Abstract
Microtubules define the architecture and internal organisation of cells by positioning organelles and activities, as well as by supporting cell shape and mechanics. One of the major functions of microtubules is the control of polarized cell motility. In order to support the asymmetry of polarized cells, microtubules have to be organised asymmetrically themselves. Asymmetry in microtubule distribution and stability is regulated by multiple molecular factors, most of which are microtubule-associated proteins that locally control microtubule nucleation and dynamics. At the same time, the dynamic state of microtubules is key to the regulatory mechanisms by which microtubules regulate cell polarity, modulate cell adhesion and control force-production by the actin cytoskeleton. Here, we propose that even small alterations in microtubule dynamics can influence cell migration via several different microtubule-dependent pathways. We discuss regulatory factors, potential feedback mechanisms due to functional microtubule-actin crosstalk and implications for cancer cell motility.
Keywords: cell migration, cell motility, microtubule dynamics, microtubule associated proteins, focal adhesion turnover, actin cytoskeleton
1. What is microtubule dynamics?
The microtubule cytoskeleton serves various vital cellular functions. Microtubules provide the tracks for intracellular long-distance transport, positioning of organelles and intracellular activities, thereby defining interphase cellular architecture and ensuring precise chromosome segregation in mitosis. Microtubules also function to support cell shape and mechanics due to their ability to resist high compressive loads [1]. Microtubules are composed of alpha and beta tubulin heterodimers that bind head-to-tail to form protofilaments, 13 of which form a hollow tubule. This architecture gives microtubules an intrinsic polarity with assembly and disassembly occurring exclusively at their ends. The observation that in a population of microtubules, some ends grow while others shrink led to the GTP cap model of dynamic instability [2]. In this model, GTP tubulin incorporates at the end of the microtubule and forms a stabilising GTP cap at the growing end. Subsequent GTP hydrolysis leads to the lattice of the microtubule consisting mainly of GDP tubulin, which prefers an outward bent conformation and thus promotes microtubule depolymerisation when exposed at the microtubule end [3]. Thus each microtubule end switches between periods of growth and shrinkage, which are governed by the presence or loss of the GTP cap [4]. The two ends of a microtubule are not equal. The plus end, where beta tubulin is exposed, grows and shrinks faster and is thus also called the dynamic end.
2. How is microtubule dynamics regulated?
In cells, the microtubule minus end is usually embedded in the main microtubule-organizing centre (MTOC) and does not grow [5], while the plus end explores the cellular space and tends ultimately to come into contact with the cell edges. Microtubules do not show stochastic switching between growth and shrinkage in cells. Instead, transitions appear to be spatially and temporally regulated. Microtubules generally grow persistently in the cytoplasm and most catastrophes are induced at the cell edges [6, 7]. This results in most microtubules reaching the cell edge, thus ensuring efficient cargo transport. At the cell edges, microtubule ends often dwell for some time. This state usually involves fast transitions between growth and shrinkage phases, even though microtubules doing this are frequently referred to as paused or captured [8]. The observed dynamics of microtubule plus ends in cells suggests extensive regulation. Many microtubule regulatory factors are known, some of which promote assembly and some of which induce disassembly (see [9] for a recent review). Mechanistically, microtubule dynamics can be regulated in various ways, for example XMAP215 family proteins are thought to catalyse microtubule growth by stabilising a transitional state and thus favour tubulin subunit incorporation [10], while Kinesin-13s probably induce microtubule disassembly by promoting a bent conformation of tubulin subunits, thereby destabilising lateral protofilament interactions [11, 12]. The fast transitions between growth and shrinkage observed at the cell boundaries are likely to require the action of both a catastrophe inducer and a rescue factor, whose activities are somehow coordinated. How such regulatory crosstalks operate remains to be understood and is a major challenge for the future. Recent perturbation experiments suggest that CLASPs can act as cortical rescue factors [13] and that EB3 is involved in the regulation of both catastrophe induction as well as rescue promotion at cortical sites [14].
