Abstract
Background
Adiponectin is linked to reduced diabetes risk and may be anti-atherogenic, yet clinical data show no consistent relationship with incident cardiovascular events, especially among women. To our knowledge, no prior prospective studies have evaluated adiponectin, including high-molecular-weight (HMW) adiponectin, and incident peripheral artery disease (PAD).
Methods & Results
We evaluated the relationship of total, HMW and the HMW-to-total adiponectin ratio with incident symptomatic PAD in a prospective, nested case-control study conducted within the Women’s Health Study (n=110 cases, n=230 controls, frequency matched in strata defined by five-year age categories, smoking, fasting status and follow-up time; median cohort follow-up=13.2 yrs). Baseline median levels of HMW and total adiponectin were significantly lower in women developing PAD than those remaining event-free(HMW: 3.3 vs. 3.8 μg/mL, P=0.0005; total: 5.6 vs. 7.4 μg/mL, P<0.0001). The ratio did not differ significantly between groups. Age-adjusted PAD odds ratios (95% CI) across tertiles were 1.0, 0.66 (0.39–1.13) and 0.40 (0.22–0.74)for HMW and 1.0, 0.74 (0.43–1.25) and 0.35 (0.18–0.65) for total adiponectin (P-trend=0.004 and 0.001, respectively). Results were similar after adjustment for traditional cardiovascular risk factors, use of post-menopausal hormone therapy, high-sensitivity C-reactive protein, soluble intercellular adhesion molecule-1, leptin, hemoglobin A1c and fasting insulin [adjusted OR and 95% CI for HMW: 1.0, 0.62 (0.29–1.34), 0.30 (0.12–0.74); total: 1.0, 0.46 (0.22–1.00), 0.30 (0.12–0.76 );Ptrend=0.01 for both].
Conclusion
Total and HMW adiponectin are inversely associated with incident PAD among initially healthy women. These prospective data support a protective role for this adipokine in peripheral atherosclerosis development.
Keywords: adiponectin, biomarker, epidemiology, peripheral artery disease, women
Introduction
In recent years, the role of adipose tissue as an endocrine organ capable of elaborating adipose-specific or adipose-enriched hormones involved in glucose metabolism and inflammation has gained greater appreciation. Adiponectin, the most abundant circulating adipokine, is paradoxically decreased in obesity, with the HMW form considered to be the biologically active moiety.1,2 High levels are associated with greater insulin sensitivity, anti-oxidative and anti-inflammatory effects3,4 and consistently lower risk of future type 2 diabetes (T2D) in prospective analyses.5,6 However, despite favorable effects on atherosclerotic plaque initiation and progression in basic laboratory investigations,7–9 population-based studies examining the role of adiponectin in cardiovascular disease (CVD)development have yielded conflicting results. Among men, several studies have found varying degrees of reduced CVD risk with elevated adiponectin,10–12 while others have found increased risk.13,14 Among several large studies conducted solely in women or in subgroup analyses confined to women, no effect has been found,12,15–18 with one major exception: among female participants in the Hoorn Study, elevated adiponectin was associated with 28% reduced risk of nonfatal CVD.19 Apart from potential gender differences in risk association, these divergent findings may also relate to heterogeneity in the outcome of interest, e.g. myocardial infarction, stroke or heart failure, and the variable inclusion of subjects with pre-existing CVD.
The disease-specific association of adiponectin with incident peripheral artery disease (PAD) has not been previously evaluated, with no published prospective data currently available. In two cross-sectional studies of individuals with diagnosed PAD, lower adiponectin was associated with lower ankle-brachial index (ABI)and reduced exercise performance, indicating more severe disease.20,21 As low adiponectin is closely linked to insulin resistance and type 2 diabetes, two potent risk factors for PAD, we evaluated the association of baseline plasma levels of high-molecular-weight (HMW), total, and the HMW-to-total adiponectin ratio with incident symptomatic PAD (intermittent claudication and peripheral artery revascularization) in the Women’s Health Study, a large cohort of relatively healthy middle-aged and older American women followed for a median of 13.2 years. Furthermore, we assessed whether potential risk associations may be explained by the presence of underlying subclinical inflammation as measured by high-sensitivity C-reactive protein (hsCRP) and soluble intercellular adhesion molecule-1 (sICAM-1).
Materials & Methods
Study Population
We designed a prospective, nested case-control study involving participants in the Women’s Health Study (WHS), a randomized clinical trial evaluating low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. The WHS study population, design and clinical trial results have been previously described.22,23 In brief, between November 1992 and July 1995, a total of 39 876 female health professionals aged 45 or older without prior cancer or cardiovascular disease (including myocardial infarction, stroke, coronary and peripheral arterial revascularization) were enrolled and randomized into the study. Among women enrolled, 28 345 (71%) provided baseline blood samples, which were centrifuged and stored in liquid nitrogen until analysis. Mailed questionnaires collected baseline and follow-up information every six months during the first year and every 12 months thereafter. The median cohort follow-up at the time of case-control sampling was 13.2 years.
The study was approved by an institutional review board and all subjects provided informed consent.
