Abstract
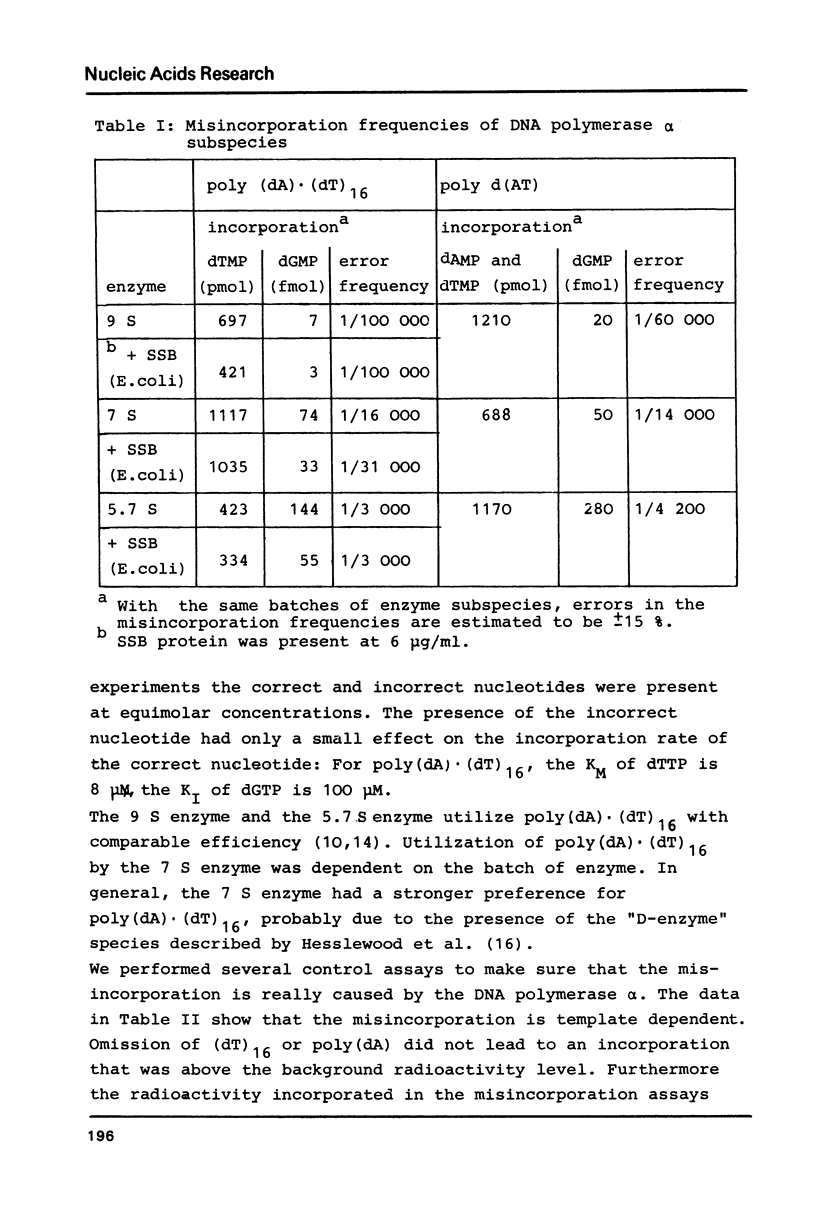
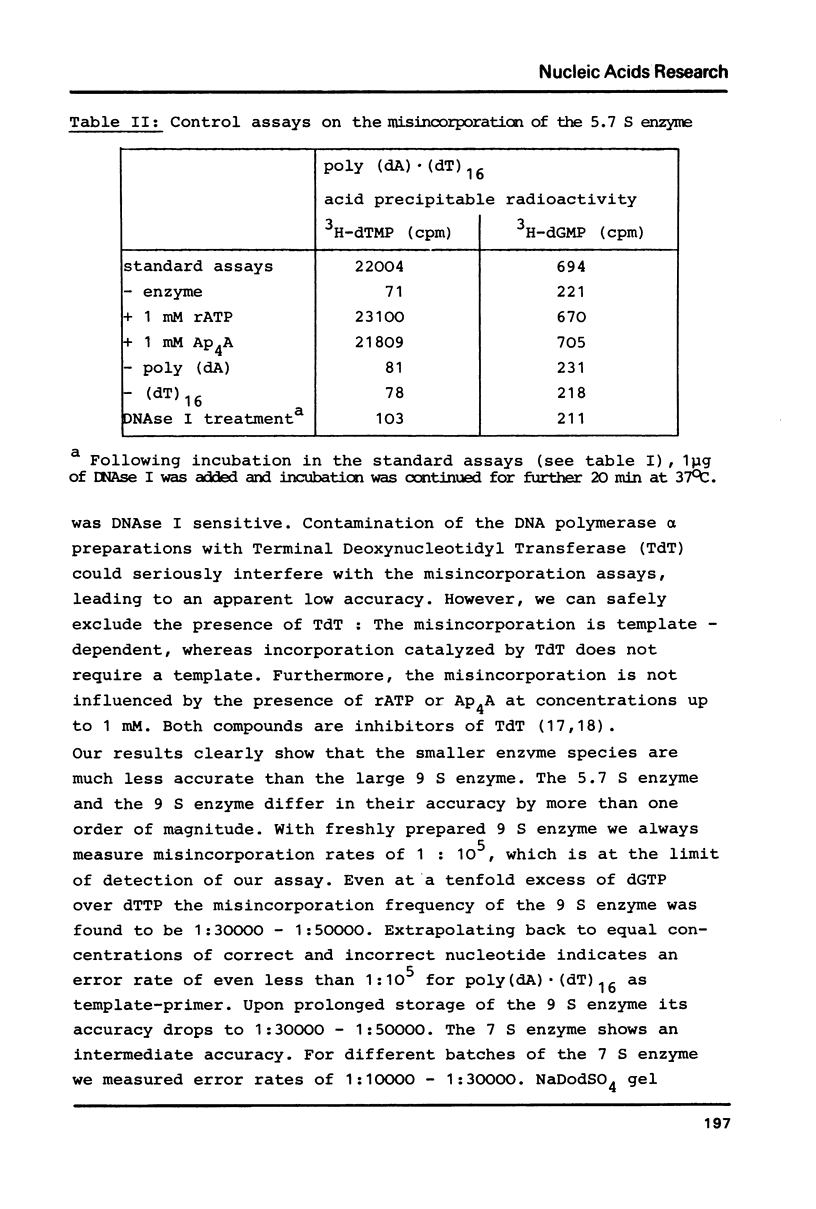
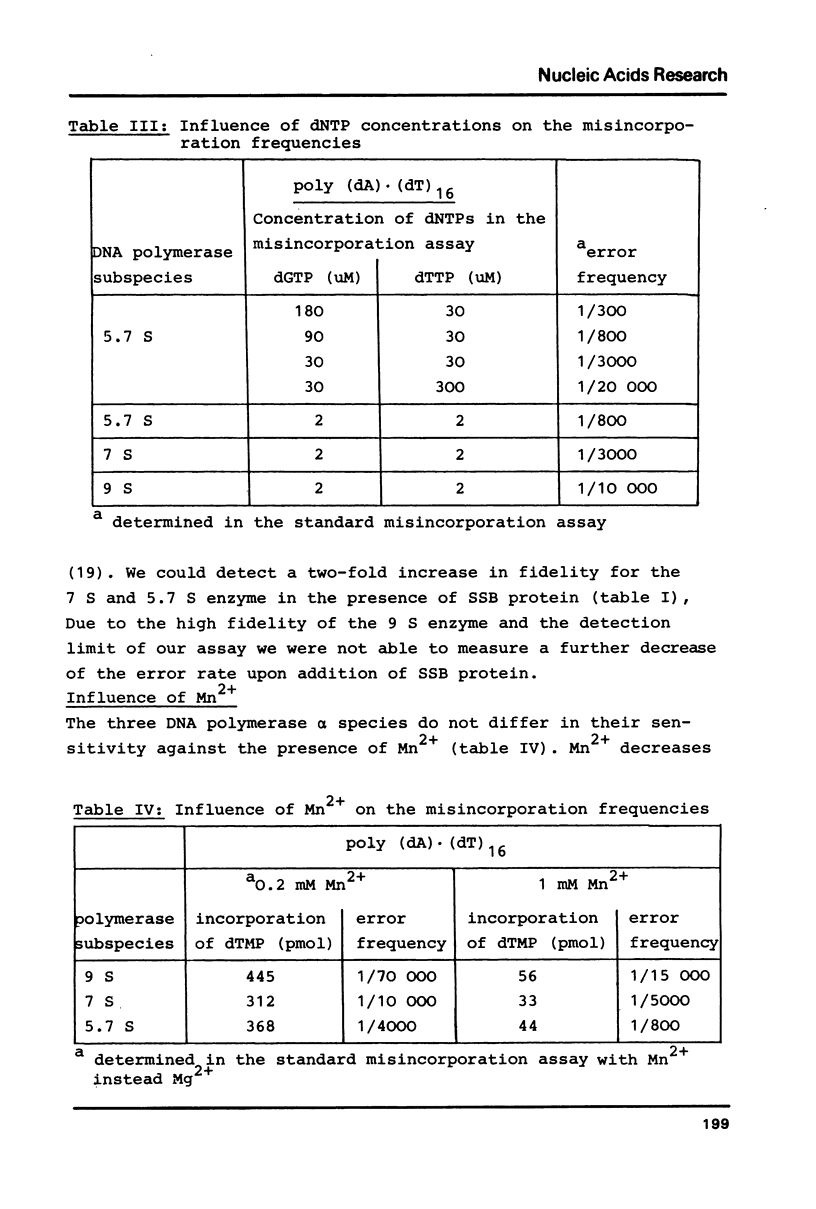
Three different subspecies of DNA polymerase alpha from calf thymus sedimenting at 9 S, 7 S and 5.7 S have been investigated with respect to their accuracy of in vitro DNA synthesis on poly (dA) (dT)16 and poly d(AT) as template-primers. Our results indicate that the structure of DNA polymerase alpha has a strong influence on the accuracy of DNA synthesis. The 9 S enzyme shows a misincorporation frequency of about 1:100 000. An error rate of 1:15 000 is measured for the 7 S species. The 5.7 S enzyme for which an error rate of 1:3 000 is determined, has to be considered as error prone. Lowering the rate of DNA synthesis leads to a decrease in fidelity. The single stranded DNA binding protein from E.coli increases the accuracy of the 5.7 S and the 7 S enzyme by a factor of two. Mn2+ decreases the fidelity of all three subspecies in a concentration dependent manner.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S. S., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Accuracy of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):101–106. [PubMed] [Google Scholar]
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bhalla R. B., Schwartz M. K., Modak M. J. Selective inhibition of terminal deoxynucleotidyl transferase (TdT) by adenosine ribonucleoside triphosphate (ATP) and its application in the detection of TdT in human leukemia. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1056–1061. doi: 10.1016/0006-291x(77)90963-9. