNVP | 67 (93) | 64 (90) |
| ZDV | 51 (71) | 44 (62) |
| Lamivudine | 7 (5) | 4 (6) |
| Infant delivery characteristics | (n = 72) | (n = 71) |
| Birth weight (kg)j | 3.2 (2.9–3.5) | 3.0 (2.9–3.5) |
| Female | 38 (53) | 47 (66) |
| Breastfeedingk | 67 (93) | 66 (100) |
| Antiretrovirals receivedh | 69 (96) | 71 (100) |
| sdNVP | 67 (93) | 70 (99) |
| ZDV | 40 (56) | 38 (54) |
| Lamivudine | 12 (17) | 8 (11) |
Abbreviations: IQR, interquartile range; sdNVP, single dose nevirapine; ZDV, zidovudine.
$1 = 75 Kenyan shillings.
Excludes 1 valacyclovir.
Excludes 1 placebo and 3 valacyclovir with unknown status.
Excludes 3 placebo and 8 valacyclovir who had no partner at enrollment.
WHO stage 3 and 4 excluded from enrollment.
Indicates means and 95% confidence interval reported.
Indicates range reported.
Antiretrovirals received are not mutually exclusive.
Only reported for live births.
Excludes 3 placebo and 3 valacyclovir.
As reported at 2 week postpartum visit (missing data for 5 placebo).
Effect of Valacyclovir on Plasma and Cervical HIV-1 RNA and Genital HSV DNA During Pregnancy
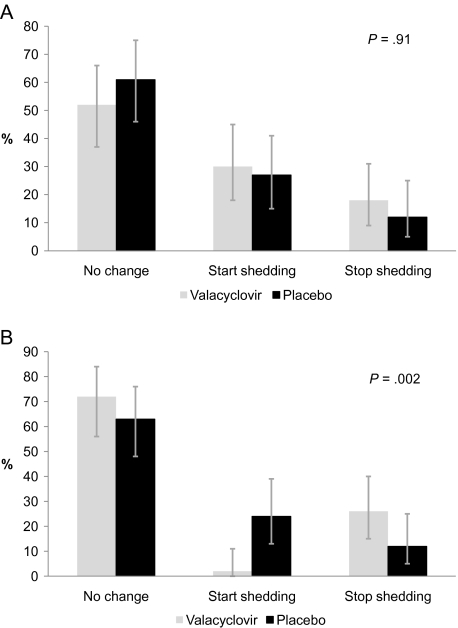
The mean change in HIV-1 plasma RNA levels between enrollment and follow-up at 38 weeks gestation was −0.53 and 0.03 log10 copies/mL in the valacyclovir and placebo arms, respectively. The overall difference in mean quantity of plasma HIV-1 RNA was 0.56 log10 copies/mL (95% confidence interval [CI], 0.34–0.77 log10 copies/mL; P < .001) lower in the valacyclovir arm than in the placebo arm. At enrollment, 28 (56%) and 25 (51%) of women were shedding HIV-1 RNA in cervical secretions in the valacyclovir and placebo arms, respectively. Between 34 and 38 weeks gestation there were no significant differences between cervical HIV-1 RNA shedding: in the valacyclovir arm 9 (18%) women stopped and 15 (30%) started shedding, and in the placebo arm 6 (12%) stopped and 13 (27%) started shedding (P = .91) (Figure 2A). Median cervical HIV-1 RNA levels at 34 and 38 weeks gestation were similar (2.14 log10 copies/mL in the valacyclovir arm vs 2.25 log10 copies/mL in placebo arm; P = .12). There was also no difference in median cervical HIV-1 RNA levels in exploratory subgroup analysis of women with detectable cervical HIV-1 at baseline (P = .13).
Figure 2.