Outcome Ascertainment
Participants were surveyed annually for the occurrence of a number of incident health events including symptomatic PAD, defined as new report of intermittent claudication and/or PAD revascularization (surgery or catheter-based interventions). Case confirmation occurred through telephone interview conducted by a cardiovascular physician every 1–2 years during the conduct of the study. A confirmation of intermittent claudication was made after physician administration of the Edinburgh Claudication Questionnaire(ECQ). This instrument is a modified version of the World Health Organization/Rose Claudication Questionnaire, which has previously been validated in a community outpatient setting with 92% sensitivity and 99% specificity for physician-diagnosed intermittent claudication.24 In addition, we obtained medical records to assess the concordance of reported symptoms with diagnostic testing when available. Reports of peripheral arterial surgery or peripheral angioplasty were confirmed after review of operative notes or procedural reports, respectively. Cases were thus validated based upon response to the claudication questionnaire and medical record documentation of diagnostic procedures or vascular intervention. As of November 23, 2007, among 28345 subjects providing baseline blood specimens, there were 556 self-reported PAD events; of these, 117 were confirmed through the methods described above. Venous disease, lower extremity arthritis, lumbar disc disease and peripheral neuropathy were the main causes of non-ischemic leg pain in disconfirmed events. Only individuals with confirmed events were considered in the current analysis.
Case-Control Selection and Matching
Control subjects (n=234) were frequency-matched to the 117 WHS participants who developed confirmed PAD in a 2:1 ratio by strata defined according to five-year age categories, smoking status (current, former or never smoked), fasting status of submitted blood specimens and follow-up time. Fasting was defined as 8 or more hours since last meal prior to sample collection; 75% of samples in this analysis were fasting. Of the 117 case subjects included in the initial sample for laboratory analysis, two subjects with unavailable adiponectin level and five with confirmed pre-randomization PAD events were excluded. As a result, some strata had more than 2 control subjects for each case subject, but were retained in the analysis due to the frequency matched design. Re-assessment of each matched stratum also revealed four control subjects that no longer had matched case subjects; these individuals were therefore removed from the analysis. Thus, the final study sample comprised 110 case and 230 control subjects.
Laboratory Analysis
Baseline plasma samples from case and controls subjects were thawed and assayed. Samples had not previously been thawed for other studies. Total and high-molecular-weight adiponectin levels were measured separately using a sandwich ELISA method (ALPCO Diagnostics, Salem, NH).The day-to-day variabilities of the assay for total adiponectin at 9.1 and 3.9 μg/mL and high-molecular weight adiponectin at 4.8 and 1.17 μg/mL were 9.8, 10.2, 11.4 and 12.6% respectively. The percent recoveries of total and HMW adiponectin after one freeze-thaw cycle as reported by the assay manufacturer are 99 and 97%, respectively. Insulin was measured with an electrochemiluminescence immunoassay(Roche Diagnostics, Indianapolis, IN) with a cross-reactivity of less than 0.05% with proinsulin. Leptin was measured by an ultra-sensitive enzymatically amplified two-step sandwich immunoassay (R&D Systems, Minneapolis, MN). High-density lipoprotein (HDL), hsCRP, sICAM-1 and hemoglobin A1c (HbA1c) were measured as previously described.25
Statistical Analysis
We used repeated measures analysis (SAS PROC mixed) to evaluate differences in means, the van Elteren test for differences in medians and a matched χ2 statistic to assess for differences in proportions, each approach accounting for frequency matching in the study design. Continuous measures are presented as means or medians based on normality of their distribution. Categorical data are represented as percentages. Age-adjusted Spearman partial correlation coefficients were calculated among control subjects to assess the associations between HMW adiponectin, total adiponectin, the HMW-to-total ratio, BMI, fasting insulin, HbA1c and the subclinical markers of inflammation sICAM-1 and hsCRP.
We then assessed the relationship between HMW, total adiponectin and the HMW-to-total ratio and incident PAD, after dividing the sample into tertiles based upon the control distribution. Conditional logistic regression models were then constructed to estimate tertile-specific odds ratios and 95% confidence intervals, with the lowest tertile as the referent group. Median values were used to test for a linear trend in increasing tertiles. As adiponectin (and leptin) levels are not significantly altered by short-term (<48 hours) fasting,26,27 the primary analyses were not restricted to fasting subjects until adjustment for fasting insulin.
Initial models adjusted for age, the randomized treatment arms of the original WHS trial, and accounted for matched factors of five-year age categories, smoking, follow-up time and fasting status (Model 1). Model 2 additionally adjusted for race, traditional cardiovascular disease risk factors and use of postmenopausal hormone replacement therapy (HRT). Subsequent models additionally adjusted for markers of subclinical inflammation including hsCRP, sICAM-1 and leptin (Model 3), hemoglobin A1c and fasting insulin(Model 4) and HDL in place of history of high cholesterol (Model 5). HDL was chosen as prior data in this cohort have demonstrated HDL to be the lipid marker most strongly associated with PAD risk.28 Sensitivity analyses were performed restricting the analysis to non-diabetic subjects (no baseline diagnosed diabetes and baseline HbA1c<6.5%).Model fit was compared by the likelihood ratio chi-squared statistic, with a higher value indicating superior fit.
