Abstract
Myc is a crucial regulator of growth and proliferation during animal development. Many signals and transcription factors lead to changes in the expression levels of Drosophila myc, yet no clear model exists to explain the complexity of its regulation at the level of transcription. In this study we used Drosophila genetic tools to track the dmyc cis-regulatory elements. Bioinformatics analyses identified conserved sequence blocks in the noncoding regions of the dmyc gene. Investigation of lacZ reporter activity driven by upstream, downstream, and intronic sequences of the dmyc gene in embryonic, larval imaginal discs, larval brain, and adult ovaries, revealed that it is likely to be transcribed from multiple transcription initiation units including a far upstream regulatory region, a TATA box containing proximal complex and a TATA-less downstream promoter element in conjunction with an initiator within the intron 2 region. Our data provide evidence for a modular organization of dmyc regulatory sequences; these modules will most likely be required to generate the tissue-specific patterns of dmyc transcripts. The far upstream region is active in late embryogenesis, while activity of other cis elements is evident during embryogenesis, in specific larval imaginal tissues and during oogenesis. These data provide a framework for further investigation of the transcriptional regulatory mechanisms of dmyc.
Keywords: dmyc, cis-regulatory module, enhancer, promoter, downstream promoter element, Drosophila
Introduction
In the early stages of organ development, the expression patterns of genes must be tightly spatiotemporally controlled. This function requires a set of complex interactions between the cis regulatory modules of each gene and the gene’s regulatory proteins, which bind to these elements to modulate transcription. Indeed, the interactions between cis elements and their binding factors is well established as a key mechanism controlling expression of the developmental genes required for establishing the anteroposterior and dorsoventral axes in Drosophila.1,2
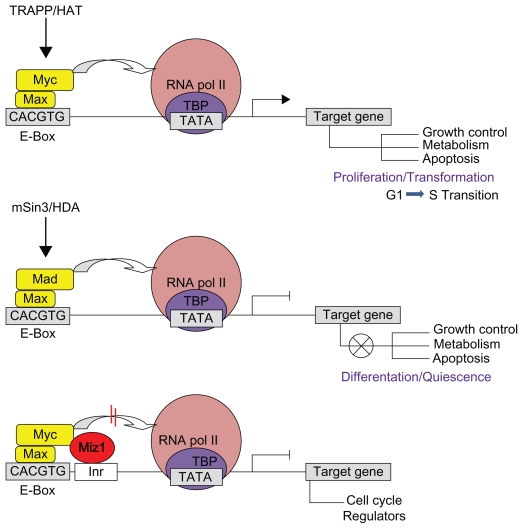
Myc is an important developmental gene requiring tight transcriptional regulation.3–6 As an evolutionarily conserved bHLHZ (basic-helix-loop-helix leucine zipper) transcription factor, Myc is a master regulator of cell growth and proliferation.7 Upon dimerization with Max, another bHLH protein, Myc binds to the E-box sequences of target genes to activate cellular growth and cell cycle progression.8–10 Conversely, heterodimers of Myc and other Myc-associated zinc finger proteins, such as Miz1, can act negatively to regulate transcription of genes responsible for cell cycle arrest11–13 (Fig. 1).
Figure 1.
Simplified schematic interaction of Myc/Myc-associated bHLH proteins at the promoters of target genes. Upon binding to E-box sequences in the promoter region of target genes, heterodimers of Myc/Max can recruit chromatin remodeling complex TRAPP/Histone acetyl-transferase (HAT) and interact with the bound basal transcription machinery at the TATA region of target genes to activate transcription. Conversely, heterodimers of Mad/Max transcription factors recruit mSin3/Histone deacetylases to counteract Myc activity and repress Myc target genes by regulating differentiation and cell cycle arrest. The binding of Myc/Max dimers can interfere with the function of transcription activator Miz-1 to inhibit the recruitment of cofactor proteins like p300 to the promoters of genes responsible for cell cycle regulation.
Myc proteins can link growth with cell cycle progression via activation of the S phase cyclins, which are required for DNA replication.6,8,14 Regulation of cell growth and division is critical for animal development because too little growth leads to small organs and small body size, whilst excessive growth can lead to tissue overgrowth and initiation of cancer. For Myc, its misexpression during development perturbs normal growth; too much Myc can facilitate malignant transformation and too little Myc leads to retarded growth.12,15–29
Functional conservation between Drosophila dMyc and human c-Myc has been demonstrated in a variety of biological activities, such as the ability of dMyc to drive the cell cycle in c-myc null fibroblasts and to transform primary mammalian cells.30,31 Conversely, c-Myc can rescue lethal mutations of dmyc.32
The evolutionarily conserved structure and function between dMyc and c-Myc7 has prompted the use of Drosophila as a model to gain insight into many aspects of mammalian Myc biology. In particular, Drosophila genetic models have demonstrated that dMyc controls cell growth and cell division by regulating its direct targets involved in protein biogenesis and metabolism.8,33–38 In response to developmental signals and mitogenic stimuli, patterned dmyc expression is required for a developing organ to reach its appropriate size and shape. For example, Wingless, Dpp, and Notch signaling pathways are key regulators of dmyc expression that are required to keep the balance between cell growth/division and differentiation.35,37 A variety of tumor suppressor factors, including Half pint and Lethal giant larvae, negatively regulate dmyc transcription to achieve cell cycle inhibition and promote differentiation.39,40 In addition, dmyc target genes can influence the transcription of dmyc in a regulatory feedback manner. For instance, the Hippo pathway transcriptional coactivator, Yorkie (yki), upregulates dmyc, and high levels of dMyc in turn repress yki.36
However, despite a growing list of pathways leading to altered dmyc transcription, we are far from unraveling the many complex interactions required at the dmyc promoter for patterning of myc transcription throughout development. Here we dissected the dmyc promoter and other potential regulatory regions and have drawn connections between certain domains and spatial and temporal patterning of dmyc expression throughout development of Drosophila. We find that its high expression in early embryos, larval discs and brain, and ovary is achieved through initiation of transcription at multiple sites. Additionally, dmyc utilizes multiple polyadenylation signals to terminate transcription and promote 3′-end formation. These findings raise the possibility that modularly structured regulatory elements of the dmyc gene play a key role in controlling both its high expression in growing and dividing cells as well as its downregulation during differentiation.
Our computational analyses of the dmyc locus reveal multiple conserved sequence blocks within the noncoding regions that show reporter activity in the tested tissues. This work will provide a basis for understanding the regulatory clusters likely to be important downstream of the developmental signals previously implicated in myc regulation.
Materials and Methods
Cloning and sequencing of the dmyc gene
The RP98-2A13 BAC clone containing the dmyc locus (obtained from the Children’s Hospital Oakland Research Institute, Oakland, CA) was triple digested with NotI/Asp718/DraIII. The resulting genomic fragment RP27, a 27-kb insert harboring the dmyc locus of 12.83-kb, was cloned into the 5′ NotI and 3′ ASP718 sites in the fly transforming vector pCaSpeR4 (Drosophila Genomic Resources Center, DGRC) to obtain pC-RP27. The genomic fragment in the BAC clone RP98–2A13 harboring the dmyc gene was sequenced at the beginning and at the end of the dmyc gene, each time towards the end of the dmyc gene with primers BAC-F and BAC-R. The 27-kb genomic fragment in the pC-RP27, including the dmyc gene, was sequenced at its distal site with the primer pcaF and at its proximal end with the primer pcaR, each time towards the dmyc gene. Sequences for all the polymerase chain reaction and sequencing primers are listed in Supplemental Table 1.
Generation of LacZ reporter strains
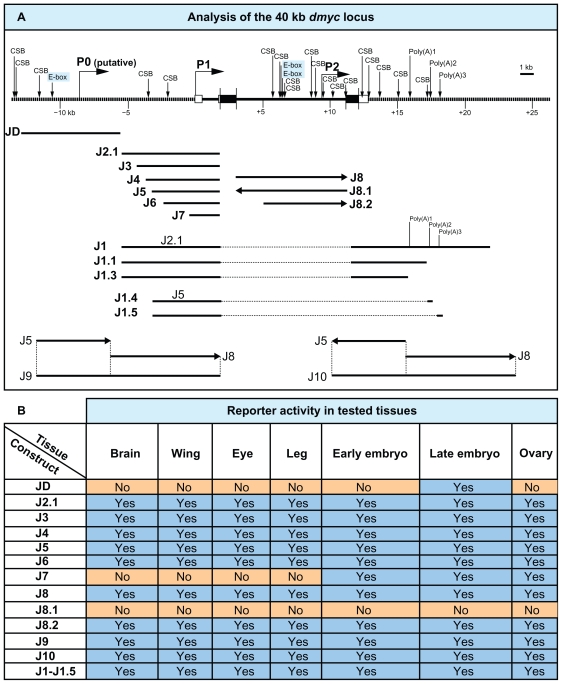
For random P-element transformation, the ready-to-use transforming vector pCaSpeR-NLSlacZ (Drosophila Genomic Resources, originally from Tummel’s laboratory) was used. The pCaSpeR4-NLSlacZ vector, free from hsp70 promoter or any other regulatory elements, contains the eye marker white gene, the reporter NLSlacZ, SV40 poly (A) tail, and the ampicillin resistance gene. Inserts for the reporter constructs, J2.1–J7, J1–J1.5, J8, J8.2, J9 (Figs. 5–8), J8.1, and J10 (Supplemental Figs. 3 and 4), are derived from the genomic sequences in pC-RP27 and were subcloned either into pCaSpeR-NLSlacZ and/or into the site-specific pattB-temp del LoxP reporter plasmid (Rainbow Transgenic Flies, original pUASTattB vector was a gift from Basler’s laboratory). The original vector pUAST-attB was engineered to remove the 5 × UAS-hsp70 and LoxP site sequences, followed by self-ligation to obtain the ready-to-use pattB-temp del LoxP transforming vector. pC-RP27 was digested with BamHI and run on 0.5% agarose gel to isolate the 7.9-kb BamHI-BamHI dmyc 5′ and 8.9-kb BamHI-BamHI dmyc large intron fragments. Each fragment was subcloned into pBS-SKII (+) (which was a generous gift from Oleg Georgiev, Walter Schaffner’s laboratory) to obtain SKII-dmyc5 and SKII-dmIn2 intermediate plasmids. For subcloning the dmyc 5′ promoter/enhancer into pCaspeR4-NLSlacZ, the 7.9-kb BamHI-BamHI fragment in SKII-dmyc5 was first mutated by polymerase chain reaction to remove the approximately 800 bp open reading frame sequence and to introduce an Acc65I restriction site at the 3′ end of the fragment. Polymerase chain reaction amplification of the dmyc promoter/enhancer, the BAC clone RP98–2A13 served as the template, the amplified fragment was 2601 bp in size, and the amplifying primers had the names dm5E2F and dm5E2R (primers 17 and 18 in Supplemental Table 1). Polymerase chain reaction conditions in the thermal cycler were one cycle of initial denaturation at 98 °C for 30 seconds; 30 cycles of denaturation at 98 °C for 10 seconds, annealing at 60 °C for 30 seconds, and extension at 72 °C for 60 seconds; and one final extension cycle at 72 °C for 5 minutes, held at 4 °C. Plasmids SKII-dmyc5 and the polymerase chain reaction fragment were each separately digested with AatII and Acc65I (AatII is located 1550 bp downstream of the transcription start and Acc65I is in the SKII-dmyc5, 60 bp downstream of the dmyc fragment in the multiple cloning site). The vector SKII-dmyc5 was digested to excise an approximately 1150 bp AatII/Acc65I fragment. The polymerase chain reaction fragment was digested to isolate the approximately 296 bp AatII/Acc65I fragment. After isolation on 1.5% agarose gel, the vector and insert were ligated overnight to create plasmid SKIIdmyc5, free from open reading frame (10,035 bp in size). The 5′ NotI-Acc65I 3′ dmyc 5′ fragment in the plasmid SKII-dmyc5, free from open reading frame, was excised and subcloned in 5′ NotI-Acc65I 3′ linearized/ dephosphorylated pCaspeR4-NLSlacZ in front of reporter lacZ to obtain the transgene J2.1. Constructs J3–J7 contain successive restriction deletions of the J2.1 insert, using either J2.1 or pC-RP27 as templates and digesting with NotI and another suitable enzyme. For the creation of J1, the 5′ BamHI-XbaI 3′ SV40 trailer in transgene J2.1 was replaced with an approximately 10.5-kb fragment from the 3′ end of dmyc in pC-RP27. The genomic fragment for JD was obtained by polymerase chain reaction amplification of the RP98–34B12 BAC clone (obtained from the Children’s Hospital Oakland Research Institute) in two steps to create products D1 and D2 (using a high fidelity polymerase chain reaction kit, Finnzymes Inc, Lafayette, CO). The two polymerase chain reaction fragments were combined by blunt ligation (CIAP, Roche Diagnostics, Indianapolis, IN) into BlueScript, then removed and exchanged for the J2.1 insert by 5′ NotI and 3′ Acc65I digestion. For the creation of J8, SKII-dmIn2 served as the template. As for the creation of J2.1, the open reading frame sequences flanking the intron 2 sequence were first removed by the polymerase chain reaction, for which plasmid pC-RP27 served as a template. The resulting 5′ NotI and 3′ ASP718 full length intron 2 sequence, free from open reading frames, was exchanged for the J2.1 insert by 5′ NotI and 3′ ASP718 restriction digestion. The insert of J5 transgene was either fused proximal to distal relative to the 5′ end of the J8 fragment to obtain the J9 reporter construct, or it was combined distal to proximal with the 5′ end of the J8 construct to generate the J10 transgene. Reporters J1–J1.5 terminate transcription by full length or truncated forms of dmyc 3′ sequences; all the other constructs contain the SV40 poly (A) signal.