3. Microtubule dynamics is asymmetric to polarize a motile cell
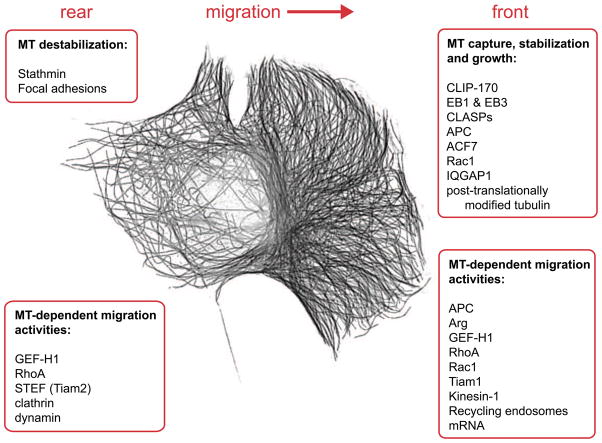
In order to support asymmetry of cellular activities, the microtubule network itself has to be asymmetric. Microtubule asymmetry in a motile cell includes both asymmetric microtubule distribution and microtubule dynamics. In most cells, like fibroblasts and neurons, more microtubules extend to the cell front than to the cell rear. Such difference is due to a combination of cell front-specific activities that result in increasing microtubule numbers and selective de-stabilization of microtubules at the cell rear (see Figure 1). Front activities include selective capture and stabilization of microtubules at cortical sites [13–17], selective support of persistent microtubule growth by local Rac1-dependent tubulin polymerization [18], inactivation of catastrophe factors like stathmin [19], and the asymmetric nucleation of microtubules at non-centrosomal Golgi-associated MTOCs [20]. Moreover, in many cases microtubule motors contribute to the polarized organization of the microtubule network either by transporting or by crosslinking microtubules. Recent studies implicate a collaborative effort of kinesin-5 and kinesin-12 in neuronal outgrowth and growth cone guidance, potentially by using their microtubule cross-bridging activity to prevent microtubules invading the growth cone equally, thus supporting asymmetry [21–23]. In contrast, kinesin-1 can actively slide microtubules along each other to support the formation of parallel microtubule arrangements in cellular protrusions in multiple cell types [24].
Figure 1.
Polarized regulation of microtubule dynamics and molecular factors asymmetrically regulated and/or positioned by microtubules at the front or rear of motile cell. Background: asymmetric microtubule cytoskeleton of a Swiss 3T3 fibroblast visualised by tubulin immunostaining.
Long-lived microtubules that extend to the cell front are often posttranslationally modified [25]. It is thought that stable microtubules accumulate tubulin modifications such as acetylation and detyrosination over time [26], but also that tubulin acetylation protects microtubules from depolymerisation and thus may reinforce stability [27, 28]. Tubulin modifications selectively increase the affinity of certain molecular motors to their tracks (e.g. kinesin-1 [29, 30]) and could thereby serve as signposts to facilitate directional transport to the cell front. At the rear part of the cell, frequent catastrophes [31] are observed. Preferential microtubule destabilization at the cell rear is likely to be triggered by an excess of active stathmin that is inhibited by phosphorylation at the cell front but not in the rear [19]. Catastrophes are also specifically induced at adhesion sites [32], many of which are positioned in the trailing part of a cell. On the other hand, early adhesions at the cell front and their surroundings have been implicated in capturing microtubules and increasing their life times [33, 34], and how these two opposite activities are differentiated is not yet known. One possible hypothesis predicts the existence of a regulatory mechanism that results in catastrophe-inducing adhesions, which are possibly mature adhesion sites that are associated with paxillin [32], and stabilizing adhesion sites of a different, to-be-defined composition. In this case, these two groups of adhesions should be differentially distributed in a polarized motile cell. Even though more microtubules grow towards the cell front, the density of microtubules close to the cell cortex is lower at the protruding front than at the retracting rear [35]. This is probably caused by the speed of membrane protrusion exceeding that of microtubule polymerisation together with the active rearward transport of microtubules by the actin retrograde flow [36]. Thus, differences in microtubule dynamics between the cell front and the cell rear are quite significant on their own and, in addition, underlie the differences in microtubule distribution observed.
4. Microtubules regulate cell motility
The role of microtubules in cell migration has been established since Vasiliev and Gelfand observed that fibroblasts in culture cease motility when treated with the microtubule depolymerising drug colcemide [37]. Subsequent studies showed that not only full microtubule depolymerisation but also abolishing microtubule dynamics using Taxol or low doses of nocodazole interferes with cell motility [38]. The major question arising from these studies was which mechanistic component of cell migration is regulated by dynamic microtubules. Directional cell migration requires protrusion of the cell front and retraction of the rear, processes that are driven by actin polymerisation and acto-myosin contraction. Furthermore, traction is provided through integrin-mediated links between the extracellular substrate and the cytoskeleton. These activities do not require microtubules per se (see also section 7). However, many processes essential to cell motility are regulated by microtubules and depend on distinct modes of microtubule dynamics (see Figure 1).