Effect of valacyclovir on change in cervical human immunodeficiency virus type 1 (HIV-1) RNA and genital herpes simplex virus (HSV) DNA shedding status at 38 weeks gestation. A, Change in cervical HIV-1 shedding, by treatment arm. B, Change in genital HSV shedding, by treatment arm. Change in HIV-1 RNA and HSV DNA is for the period between the 34th and 38th week of gestation and was calculated as shedding at follow-up minus shedding at enrollment. Changes in shedding were assigned a value on an ordinal scale and compared between arms using Wilcoxon rank-sum tests. HIV-1 and HSV shedding were defined as having HIV-1 RNA or HSV DNA copies/mL above the lower limit of detection of the assay, respectively. Women with virus detected at 38 weeks gestation but not at 34 weeks gestation were classified in the group “start shedding”; women with virus detected at 34 weeks gestation but not at 38 weeks gestation were classified in the group “stop shedding.” Data exclude 1 valacyclovir due to missing swabs at 38 weeks gestation.
Between 34 and 38 weeks gestation, the proportion of women shedding HSV DNA dropped from 26% to 2% in the valacyclovir arm but increased from 16% to 29% in the placebo arm. Women in the valacyclovir arm were more likely than women in the placebo arm to stop shedding (26% vs 12%) and less likely to start shedding (2% vs 24%) (P = .002, χ2) (Figure 2B). Mean plasma HIV-1 RNA was 0.45 log10 copies/mL higher among women shedding than not shedding HSV at baseline (4.20 vs 3.74 log10 copies/mL, respectively; P = .04) and at 38 weeks gestation (0.72 log10 copies/mL higher; P = .009).
Effect of Valacyclovir on Plasma and Breast Milk HIV-1 RNA Postpartum
A total of 136 women completed the study at 12 months postpartum. Duration of postpartum follow-up was similar between the two arms, 68.2 person-years in the valacyclovir arm and 64.2 in the placebo arm. Three women died during postpartum follow-up (1 valacyclovir, 2 placebo). There were no differences in adverse events between arms.
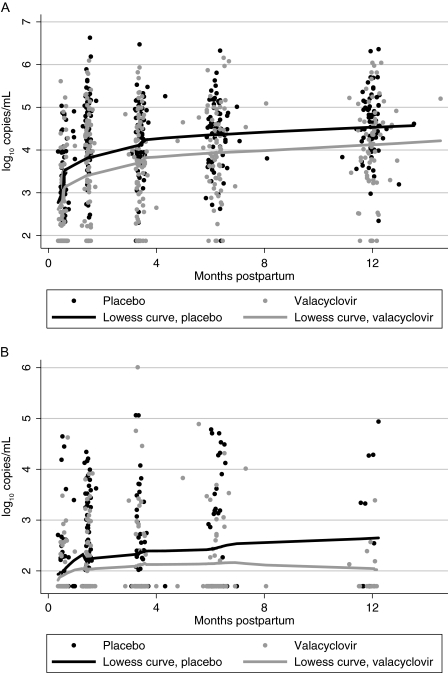
Mean plasma HIV-1 RNA at 2 weeks postpartum was lower in the valacyclovir arm compared with the placebo arm (2.83 vs 3.10 log10 copies/mL, respectively); however these were not statistically different (P = .11). The rate of change in plasma HIV-1 RNA levels was 0.60 log10 copies/mL per month lower (95% CI, −.92 to −.28) between 2 and 6 weeks postpartum in the valacyclovir arm compared with the placebo arm, after adjusting for 2 week HIV-1 RNA levels (P < .001). Between 6 weeks and 12 months postpartum, mean plasma HIV-1 RNA levels were 0.51 log10 copies/mL lower (95% CI: −.73 to −.30; P < .001) in the valacyclovir arm compared with the placebo arm (Figure 3A), but there was no difference in the rate of change (0.03 and 0.02 log10 copies/mL per month in placebo and valacyclovir arms, respectively; P = .3).
Figure 3.
The effect of valacyclovir on postpartum plasma (A) and breast milk (B) human immunodeficiency virus type 1 (HIV-1) RNA levels. Curves represent locally weighted smoothed curves of plasma and breast milk HIV-1 RNA levels over time, by treatment arm.