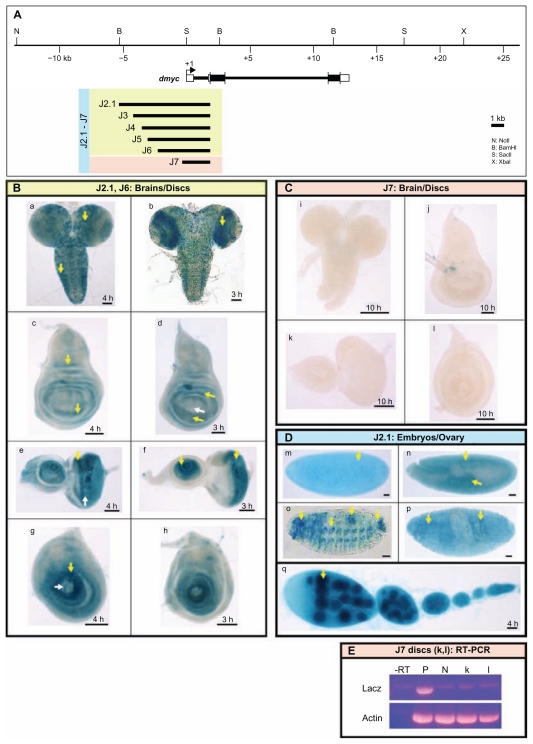
Figure 5.
dmyc 5′ regulatory region contains separable elements. (A) The 40-kb genomic locus and the location of the dmyc is shown at the top. A fragment of 7.159-kb in size (J2.1) (lacking ORF sequences) from the dmyc 5′ region and its deletion restriction fragments were tested for their ability to drive reporter activity in transgenic flies. Colors of the deletion fragments refer to their expression patterns shown in panels B, C and D. (B) J2.1 transgene (a, c, e, g) and all of its successive deletions up to 2.523-kb in the J6 construct (b, d, f, h) were able to express the reporter in a dmyc manner in the brain and discs (a, b: brain; c, d: wing discs; e, f: eye discs; g, h: leg discs). (C) Further truncation down to 1.923-kb of J7 resulted in a loss of expression in the brain and discs (i–l). (D) Shown are embryos and ovary taken from transgenic animals carrying J2.1 transgene. J2.1 retained the expression in the embryos (m–p; embryo stages: m: 2–5; n: 11–12; o, p: 13–16) and ovary (q). Except for 4D o, the smallest construct J7 recapitulated the dmyc like expression in all the other embryonic stages and in ovary. (E) RT-PCR on J7 discs (k, l shown in C), detects no lacZ transcripts beyond background level. Controls used are as follows: –RT: negative control with no transcriptase (brains from Fig. 5B, b); P: Fig. 5B, b, positive control; N: y[1] w[1118] leg discs, negative control; Actin: Drosophila actin as internal control.
Note: Yellow arrow indicates lacZ expression and white arrow indicates lack of lacZ activity. Staining time for discs and ovaries is indicated, and embryos were stained over-night. Scale bar in (a–q) indicates 50 μm.
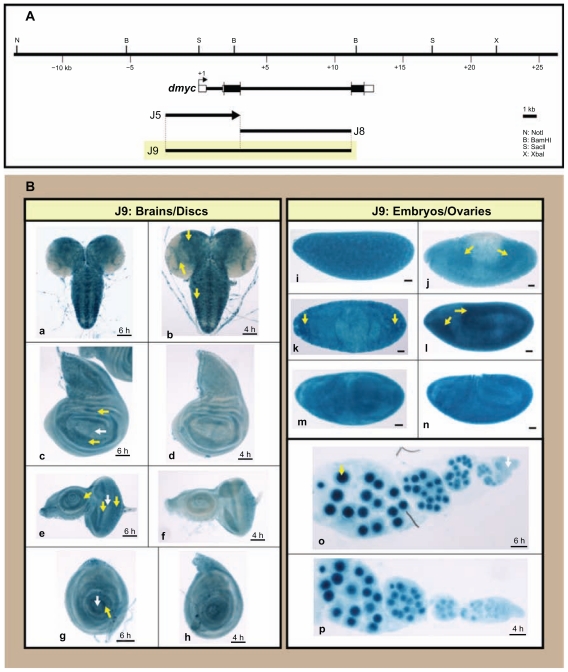
Figure 8.
J9 fusion construct (5′ J5 promoter fused to the intronic J8 promoter) has no effect on reporter expression. (A) The ~ 3.8-kb J5 promoter, the 8-kb intron 2 in J8 and their fusion product J9 are shown in respect of their relative location to the dmyc. (B) The ~ 11.1-kb chimeric restriction fragment J9 did not show any alteration in the expression of the lacZ reporter in larval tissues (a, b: brain; c, d: wing discs; e, f: eye discs; g, h: leg discs), embryos (i–n; embryo stages: i: 1–4; j–l: 9–11; m, n: 12–15) or ovaries (o, p).
Notes: Yellow arrow indicates lacZ expression and white arrow indicates lack of lacZ activity. Staining times for discs and ovaries are indicated above the scale bar, and embryos were stained over-night. Scale bar in (a–p) indicates 50 μm.
All BAC clones, intermediate plasmid constructs, engineered transforming vectors, and transgenes were sequenced by Microsynth AG, Balgach, Switzerland. Except for the standard primers (provided by Microsynth), all other oligonucleotides and sequencing primers were designed using the software tool DNASTAR Lasergene 9.1 module PrimerSelect and synthesized at Microsynth AG. Sequences for all the polymerase chain reaction and sequencing primers are listed in Supplemental Table 1.
Each reporter transgene was transfected into electrocompetent Max DH10B Escherichia coli cells (Invitrogen). Plasmid DNA for injection was isolated using a Qiagen large construct kit. Each plasmid was sequenced at the distal and proximal sites of the dmyc promoter sequences before injection. For the reporter studies based on random P-element insertion, embryos from genotype y[1] w[1118] were used and for the studies with the phage ΦC31 integrase transgenesis system we used embryos from fly lines carrying different attP attachment sites (both strains were from Bloomington Stock Center, Bloomington, IN). The attP fly stocks used in this study are listed in Supplemental Table 2.
X-Gal Staining Assays
For each construct, 4–15 independent transgenic lines (except the largest construct [J1] for which only two independent lines were obtained) were dissected and X-Gal staining was performed at standardized reaction conditions.41 The incubation temperature for different tissues was as follows: discs 29 °C, embryos 37 °C, and ovaries at room temperature. For each construct, a representative disc, brain, embryo, and ovary was chosen for presentation. dpp-lacZ fly stocks (gifted by Dragan Gligorov, Karch’s laboratory) were used as a positive control, while y[1] w[1118] and attP-fly stocks were used as negative controls.
Reverse Transcriptase Polymerase Chain Reaction Analysis
Total RNA was isolated from the sample discs (Fig. 4, c–e; Fig. 5B, b; 5C, k, l; Supplement Fig. 5g) using an aMResco phenol-free total RNA purification kit (Code N788 kit) with RNase-free DNase treatment (Promega, Basel, Switzerland) following the manufacturer’s protocol. Total RNA 1 μg was reverse transcribed into cDNA in a reaction volume of 30 μL using SuperScript™ III reverse transcriptase oligo(dT) 20 primers and reverse transcription reagents from Invitrogen (Carlsbad, CA). Semiquantitative polymerase chain reactions were performed on the resulting cDNA using a high fidelity Phusion DNA polymerase kit (Finnzymes, Bioconcept, Switzerland). Polymerase chain reaction primers were designed for the lacZ reporter gene and Drosophila actin gene using PrimerSelect from the Lasergene software suite (DNASTAR, Madison, WI). All primers were synthesized at Microsynth. Primer sequences are indicated from 5′ to 3′ in Supplemental data, Table 1. The polymerase chain reaction conditions in the thermal cycler were one cycle of initial denaturation at 98 °C for 20 seconds; 30 cycles denaturation at 98 °C for 10 seconds, annealing at 61.3 °C for 30 seconds, and extension at 72 °C for 50 seconds; one cycle final extension at 72 °C for 2 minutes, held at 4 °C. The polymerase chain reactions, 5 μL per lane, were run on 1% agarose gel for 90 minutes at a voltage of 120.