One group of such processes relates to the assembly of the actin cytoskeleton. Microtubules affect actin-driven leading edge protrusion by multiple pathways. Microtubule polymerization can activate the small Rho GTPase Rac1 [39], which is thought to occur through the guanidine exchange factor (GEF) activity of TIAM1 [40] or STEF (TIAM2) [41]. Several microtubule plus end binding proteins (+TIPs) including CLIP170 [15], APC [16] and CLASPs [42] interact with IQGAP1, an effector of Rac1 and Cdc42, that is thought to coordinate Arp2/3 and formin-dependent actin nucleation activities at cell protrusions (for a review see [43]). Assembly of actin filaments into larger structures and their functioning both at the cell front and cell rear depends on myosin II contractility regulated by RhoA signalling. RhoA, in turn, is locally controlled by GEF-H1 (Lfc) [44]. This molecule is the best-described player of microtubule-dependent actin regulation, as it is inactive when scaffolded to the microtubule lattice and is specifically activated by microtubule depolymerisation [45, 46].
The second group of microtubule-dependent processes that modulate cell migration relates to focal adhesion turnover. Microtubules directionally grow toward and target focal adhesions, thereby promoting their disassembly [33, 35]. Guidance of microtubule growth towards focal adhesions is thought to be mediated by the spektraplakin ACF7, which crosslinks microtubules and actin [47, 48]. Microtubule-induced focal adhesion disassembly is, at least partially, based on microtubule-stimulated dynamin and clathrin-dependent endocytosis of adhesion components [49, 50]. One the other hand, microtubule-dependent regulation of focal adhesions depends on activators of small GTPase-dependent pathways, such as STEF (TIAM2) [41]. Whilst the exact mechanism whereby microtubules trigger this pathway is not known, several sets of data indicate that local regulation of microtubule dynamics is important for focal adhesion disassembly. For example, focal adhesion disassembly occurs upon repetitive microtubule targeting [35] that involves multiple local microtubule catastrophes at the adhesion site and subsequent rescues in adhesion proximity [32]. Moreover, adhesion turnover is diminished upon inhibition of deacetylase HDAC6 that leads to increased tubulin acetylation and suppression of microtubule dynamics [28].
Finally, efficient cell migration requires microtubule-dependent delivery of post-Golgi carriers [51, 52], recycling endosomes [53], mRNA [54] and other functional entities to the protruding cell edge. Microtubule-driven intracellular transport supports both actin cytoskeleton organization (e.g. the localisation of mRNA encoding Arp2/3 subunits [54]) and focal adhesion turnover through kinesin-1 activity [55]. Regulation of adhesion turnover by trafficking may occur via integrin recycling as part of recycling endosome trafficking (see [56] for review) since kinesin-1 has been implicated as an essential motor for recycling endosomes [57]. Furthermore, in macrophages microtubules regulate the turnover of invasive ventral actin protrusions, called podosomes. This regulation involves activity of the kinesin-3 family member KIF1C [58] and the kinesin-9 family member KIF9 [59] although the cargos that are delivered remain to be identified. Taken together, these evidences indicate that activity of microtubule-dependent molecular motors and availability of suitable microtubule tracks for motor movement is an essential requirement for directed cell migration.