The effect of valacyclovir on breast milk HIV-1 RNA levels (Figure 3B) was similar to plasma. Median breast milk HIV-1 RNA was the same at 2 weeks postpartum (1.70 log10 copies/mL in both arms), but at 6 weeks postpartum, the median breast milk HIV-1 RNA was 0.47 log10 copies/mL lower among women in the valacyclovir arm compared with the placebo arm (1.70 vs 2.17 log10 copies/mL, respectively; P = .02). At 14 weeks postpartum the distribution of breast milk HIV-1 levels remained significantly lower among women in the valacyclovir arm, but the magnitude of median HIV-1 RNA levels was at the lower limit of detection (for both arms, 1.70 log10 copies/mL; P = .04, Wilcoxon rank-sum). Although there were no differences in median breast milk HIV-1 RNA levels at 6 and 12 months postpartum (P > .2 for both), only 74% and 19% of women, respectively, were able to express breast milk at these visits.
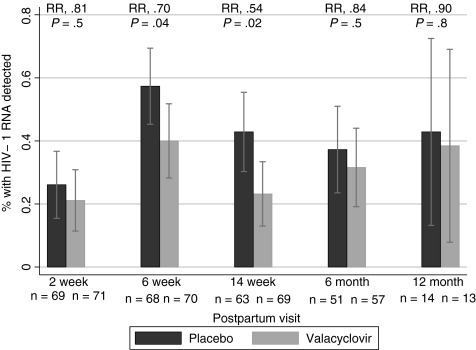
The effect of valacyclovir on HIV-1 detection in breast milk samples demonstrated a pattern similar to the effect on breast milk HIV-1 RNA levels. After receipt of sdNVP during delivery, risk of detecting HIV-1 RNA in breast milk at 2 weeks postpartum was low in the valacyclovir (45%) and placebo (55%) arms (P = .48). At 6 weeks postpartum the risk of detecting HIV-1 in breast milk was 30% lower in the valacyclovir arm than the placebo arm (relative risk [RR], 0.70; 95% CI, .49 to .99; P = .04), and at 14 weeks the risk was 46% lower (RR, 0.54; 95% CI .32 to .91; P = .02) (Figure 4), but there was no difference in detection at other postpartum visits.
Figure 4.
The effect of valacyclovir on breast milk human immunodeficiency virus type 1 (HIV-1) RNA detection. χ2 tests were used to compare breast milk HIV-1 RNA detection between study arms. Error bars represent interquartile ranges, by treatment arm. Abbreviation: RR, relative risk.
Delivery Characteristics and HIV-1 Transmission
Among 146 women followed through delivery, there were 143 live births. Nearly all women (99%) and infants (98%) received antiretrovirals during delivery or after birth for PMTCT. Maternal and infant delivery characteristics are shown in Table 1. Most infants were breastfed; the median duration of breastfeeding was 6.0 and 5.3 months in the valacyclovir and placebo arms, respectively (IQR, 3.4–6.5, both arms).
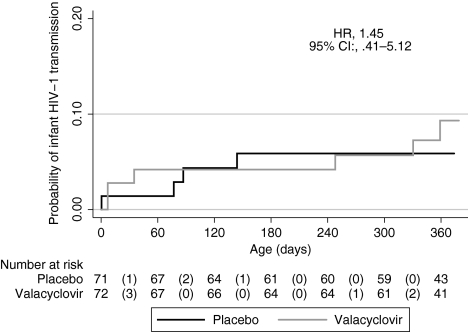
Overall, 10 infants (6 valacyclovir, 4 placebo) acquired HIV-1 by 12 months for a transmission rate of 7.0%; there was no difference in transmission between arms (hazard ratio [HR], 1.45; 95% CI, .41 to 5.12) (Figure 5). There were no significant differences in the risk of infant mortality: 2 valacyclovir arm deaths and 7 placebo arm deaths (HR, 0.28; 95% CI, .06 to 1.32). HIV-1–free survival was similar between the study arms (HR, 0.86; 95% CI, .33 to 2.22).
Figure 5.
Infant human immunodeficiency virus type 1 (HIV-1) transmission, by treatment arm. Time of infant infection was defined as the midpoint between the first positive and the last negative HIV-1 DNA test. The effect of valacyclovir on HIV-1 was evaluated using Cox proportional hazards regression. Abbreviations: CI, confidence interval; HR, hazard ratio.