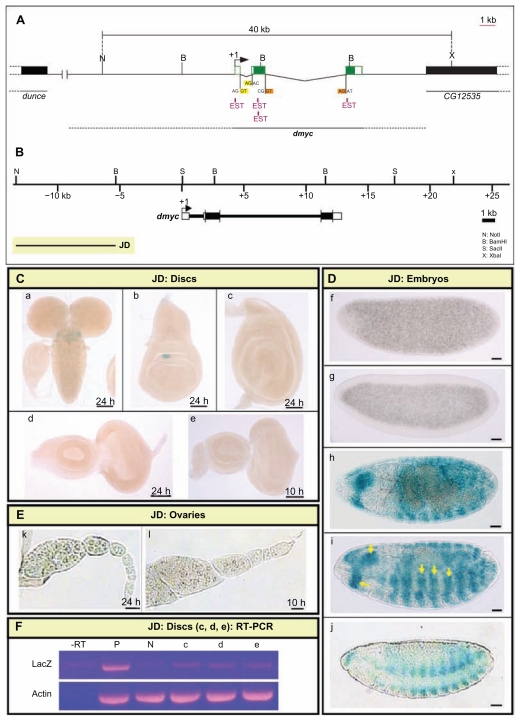
Figure 4.
Distal upstream regulatory region of dmyc is active in late embryogenesis. (A) 40-kb genomic fragment on the chromosome X of D. melanogaster between nucleotides −3253633 to +3293632, harboring the dmyc locus (nucleotides −3267216 to +3280049), is shown above. The ESTs (pink color) represent portions of the gene transcripts (not all ESTs indicated). The 5′ flanking gene dunce, also annotated as CG32498, is located ~ 33-kb upstream of the examined 40-kb sequences. The 3′ flanking gene, CG12535, is located ~ 6.5-kb to the 3′-end of the dmyc gene (nucleotide +3280049). The restriction sites at the genomic locus used in this study, splice donor sites GT (GU on mRNA) and splice acceptor sites AG (high-lighted in yellow and orange) are indicated. (B) Locations of the dmyc and the transgene JD (8-kb in size) relative to the 40-kb genomic locus are shown. (C) Activity of the JD transgene in the brain and imaginal tissues. The fragment was neither active in the brain (a) nor in imaginal discs (b: wing, c: leg, and d, e: eye discs). (D) JD fragment was not active in early embryos (f, g), however, the fragment recapitulated expression of dmyc in late embryogenesis (h–j: stage 13–16). (E) The staining in ovaries (k, l) showed no activity for JD fragment. (F) RT-PCR on JD discs (c, d, e shown in C), detects no lacZ transcripts beyond background level. Controls used are as follows: –RT: negative control with no transcriptase (brains from Fig. 5B, b); P: Fig. 5B, b, positive control; N: y[1] w[1118] leg discs, negative control; Actin: Drosophila actin as internal control.
Note: Yellow arrow indicates lacZ expression. Staining time for discs and ovaries were either 10 or 24 hours, as indicated (C, e one representative for 10 hours discs). The embryos were stained over-night. Scale bar in (a–j) indicates 50 μm.
Bioinformatics Analyses of Regulatory Elements
For defining the genomic organization of the dmyc gene in twelve sequenced Drosophila species, the bio-informatics tool DNASTAR Lasergene 9.1 MegAlign module was used to edit the sequences taken from Fly-Base. We used the Lasergene 9.1 MegAlign module with the settings “Multiple Alignment, ClustalW”.42 The dmyc 5′ end was searched with the DNASTAR Lasergene 9.1 GeneQuest module for prediction of TATA box-Inr elements, the intron 2 region for the existence of an Inr-downstream core promoter element and the 3′ end was searched for the prediction of polyadenylation sequence motifs. The phylogenetic footprinting tools, EvoPrinter and cis-Decoder43,44 were used to detect E-boxes, bHLH binding sites, and multiple conserved sequence blocks in the dmyc region common to most Drosophila species. The neural network genetic algorithm PROMOTER 2.0 was used to predict promoter regions using CCAAT or bHLH recognition motifs.
Results
Developmental expression patterns of dmyc
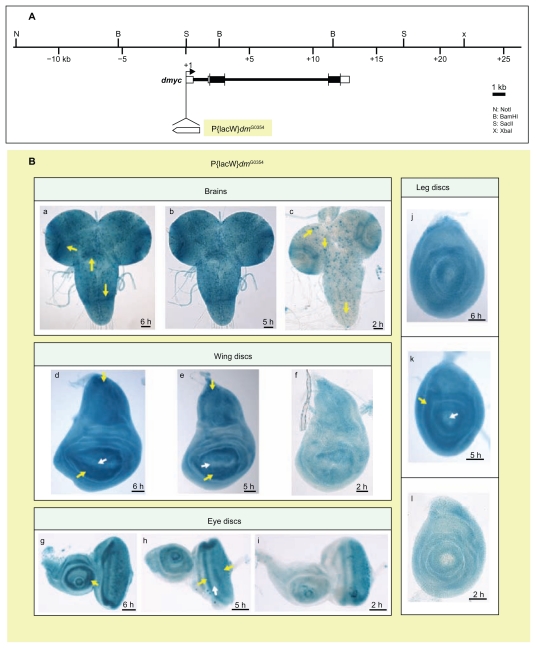
dmyc is synthesized in a dynamic spatial and temporal pattern during development of Drosophila, as determined by in situ hybridization and Northern blot analysis.7,35 We first sought to compare the pattern of dmyc promoter activity from our different dmyc deletion constructs generated from upstream, intronic, and downstream regions, with both the endogenous pattern of dmyc transcription previously reported and with the pattern resulting from the dmyc-lacZ enhancer trap line w67c23 P{lacW}dmG0354/FM7c (Fig. 2A). The enhancer trap line w67c23 P{lacW}dmG0354/FM7c has been established to give patterns of endogenous dmyc expression (Figs. 2B and 3) and is known to be responsive to dmyc regulators.6,39,45,46 Beta-gal assays for this dmyc-lacZ enhancer trap line revealed ubiquitous dmyc promoter activity in the larval brain, with an increased level of expression in the distal and middle parts of the lobes and within dividing neuroblasts in the middle parts of the ventral ganglion (Fig. 2B, a, b, yellow arrows). Additionally, lacZ activity is restricted to a limited number of cells distributed in the two proximal halves of the hemispheres and along the ventral ganglion (Fig. 2B, c, yellow arrows). The enhancer trap line detected lacZ activity around the wing pouch and in the notum region (Fig. 2B, d–f), anterior and posterior to the morphogenetic furrow in the eye disc and around the center of the antennal disc (Fig. 2B, g–i), and in the center of the leg disc (Fig. 2B, j–l). dMyc antibody staining of the tissues taken from the above enhancer trap line results in the same pattern as observed for the endogenous reporter (L Quinn, personal communication).
Figure 2.
The dmyc-lacZ enhancer trap line w67c23 P{lacW}dmG0354/FM7c reflects the endogenous expression of dmyc mRNA in larval brain and imaginal tissues. (A) Insertion site of the P-element containing reporter lacZ at the dmyc locus is shown. Breakpoints of the insertion are as follows: 3D2, X:3267141..3267197, which maps to the region 213 nucleotides upstream of dmyc exon 1 start site. (Bloomington Stock number and donor of the Stock: 11981, Ulrich Schaefer and Herbert Jackle). (B) Third instar larval brain and discs were assayed with β-gal reaction for lacZ expression. In all the tested imaginal discs and larval brain, lacZ patterning reflects the pattern reported for dmyc endogenous mRNA distribution (a–c: brain, d–f: wing discs, g–i: eye discs, j–l: leg discs). Details on dmyc patterning are explained in Discussion section.
Note: Yellow arrow indicates lacZ expression and white arrow indicates lack of lacZ activity. Staining time is indicated above the scale bar. Scale bar in (a–l) indicates 50 μm.
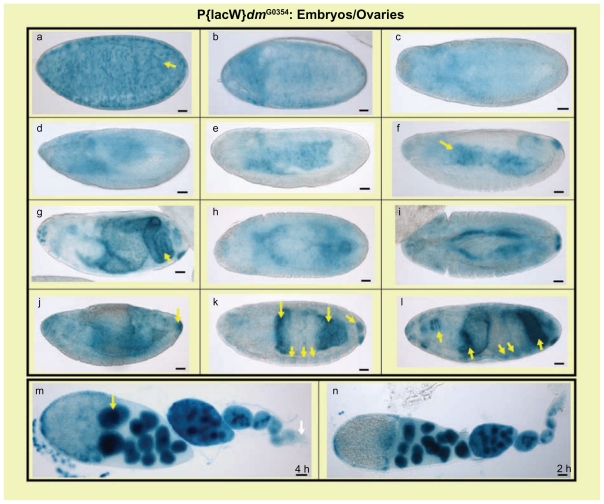
Figure 3.
The dmyc-lacZ enhancer trap line w67c23 P{lacW}dmG0354/FM7c produces the endogenous dmyc mRNA pattern during embryogenesis and in adult ovaries. In the developing embryonic tissues the dmyc-lacZ enhancer trap expresses lacZ with high similarities to dmyc mRNA distribution, predominantly in midgut, hindgut, pharynx, anal pad and partly in mesodermal tissues. a–l: embryos (embryo stages are as follows: a, b, stage 1–4, c, d, stage 6–8, e, f, stage 9–12, g–l stage 13–16). The staining in the ovaries (m, n) reflects dmyc mRNA localization, which has been reported in nurse cells, but is weakly expressed at the tip of germarium.
Note: Yellow arrow indicates lacZ expression and white arrow indicates lack of lacZ activity. Staining time for ovaries is indicated above the scale bar, embryo staining took place over-night. Scale bar in (a–n) indicates 50 μm.
Multiple conserved cis-regulatory sequences within the 40 kb dmyc locus
The occurrence of conserved regions and repetitive sequence motifs in noncoding DNA has been of great value for the identification and characterization of cis-regulatory elements. The phylogenetic footprinting tools EvoPrinter43 and cis-Decoder44 can be used to identify conserved sequence blocks in developmental genes. EvoPrinter facilitates the multialignment and rapid identification of evolutionarily conserved sequence blocks as they exist in the species of interest. The cis-Decoder then characterizes repeat motifs within the conserved sequence blocks and detects conserved elements among functionally related enhancers. It is important to mention that EvoPrinter and cis-Decoder do not detect polyadenylation signals or core promoter elements, such as TATA boxes.