5. The overall state of microtubule dynamics regulates multiple molecular factors
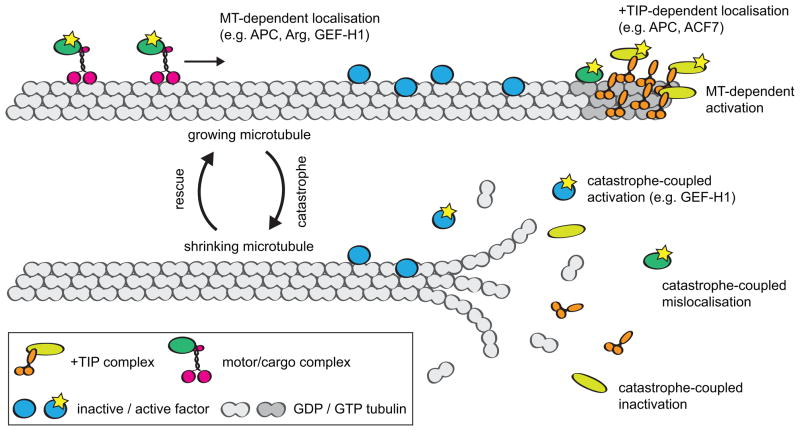
The set of factors that transduce microtubule signals to the cell migration machinery can be roughly divided into three subsets: those delivered by microtubule motors, those temporarily concentrated as components of the +TIP complex and those accumulated along the whole microtubule lattice. The activity of members of each group strongly depends on microtubule dynamics (see Figure 2). Microtubule motors, such as kinesin-1, prefer reliable tracks composed of post-translationally modified tubulin [29] or associated with specific stabilizing MAPs (e.g. ensconsin [60]) while other MAPs can reduce motor attachment [61]. +TIPs (see [62] for a recent review) can concentrate their activities locally at consistently growing microtubule ends, or be released acutely for action at a site of induced microtubule catastrophe. Certain factors of potentially high significance, like APC, possibly depend on both kinesin motor activity [63] and interaction with the +TIP complex [64] for accumulation at the microtubule plus end. Microtubule lattice binding proteins such as GEF-H1, can be sequestered and inactivated by a growing microtubule or, similarly to +TIPs, be acutely released and activated by a catastrophe [45, 46]. Other microtubule lattice binding active proteins, such as tyrosine kinase Arg [65], may be localised by growing or stable microtubules but diffuse away upon catastrophe. Thus, overall changes in microtubule dynamics, such as an increase in microtubule dynamicity or, alternatively, microtubule stabilization, could cause a distinct signalling response by changing the status of all the above-mentioned factors. Additionally, an overall shift in microtubule dynamics properties may alter the distribution of microtubule dynamics events in the cell, so as to make it more or less asymmetric. Altogether, these ideas prompt the hypothesis that mis-regulation of microtubule dynamics by generic means would produce a system-level response that alters cell polarity and migration via multiple pathways.
Figure 2.
Putative mechanisms of microtubule dynamics-mediated control of protein localisation and activity.
6. Microtubule-dependent alteration of cell migration implies changes in microtubule dynamics
Various microtubule-associated proteins (MAPs) have been implicated in the regulation of cell migration direction or the polarisation of cells. In melanoma cells, loss of EB1 reduces lamellipodial protrusion, velocity and persistence of cell migration [66]. APC also promotes cell front protrusion [67]. That the localisation of APC to microtubule ends depends on EB1 [64, 68] suggests that APC acts downstream of EB1. CLASPs are required for directionally persistent cell migration [52, 69] as well as to contribute to the formation of stable microtubules that accumulate acetylated and detyrosinated tubulin [70]. ACF7 has been shown to contribute to the localisation of CLASP2 [69], but also to be involved in epidermal migration directly using its actin-regulated ATPase function, which is required for targeting microtubules to focal adhesions [47]. Depletion of the microtubule-associated tumor suppressor RASSF1A results in extreme protrusion activity and excessive migration in HeLa cells [71]. In neurons, MAP1b regulates axonal outgrowth through GEF TIAM1 [40]. Phosphorylation of CLIP-170 by the energy-sensing kinase AMPK regulates the rapid turnover of CLIP-170 at microtubule ends, which is important for fast microtubule growth, microtubule stabilisation, focal adhesion turnover and directional cell migration [72].
It is likely that all these functions of microtubule-associated proteins relate to their function in regulating microtubule dynamics. All MAPs implicated in cell migration thus far stabilise microtubules: EB1 and APC have been shown to act downstream of Rho and mDia in the stabilisation of microtubules to the front of migrating cells [73], EB1 and EB3 promote persistent microtubule growth in the cytoplasm [74], and APC stabilises microtubules by promoting growth, slowing shrinkage and decreasing transition frequencies [75]. MAP1b stabilizes microtubules and serves as a major scaffolding factor for microtubule-related activities in neurons [76, 77]. RASSF1a acts as a microtubule lattice-associated MAP acting in conjunction with small GTPase RAN to cause microtubule over-stabilization [78]. CLIP-170 and CLASPs possess rescue factor activity [79–81] that increases the cortical dwell time of microtubules [13]. A loss of stable (cold or drug-resistant) microtubules is frequently observed when these microtubule stabilisers are deleted from cells [69, 73, 76]. Although each of these proteins enhances microtubule stability by a specific mechanism, it is plausible to suggest that disturbance of this regulation would cause similar changes in overall microtubule dynamics. As we discussed above, such changes could result in a system-level disturbance in cell polarity and motility via multiple microtubule-dependent pathways.