Conditional logistic regression was also used to investigate the joint association of inflammatory markers (hsCRP and sICAM-1)and HMW or total adiponectin with future PAD. The primary sample was divided into above-and below-median groups for hsCRP and sICAM-1, and tertiles for total and HMW adiponectin. For this analysis, the adjustment variables used in model 2 were chosen to reflect inclusion of traditional cardiovascular risk factors. Those with low HMW or total adiponectin and above-median inflammatory marker level were made the referent group.
Statistical analyses were performed using SAS version 9.2(SAS Institute, Cary, NC). All confidence intervals are 2-tailed and calculated at p=0.05 level of significance.
Results
Baseline characteristics of women who developed symptomatic PAD (case subjects) and those remaining free of symptomatic PAD (control subjects) are shown in Table 1. The majority of participants in this study were non-Hispanic Whites. As might be expected, individuals who experienced PAD events were significantly more likely to have hypertension, hypercholesterolemia or diabetes at baseline. Notably, the proportion of participants reporting family history of MI did not differ significantly between groups. Baseline prevalence of other characteristics, including BMI, pre-and post-menopausal status with or without use of hormone replacement therapy, exercise frequency and alcohol use were also comparable.
Table 1.
Baseline characteristics of individuals who developed PAD (cases) versus those who did not (controls).
| Characteristic | Cases (n=110) | Controls (n=230) | P-value | |
|---|---|---|---|---|
| Age (years) | 59.0 (7.7) | 58.7 (7.5) | 0.75 | |
| White | 106 (98.2%) | 214(95.1%) | 0.18 | |
| BMI (kg/m2) | 25.6 (4.4) | 25.3 (4.5) | 0.45 | |
| Smoking | Current | 49 (44.6%) | 106 (46.1%) | …* |
| Past | 41 (37.3%) | 82(35.7%) | ||
| Never | 20 (18.2%) | 42(18.3%) | ||
| Diabetes | Yes | 7 (6.4%) | 3 (1.3%) | 0.01 |
| Hypertension | Yes | 44 (40.0%) | 64(27.8%) | 0.02 |
| Hypercholesterolemia | Yes | 50 (45.5%) | 79 (34.4%) | 0.05 |
| Family History of MI | Yes | 17 (15.5%) | 31(13.5%) | 0.63 |
| Postmenopausal | Yes + current HRT | 30 (27.3%) | 92(40.0%) | 0.42 |
| Yes − current HRT | 44 (40.0%) | 81(35.2%) | ||
| No | 18 (16.4%) | 27 (11.7%) | ||
| Unknown | 18 (16.4%) | 30 (13.0%) | ||
| Exercise at least once a week | 45 (40.9%) | 91(39.6%) | 0.84 | |
| Alcohol Use | Rare/never | 51 (46.4%) | 90(39.1%) | 0.14 |
| 1–3/mo | 11 (10.0%) | 23(10.0%) | ||
| 1–6/wk | 34 (30.9%) | 78(33.9%) | ||
| 1+/day | 14 (12.7%) | 39 (17.0%) | ||
| Biomarker Level | ||||
| Adiponectin (μg/mL) | HMW | 3.3 (0.5–12.3) | 3.8 (0.4–16.7) | 0.0005 |
| Total | 5.6 (1.5–23.7) | 7.4 (1.6–27.4) | <0.0001 | |
| HMW:Total Adiponectin ratio | 0.53 (0. 1) | 0.53 (0.1) | 0.57 | |
| Leptin (ng/mL) | 19.8 (4.2–74.2) | 19.4(1.6–85.9) | 0.78 | |
| CRP (mg/L) | 3.1 (0.1–48.4) | 2.2 (0.1–52.9) | 0.001 | |
| sICAM-1 (ng/mL) | 418.5 (181.9–785.0) | 370.1 (158.5–835.6) | 0.0002 | |
| Hemoglobin A1C (%) | 5.1 (4.5–11.8) | 5.0 (4.3–9.0) | 0.01 | |
| Fasting Insulin† (μIU/mL) | 7.9(1.7–50.9) | 6.6 (1.9–93.3) | 0.32 | |
Data shown are median values (range) or percentages. P-values for medians were computed using the van Elteren test for continuous variables and a matched χ2 test for categorical variables. Means and standard deviations are reported for age, BMI and HMW:total adiponectin, as these levels were normally distributed. P-values for these three variables were determined using repeated measures analysis.
Matching factor. 5-year age groups were also a matching factor, but not age as a continuous
variable, which is presented above.
Restricted to individuals with fasting blood draws (n=83 cases, n=172 matched controls)
Both HMW and total adiponectin were significantly decreased in case subjects despite a similar mean BMI(P<0.001 for both). The ratio of HMW to total adiponectin, however, was not significantly different between groups with the average ratio being 0.53 in this study population (P=0.69). High-sensitivity CRP and sICAM-1, previously shown to be independent predictors of peripheral atherosclerosis in this cohort,28 were significantly increased in cases, as were hemoglobin A1c and fasting insulin levels. Leptin levels were comparable between the two groups.