To identify cis-regulatory regions for dmyc, a 40-kb fragment on the X chromosome harboring the dmyc gene of Drosophila melanogaster (Fig. 4A) was used as the reference DNA to align orthologous sequences from 12 sequenced Drosophilids to test for the existence of multispecies-conserved sequence blocks in the noncoding regions. We identified several putative enhancer regions with conserved sequences, including several conserved E-boxes, which are preferred Myc binding sites47–49 of both the CACGTG and CACTTG type (Supplemental Figs. 1 and 2). Like the c-myc promoter,50,51 dmyc has previously been shown to undergo autoregulation.52 The identified E-box sequences may represent the basis for the previously demonstrated ability of dMyc to undergo autoregulation.53
Comparison between the sequences from intron 2 identified multiple clusters of conserved sequence blocks upstream of the predicted intronic promoter (Supplemental Fig. 2). In addition to two conserved E-box sequences (CACGTG and CACTTG), we identified a repeat sequence element (ATGTTGCCA) where the core TGTTGC is repeated three times (Supplemental Fig. 2). In the large, approximately 10-kb 3′-UTR region, we identified clusters of conserved sequence blocks, but no E-boxes as was the case for the intronic region (Supplemental Fig. 2).
Far upstream region determines activity of dmyc in late embryogenesis
As noted above, we have identified a diverse array of potential promoter regions and enhancer elements, which represent sequences that are likely to be required to achieve the patterning of dmyc transcription throughout development. To determine which of these domains participate in the regulation of dmyc expression, we generated overlapping deletion constructs spanning the dmyc gene and examined the activity of each fragment throughout development. The creation of overlapping deletions was based on the sequence conservation and the existence of suitable exonuclease recognition sites in the region. We decided to first test the 8-kb far upstream fragment (JD, Fig. 4B) for its ability to self-initiate transcription, as reported for the far upstream P0 promoter in human c-myc.51,54,55
No activity was detected in the tissues known to normally express dmyc, including larval brain and imaginal tissues (Fig. 4C, a–e), the earlier embryonic stages (Fig. 4D, f, g), and adult ovaries (Fig. 4E, k, l). Reverse transcription polymerase chain reaction on the discs shown in Figure 4C (c–e), did not detect any lacZ transcripts beyond background level in these tissues that were negative for lacZ staining (Fig. 4F, lanes –RT-e). Activity of the JD-lacZ reporter was confined to presumptive mesodermal tissues in body segments and the head regions of embryos during late development (Fig. 4D, h–j, arrow heads). Previous studies have reported dmyc activity in putative neuromuscular tissues by in situ hybridization experiments on dmyc endogenous mRNA. However, the enhancer trap line used as the control in this study shows only partial expression in these tissues (Fig. 3, k, l). This result suggests that the regulatory sequences in this far upstream region are only sufficient for activation of dmyc expression during late embryogenesis. Due to its far upstream position, we have dubbed the remote cis-regulatory elements in this region “P0 (putative)”, analogous to the human c-myc P0 promoter.
Proximal upstream region controls dmyc expression in larval and adult female tissues
The two main promoters required for activation of mammalian c-myc transcription, P1 and P2, are located in the 5′-UTR, where the majority of transcripts are initiated.51,56–58 Thus, our first efforts were directed toward identifying regulatory elements in the dmyc 5′-UTR, capable of activating endogenous patterns of dmyc gene expression during development. Analysis of the 7.2-kb region between the dmyc translation unit and the 3′ end of the JD transgene (Fig. 5A) revealed that when most of the conserved sequences were removed by successive distal to proximal deletions of the J2.1 fragment, a loss of reporter expression in both the brain and imaginal discs was observed (Fig. 5B and C). Interestingly, by staining embryonic and ovarian tissues taken from the largest construct (J2.1) and the smallest deletion (J7), we identified a proximal promoter region within the 5′-UTR, which recapitulated almost all aspects of early embryonic and ovarian dmyc expression (Fig. 5D). This observation suggests that the 2-kb 5′-UTR and a further 100 bp upstream of the 5′-UTR region contain regulatory elements important for embryogenesis and oogenesis, but insufficient for patterning in the larval brain or imaginal tissues. It further suggests that the sequences 5.1-kb upstream of 5′-UTR contain tissue-specific enhancer regions responsible for the endogenous pattern of dmyc expression in the brain and larval imaginal tissues (Fig. 5B).
Full length intron 2 with downstream core promoter element activated in dmyc pattern
In most protein-coding genes of Drosophila, there is a downstream core promoter element that functions cooperatively with an initiator to facilitate the binding of transcription factors in the absence of a TATA box.59 The high throughput expression data for the D. melanogaster transcriptome, generated by tilling arrays, has shown that most of introns are transcriptionally active within the early hours of development at specific time points.60 Scanning of the intron 2 region with cis-Decoder initially revealed clusters of multiple conserved sequence blocks common to most Drosophila species. A search using the Lasergene GeneQuest module identified a downstream promoter region with no TATA box, comparable with the Drosophila consensus sequence (Supplemental Fig. 2).
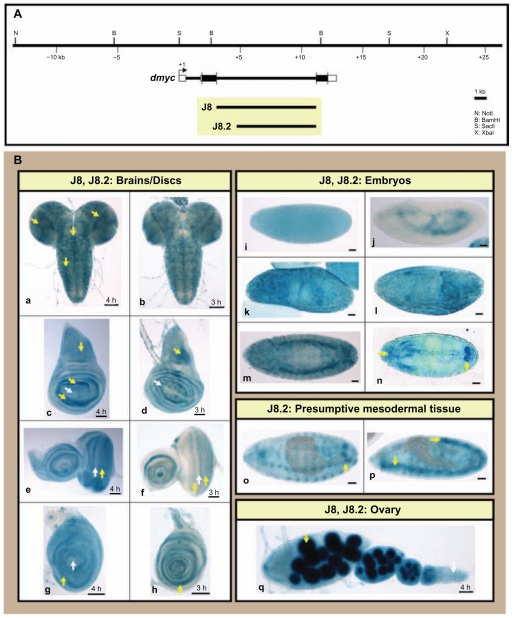
To analyze the intragenic region of dmyc, we generated transgenic animals carrying the 8-kb intron 2 full length fragment (J8), or the subfragment (J8.2) lacking the 2-kb distal sequences (Fig. 6A). The J8 and the J8.2 transgenes both showed dmyc-like expression in imaginal discs and brain tissues (Fig. 6B, a–h). During embryogenesis, both transgenes are active in the early and later stages (Fig. 6B, i–n). However, only the J8.2 transgenic animals, which lack the 2-kb upstream sequences, showed strong patterning of dmyc in mesodermal tissues (Fig. 6B, o, p). This suggests that the upstream sequences might contain elements that influence dmyc expression in presumptive mesodermal tissues. Unlike the difference observed in embryonic activity, the expression of LacZ in ovarian tissues remains unchanged for both transgenes (Fig. 6B, q). The reversed full length intron 2 fragment fused with its 5′ end to the 5′ end of the reporter gene (subfragment J8.1 in Supplemental Fig. 3A), causes the abolition of reporter activity in virtually all tested tissues (Supplemental Fig. 3B, a–g). This observation suggests that the core promoter element only functions unidirectionally. The experimental identification of binding sites for transcriptional regulators in this region remains unresolved.
Figure 6.
Analysis of the dmyc intron 2 identifies a downstream core promoter element, DPE. (A) The 8-kb intron 2 full length sequence in the J8 transgene and its truncation, J8.2 (6.1-kb) and their relative location with respect to the dmyc locus and the genomic organization are shown. (B) The full fragment J8 and its derived sub-fragment were assayed in different tissues during early developmental stages. Both transgenes (J8: a, c, e, g; J8.2: b, d, f, h) were capable of reflecting dmyc expression in the brain (a, b) and discs (c, d: wing; e, f: eye; g, h: leg). The transgenes J8 and J8.2 both express lacZ in the early and late embryogenesis (i–n) (embryo stages: i, j: 2–6; k–n: 9–13). However, only the shorter transgene J8.2 shows expression in mesodermal tissues (o, p; embryo stages: 12–15). In the ovary (q) both transgenes are active.
Note: Yellow arrow indicates lacZ expression and white arrow indicates lack of lacZ activity. Staining time for discs and ovary is indicated, and embryos were stained over-night. Scale bar in (a–q) indicates 50 μm.
Analysis of 3′ dmyc sequences reveals multiple poly (A) sites
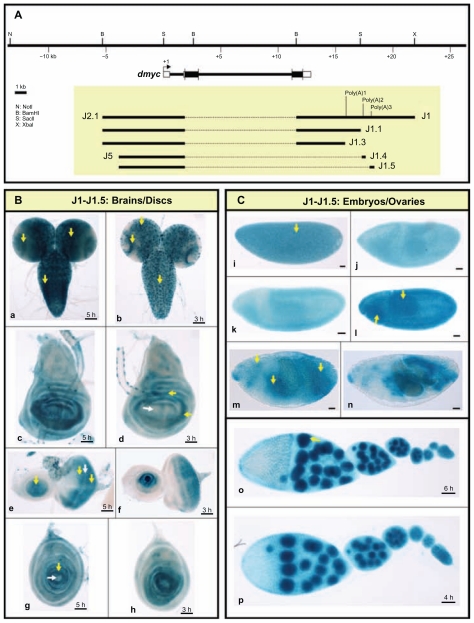
In addition to understanding transcriptional activation of dmyc, we are interested in deciphering the mechanism of transcript termination. In animal cells, there is a hexanucleotide-sequence of AAUAAA (occasionally AUUAAA) that is located 10–35 nucleotides upstream of the polyadenylation signal.61,62 In addition, there is a U/GU-rich region (cleavage stimulation factor binding site) 14–70 nucleotides downstream of the polyadenylation signal.63 We searched for polyadenylation signals in the dmyc 3′ end comparable with the consensus sequence using the Lasergene GeneQuest module with a threshold of 100% (no errors allowed). The threshold for the T-rich region was set at 80%. Analysis with the AATAAA sequence identified two potential polyadenylation signals, highly similar to the polyadenylation consensus sequence found in animal cells.61 The first A at the 5′ end of the polyadenylation signal, poly (A)1, corresponds to nucleotide +3761 downstream of the 3′-UTR and the first A at the 5′ end of poly (A)3 corresponds to nucleotide +5952 relative to the 3′-UTR. A search for ATTAAA sequences on the DNA sense strand detected poly (A)2, with a high degree of homology to the consensus sequence. The first A at the 5′ end corresponds to nucleotide +5245 downstream of the 3′-UTR. In order from proximal to distal relative to the transcription start, we named them poly (A)1, poly (A)2, and poly (A)3 (Fig. 7A). To test the detected polyadenylation signals separately, we performed successive deletions from distal towards proximal on the 10.3-kb full length construct (J1, Fig. 7A). The activity of each transgene was invariably high in all the examined tissues (Fig. 7B and C), suggesting that more than the three predicted polyadenylation signals are involved in the formation of the 3′ end of the dmyc gene. This finding is consistent with the observation that most developmentally active genes use more than one poly (A) signal to terminate transcription.64 Direct determination of the efficiency of utilization of polyadenylation signals on the 3′ end of dmyc transcripts remains to be resolved.
Figure 7.