7. Cell-type specificity or: size does matter
That said, paradoxically, none of the major motility processes of the cell absolutely require microtubules. Actin polymerization, focal adhesion turnover and delivery of post-Golgi carriers to the cell edge can proceed in the absence of microtubules or their proper dynamics. Rather, the polarisation of motility, in other words, the asymmetric distribution of motility processes requires microtubule regulation. Thus the main function of microtubules is to manage the overall organization and proper positioning of multiple activities within a cell in order to enable the persistent directional relocation of the whole cell. Microtubule control is more important for large cells than for small ones. A large number of studies have sought to understand how relevant microtubule control is for motility of diverse cell types. An often-cited example of a cell which can move directionally without microtubules is a fish keratocyte [82]. Similarly, directional migration of small chemotactic cells of hematopoetic origin is not abolished in the absence of microtubules. For example, neutrophils can efficiently polarize and initiate movement in the absence of microtubules [83] though the efficiency and directional persistence of migration toward the chemo-attractant is decreased [84]. Similarly, T cells reduce but do not entirely lose the directionality of their migration if microtubules are destroyed [85]. Thus it appears that small cells can overcome a lack of microtubule-based cellular organization better than large ones. In particular, directional trafficking of required components can be more easily compensated in smaller cells where actin-based transport or diffusion can provide a sufficient supply. In simple systems, a persistent leading edge can be maintained after initial symmetry breakage by actin polymerization and acto-myosin contractility without additional stimulation [87, 88]. It is likely that such mechanoregulatory response underlies migration of small cells without microtubules [85–86, 88]. Moreover, small cells do not employ complicated adhesion machinery. Migration of immune cells is integrin-dependent but these cells likely don’t form distinct adhesion sites (see [89–91] for reviews). Fish keratocytes have transient, dot-like adhesions [92], that are probably capable to disassemble without additional microtubule-introduced stimuli just like nascent focal complexes in bigger cells. In contrast, large systems like fibroblasts, motile epithelial cells and neurons are rendered disorganized and unable to move directionally in the absence of proper microtubule-mediated management of adhesion turnover, actin dynamics and membrane trafficking.
8. Crosstalk between actin and microtubule cytoskeleton
To describe the influence of microtubule dynamics on cell motility, we present a simple model whereby MAPs regulate microtubule dynamics, which, in turn, influences actin assembly, adhesion and cell polarity. This model is incomplete because does not take into account various possible feedback and cooperativity effects. The polarity of the microtubule network is to a large extent defined by cortical interactions with the actin cytoskeleton. In a polarized cell, there is it the potential for a positive feedback loop whereby actin and microtubule polarity stimulate each other.
Additionally, some microtubule stabilisers act directly on actin dynamics and vice versa. APC can both stabilize microtubules and nucleate actin filaments, with nucleation further stimulated by cooperation with the formin mDia1 [75, 93]. An additional role as a microtubule stabiliser has been reported for the formin mDia2, which is independent of its actin-nucleating activity [94]. Synergistic effects of such dual-acting proteins would be likely to be enhanced by the actin-microtubule crosslinking function of ACF7 [47] and Arg [65], which ensure a close proximity of both filament systems. Thus, crosstalk between the actin and microtubule cytoskeleton to orchestrate cell polarity and migration is manifold and extends from dual-function molecules to complex feedback mechanisms in signalling networks.
9. Microtubule-dependent regulation of cell migration is impaired in cancers
While the coordination of cell migration is of crucial importance during embryonic development, wound healing and immune response, the deregulation of the migration machinery allows tissue invasion and metastasis by cancer cells. Thus understanding the regulation of cell migration might open new avenues for therapeutic approaches. Interestingly, microtubule-stabilizing factors implicated in the control of cell motility, such as APC, Dlg1 and RASSF1A, act as tumour suppressors and their loss is frequently associated with human cancers (see [95–97]). Moreover, non-specific microtubule stabilization by Taxol does inhibit cancer cell migration [98]. These observations suggest that a loss of microtubule-associated proteins and subsequent alteration of interphase microtubule dynamics stimulates uncontrolled motility in cancer cells that is associated with invasiveness and poor prognosis in cancer patients.
Highlights.
Microtubule-dependent regulation of cell migration is particularly important in large cells.
Microtubules transport post-Golgi carriers, mRNA and recycling endosomes to cortical locations.