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F. Decreased fidelity of DNA polymerase activity during N-2-fluorenylacetamide hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1979 Feb;76(2):814–818. doi: 10.1073/pnas.76.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., Allen E. F., Forsberg S. A., Preparata R. M., Greening E. O. Genetic control of mutation rates in bacteriophageT4. Nature. 1969 Mar 22;221(5186):1128–1132. [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler R. G., Degnen G. E., Cox E. C. Mutational specificity of a conditional Escherichia coli mutator, mutD5. Mol Gen Genet. 1974;133(3):179–191. doi: 10.1007/BF00267667. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. Control of mutation frequency by bacteriophage T4 DNA polymerase. II. Accuracy of nucleotide selection by the L88 mutator, CB120 antimutator, and wild type phage T4 DNA polymerases. J Biol Chem. 1976 Sep 10;251(17):5225–5232. [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification and partial characterization of a DNA polymerase alpha species from calf thymus. Nucleic Acids Res. 1980 Dec 11;8(23):5703–5714. doi: 10.1093/nar/8.23.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification of a 9S DNA polymerase alpha species from calf thymus. Biochemistry. 1981 Sep 15;20(19):5470–5475. doi: 10.1021/bi00522a019. [DOI] [PubMed] [Google Scholar]
- Hesslewood I. P., Holmes A. M., Wakeling W. F., Johnston I. R. Studies on the purification and properties of a 6.8-S DNA polymerase activity found in calf-thymus DNA polymerase-alpha fraction. Eur J Biochem. 1978 Mar;84(1):123–131. doi: 10.1111/j.1432-1033.1978.tb12148.x. [DOI] [PubMed] [Google Scholar]