Analysis of the dmyc 3′ region reveals multiple functional polyadenylation sites. (A) The dmyc gene and its location in the genomic region, the 10.3-kb full length construct (J1) and different deletions are shown. In the J1 fragment three potential Poly (A) signals with a high degree of homology to the consensus polyadenylation signal were detected computationally. The transgenes J1, J1.1 and J1.3 are under the control of the full length 5′-promoter (see also Fig. 5 part A). The constructs J1.4 and J1.5 contain the promoter of J5 in Figure 5. J1.1 only contain Poly (A)1, J1.3 does not include the predicted Poly (A) sites, J1.4 contains Poly (A)2 and J1.5 contains Poly (A)3. (B) All the constructs J1-J1.5 are capable of mediating regulated expression of the reporter in a manner similar to dmyc expression in the third instar larval brain (a, b) and discs (c, d: wing; e, f: eye; g, h: leg). (C) All the transgenes are active during different stages of embryogenesis (i–n; embryo stages: i: 2–5; j–l: 9–11; m, n: 12–15) and in ovaries (o, p).
Notes: Yellow arrow indicates lacZ expression and white arrow indicates lack of lacZ activity. Staining times for discs and ovaries are indicated above the scale bar, and embryos were stained over-night. Scale bar in (a–p) indicates 50 μm.
Effect of Upstream Promoter Region on Downstream Core Promoter Element
It has been shown that parameters such as stoichiometry, affinity, spacing, and arrangement of binding sites within the cis-regulatory regions influence the output of the transcriptional regulatory sequences.65 For example, depending on the total number of binding sites for a certain regulator in the same promoter region, stage-specific and tissue-specific expression patterns can be achieved for the same gene during development.65
Here we have shown that truncations of conserved sequence blocks and putative regulatory protein binding sites at the dmyc proximal 5′ promoter resulted in a loss of expression in larval brain and different imaginal discs (Fig. 5C). Conversely, removal of upstream sequences in the intron 2 region intensified expression in embryonic mesodermal tissues (Fig. 6B, o, p).
We tested the effect of the combination of these two regulatory regions on reporter activity. Fusion of the fully functional 5′ deletion (J5) inframe to the full length intron 2 transgene (J8) to generate the fusion promoter (J9, Fig. 8A), had no effect on the activity of the reporter in the tested tissues (Fig. 8B). However, the reversed (J5) transgene, fused with its 5′ end to the 5′ end of the (J8) fragment (Supplemental Fig. 4A), caused partial attenuation of dmyc embryonic activity (Supplemental Fig. 4B). The mode of action of enhancer elements responsible for embryonic development was dependent on the spacing and arrangement of binding sites, with the activity in imaginal tissues and ovaries remaining unaffected.
Discussion
The dynamic expression of dmyc is initiated from multiple transcription start sites, as summarized in Figures 9 and 10. Tight regulation of dMyc is crucial for cell growth and division during the early phases of development and cell fate specification. In third instar larvae, dmyc mRNA is detected around the wing pouch and in the notum.66 dmyc activity is absent from the cell cycle-arrested nonproliferating cells that surround the dorsoventral boundary in the wing pouch.66 In the eye disc, dmyc is synthesized in the proliferating cells posterior and anterior to the morphogenetic furrow, but not in the cell cycle-delayed cells of the morphogenetic furrow.7,35 In antennal discs, dmyc mRNA is mainly detected in the central ring of proliferating cells.7 In the leg disc, endogenous dmyc is expressed around the middle of the disc, with the central cells lacking dmyc activity.35 Maternal transcripts are detected in the nurse cell cytoplasm of the adult ovary, and are subsequently dumped into the oocyte, and can be detected in early embryos.7 Zygotically derived transcripts can be detected during preblastoderm, with the highest level of dmyc mRNA in the anterior and posterior termini. At later stages, dmyc mRNA can be detected in the presumptive mesoderm along the ventral midline. During germ band extension, dmyc expression intensifies in the mesoderm and endoreplicating cells of the midgut, where expression continues until mid-embryogenesis.7 The majority of developmental genes achieve patterning via large noncoding regulatory regions containing numerous cis-regulatory elements and other diverse regulatory sequences.67,68 Thus, we set out to determine whether the pattern of dmyc expression might be similarly regulated. First, using computational comparative searches of the 40-kb region spanning the dmyc gene, we detected multiple conserved sequence blocks. Our subsequent analysis of the dmy-clacZ reporter constructs, containing all of the conserved sequence blocks, suggested that they were transcriptionally active and generated similar patterns of reporter activity as that described for the endogenous dmyc-lacZ enhancer trap.6,39,45,46
Figure 9.
Summary of the computational analyses of the dmyc locus and reporter activity studies in the tested tissues. (A) Top: The 40-kb dmyc locus was scanned with EvoPrinter and cis-Decoder to detect multiple conserved sequence blocks (CSB) and E-boxes within the noncoding DNA sequences. The neural network genetic algorithm PROMOTER 2.0 predicted three potential promoter regions, P0 (putative), P1 and P2. The scanning with Lasergene module GeneQuest detected three potential polyadenylation signals (Poly(A)1, Poly(A)2, Poly(A)3). Bottom: Deletion constructs derived from the 40-kb locus and their names are given. (B) The table shows the summary of the activity of lacZ reporter constructs in the tested tissues (For details see Fig. 4–Fig. 8).
Figure 10.
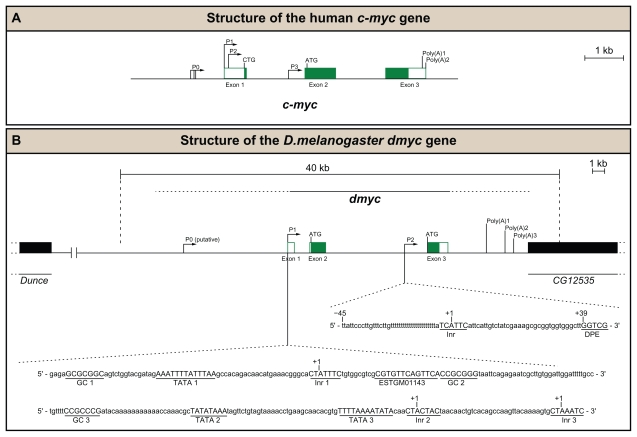
During development, the activity of the dmyc regulatory region is integrated by multiple transcription start sites. (A) Human c-myc gene contains three exons (white boxes indicate noncoding regions, the green boxes represent coding regions), four promoters (P0, P1, P2, P3), two major translation start sites (CTG, ATG) and two polyadenylation signals (Poly(A)1, Poly(A)2). (B) Drosophila myc gene contains three exons (white boxes noncoding, the green boxes coding regions), two main promoters (P1, P2), one putative promoter (P0), two major translation start sites (ATG, ATG) and three potential polyadenylation signals (Poly(A)1, Poly(A)2, Poly(A)3). The specified regulatory elements are plotted on the basis of our experimental data (see Results section) and the computational analyses of the dmyc locus. The sequences of the major elements for the promoters P1 and P2, shown at the bottom, were identified by search with Lasergene GeneQuest module.
Dissection of the dmyc promoter using constructs spanning defined domains of the dmyc gene revealed the regions likely required for tissue-specific patterning of dmyc transcription. For instance, the lacZ reporter for the far upstream 8-kb fragment produced an expression pattern restricted to late embryogenesis in body segments and in presumptive neuromuscular tissues (Fig. 4D). In silico analysis revealed possible regulatory motifs in this region, including core promoter elements and conserved sequence blocks. Regulation by these cis elements may be required during embryogenesis, where dMyc is required to specify neuronal fate and facilitate neuroblast proliferation69 and in control of mesodermal fate determination.70 In light of this finding, further analysis of dmyc transcriptional regulation in this region in response to developmental signals will be of great interest.
Analysis of the 5′ dmyc-lacZ deletion construct, containing intron 1, the 5′-UTR, and 100 bp upstream of the predicted transcription start site (J7), revealed that this minimal region was sufficient to give reporter activity in a dmyc-like pattern in both ovarian nurse cells and in the embryo, but not in larval tissues (Fig. 5C and D). Therefore, we inspected the region extending from nucleotide 100 upstream of the 5′-UTR to nucleotide +187 for initiator consensus sequences. In most mammalian protein-coding genes, there is a TATA box located 25–30 bp upstream of the transcription start site, an initiator element (Inr) overlapping the start site,71–74 and/or a GC-box (SP1 binding site) 60–100 nucleotides upstream of the transcription start site.75–77 Experiments with vertebrate cell lines and Drosophila embryonic extracts have revealed strict conservation of the Inr consensus sequence, Py Py A+1 N T/A Py Py among vertebrates and invertebrates.73,78
Analysis of J7 (the region 1914 bp upstream of the predicted translation start site) and the expressed sequence tag (ESTGM01143; beginning at 1812 bp upstream of the translation start) revealed that the 102 bp sequence between the 5′ end of the expressed sequence tag and the 5′ end of the J7 genomic sequence contains a perfect Inr consensus sequence, a TATA box and a GC box (Fig. 10B). We named it the GC box/TATA box/Inr promoter 1 (P1, Fig. 10B). The TATA box is located 39 bp upstream of A+1 in the initiator element. Previous reports have shown that occurrence of a TATA box 25–30 bp upstream of the Inr in the same core promoter leads to cooperation between the two elements to enhance promoter strength.73 Although the distance of 39 bp in the dmyc promoter is on the edge of this optimum, cooperation between the TATA box and the Inr element has been shown to extend up to 90 bp in yeast promoters (W Schaffner, personal communication). Together, this suggests that the predicted regulatory elements (TATA box, Inr, and GC box) correspond to the cis-regulatory elements that may be responsible for correct developmental dmyc expression in larval tissue, the embryo, and the ovary. In addition to the TATA box, Inr, and GC box, two putative TATA boxes (TATA2, TATA3), two GC boxes (GC2, GC3), and two Inr elements (Inr2, Inr3), as shown in Figure 10B, were identified 180 bp downstream of the expressed sequence tag start site. The putative Inr2 element shows one deviation from the Inr consensus sequence at position +4, but the critical positions for Inr activity are +1 and +3, and single bp substitutions at the −2, −1, +4, and +5 positions can still produce an active Inr,79 suggesting the second Inr element might be functional. The putative Inr3 element shows no deviation from the Inr consensus sequence, suggesting that Inr3 might be as functional as Inr1. TATA box 2 is located 57 bp upstream of A+1 in the Inr2 element and TATA box 3 is located 55 bp upstream of A+1 in the Inr 3 element, both distances less than 90 bp in yeast promoters.