Microtubules regulate actin assembly kinetics and focal adhesion turnover.
Microtubule functions in cell migration require proper regulation of microtubule dynamics.
Changes in microtubule dynamics affect cell migration through multiple pathways.
Acknowledgments
We would like to thank Rob Cross and Ulrike Theisen for critical reading of the manuscript. This work was supported by an NIH NIGMS grant R01 GM078373 and an American Heart Association grant 10GRNT4230026 to I.K., a Marie Curie Cancer Care Programme Grant to A.S. and a University of Warwick Strategic Partnership Fund award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Irina Kaverina, Email: Irina.Kaverina@Vanderbilt.Edu.
Anne Straube, Email: anne@mechanochemistry.org.
References
- 1.Brangwynne CP, MacKintosh FC, Weitz DA. Force fluctuations and polymerization dynamics of intracellular microtubules. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16128–33. doi: 10.1073/pnas.0703094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschner MW, Mitchison T. Microtubule dynamics. Nature. 1986;324:621. doi: 10.1038/324621a0. [DOI] [PubMed] [Google Scholar]
- 3.Mandelkow EM, Mandelkow E, Milligan RA. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol. 1991;114:977–91. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard J, Hyman AA. Growth, fluctuation and switching at microtubule plus ends. Nat Rev Mol Cell Biol. 2009;10:569–74. doi: 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- 5.Dammermann A, Desai A, Oegema K. The minus end in sight. Curr Biol. 2003;13:R614–24. doi: 10.1016/s0960-9822(03)00530-x. [DOI] [PubMed] [Google Scholar]
- 6.Komarova YA, Vorobjev IA, Borisy GG. Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary. J Cell Sci. 2002;115:3527–39. doi: 10.1242/jcs.115.17.3527. [DOI] [PubMed] [Google Scholar]
- 7.Drummond DR, Cross RA. Dynamics of interphase microtubules in Schizosaccharomyces pombe. Curr Biol. 2000;10:766–75. doi: 10.1016/s0960-9822(00)00570-4. [DOI] [PubMed] [Google Scholar]
- 8.Straube A. How to measure microtubule dynamics? Methods Mol Biol. 2011;777:1–14. doi: 10.1007/978-1-61779-252-6_1. [DOI] [PubMed] [Google Scholar]
- 9.van der Vaart B, Akhmanova A, Straube A. Regulation of microtubule dynamic instability. Biochem Soc Trans. 2009;37:1007–13. doi: 10.1042/BST0371007. [DOI] [PubMed] [Google Scholar]
- 10.Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, et al. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, et al. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell. 2003;11:445–57. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa T, Nitta R, Okada Y, Hirokawa N. A common mechanism for microtubule destabilizers-M type kinesins stabilize curling of the protofilament using the class-specific neck and loops. Cell. 2004;116:591–602. doi: 10.1016/s0092-8674(04)00129-1. [DOI] [PubMed] [Google Scholar]
- 13.Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168:141–53. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straube A, Merdes A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr Biol. 2007;17:1318–25. doi: 10.1016/j.cub.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–85. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–83. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Manneville JB, Jehanno M, Etienne-Manneville S. Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity. J Cell Biol. 2010;191:585–98. doi: 10.1083/jcb.201002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J Cell Biol. 2003;161:845–51. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niethammer P, Bastiaens P, Karsenti E. Stathmin-tubulin interaction gradients in motile and mitotic cells. Science. 2004;303:1862–6. doi: 10.1126/science.1094108. [DOI] [PubMed] [Google Scholar]
- 20.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12:917–30. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Nadar VC, Kozielski F, Kozlowska M, Yu W, Baas PW. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J Neurosci. 2010;30:14896–906. doi: 10.1523/JNEUROSCI.3739-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadar VC, Ketschek A, Myers KA, Gallo G, Baas PW. Kinesin-5 is essential for growth-cone turning. Curr Biol. 2008;18:1972–7. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J Cell Biol. 2007;178:1081–91. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, Gelfand VI. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12151–6. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988;85:5946–50. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulinski JC, Gundersen GG. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991;13:285–93. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- 27.Matov A, Applegate K, Kumar P, Thoma C, Krek W, Danuser G, et al. Analysis of microtubule dynamic instability using a plus-end growth marker. Nat Methods. 2010;7:761–8. doi: 10.1038/nmeth.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran AD, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120:1469–79. doi: 10.1242/jcs.03431. [DOI] [PubMed] [Google Scholar]
- 29.Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–83. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–72. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Salaycik KJ, Fagerstrom CJ, Murthy K, Tulu US, Wadsworth P. Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J Cell Sci. 2005;118:4113–22. doi: 10.1242/jcs.02531. [DOI] [PubMed] [Google Scholar]