- Hockensmith J. W., Bambara R. A. Kinetic characteristics which distinguish two forms of calf thymus DNA polymerase alpha. Biochemistry. 1981 Jan 6;20(1):227–232. doi: 10.1021/bi00504a038. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Krauss G., Sindermann H., Schomburg U., Maass G. Escherichia coli single-strand deoxyribonucleic acid binding protein: stability, specificity, and kinetics of complexes with oligonucleotides and deoxyribonucleic acid. Biochemistry. 1981 Sep 1;20(18):5346–5352. doi: 10.1021/bi00521a040. [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Fidelity of fractionated deoxyribonucleic acid polymerases from human placenta. Biochemistry. 1980 Jan 8;19(1):220–228. doi: 10.1021/bi00542a033. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. Fidelity of mammalian DNA polymerases. Science. 1981 Aug 14;213(4509):765–767. doi: 10.1126/science.6454965. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Meyer R. R., Loeb L. A. Single-strand binding protein enhances fidelity of DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6331–6335. doi: 10.1073/pnas.76.12.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe P., Baril B., Chi A., Lee L., Baril E. Accessory proteins for DNA polymerase alpha activity with single-strand DNA templates. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4723–4727. doi: 10.1073/pnas.78.8.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Kairis M., Holliday R. Decreased fidelity of DNA polymerase activity isolated from aging human fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2818–2822. doi: 10.1073/pnas.73.8.