Spearman partial correlation coefficients adjusting for age are shown in Table 2 (restricted to control subjects). HMW and total adiponectin were very highly correlated (correlation coefficient=0.95, P<0.0001). Both were moderately inversely correlated with BMI, hsCRP, leptin and fasting insulin, but not with sICAM-1. The HMW-to-total ratio was strongly correlated with HMW adiponectin, modestly with total adiponectin (correlation coefficients=0.61 and 0.36 respectively; P<0.0001 for both),mildly inversely correlated with BMI, and not correlated with the remaining biomarkers.
Table 2.
Spearman Partial Correlation Coefficients among controls, adjusting for age
| Total | HMW:Total Ratio | BMI | hsCRP | sICAM-1 | Leptin | Fasting Insulin | HbA1c | |
|---|---|---|---|---|---|---|---|---|
| HMW | 0.95* | 0.61* | −0.40* | −0.34* | −0.07 | −0.33* | −0.41* | −0.19† |
| Total | 0.36* | −0.41* | −0.36* | −0.11 | −0.38* | −0.47* | −0.18† | |
| Ratio | −0.19† | −0.12 | 0.06 | −0.06 | −0.06 | −0.11 | ||
P<0.0001
P<0.05
Table 3 shows the crude and multivariable-adjusted estimated odds ratios for increasing tertiles of HMW, total adiponectin and HMW-to-total adiponectin ratio. In analyses matched on five-year age groups, smoking, fasting status, and follow-up time, additionally adjusting for continuous age and the WHS treatment arms, increasing tertiles of both HMW and total adiponectin were inversely related to the risk of PAD (OR for increasing tertiles: 1.0, 0.66, 0.40 for HMW; 1.0, 0.74, 0.35 for total; Ptrend=0.004 and 0.001, respectively). Further adjustment for traditional cardiovascular risk factors (Model 2)did not materially alter these results, and there was no major impact of additional adjustment for markers of subclinical inflammation, sICAM-1, hsCRP and leptin (Model 3). Results were also similar with adjustment for hemoglobin A1c and fasting insulin in the subgroup of women providing fasting samples (Model 4). There was no impact of substituting HDL for history of hypercholesterolemia (Model 5). To assess whether our results might have been unduly influenced by inclusion of baseline diabetic patients, we conducted sensitivity analyses in which subjects with either baseline diabetes or HbA1c ≥6.5% were excluded (n=103 cases, n=226 controls remaining). In these analyses, results of every model were essentially identical(data not shown). Additional analyses explored the potential effect of adjustment for pack-years of smoking, renal dysfunction via estimated creatinine clearance and type of hormone therapy used (estrogen only versus estrogen and progesterone) by adding these variables individually to Model 2. Results were essentially unchanged (data not shown)and demonstrated a consistent risk decline with increasing tertiles of both HMW and total adiponectin.
Table 3.
Odds ratios for incident PAD by increasing tertiles of HMW, non-HMW, total and HMW:total adiponectin ratio
| Odds Ratios by Tertile (95% CI)
|
||||
|---|---|---|---|---|
| Tertile 1 (lowest) | Tertile 2 | Tertile 3 | P-trend | |
| HMW Adiponectin | ||||
| Median (μg/mL) | 1.8 | 3.8 | 6.9 | |
| Range | <2.91 | 2.91–5.00 | >5.00 | |
| No. Cases | 51 | 35 | 24 | |
| Model 1 | 1.0 | 0.66 (0.39–1.13) | 0.40 (0.22–0.74) | 0.004 |
| Model 2 | 1.0 | 0.61 (0.33–1.12) | 0.41 (0.20–0.85) | 0.02 |
| Model 3 | 1.0 | 0.57 (0.31–1.07) | 0.39 (0.19–0.81) | 0.01 |
| Model 4* | 1.0 | 0.62 (0.29–1.34) | 0.30 (0.12–0.74) | 0.01 |
| Model 5* | 1.0 | 0.64 (0.29–1.39) | 0.32 (0.12–0.85) | 0.02 |
| Total Adiponectin | ||||
| Median (μg/mL) | 4.1 | 7.3 | 12.0 | |
| Range | <5.44 | 5.44–9.67 | >9.67 | |
| No. Cases | 51 | 39 | 20 | |
| Model 1 | 1.0 | 0.74 (0.43–1.25) | 0.35 (0.18–0.65) | 0.001 |
| Model 2 | 1.0 | 0.68 (0.37–1.26) | 0.37 (0.18–0.76) | 0.007 |
| Model 3 | 1.0 | 0.66 (0.35–1.24) | 0.35 (0.17–0.74) | 0.006 |
| Model 4* | 1.0 | 0.46 (0.22–1.00) | 0.30 (0.12–0.76) | 0.01 |
| Model 5* | 1.0 | 0.48 (0.22–1.05) | 0.33 (0.13–0.87) | 0.03 |
| HMW:Total Ratio | ||||
| Median | 0.43 | 0.52 | 0.62 | |
| Range | ≤0.48 | 0.48–0.57 | >0.57 | |
| No. Cases | 42 | 31 | 37 | |
| Model 1 | 1.0 | 0.76 (0.43–1.33) | 0.87 (0.50–1.53) | 0.62 |
| Model 2 | 1.0 | 0.91 (0.49–1.68) | 1.02 (0.55–1.86) | 0.96 |
| Model 3 | 1.0 | 0.88 (0.47–1.64) | 0.87 (0.47–1.63) | 0.67 |
| Model 4* | 1.0 | 1.05 (0.49–2.25) | 0.83 (0.39–1.74) | 0.60 |
| Model 5* | 1.0 | 1.09 (0.51–2.36) | 0.91 (0.42–1.95) | 0.79 |
Median calculations include cases + controls. Ranges have been rounded for ease of interpretation.