Most developmentally expressed genes contain separable cis regulatory units, which allow patterned expression for tissue-specific roles.68,80 Indeed, previous work has suggested that both Drosophila36 and mammalian51 myc transcription is also regulated via intronic promoter sequences. In support of these findings, we demonstrated that the J8 transgene, which contains just the intron 2 sequence of the dmyc gene, results in lacZ reporter activity in all tissues examined (Fig. 6B). Thus we searched for Inr and downstream promoter elements, a sequence motif common to all Drosophila downstream promoters in this region. Most protein-coding genes of Drosophila contain a downstream core promoter element that functions cooperatively with an initiator to facilitate the binding of transcription factors in the absence of a TATA box.59,81 A search for consensus Drosophila Inr (T-C-A+1-G/T-T-T/C) and downstream core promoter elements (G-A/T-C-G) using DNASTAR Lasergene 9.1, GeneQuest module, revealed the presence of downstream promoter sequence motifs comparable with the Drosophila consensus Inr/downstream core promoter element.59 Sequence motifs were typed in GeneQuest “type in pattern function” and searched for with a threshold of 100% (no errors allowed). The Inr and downstream promoter element motifs at the 3′ end of the intron 2 DNA sequence (Fig. 10B) met all the strict criteria for such elements, in that the Inr sequence motif (T-C-A+1-T-T-C) does not deviate from the consensus, the downstream core promoter element (G-G-T-C-G) is identical to the core consensus, the spacing between the downstream core promoter element and the Inr (34 nucleotides) is appropriate, and a G nucleotide is correctly positioned between the Inr and the downstream core promoter element (Fig. 10B).
Post-transcriptional 3′ end formation or polyadenylation of the mRNA precursor is a crucial step in mRNA maturation, in which most eukaryotic mRNAs acquire a poly (A) tail at their 3′ ends to promote transcription termination,62 transport of the mature mRNA from the nucleus,82 and to enhance the translation and stability of mRNA.83 Analysis of the entire dmyc 3′-UTR for polyadenylation signals and polyadenylation sites revealed three potential consensus sequences, i.e., poly (A)1, poly (A)2, and poly (A)3, which are all capable of terminating transgene transcription. In addition, the 4.362-kb DNA sequence upstream of poly (A)1 at the dmyc 3′ end leads to reporter activity in the pattern predicted for dmyc expression. Therefore, the dmyc gene would be predicted to produce different transcripts with shorter and longer lengths, consistent with the previous analysis of dmyc mRNA in which genomic probes derived from this region revealed three alternative transcripts.7 Comparative analysis of the 3′ end of dmyc across 12 sequenced Drosophila species revealed multiple conserved sequence blocks in this region. Given that c-myc is regulated at the level of mRNA stability84 via conserved sequences in its 3′-UTR,85 it will be of interest in the future to determine whether the stability of dmyc transcripts depends upon the presence of regulatory domains in its 3′-UTR. The conserved sequence blocks may contain potential microRNA target sites to serve for posttranscriptional modifications, as is the case for the majority of developmental control genes.86,87 Indeed, dMyc has an active role in microRNA biology,87,88 although regulation of c-myc by microRNAs has been reported,89–92 the evidence for direct regulation of dmyc requires investigation. This work provides a starting point for investigating the putative microRNA binding sites and the mechanisms for the interactions between these motifs and their targets.
Because c-Myc is a potent mitogen, the level of c-myc transcription must be tightly regulated. Myc transcription responds to developmental signaling molecules,33,93,94 which are likely to modulate the complement of a wide variety of transcription factors at the myc promoter.95,96 The evidence presented in this work reinforces the idea that dmyc represents a tightly and dynamically regulated gene. Further genetic studies combined with genomic approaches will be required to identify the molecular mechanism controlling dmyc transcription via the regulatory elements identified here.
Supplementary Materials
Table S1.
Analytical primer pairs.
Table S2.
List of the Fly Stocks used in the study.
| No. | Bloomington stock # | Name | Genotype with FlyBase links |
|---|---|---|---|
| 1 | 6598 | y w | y1 w1118 |
| 2 | 23648 | attp-86F | P{hsp70-flp}1, y1 w*; M{3xP3-RFP.attP}ZH-86Fb; M{vas-int.B}ZH-102D |
| 3 | 24480 | attp-2A | y1 M{3xP3-RFP.attP}ZH-2A w*; M{vas-int.Dm}ZH-102D |
| 4 | 24482 | attp-51C | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP′}ZH-51C |
| 5 | 24485 | attp-68E | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP′}ZH-68E |
| 6 | 24871 | attp-VK00033 | y1 M{vas-int.Dm}ZH-2A w*; PBac{y+-attP-3B}VK00033 |
| 7 | 24872 | attp-VK00037 | y1 M{vas-int.Dm}ZH-2A w*; PBac{y+-attP-3B}hafVK00037 |
| 8 | 2376 | Oregon | Oregon-R-P2 |
| 9 | 2475 | double balancer | w*; T(2;3)apXa, apXa/CyO; TM3, Sb1 |
| 10 | 11108 | blue balancer | Cyo, P{lArB}A66.2F2/b1 Adh* cn* l(2)**; ry506 |
| 11 | 8412 | dpp-lacZ | y1 w1118; P{dpp-lacZ.Exel.2}3 |
Notes: The attp-lines, 86F, 2A, VK00033 and VK000037 show strong activity of the reporter lacZ. The expression of transgene in the attp-strains 51C and 68E is weak. For random P-element mediated transgenesis embryos were taken from the y w (6598) flies. Oregon flies were used for dmyc in situ hybridization in different imaginal tissues. The fly stock “blue balancer” that expresses lacZ in embryos and ovaries, was used as a source for positive control in the embryos and ovaries staining. The dpp-lacZ line was used as positive control for the imaginal discs staining.
EvoDifference analysis of the distal 8-kb upper strand of the 40-kb D. melanogaster dmyc locus detects conserved sequence blocks. Each block of uppercase bases represents a cluster of conserved sequences. Black capital letter bases are conserved in all or in all but one species including D. sechellia, D. yakuba, D. erecta, D. ananassae, D. pseudoobscura, D. willistoni or D. virilis. There is one E-Box, conserved in all species except D. virilis (white text with black highlight). The neural network genetic algorithm PROMOTER 2.0 predicts three possible promoter regions using ccaat or bHLH recognition motifs (lower case black letters). The yellow highlighted sequence includes a highly likely promoter region (dubbed as P0 putative). Sequence orientation is 5′ → 3′.
EvoDifference analysis of the 40-kb top strand D. melanogaster dmyc locus. The 12.834-kb dmyc gene resides within the 40-kb fragment. Each block of uppercase bases represents a cluster of conserved sequences. Uppercase black nucleotides represent bases in the D. melanogaster dm EvoP 40-kb reference sequence that are conserved in all or all but one of the other 5 orthologous DNAs, D. sechellia, D. yakuba, D. erecta, D. willistoni or D. virilis. The D. melanogaster dmyc transcribed sequence is annotated according to FlyBase as follows: 5′-UTR (light grey), protein-coding sequence (dark grey) and 3′-UTR (light grey). The conserved bHLH binding sites and E-boxes are in white text with black highlight, as is the repeat motif ATGTTGCCA and the nearby sub-repeat TGTTGC. PolyA1, 2, 3 in the 3′ UTR (yellow highlights), detected by Lasergene GeneQuest module, is indicated. The DPE in the intron 2 region is lower case bold and underlined. ORF orientation is 5′ → 3′.
The TATA-less downstream promoter element DPE functions unidirectionally. (A) The 8-kb intron 2 full length sequence in the J8.1 transgene and its relative location with respect to the dmyc locus and genomic organization is shown. (B) The J8.1 fragment was examined for its ability to drive reporter expression in different tissues during early developmental stages. Except for a minimal basal expression in the brain (a) the activity of the reporter was abolished in imaginal discs (b–d), embryos (e, stage 1–2; f, stage 13–16) and ovary (g), emphasizing the unidirectionality of the minimal core promoter within the J8.1 fragment responsible for full activity in all the tissues (compared to the J8 activity in Fig. 6).
Note: Staining times for the discs and ovary are indicated above the scale bars. Embryo staining took place over-night. Scale bar in (a–g) indicates 50 μm.
The fusion of J5 in the direction opposite to J8 attenuates expression in embryos. (A) The approximately 4.1-kb J5 promoter, the 8-kb intron 2 full length sequence in the J8 transgene, their fusion product J10 and their relative location with respect to the dmyc locus and genomic organization are shown. (B) The approximately 12.1-kb chimeric restriction fragment in J10 results in reporter activity in the brain (a), discs (b–d) in a manner similar to fragments J2.1 to J6 in Figure 5 or intron 2 transgenes in Figure 6. However, the expression is weak during different stages of embryogenesis (e, stage 1–3; f, g, stage 9–12). The expression in ovary remained similar to J2.1 and its deletions and J8, J8.2 transgenes.
Note: Staining times for the discs and ovary are indicated above the scale bars. Embryo staining took place over-night. Scale bar in (a–h) indicates 50 μm.
Positive and negative controls for lacZ staining. For each lacZ staining of transgenes negative and positive control stainings were performed. As negative control different tissues were taken from the “y[1] w[1118] “ and “attp-flies” listed in Table 2. dpp-lacZ flies served as source for positive control. Depicted are negative controls (a–n) and positive controls (o–u), (a, b, o, brain; c–h, p–r, discs; i–m, s, t, embryos; and n, u ovaries).
Note: Staining times for the discs and ovary are indicated above the scale bars. Embryo staining took place over-night. Scale bar in (a–u) indicates 50 μm.