- 32.Efimov A, Schiefermeier N, Grigoriev I, Ohi R, Brown MC, Turner CE, et al. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J Cell Sci. 2008;121:196–204. doi: 10.1242/jcs.012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–90. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006;11:21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–34. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970;24:625–40. [PubMed] [Google Scholar]
- 38.Liao G, Nagasaki T, Gundersen GG. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J Cell Sci. 1995;108 ( Pt 11):3473–83. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- 39.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 40.Montenegro-Venegas C, Tortosa E, Rosso S, Peretti D, Bollati F, Bisbal M, et al. MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol Biol Cell. 2010;21:3518–28. doi: 10.1091/mbc.E09-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooney C, White G, Nazgiewicz A, Woodcock SA, Anderson KI, Ballestrem C, et al. The Rac activator STEF (Tiam2) regulates cell migration by microtubule-mediated focal adhesion disassembly. EMBO Rep. 2010;11:292–8. doi: 10.1038/embor.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, et al. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci. 2009;122:2969–79. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- 43.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–23. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell. 2009;20:4070–82. doi: 10.1091/mbc.E09-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19:2147–53. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates crosstalk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 47.Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135:137–48. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, et al. Skin Stem Cells Orchestrate Directional Migration by Regulating Microtubule-ACF7 Connections through GSK3beta. Cell. 2011;144:341–52. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–47. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–90. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 51.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–36. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller PM, Folkmann AW, Maia AR, Efimova N, Efimov A, Kaverina I. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat Cell Biol. 2009;11:1069–80. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–47. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 54.Mingle LA, Okuhama NN, Shi J, Singer RH, Condeelis J, Liu G. Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts. J Cell Sci. 2005;118:2425–33. doi: 10.1242/jcs.02371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krylyshkina O, Kaverina I, Kranewitter W, Steffen W, Alonso MC, Cross RA, et al. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J Cell Biol. 2002;156:349–59. doi: 10.1083/jcb.200105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pellinen T, Ivaska J. Integrin traffic. J Cell Sci. 2006;119:3723–31. doi: 10.1242/jcs.03216. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt MR, Maritzen T, Kukhtina V, Higman VA, Doglio L, Barak NN, et al. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15344–9. doi: 10.1073/pnas.0904268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopp P, Lammers R, Aepfelbacher M, Woehlke G, Rudel T, Machuy N, et al. The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol Biol Cell. 2006;17:2811–23. doi: 10.1091/mbc.E05-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornfine S, Himmel M, Kopp P, El Azzouzi K, Wiesner C, Kruger M, et al. The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes. Mol Biol Cell. 2011;22:202–15. doi: 10.1091/mbc.E10-05-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung HH, Telley IA, Papadaki P, Ephrussi A, Surrey T, Rorth P. Drosophila ensconsin promotes productive recruitment of Kinesin-1 to microtubules. Dev Cell. 2008;15:866–76. doi: 10.1016/j.devcel.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Seitz A, Kojima H, Oiwa K, Mandelkow EM, Song YH, Mandelkow E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. Embo J. 2002;21:4896–905. doi: 10.1093/emboj/cdf503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gouveia SM, Akhmanova A. Cell and molecular biology of microtubule plus end tracking proteins: end binding proteins and their partners. Int Rev Cell Mol Biol. 2010;285:1–74. doi: 10.1016/B978-0-12-381047-2.00001-3. [DOI] [PubMed] [Google Scholar]
- 63.Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, et al. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol. 2002;4:323–7. doi: 10.1038/ncb779. [DOI] [PubMed] [Google Scholar]