CONCLUSIONS
In our study, twice daily 500 mg valacyclovir reduced plasma HIV-1 RNA levels by 0.54 log10 copies/mL during pregnancy and by 0.51 log10 copies/mL after 6 weeks postpartum among pregnant Kenyan women ineligible for HAART and receiving ZDV plus sdNVP prophylaxis for PMTCT. The proportion and levels of HIV-1 RNA in breast milk were also lower at 6 and 14 weeks postpartum among women randomized to valacyclovir but were similar between the two arms at 2 weeks postpartum after maternal sdNVP administered at the time of delivery. At 12 months, 7.0% of infants were infected with HIV-1; there were no differences in HIV-1 transmission between arms.
Previous studies have estimated the risk of HIV-1 infection through breastfeeding to be 1%–2% per month in the absence of postnatal antiretroviral prophylaxis [32, 33]. The significant reduction in breast milk HIV-1 RNA we observed prior to 6 months corresponds to a period when infants were at risk of postnatal MTCT. By 5–6 months the cumulative risk of postnatal infection is estimated to be 8%–10% with a ZDV plus sdNVP regimen and 3%–5% with extended infant NVP or maternal HAART [34–36]. In addition, cumulative exposure to HIV-1 RNA in breast milk was shown to be a more important predictor of breast milk transmission than duration of feeding [37]. Thus, if HSV-2 suppressive therapy can reduce breast milk HIV-1 RNA, it may provide an additional intervention to decrease HIV-1 transmission via breastfeeding.
We also observed a ∼0.5 log10 copies/mL reduction in plasma HIV-1 RNA levels both during and after pregnancy, consistent with previous studies of HSV-2 suppression among men and nonpregnant women [12–17]. Women in the valacyclovir arm continued to benefit from suppressive therapy, despite the effect of ZDV on HIV-1 RNA levels during pregnancy. It is possible that the additional viral suppression during pregnancy was due to synergy between ZDV and valacyclovir. However, although in vitro studies suggest that acyclovir may potentiate the action of ZDV [38, 39], an in vivo study did not support these findings [40]. During the postpartum period the effect of suppressive therapy on HIV-1 RNA continued and was sustained for 12 months after the effect of sdNVP on plasma and breast milk HIV-1 RNA waned.
Our study had some limitations. We were unable to evaluate the effect of valacyclovir on MTCT or on change in HIV-1 RNA among women who were eligible for HAART. We were also underpowered to detect an association between valacyclovir and breast milk HIV-1 RNA levels or detection at 6 and 12 months postpartum due to small numbers of women who were able to express breast milk at these visits.
The reduction in plasma HIV-1 RNA levels we observed with valacyclovir suppressive therapy may provide some protection against in utero, intrapartum, or postnatal MTCT; however, our study was not designed to evaluate the effect of valacyclovir on MTCT. In order to detect a 2-fold difference in transmission between study arms, assuming a transmission rate of 7% in the placebo arm, we would need to enroll nearly 1400 mother-infant pairs. Although a 0.25 log10 copies/mL reduction in plasma HIV-1 RNA levels did not correspond to a decreased risk of heterosexual HIV-1 transmission, we observed a viral load effect twice as large, and a 0.4 log10 lower HIV-1 RNA level in breast milk and plasma has previously been associated with lower risk of postnatal MTCT [37]. The impact of a reduction in plasma HIV-1 RNA levels on MTCT may differ from the impact on heterosexual transmission due to prolonged exposure to HIV-1 containing bodily fluids during pregnancy, labor and delivery, and breastfeeding. Additional research is needed to determine the effect of valacyclovir on postnatal and peripartum HIV-1 transmission through breast milk.