Model 1: Matched on 5-year age categories, smoking and fasting status, adjusted for age and WHS treatment arms (vitamin E and low-dose aspirin)
Model 2: Model 1 + race (white or non-white), BMI (linear continuous), diabetes (yes or no), HTN (≥140/90, yes or no) hyperlipidemia(>240mg/dL, yes or no), family history of MI (yes, no or unknown) and use of HRT (yes or no)
Model 3: Model 2 + hsCRP, sICAM-1 and leptin
Model 4: Model 3 + hemoglobin A1C and fasting insulin
Model 5: Model 4 + with HDL in place of history of high cholesterol
Restricted to individuals with fasting blood draws (n = 83 cases, n = 172 matched controls).
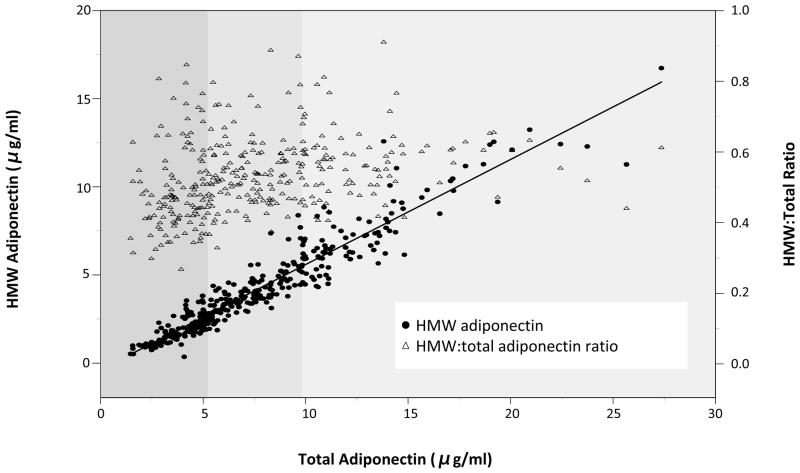
In contrast, the HMW-to-total adiponectin ratio was not associated with future PAD (Model 2 adjusted OR for increasing tertiles: 1.0, 0.91, 1.02; Ptrend=0.96. Given previously reported strong associations of the ratio with insulin resistance and incident type 2 diabetes,29,30 we further explored potential explanations for this lack of association in our data for PAD. Figure 1 shows a scatter plot of HMW and total adiponectin levels in control subjects, with HMW adiponectin plotted on the left vertical axis, total adiponectin on the horizontal axis and tertiles of total adiponectin indicated by shading in grey. Data points are closely clustered around the regression line, reflecting the high correlation between the two variables. The HMW-to-total ratio for each point is also represented on the figure as triangles with values indicated by the right vertical axis. There is little variability of the ratio across tertiles of total adiponectin within this population, with values predominantly distributed between 0.4 and 0.6. While our analyses indicate that high HMW and total adiponectin are individually related to lower incidence of PAD, their strong correlation and largely fixed ratio may explain alack of ability of the ratio to delineate risk in this study.
Figure 1. Relationship between HMW, total and the HMW-to-Total adiponectin ratio.
Closed circles in blue indicate values of total adiponectin plotted against HMW adiponectin (left vertical axis) for each control subject. Values cluster tightly around the regression line. Tertiles of total adiponectin are denoted by shading. Triangles in red indicate the HMW-to-total ratio (right vertical axis) for each individual.
Comparison of the model likelihood ratio chi-square statistic, a global measure of model fit with larger values indicating superior fit, showed that the model with total adiponectin had the highest value (16.8, df=5) followed by HMW adiponectin (14.1, df=5). There was no significant improvement in fit with addition of HMW adiponectin to a model with total adiponectin (LRT for nested models=0.35, P=0.16) and similarly no evidence of improved fit with addition of total adiponectin to a model with HMW adiponectin alone (LRT for nested models=3.06; P=0.78).