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Murray V. Properties of DNA polymerases from young and ageing human fibroblasts. Mech Ageing Dev. 1981;16(4):327–343. doi: 10.1016/0047-6374(81)90017-8. [DOI] [PubMed] [Google Scholar]
- Ono K., Iwata Y., Nakamura H., Matsukage A. Selective inhibition of terminal deoxynucleotidyl transferase by diadenosine 5',5"-P1,P4-tetraphosphate. Biochem Biophys Res Commun. 1980 Jul 16;95(1):34–40. doi: 10.1016/0006-291x(80)90700-7. [DOI] [PubMed] [Google Scholar]
- Salisbury J. G., O'Connor P. J., Saffhill R. Molecular size and fidelity of DNA polymerase alpha from the regenerating liver of the rat. Biochim Biophys Acta. 1978 Jan 26;517(1):181–185. doi: 10.1016/0005-2787(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Replication of ultraviolet-irradiated simian virus 40 in monkey kidney cells. J Mol Biol. 1980 Apr;138(2):299–319. doi: 10.1016/0022-2836(80)90288-0. [DOI] [PubMed] [Google Scholar]
- Seal G., Shearman C. W., Loeb L. A. On the fidelity of DNA replication. Studies with human placenta DNA polymerases. J Biol Chem. 1979 Jun 25;254(12):5229–5237. [PubMed] [Google Scholar]
- Sloan D. L., Loeb L. A., Mildvan A. S. Conformation of deoxynucleoside triphosphate substrates on DNA polymerase I from Escherichia coli as determined by nuclear magnetic relaxation. J Biol Chem. 1975 Dec 10;250(23):8913–8920. [PubMed] [Google Scholar]
- Villani G., Fay P. J., Bambara R. A., Lehman I. R. Elongation of RNA-primed DNA templates by DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1981 Aug 10;256(15):8202–8207. [PubMed] [Google Scholar]