We also found that women randomized to valacyclovir had a lower risk of genital HSV DNA shedding, consistent with previous studies [41, 42]. In contrast, we did not detect a significant association between valacyclovir and cervical HIV-1 RNA levels or shedding, which is consistent with results from a cohort receiving HAART reported by Ouedraogo et al [43] and an antiretroviral-naive cohort reported by Delany et al [17] but in conflict with 3 other randomized trials conducted in antiretroviral-naive cohorts [12–14]. One potential explanation for the lack of association in our study is that a high proportion (>40%) of women in our cohort were not shedding HIV-1 in cervical secretions and had low baseline cervical HIV-1 RNA levels after initiation of ZDV, which reduces cervical HIV-1 RNA levels by ∼1 log10 copies/mL after 1 week of ZDV treatment [44]. Other potential explanations include short duration of valacyclovir suppression, use of cervical swabs rather than cervicovaginal lavages, and hormonal changes associated with pregnancy.
Valacyclovir has many features that make it an appealing intervention. It is commercially available in generic form, has a good safety profile during and after pregnancy, and could be used as an intervention to reduce infant exposure to HIV-1 during breastfeeding. Furthermore, HSV-2 suppressive therapy can be safely used without inducing HIV-1 resistance, as has been shown in cohorts receiving acyclovir or valacyclovir [45]. Our study demonstrates that valacyclovir significantly reduces plasma and breast milk HIV-1 RNA and the risk of genital HSV DNA shedding during pregnancy and can be easily administered within the existing PMTCT infrastructure. In addition, the effect of valacyclovir was sustained after PMTCT antiretroviral effects had waned, which may also lower the risk of early breast milk HIV-1 transmission. Valacyclovir suppressive therapy, in conjunction with PMTCT antiretrovirals, should be further evaluated in a larger trial as a combination intervention for reducing MTCT and improving maternal health.
Notes
Acknowledgments.
We would like to acknowledge the members of our data safety and monitoring committee for their review throughout the study: Dalton Wamalwa (chair), Brandon Guthrie, Irene Inwani, and John Ong’ech. We would also like to thank our participants for their time and dedication to this study.
Financial support.
This work was supported by the National Institutes of Health (NIH) (1R03 HD057773–01, R03 HD057773-02S1, R01 AI076105, and K24 AI087399 to C. F.; K24 HD054314 to G. J. S.; K24 AI071113 to A. W.; University of Washington Center for AIDS Research [UW CFAR] P30 AI027757; International AIDS Research and Training Program by the Fogarty International Center [FIC] D43 TW00007; FIC R24 TW007988 to A. C. R. and F. O. O.; UW CFAR T32 AI0714032 to A. L. D.), Puget Sound Partners for Global Health Research Technology Grant to B.A.R., and University of Washington Royalty Research Fund Grant (4027) to C. F. GlaxoSmithKline donated study drugs.
Potential conflicts of interest.
A. W. has received grant support from GlaxoSmithKline and has been a consultant for AiCuris. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. UNAIDS report on the global HIV/AIDS epidemic, 2010. Available at: http://www.unaids.org/globalreport/Global_report.htm. Accessed 25 November 2011.
- 2. WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a public health approach. 2010. Available at: http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/index.html. Accessed 25 November 2011.
- 3. WHO. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. Available at: http://www.who.int/hiv/pub/2010progressreport/en/. Accessed 25 November 2011.
- 4.Drake AL, John-Stewart GC, Wald A, et al. Herpes simplex virus type 2 and risk of intrapartum human immunodeficiency virus transmission. Obstet Gynecol. 2007;109:403–9. doi: 10.1097/01.AOG.0000251511.27725.5c. [DOI] [PubMed] [Google Scholar]
- 5.Mbopi-Keou FX, Gresenguet G, Mayaud P, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–6. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 6.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 7.Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis. 1998;178:1616–22. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 8.Gray RH, Li X, Wawer MJ, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis. 2004;189:1209–15. doi: 10.1086/382750. [DOI] [PubMed] [Google Scholar]
- 9.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 10.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–70. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 11.LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS. 2007;21:1569–78. doi: 10.1097/QAD.0b013e32825a69bd. [DOI] [PubMed] [Google Scholar]
- 12.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 13.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)–suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2–infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 15.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2–seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 17.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 coinfected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–8. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John GC, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–12. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 20.Chen KT, Segu M, Lumey LH, et al. Genital herpes simplex virus infection and perinatal transmission of human immunodeficiency virus. Obstet Gynecol. 2005;106:1341–8. doi: 10.1097/01.AOG.0000185917.90004.7c. [DOI] [PubMed] [Google Scholar]
- 21.Bollen LJ, Whitehead SJ, Mock PA, et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: possibility to further decrease transmission? AIDS. 2008;22:1169–76. doi: 10.1097/QAD.0b013e3282fec42a. [DOI] [PubMed] [Google Scholar]
- 22. Kenya Ministry of Health, National AIDS and STI Control Programme. Guidelines for prevention of mother to child transmission (PMTCT) of HIV/AIDS in Kenya. 3rd ed. 2009. Available at http://www.aidstar-one.com/focus_areas/pmtct/resources/pmtct_country_guidelines. Accessed 25 November 2011.