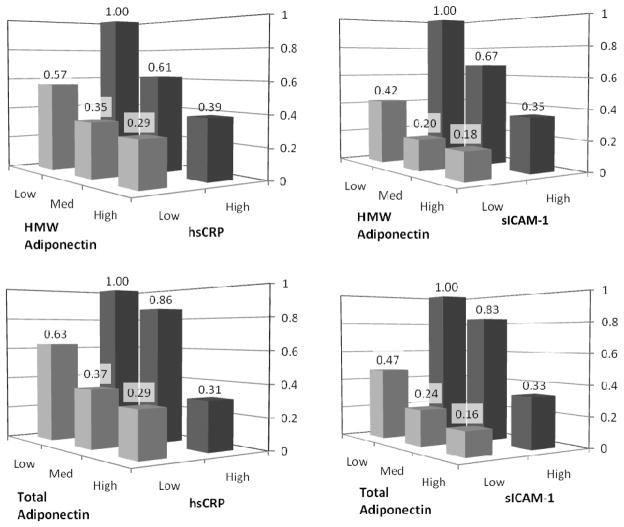
We then sought to investigate potential joint effects of adiponectin when combined with levels of hsCRP and sICAM-1 (Figure 2). Low adiponectin and high inflammatory marker levels were chosen as the high risk referent group. A risk gradient was evident according to baseline adiponectin (total and HMW) irrespective of inflammatory biomarker level (all four interaction p-values >0.5). In all comparisons, the lowest risk of future PAD was seen in the group with high adiponectin and low inflammatory marker levels, and, conversely, highest risk in those with low adiponectin and high inflammatory marker levels. The effect was particularly pronounced in comparisons of adiponectin with sICAM-1, which was also the biomarker least correlated with adiponectin (Table 2).
Figure 2. Odds ratios for incident PAD by tertiles of HMW and total adiponectin, and below-and above-median levels of hsCRP and sICAM-1.
Data shown are multivariable-adjusted odds ratios estimated by conditional logistic regression, adjusting for age, WHS treatment arms, race, BMI, diabetes, HTN, hyperlipidemia, family history of MI and use of HRT(Model 2). The referent is the high risk group (OR=1.0) with low adiponectin and above-median inflammatory marker level.
Discussion
To our knowledge, this is the first prospective investigation of adiponectin with incident PAD. Baseline HMW and total adiponectin levels were found to be inversely associated with future symptomatic PAD in women independent of traditional cardiovascular risk factors, fasting insulin levels and evidence of subclinical inflammation. Our findings were robust in analyses limited to non-diabetic subjects (by history and HbA1c<6.5%) and we found a persistent effect irrespective of baseline levels of hsCRP or sICAM-1. We also found no association for the ratio of HMW to total adiponectin, likely due to the high correlation of the two variables in this population of healthy women.
While experimental data support anti-atherosclerotic properties of adiponectin, epidemiological studies of adiponectin and incident CVD have yielded conflicting results. These inconsistencies may relate to a number of factors including the 1) limited assessment of HMW adiponectin, 2) use of diverse CVD endpoints with potentially distinct pathophysiology, including combinations of non-fatal MI, heart failure, or stroke and, importantly, 3) variable inclusion of subjects with pre-existing atherosclerotic disease. In patients with extant CVD, elevated adiponectin may result from compensatory upregulation, adiponectin resistance, and/or pathologic weight loss,19 all possibilities supported by data demonstrating a positive association between adiponectin and adverse outcomes in this group.13,14,19,31,32 Similarly, in advanced PAD (PAD hospitalization or post-surgery), elevated total adiponectin was recently shown to portend reduced survival33,34: Dieplinger et al. showed a 3% increase in all-cause mortality per 1μg/mL increase in baseline total adiponectin [risk ratio 1.03 (95% CI 1.00–1.05); P=0.03].31 In contrast, among subjects with milder forms of symptomatic PAD, elevated levels are associated with higher ankle-brachial index (ABI) and improved exercise performance.20,21 Thus, the observed associations may differ during periods of early versus late stage clinical disease.
In the current study among a relatively homogenous population of women without prior diagnosed CVD, we found a strong association between elevated adiponectin and reduced incidence of symptomatic PAD. Our findings suggest that adiponectin may be biologically relevant to pathogenesis of this disease. Although the main underlying mechanism in peripheral as in coronary atherosclerosis is atheroma initiation with progressive vascular occlusion and attendant reduction in blood flow, the severity of PAD symptoms does not correlate well with the degree of hemodynamic obstruction, and revascularization does not completely normalize exercise performance.35–38 In this regard, mitochondrial dysfunction, impaired oxidative capacity, and enhanced oxidative stress are “downstream” features of altered skeletal muscle metabolism which do correlate with functional limitation in patients with PAD,39 and which may be directly modulated by adiponectin. Of the two known adiponectin receptors, the adiponectin receptor 1 (AdipoR1) is abundant in skeletal muscle and liver whereas AdipoR2 is predominantly expressed in liver.40 It has recently been demonstrated41 that adiponectin binding to AdipoR1 induces calcium-dependent mitochondrial biogenesis. Furthermore, skeletal muscle-specific disruption of AdipoR1 in mice in vivo produces a phenotype characterized by impaired oxidative capacity, decreased mitochondrial DNA content, and importantly, reduced exercise performance when compared to controls. Thus, beyond direct anti-atherogenic effects on plaque development, these experimental data coupled with our prospective findings support a secondary mechanism by which adiponectin may contribute to the symptomatic expression of this disease.