Acknowledgements
We thank W McGinnis and D Bopp for critical reading of the manuscript, and E Kubli and E Künnemann for the original editing of the manuscript. The plasmid BlueScript was a generous gift from O Georgiev at the W Schaffner laboratory, and the pCaSpeR4 vector containing lacZ sequences was obtained from Drosophila Genomic Resources. The enhancer trap line dpp-lacZ used as the positive control was a gift from D Gligorov at the F Karch laboratory, University of Geneva. All the other fly strains were received from Bloomington Stock Center and Kevin Cook assisted JK with the fly genetics. The injections were done at Rainbow Transgenic Flies Inc, CA. The photographs were taken at the Center for Microscopy, University of Zurich. This study was partly supported by a scholarship from the “Bildungsdirektion Kanton Zürich, Abteilung Stipendien” to JK.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Kulkarni MM, Arnosti DN. Information display by transcriptional enhancers. Development. 2003 Dec;130(26):6569–75. doi: 10.1242/dev.00890. [DOI] [PubMed] [Google Scholar]
- 2.Clyde DE, Corado MS, Wu X, Pare A, Papatsenko D, Small S. A self-organizing system of repressor gradients establishes segmental complexity in Drosophila. Nature. 2003 Dec 18;426(6968):849–53. doi: 10.1038/nature02189. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Edgar BA, Grewal SS. Nutritional control of gene expression in Drosophila larvae via TOR, Myc and a novel cis-regulatory element. BMC Cell Biol. 2010;11:7. doi: 10.1186/1471-2121-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orian A, Grewal SS, Knoepfler PS, Edgar BA, Parkhurst SM, Eisenman RN. Genomic binding and transcriptional regulation by the Drosophila Myc and Mnt transcription factors. Cold Spring Harb Symp Quant Biol. 2005;70:299–307. doi: 10.1101/sqb.2005.70.019. [DOI] [PubMed] [Google Scholar]
- 5.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010 Apr;10(4):301–9. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 6.Siddall NA, Lin JI, Hime GR, Quinn LM. Myc—what we have learned from flies. Curr Drug Targets. 2009 Jul;10(7):590–601. doi: 10.2174/138945009788680400. [DOI] [PubMed] [Google Scholar]
- 7.Gallant P, Shiio Y, Cheng PF, Parkhurst SM, Eisenman RN. Myc and Max homologs in Drosophila. Science. 1996 Nov 29;274(5292):1523–7. doi: 10.1126/science.274.5292.1523. [DOI] [PubMed] [Google Scholar]
- 8.de la Cova C, Johnston LA. Myc in model organisms: a view from the flyroom. Semin Cancer Biol. 2006 Aug;16(4):303–12. doi: 10.1016/j.semcancer.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–7. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 10.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–99. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 11.Herkert B, Eilers M. Transcriptional repression: the dark side of myc. Genes Cancer. 2011 Jun;1(6):580–6. doi: 10.1177/1947601910379012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005 Aug;6(8):635–45. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 13.Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008 Oct 15;22(20):2755–66. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez-Sola D, Ying CY, Grandori C, et al. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007 Jul 26;448(7152):445–51. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- 15.Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene. 2001 Sep 10;20(40):5595–610. doi: 10.1038/sj.onc.1204595. [DOI] [PubMed] [Google Scholar]
- 16.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999 Jan;19(1):1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenman RN. Deconstructing myc. Genes Dev. 2001 Aug 15;15(16):2023–30. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Roman N, Felton-Edkins ZA, Kenneth NS, et al. Activation by c-Myc of transcription by RNA polymerases I, II and III. Biochem Soc Symp. 2006;(73):141–54. doi: 10.1042/bss0730141. [DOI] [PubMed] [Google Scholar]
- 19.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997 Jan 24;88(2):213–22. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 20.Levens D. Disentangling the MYC web. Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):5757–9. doi: 10.1073/pnas.102173199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz W, Leon J, Eilers M. Contributions of Myc to tumorigenesis. Biochim Biophys Acta. 2002 Mar 14;1602(1):61–71. doi: 10.1016/s0304-419x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 22.Mai S, Mushinski JF. c-Myc-induced genomic instability. J Environ Pathol Toxicol Oncol. 2003;22(3):179–99. doi: 10.1615/jenvpathtoxoncol.v22.i3.30. [DOI] [PubMed] [Google Scholar]
- 23.Nasi S, Ciarapica R, Jucker R, Rosati J, Soucek L. Making decisions through Myc. FEBS Lett. 2001 Feb 16;490(3):153–62. doi: 10.1016/s0014-5793(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 24.Oskarsson T, Trumpp A. The Myc trilogy: lord of RNA polymerases. Nat Cell Biol. 2005 Mar;7(3):215–7. doi: 10.1038/ncb0305-215. [DOI] [PubMed] [Google Scholar]
- 25.Oster SK, Ho CS, Soucie EL, Penn LZ. The myc oncogene: MarvelouslY Complex. Adv Cancer Res. 2002;84:81–154. doi: 10.1016/s0065-230x(02)84004-0. [DOI] [PubMed] [Google Scholar]
- 26.Pelengaris S, Khan M. The c-MYC oncoprotein as a treatment target in cancer and other disorders of cell growth. Expert Opin Ther Targets. 2003 Oct;7(5):623–42. doi: 10.1517/14728222.7.5.623. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999 May 13;18(19):2988–96. doi: 10.1038/sj.onc.1202751. [DOI] [PubMed] [Google Scholar]
- 28.Soucek L, Evan G. Myc-Is this the oncogene from Hell. Cancer Cell. 2002 Jun;1(5):406–8. doi: 10.1016/s1535-6108(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 29.Wade M, Wahl GM. c-Myc, genome instability, and tumorigenesis: the devil is in the details. Curr Top Microbiol Immunol. 2006;302:169–203. doi: 10.1007/3-540-32952-8_7. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber-Agus N, Stein D, Chen K, Goltz JS, Stevens L, DePinho RA. Drosophila Myc is oncogenic in mammalian cells and plays a role in the diminutive phenotype. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1235–40. doi: 10.1073/pnas.94.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trumpp A, Refaeli Y, Oskarsson T, et al. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001 Dec 13;414(6865):768–73. doi: 10.1038/414768a. [DOI] [PubMed] [Google Scholar]
- 32.Benassayag C, Montero L, Colombie N, Gallant P, Cribbs D, Morello D. Human c-Myc isoforms differentially regulate cell growth and apoptosis in Drosophila melanogaster. Mol Cell Biol. 2005 Nov;25(22):9897–909. doi: 10.1128/MCB.25.22.9897-9909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duman-Scheel M, Johnston LA, Du W. Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc Natl Acad Sci USA. 2004 Mar 16;101(11):3857–62. doi: 10.1073/pnas.0400526101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston LA, Gallant P. Control of growth and organ size in Drosophila. Bioessays. 2002 Jan;24(1):54–64. doi: 10.1002/bies.10021. [DOI] [PubMed] [Google Scholar]
- 35.Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999 Sep 17;98(6):779–90. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neto-Silva RM, de Beco S, Johnston LA. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell. 2010 Oct 19;19(4):507–20. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herranz H, Perez L, Martin FA, Milan M. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J. 2008 Jun 4;27(11):1633–45. doi: 10.1038/emboj.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 2009 Jun;16(6):797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell NC, Johanson TM, Cranna NJ, et al. Hfp inhibits Drosophila myc transcription and cell growth in a TFIIH/Hay-dependent manner. Development. 2010 Jul 28; doi: 10.1242/dev.049585. [DOI] [PubMed] [Google Scholar]
- 40.Froldi F, Ziosi M, Garoia F, et al. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 2010;8:33. doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H, Hasson P, Paroush Z, Courey AJ. Groucho oligomerization is required for repression in vivo. Mol Cell Biol. 2004 May;24(10):4341–50. doi: 10.1128/MCB.24.10.4341-4350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yavatkar AS, Lin Y, Ross J, Fann Y, Brody T, Odenwald WF. Rapid detection and curation of conserved DNA via enhanced-BLAT and EvoPrinterHD analysis. BMC Genomics. 2008;9:106. doi: 10.1186/1471-2164-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brody T, Rasband W, Baler K, Kuzin A, Kundu M, Odenwald WF. cis-Decoder discovers constellations of conserved DNA sequences shared among tissue-specific enhancers. Genome Biol. 2007;8(5):R75. doi: 10.1186/gb-2007-8-5-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cranna N, Quinn L. Impact of steroid hormone signals on Drosophila cell cycle during development. Cell Div. 2009;4:3. doi: 10.1186/1747-1028-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cranna NJ, Mitchell NC, Hannan RD, Quinn LM. Hfp, the Drosophila homolog of the mammalian c-myc transcriptional-repressor and tumour suppressor FIR, inhibits dmyc transcription and cell growth. Fly (Austin) 2011 Apr 1;5(2) doi: 10.4161/fly.5.2.14482. [DOI] [PubMed] [Google Scholar]
- 47.Jones RM, Branda J, Johnston KA, et al. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol Cell Biol. 1996 Sep;16(9):4754–64. doi: 10.1128/mcb.16.9.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blackwell TK, Huang J, Ma A, et al. Binding of myc proteins to canonical and noncanonical DNA sequences. Mol Cell Biol. 1993 Sep;13(9):5216–24. doi: 10.1128/mcb.13.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackwell TK, Kretzner L, Blackwood EM, Eisenman RN, Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–51. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- 50.Cleveland JL, Huleihel M, Bressler P, et al. Negative regulation of c-myc transcription involves myc family proteins. Oncogene Res. 1988;3(4):357–75. [PubMed] [Google Scholar]
- 51.Wierstra I, Alves J. The c-myc promoter: still MysterY and challenge. Adv Cancer Res. 2008;99:113–333. doi: 10.1016/S0065-230X(07)99004-1. [DOI] [PubMed] [Google Scholar]
- 52.Goodliffe JM, Wieschaus E, Cole MD. Polycomb mediates Myc autorepression and its transcriptional control of many loci in Drosophila. Genes Dev. 2005 Dec 15;19(24):2941–6. doi: 10.1101/gad.1352305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan A, Shover W, Goodliffe JM. Su(z)2 antagonizes auto-repression of Myc in Drosophila, increasing Myc levels and subsequent trans-activation. PLoS One. 2009;4(3):e5076. doi: 10.1371/journal.pone.0005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentley DL, Groudine M. Novel promoter upstream of the human c-myc gene and regulation of c-myc expression in B-cell lymphomas. Mol Cell Biol. 1986 Oct;6(10):3481–9. doi: 10.1128/mcb.6.10.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bentley DL, Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12–18;321(6071):702–6. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- 56.Bareket-Samish A, Cohen I, Haran TE. Signals for TBP/TATA box recognition. J Mol Biol. 2000 Jun 16;299(4):965–77. doi: 10.1006/jmbi.2000.3797. [DOI] [PubMed] [Google Scholar]
- 57.Yean D, Gralla J. Transcription reinitiation rate: a special role for the TATA box. Mol Cell Biol. 1997 Jul;17(7):3809–16. doi: 10.1128/mcb.17.7.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart TA, Bellve AR, Leder P. Transcription and promoter usage of the myc gene in normal somatic and spermatogenic cells. Science. 1984 Nov 9;226(4675):707–10. doi: 10.1126/science.6494906. [DOI] [PubMed] [Google Scholar]