- 23.John GC, Sheppard H, Mbori-Ngacha D, et al. Comparison of techniques for HIV-1 RNA detection and quantitation in cervicovaginal secretions. J Acquir Immune Defic Syndr. 2001;26:170–5. doi: 10.1097/00042560-200102010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–50. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 25.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVange Panteleeff D, Emery S, Richardson BA, et al. Validation of performance of the gen-probe human immunodeficiency virus type 1 viral load assay with genital swabs and breast milk samples. J Clin Microbiol. 2002;40:3929–37. doi: 10.1128/JCM.40.11.3929-3937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–95. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–20. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–3. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chohan BH, Emery S, Wamalwa D, et al. Evaluation of a single round polymerase chain reaction assay using dried blood spots for diagnosis of HIV-1 infection in infants in an African setting. BMC Pediatr. 2011;11:18. doi: 10.1186/1471-2431-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn L, Sinkala M, Kankasa C, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One. 2007;2:e1363. doi: 10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission through breastfeeding: a study in Malawi. JAMA. 1999;282:744–9. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 34.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 35.The Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 36.Taha TE, Kumwenda J, Cole SR, et al. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis. 2009;200:1490–7. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 37.Neveu D, Viljoen J, Bland RM, et al. Cumulative exposure to cell-free HIV in breast milk, rather than feeding pattern per se, identifies postnatally infected infants. Clin Infect Dis. 2011;52:819–25. doi: 10.1093/cid/ciq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown SD, Bartlett MG, White CA. Pharmacokinetics of intravenous acyclovir, zidovudine, and acyclovir-zidovudine in pregnant rats. Antimicrob Agents Chemother. 2003;47:991–6. doi: 10.1128/AAC.47.3.991-996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsuya H, Broder S. Strategies for antiviral therapy in AIDS. Nature. 1987;325:773–8. doi: 10.1038/325773a0. [DOI] [PubMed] [Google Scholar]
- 40.Collier AC, Bozzette S, Coombs RW, et al. A pilot study of low-dose zidovudine in human immunodeficiency virus infection. N Engl J Med. 1990;323:1015–21. doi: 10.1056/NEJM199010113231502. [DOI] [PubMed] [Google Scholar]
- 41.Hollier L, Wendel G. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008;1:CD004946. doi: 10.1002/14651858.CD004946.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Sheffield JS, Hill JB, Hollier LM, et al. Valacyclovir prophylaxis to prevent recurrent herpes at delivery: a randomized clinical trial. Obstet Gynecol. 2006;108:141–7. doi: 10.1097/01.AOG.0000219749.96274.15. [DOI] [PubMed] [Google Scholar]
- 43.Ouedraogo A, Nagot N, Vergne L, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. AIDS. 2006;20:2305–13. doi: 10.1097/QAD.0b013e328010238d. [DOI] [PubMed] [Google Scholar]
- 44.Mbori-Ngacha D, Richardson BA, Overbaugh J, et al. Short-term effect of zidovudine on plasma and genital human immunodeficiency virus type 1 and viral turnover in these compartments. J Virol. 2003;77:7702–5. doi: 10.1128/JVI.77.13.7702-7705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baeten JM, Lingappa J, Beck I, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2011;203:117–21. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]