Our study found no association between the baseline HMW-to-total adiponectin ratio and future PAD, although prior data linking the ratio to insulin resistance and incident T2D are strong.29,30 Total adiponectin levels and the relative proportions of adiponectin forms differ between men and women, with women having generally higher total and HMW levels as well as higher ratios.42 For instance, in the Atherosclerosis Risk In Community Study using the same quantitative assay, after adjustment for age and ethnicity, the mean level of HMW adiponectin was 3.16 and 1.75 μg/mL with a HMW-to-total ratio of 0.44 and 0.37 in women and men, respectively (both p-values <0.001).6 The mechanism underlying for this gender difference is unclear, although testosterone may selectively inhibit HMW adiponectin secretion.42,43 Some have suggested that the estrogen-androgen balance and thus variation not only by gender but menopausal status may also be important for regulating adiponectin multimer distribution.12,12,42 Although we could not assess the impact of sex hormone levels, in this population of relatively healthy women, variation in the HMW-to-total ratio was not associated with PAD risk.
Strengths of the present study include the prospective design, large sample size, long-term follow-up and the homogeneity of our study participants, which may reduce confounding. However, several potential limitations of this study should be considered. First, the WHS included mainly Caucasian women who were healthy at baseline, and thus our conclusions may not be generalizable to other populations. Second, as our study is observational, residual unmeasured confounding may persist. However, we were able to adjust for a broad range of established and emerging cardiovascular risk factors and found no material difference in our results. Third, the use of symptomatic PAD as the primary endpoint by definition excludes subclinical disease that might otherwise have been detected using ankle-brachial index or abnormal pulse examination; however, we believe our data are not only mechanistically relevant but also clinically important as claudication and revascularization of an ischemic limb are the principal clinical manifestations of PAD. Importantly, each case included in this analysis was confirmed through rigorous methods using a validated claudication questionnaire, cardiovascular physician interview, and medical record review. It should be noted that the sensitivity of the self-administered ECQ for ABI-diagnosed PAD is reported to be 29% albeit with a high specificity of 90%.44 In the current study, the ECQ was physician-administered to subjects with a reported prior clinical diagnosis and thus the accuracy is expected to be somewhat higher although data are not available in this regard. Nonetheless, our focus on symptomatic disease may have reduced the likelihood of endpoint misclassification as would the characteristics of our study population comprised of health professionals. Whether or not women with PAD are more likely to have atypical leg symptoms or be more often asymptomatic is controversial,45,46 especially as some studies suggest a lower (less stringent) ABI cut-off may be more appropriate in women.47,48 Regardless, misclassification of women with atypical PAD symptoms as non-cases in our study would, if anything, have tended to bias our results towards the null.
Despite strong preclinical data supporting anti-atherosclerotic properties of adiponectin, previous epidemiologic studies have been somewhat inconsistent. Our findings indicate a strong inverse association between both HMW and total adiponectin and the development of symptomatic peripheral atherosclerosis in women. Our data require confirmation, yet a biologic role is not only plausible but raises the intriguing possibility that therapeutic modulation of adiponectin as already possible by use of certain currently available anti-diabetic, anti-hypertensive agents and nutritional agents49 may hold promise in prevention and treatment of this disease.
Clinical Perspective.
Lower-extremity peripheral artery disease (PAD) is a manifestation of atherosclerosis that has received considerably less clinical and research attention than coronary or cerebrovascular disease. PAD shares many risk factors with other cardiovascular diseases, including smoking, diabetes, hypertension and hyperlipidemia; however, less is known about how PAD differs from atherosclerosis of other vascular territories. Studies of biomarkers and future disease risk can improve our ability to detect patients at heightened risk, our understanding of disease pathogenesis, and, by extension, may identify potential novel modalities for treatment. Adiponectin is secreted from adipose tissue and is known to be inversely correlated with future diabetes risk. It may also be anti-atherogenic. This study is the first to examine the relationship between adiponectin and PAD as a specific vascular endpoint. A large population of initially healthy women aged 45 years or older without existing cardiovascular disease was studied. After taking into account traditional cardiovascular risk factors, women with HMW or total adiponectin levels in the highest tertile had a 59% (HMW) or 63% (total) reduced risk for future symptomatic PAD (intermittent claudication or lower extremity revascularization) when compared to women with levels in the lowest tertile. Given the lack of a consistently demonstrated relationship between adiponectin and other cardiovascular endpoints, this striking result, if confirmed, suggests a unique relationship of adiponectin in PAD development which may reflect a more prominent role of adipokines in peripheral atherosclerosis.
Acknowledgments
FUNDING SOURCES
This study was supported by research grants HL080467, HL 043851, HL 082740, HL 075771 and CA 047988 from the National Institutes of Health, Bethesda, MD. Deborah Ho was supported through a research fellowship awarded by the Sarnoff Cardiovascular Research Foundation.
Footnotes
DISCLOSURES
Dr. Creager is a consultant relevant to PAD for Astra-Zeneca, Genzyme, Merck, NormOxys and Provasculon. Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease.