- 64.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–76. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 65.Miller AL, Wang Y, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J Cell Biol. 2004;165:407–19. doi: 10.1083/jcb.200308055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schober JM, Cain JM, Komarova YA, Borisy GG. Migration and actin protrusion in melanoma cells are regulated by EB1 protein. Cancer Lett. 2009;284:30–6. doi: 10.1016/j.canlet.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Kroboth K, Newton IP, Kita K, Dikovskaya D, Zumbrunn J, Waterman-Storer CM, et al. Lack of adenomatous polyposis coli protein correlates with a decrease in cell migration and overall changes in microtubule stability. Mol Biol Cell. 2007;18:910–8. doi: 10.1091/mbc.E06-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura M, Zhou XZ, Lu KP. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol. 2001;11:1062–7. doi: 10.1016/s0960-9822(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 69.Drabek K, van Ham M, Stepanova T, Draegestein K, van Horssen R, Sayas CL, et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol. 2006;16:2259–64. doi: 10.1016/j.cub.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 70.Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–35. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 71.Dallol A, Agathanggelou A, Tommasi S, Pfeifer GP, Maher ER, Latif F. Involvement of the RASSF1A tumor suppressor gene in controlling cell migration. Cancer Res. 2005;65:7653–9. doi: 10.1158/0008-5472.CAN-05-0247. [DOI] [PubMed] [Google Scholar]
- 72.Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–90. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 73.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 74.Komarova Y, De Groot CO, Grigoriev I, Gouveia SM, Munteanu EL, Schober JM, et al. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol. 2009;184:691–706. doi: 10.1083/jcb.200807179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kita K, Wittmann T, Nathke IS, Waterman-Storer CM. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell. 2006;17:2331–45. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci. 1992;103 ( Pt 4):953–64. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- 77.Riederer BM. Microtubule-associated protein 1B, a growth-associated and phosphorylated scaffold protein. Brain Res Bull. 2007;71:541–58. doi: 10.1016/j.brainresbull.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 78.Dallol A, Hesson LB, Matallanas D, Cooper WN, O'Neill E, Maher ER, et al. RAN GTPase is a RASSF1A effector involved in controlling microtubule organization. Curr Biol. 2009;19:1227–32. doi: 10.1016/j.cub.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 79.Al-Bassam J, Kim H, Brouhard G, van Oijen A, Harrison SC, Chang F. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev Cell. 2010;19:245–58. doi: 10.1016/j.devcel.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Komarova YA, Akhmanova AS, Kojima S, Galjart N, Borisy GG. Cytoplasmic linker proteins promote microtubule rescue in vivo. J Cell Biol. 2002;159:589–99. doi: 10.1083/jcb.200208058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arnal I, Heichette C, Diamantopoulos GS, Chretien D. CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr Biol. 2004;14:2086–95. doi: 10.1016/j.cub.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 82.Euteneuer U, Schliwa M. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature. 1984;310:58–61. doi: 10.1038/310058a0. [DOI] [PubMed] [Google Scholar]
- 83.Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813–22. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- 84.Xu J, Wang F, Van Keymeulen A, Rentel M, Bourne HR. Neutrophil microtubules suppress polarity and enhance directional migration. Proc Natl Acad Sci U S A. 2005;102:6884–9. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One. 2010;5:e8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, et al. Mechanism of shape determination in motile cells. Nature. 2008;453:475–80. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- 88.Barnhart EL, Allen GM, Julicher F, Theriot JA. Bipedal locomotion in crawling cells. Biophys J. 2010;98:933–42. doi: 10.1016/j.bpj.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, et al. Integrins in immunity. J Cell Sci. 2009;122:215–25. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 90.Chen D, Bromberg JS. T regulatory cells and migration. Am J Transplant. 2006;6:1518–23. doi: 10.1111/j.1600-6143.2006.01372.x. [DOI] [PubMed] [Google Scholar]
- 91.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116:4695–705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 92.Anderson KI, Cross R. Contact dynamics during keratocyte motility. Curr Biol. 2000;10:253–60. doi: 10.1016/s0960-9822(00)00357-2. [DOI] [PubMed] [Google Scholar]
- 93.Okada K, Bartolini F, Deaconescu AM, Moseley JB, Dogic Z, Grigorieff N, et al. Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J Cell Biol. 2010;189:1087–96. doi: 10.1083/jcb.201001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–36. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donninger H, Vos MD, Clark GJ. The RASSF1A tumor suppressor. J Cell Sci. 2007;120:3163–72. doi: 10.1242/jcs.010389. [DOI] [PubMed] [Google Scholar]
- 96.van Es JH, Giles RH, Clevers HC. The many faces of the tumor suppressor gene APC. Exp Cell Res. 2001;264:126–34. doi: 10.1006/excr.2000.5142. [DOI] [PubMed] [Google Scholar]
- 97.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 98.Pienta KJ, Coffey DS. Cell motility as a chemotherapeutic target. Cancer Surv. 1991;11:255–63. [PubMed] [Google Scholar]