- 59.Kutach AK, Kadonaga JT. The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol Cell Biol. 2000 Jul;20(13):4754–64. doi: 10.1128/mcb.20.13.4754-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daines B, Wang H, Wang L, et al. The Drosophila melanogaster transcriptome by paired-end RNA sequencing. Genome Res. 2011 Feb;21(2):315–324. doi: 10.1101/gr.107854.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson S, Bankier AT, Barrell BG, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Hyman L, Moore C. Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol Mol Biol Rev. 1999 Jun;63(2):405–45. doi: 10.1128/mmbr.63.2.405-445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lutz CS. Alternative polyadenylation: a twist on mRNA 3′ end formation. ACS Chem Biol. 2008 Oct 17;3(10):609–17. doi: 10.1021/cb800138w. [DOI] [PubMed] [Google Scholar]
- 64.Neilson JR, Sandberg R. Heterogeneity in mammalian RNA 3′ end formation. Exp Cell Res. 2010 May 1;316(8):1357–64. doi: 10.1016/j.yexcr.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulkarni MM, Arnosti DN. cis-regulatory logic of short-range transcriptional repression in Drosophila melanogaster. Mol Cell Biol. 2005 May;25(9):3411–20. doi: 10.1128/MCB.25.9.3411-3420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu DC, Johnston LA. Control of wing size and proportions by Drosophila myc. Genetics. 2010 Jan;184(1):199–211. doi: 10.1534/genetics.109.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeitlinger J, Stark A. Developmental gene regulation in the era of genomics. Dev Biol. 2010 Mar 15;339(2):230–9. doi: 10.1016/j.ydbio.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 68.Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008 Jul 11;134(1):25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 69.Orian A, Delrow JJ, Rosales Nieves AE, et al. A Myc-Groucho complex integrates EGF and Notch signaling to regulate neural development. Proc Natl Acad Sci U S A. 2007 Oct 2;104(40):15771–6. doi: 10.1073/pnas.0707418104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demontis F, Perrimon N. Integration of Insulin receptor/Foxo signaling and dMyc activity during muscle growth regulates body size in Drosophila. Development. 2009 Mar;136(6):983–93. doi: 10.1242/dev.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kollmar R, Farnham PJ. Site-specific initiation of transcription by RNA polymerase II. Proc Soc Exp Biol Med. 1993 Jun;203(2):127–39. doi: 10.3181/00379727-203-43583. [DOI] [PubMed] [Google Scholar]
- 72.Breathnach R, Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–83. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- 73.Smale ST, Kadonaga JT. The RNA polymerase II core promoter. Annu Rev Biochem. 2003;72:449–79. doi: 10.1146/annurev.biochem.72.121801.161520. [DOI] [PubMed] [Google Scholar]
- 74.Juven-Gershon T, Hsu JY, Kadonaga JT. Perspectives on the RNA polymerase II core promoter. Biochem Soc Trans. 2006 Dec;34(Pt 6):1047–50. doi: 10.1042/BST0341047. [DOI] [PubMed] [Google Scholar]
- 75.Liu H, Zhou M, Luo X, et al. Transcriptional regulation of BRD7 expression by Sp1 and c-Myc. BMC Mol Biol. 2008;9:111. doi: 10.1186/1471-2199-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001 Aug;188(2):143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 77.Lang JC, Whitelaw B, Talbot S, Wilkie NM. Transcriptional regulation of the human c-myc gene. Br J Cancer Suppl. 1988 Dec;9:62–6. [PMC free article] [PubMed] [Google Scholar]
- 78.Lo K, Smale ST. Generality of a functional initiator consensus sequence. Gene. 1996 Dec 5;182(1–2):13–22. doi: 10.1016/s0378-1119(96)00438-6. [DOI] [PubMed] [Google Scholar]
- 79.Javahery R, Khachi A, Lo K, Zenzie-Gregory B, Smale ST. DNA sequence requirements for transcriptional initiator activity in mammalian cells. Mol Cell Biol. 1994 Jan;14(1):116–27. doi: 10.1128/mcb.14.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirchhamer CV, Bogarad LD, Davidson EH. Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different genes. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13849–54. doi: 10.1073/pnas.93.24.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke TW, Willy PJ, Kutach AK, Butler JE, Kadonaga JT. The DPE, a conserved downstream core promoter element that is functionally analogous to the TATA box. Cold Spring Harb Symp Quant Biol. 1998;63:75–82. doi: 10.1101/sqb.1998.63.75. [DOI] [PubMed] [Google Scholar]
- 82.Huang Y, Carmichael GG. A suboptimal 5′ splice site is a cis-acting determinant of nuclear export of polyomavirus late mRNAs. Mol Cell Biol. 1996 Nov;16(11):6046–54. doi: 10.1128/mcb.16.11.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Preiss T, Hentze MW. Dual function of the messenger RNA cap structure in poly(A)-tail-promoted translation in yeast. Nature. 1998 Apr 2;392(6675):516–20. doi: 10.1038/33192. [DOI] [PubMed] [Google Scholar]
- 84.Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–6. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lemm I, Ross J. Regulation of c-myc mRNA decay by translational pausing in a coding region instability determinant. Mol Cell Biol. 2002 Jun;22(12):3959–69. doi: 10.1128/MCB.22.12.3959-3969.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herranz H, Hong X, Perez L, et al. The miRNA machinery targets Mei-P26 and regulates Myc protein levels in the Drosophila wing. EMBO J. 2010 May 19;29(10):1688–98. doi: 10.1038/emboj.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell. 2011 Jan 18;20(1):72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19678–83. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bueno MJ, Malumbres M. MicroRNAs and the cell cycle. Biochim Biophys Acta. 2011 Feb 17; doi: 10.1016/j.bbadis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 91.Bueno MJ, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008 Oct;7(20):3143–8. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 92.Cannell IG, Bushell M. Regulation of Myc by miR-34c: A mechanism to prevent genomic instability. Cell Cycle. 2010 Jul 15;9(14):2726–30. [PubMed] [Google Scholar]
- 93.Johnston LA, Edgar BA. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature. 1998;394(6688):82–4. doi: 10.1038/27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004 Apr 2;117(1):107–16. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 95.Liu J, Kouzine F, Nie Z, et al. The FUSE/FBP/FIR/TFIIH system is a molecular machine programming a pulse of c-myc expression. Embo J. 2006 May 17;25(10):2119–30. doi: 10.1038/sj.emboj.7601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hallikas O, Palin K, Sinjushina N, et al. Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell. 2006 Jan 13;124(1):47–59. doi: 10.1016/j.cell.2005.10.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Analytical primer pairs.
Table S2.
List of the Fly Stocks used in the study.
| No. | Bloomington stock # | Name | Genotype with FlyBase links |
|---|---|---|---|
| 1 | 6598 | y w | y1 w1118 |
| 2 | 23648 | attp-86F | P{hsp70-flp}1, y1 w*; M{3xP3-RFP.attP}ZH-86Fb; M{vas-int.B}ZH-102D |
| 3 | 24480 | attp-2A | y1 M{3xP3-RFP.attP}ZH-2A w*; M{vas-int.Dm}ZH-102D |
| 4 | 24482 | attp-51C | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP′}ZH-51C |
| 5 | 24485 | attp-68E | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP′}ZH-68E |
| 6 | 24871 | attp-VK00033 | y1 M{vas-int.Dm}ZH-2A w*; PBac{y+-attP-3B}VK00033 |
| 7 | 24872 | attp-VK00037 | y1 M{vas-int.Dm}ZH-2A w*; PBac{y+-attP-3B}hafVK00037 |
| 8 | 2376 | Oregon | Oregon-R-P2 |
| 9 | 2475 | double balancer | w*; T(2;3)apXa, apXa/CyO; TM3, Sb1 |
| 10 | 11108 | blue balancer | Cyo, P{lArB}A66.2F2/b1 Adh* cn* l(2)**; ry506 |
| 11 | 8412 | dpp-lacZ | y1 w1118; P{dpp-lacZ.Exel.2}3 |
Notes: The attp-lines, 86F, 2A, VK00033 and VK000037 show strong activity of the reporter lacZ. The expression of transgene in the attp-strains 51C and 68E is weak. For random P-element mediated transgenesis embryos were taken from the y w (6598) flies. Oregon flies were used for dmyc in situ hybridization in different imaginal tissues. The fly stock “blue balancer” that expresses lacZ in embryos and ovaries, was used as a source for positive control in the embryos and ovaries staining. The dpp-lacZ line was used as positive control for the imaginal discs staining.
EvoDifference analysis of the distal 8-kb upper strand of the 40-kb D. melanogaster dmyc locus detects conserved sequence blocks. Each block of uppercase bases represents a cluster of conserved sequences. Black capital letter bases are conserved in all or in all but one species including D. sechellia, D. yakuba, D. erecta, D. ananassae, D. pseudoobscura, D. willistoni or D. virilis. There is one E-Box, conserved in all species except D. virilis (white text with black highlight). The neural network genetic algorithm PROMOTER 2.0 predicts three possible promoter regions using ccaat or bHLH recognition motifs (lower case black letters). The yellow highlighted sequence includes a highly likely promoter region (dubbed as P0 putative). Sequence orientation is 5′ → 3′.
EvoDifference analysis of the 40-kb top strand D. melanogaster dmyc locus. The 12.834-kb dmyc gene resides within the 40-kb fragment. Each block of uppercase bases represents a cluster of conserved sequences. Uppercase black nucleotides represent bases in the D. melanogaster dm EvoP 40-kb reference sequence that are conserved in all or all but one of the other 5 orthologous DNAs, D. sechellia, D. yakuba, D. erecta, D. willistoni or D. virilis. The D. melanogaster dmyc transcribed sequence is annotated according to FlyBase as follows: 5′-UTR (light grey), protein-coding sequence (dark grey) and 3′-UTR (light grey). The conserved bHLH binding sites and E-boxes are in white text with black highlight, as is the repeat motif ATGTTGCCA and the nearby sub-repeat TGTTGC. PolyA1, 2, 3 in the 3′ UTR (yellow highlights), detected by Lasergene GeneQuest module, is indicated. The DPE in the intron 2 region is lower case bold and underlined. ORF orientation is 5′ → 3′.
The TATA-less downstream promoter element DPE functions unidirectionally. (A) The 8-kb intron 2 full length sequence in the J8.1 transgene and its relative location with respect to the dmyc locus and genomic organization is shown. (B) The J8.1 fragment was examined for its ability to drive reporter expression in different tissues during early developmental stages. Except for a minimal basal expression in the brain (a) the activity of the reporter was abolished in imaginal discs (b–d), embryos (e, stage 1–2; f, stage 13–16) and ovary (g), emphasizing the unidirectionality of the minimal core promoter within the J8.1 fragment responsible for full activity in all the tissues (compared to the J8 activity in Fig. 6).
Note: Staining times for the discs and ovary are indicated above the scale bars. Embryo staining took place over-night. Scale bar in (a–g) indicates 50 μm.
The fusion of J5 in the direction opposite to J8 attenuates expression in embryos. (A) The approximately 4.1-kb J5 promoter, the 8-kb intron 2 full length sequence in the J8 transgene, their fusion product J10 and their relative location with respect to the dmyc locus and genomic organization are shown. (B) The approximately 12.1-kb chimeric restriction fragment in J10 results in reporter activity in the brain (a), discs (b–d) in a manner similar to fragments J2.1 to J6 in Figure 5 or intron 2 transgenes in Figure 6. However, the expression is weak during different stages of embryogenesis (e, stage 1–3; f, g, stage 9–12). The expression in ovary remained similar to J2.1 and its deletions and J8, J8.2 transgenes.
Note: Staining times for the discs and ovary are indicated above the scale bars. Embryo staining took place over-night. Scale bar in (a–h) indicates 50 μm.
Positive and negative controls for lacZ staining. For each lacZ staining of transgenes negative and positive control stainings were performed. As negative control different tissues were taken from the “y[1] w[1118] “ and “attp-flies” listed in Table 2. dpp-lacZ flies served as source for positive control. Depicted are negative controls (a–n) and positive controls (o–u), (a, b, o, brain; c–h, p–r, discs; i–m, s, t, embryos; and n, u ovaries).
Note: Staining times for the discs and ovary are indicated above the scale bars. Embryo staining took place over-night. Scale bar in (a–u) indicates 50 μm.