References
- 1.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 6.Zhu N, Pankow JS, Ballantyne CM, Couper D, Hoogeveen RC, Pereira M, Duncan BB, Schmidt MI. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab. 2010;95:5097–5104. doi: 10.1210/jc.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 9.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 10.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilz S, Maerz W, Weihrauch G, Sargsyan K, Almer G. Adiponectin serum concentrations in men with coronary artery disease: the LUdwigshafen RIsk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2006;364:251–255. doi: 10.1016/j.cccn.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. ArchIntern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Davey Smith G, Ebrahim S. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 16.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab. 2008;93:3357–3364. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattar N, Watt P, Cherry L, Ebrahim S. High molecular weight adiponectin is not associated with incident coronary heart disease in older women: a nested prospective case-control study. J Clin Endocrinol Metab. 2008;93:1846–1849. doi: 10.1210/jc.2007-2603. [DOI] [PubMed] [Google Scholar]
- 18.Ogorodnikova AD, Wassertheil-Smoller S, Mancuso P, Sowers MR, Rajpathak SN, Allison MA, Baird AE, Rodriguez B, Wildman RP. High-molecular-weight adiponectin and incident ischemic stroke in postmenopausal women: a Women’s Health Initiative Study. Stroke. 2010;41:1376–1381. doi: 10.1161/STROKEAHA.109.576546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CD, Snijder MB, Bouter LM, Matsuzawa Y, Shimomura I, Heine RJ. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93:1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 20.Iwashima Y, Horio T, Suzuki Y, Kihara S, Rakugi H, Kangawa K, Funahashi T, Ogihara T, Kawano Y. Adiponectin and inflammatory markers in peripheral arterial occlusive disease. Atherosclerosis. 2006;188:384–390. doi: 10.1016/j.atherosclerosis.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45:40–46. doi: 10.1016/j.jvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 23.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Leng GC, Fowkes FGR. The Edinburgh Claudication Questionnaire: An improved version of the WHO/Rose questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–1109. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE Women’s Genome Health Study Working Group. Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25 000 initially healthy American women. Clin Chem. 2008;54:249–255. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 26.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 27.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–831. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 29.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 30.Heidemann C, Sun Q, van Dam RM. Total and high-molecular-weight adiponectin and resistinin relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 32.Urbonaviciene G, Frystyk J, Flyvbjerg A, Henneberg EW, Lindholt JS. Association of serum adiponectin with risk for cardiovascular events in patients with peripheral arterial disease. Atherosclerosis. 2010;210:619–624. doi: 10.1016/j.atherosclerosis.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Dieplinger B, Haltmayer M, Poelz W, Mueller T. Value of adiponectin as predictor of 5-year all-cause mortality in patients with symptomatic peripheral arterial disease: results from the Linz Peripheral Arterial Disease (LIPAD) study. Clin Chim Acta. 2009;408:87–91. doi: 10.1016/j.cca.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Komai H, Shibata R, Juri M, Matsushita K, Ouchi N, Murohara T. Plasma adiponectin as a predictive factor of survival after a bypass operation for peripheral arterial disease. J Vasc Surg. 2009;50:95–99. doi: 10.1016/j.jvs.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 35.Pernow B, Zetterquist S. Metabolic evaluation of the leg blood flow in claudicating patients with arterial obstructions at different levels. Scand J Clin Lab Invest. 1968;21:277–287. doi: 10.3109/00365516809076995. [DOI] [PubMed] [Google Scholar]
- 36.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc. 1992;24:163–70. [PubMed] [Google Scholar]
- 37.Regensteiner JG, Hargarten ME, Rutherford RB, Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993;44:1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 38.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise Training for Claudication. N Engl J Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 39.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5:55–59. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 41.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1[agr] and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 42.Merki-Feld GS, Imthurn B, Rosselli M, Spanaus K. Serum concentrations of high-molecular weight adiponectin and their association with sex steroids in premenopausal women. Metabolism. 2011;60:180–185. doi: 10.1016/j.metabol.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Xu A, Chan KW, Hoo RLC, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, Lam KS. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 44.Dieter RS, Biring T, Tomasson J, Gudjonsson T, Brown RL, Vitcenda M, Einerson J, Tanke TE, McBride PE. Classic intermittent claudication is an uncommon manifestation of lower extremity peripheral arterial disease in hospitalized patients with coronary artery disease. Angiology. 2004;55:625–628. doi: 10.1177/00033197040550i603. [DOI] [PubMed] [Google Scholar]
- 45.McGrae McDermott M, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:387–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 46.Vavra AK, Kibbe MR. Women and peripheral arterial disease. Women’s Health. 2009;5:669–683. doi: 10.2217/whe.09.60. [DOI] [PubMed] [Google Scholar]
- 47.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC, Jr, Manolio TA. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–327. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease: the San Luis Valley Diabetes Study. Circulation. 1995;91:1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 49.Hui X, Lam KSL, Vanhoutte PM, Xu A. Adiponectin and Cardiovascular Health: an Update. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01395.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]