Abstract
The clustered homeobox proteins play crucial roles in development, hematopoiesis, and leukemia, yet the targets they regulate and their mechanisms of action are poorly understood. Here, we identified the binding sites for Hoxa9 and the Hox cofactor Meis1 on a genome-wide level and profiled their associated epigenetic modifications and transcriptional targets. Hoxa9 and the Hox cofactor Meis1 cobind at hundreds of highly evolutionarily conserved sites, most of which are distant from transcription start sites. These sites show high levels of histone H3K4 monomethylation and CBP/P300 binding characteristic of enhancers. Furthermore, a subset of these sites shows enhancer activity in transient transfection assays. Many Hoxa9 and Meis1 binding sites are also bound by PU.1 and other lineage-restricted transcription factors previously implicated in establishment of myeloid enhancers. Conditional Hoxa9 activation is associated with CBP/P300 recruitment, histone acetylation, and transcriptional activation of a network of proto-oncogenes, including Erg, Flt3, Lmo2, Myb, and Sox4. Collectively, this work suggests that Hoxa9 regulates transcription by interacting with enhancers of genes important for hematopoiesis and leukemia.
Introduction
Hox proteins are homeodomain-containing transcription factors (TFs) that play a vital role in establishing body plan during development. In addition to this role in body planning, Hox proteins have been implicated in limb regeneration, wound healing, adipogenesis, and hematopoietic stem cell self-renewal.1 Hoxa9, in particular, is expressed at high levels in early hematopoietic progenitor cells and promotes stem cell expansion. In contrast, Hoxa9 down-regulation is associated with hematopoietic differentiation.2,3 In keeping with this role, Hoxa9 knock-out mice show defects in B and T-cell lymphopoiesis and myelopoiesis.4
HOXA9 has been intensively studied because of its central role in human acute leukemias.5–8 Early studies of BXH2 mice, which spontaneously develop acute myeloid leukemia (AML) as a result of endogenous retroviral integration, showed that overexpression of Hoxa9, as a result of integrations at the locus, is one of the most common genetic abnormalities in these leukemias.9,10 Subsequent gene expression profiling studies showed that HOXA9 is expressed in many AMLs. Of 6817 genes tested, HOXA9 was the single most predictive marker for poor prognosis.11 Certain subtypes of acute leukemias, particularly those with rearrangements of the mixed lineage leukemia (MLL) gene, show especially high expression of A cluster HOX genes,5,6,8 which is critical for MLL fusion protein–mediated transformation.12,13 However, deregulation of Hox expression also appears to play a central role in leukemias without MLL rearrangements, including AMLs associated with the CALM-AF10 translocation, fusions of HOXA9 to the nucleoporin gene NUP98 in a subset of leukemias with the t(7;11),14,15 overexpression of CDX2 or CDX416–18 and identified T-cell acute lymphoblastic leukemia cases with translocations between the TCRβ and the HOXA9/HOXA10 locus.19
Although Hoxa9 itself is weakly oncogenic, > 90% of leukemias arising in BXH2 mice with Hoxa9 overexpression have a second integration site at the myeloid ecotropic viral integration site Meis1, which encodes a member of the 3-amino acid loop extension family of homeobox-containing proteins, resulting in high-level Meis1 expression.9,10 Hoxa9 and Meis1 synergize to produce aggressive leukemia in mice that received a transplant.20 In human leukemias that express high levels of HOXA9, either with or without MLL rearrangement, MEIS1 is consistently overexpressed.5,6,8
The mechanisms through which Hoxa9 and Meis1 function are poorly understood. To date, only a very limited number of Hoxa9 and Meis1 targets have been identified. With this paucity of binding data, it has been difficult to define the mechanisms underlying Hox protein target specificity in vivo. In vitro binding studies have shown that the 60-aa homeodomain, which is common to all Hox proteins, binds to a short TAAT or related AT-rich sequences.21 Undoubtedly, sequences surrounding this short recognition sequence contribute to the specificity of individual Hox proteins. The cofactors Meis1 and Pbx proteins increase the affinity of Hoxa9 protein binding in vitro,22 but the extent to which this contributes to specificity in vivo is not known. Therefore, it seems probable that other TFs act in concert with Hox proteins; however, only a few such “collaborators” have been identified.21 Finally, it appears that Hox proteins can have either activating or repressing effects on their targets.23 Presumably, this is governed by the collaborating proteins that either physically interact with the Hox or Meis1 proteins or bind to adjacent cis-acting sequences, but these mechanisms are poorly understood.
In this study, we used ChIP-Seq and ChIP-chip to identify the in vivo binding sites and associated epigenetic signatures for Hoxa9 and Meis1, along with microarray expression profiling to define their target genes. We then explored what TFs interact with Hoxa9 and Meis1 and whether association of these TFs is modulated by Hoxa9. Collectively, our results suggest that Hoxa9 associates with enhanceosomes (Mann et al21 have termed “Hoxasomes”) through interactions with DNA sequences in cis, Hox cofactors, and lineage-restricted TFs that play crucial roles in hematopoiesis and leukemia.
Methods
Cell line generation
BM cells were harvested from 5-fluorouracil–treated female 6- to 8-week-old C57BL/6 mice and transformed into myeloblastic cell lines with a murine stem cell virus –based retrovirus expressing HA-tagged or untagged Hoxa9 or Meis1 (HM2 cells). All animal experiments were approved by the University of Michigan Committee on Use and Care of Animals. For conditional activation of Hoxa9, cells were transformed with Hoxa9 fused to a modified estrogen receptor ligand-binding domain (Hoxa9-ER). Cells were cultured in IMDM with 15% FBS (StemCell Technologies) and penicillin/streptomycin. IL-3 (R&D Systems) was added to media; alternatively, cells were transduced with an IL-3–expressing retroviral vector (pMFGmIL3, obtained from RIKEN DNA Bank with consent of Dr Hirofumi Hamada); Hoxa9-ER cells were also supplemented with 4-hydroxytamoxifen (4-OHT; Sigma-Aldrich). Hoxa9 is required for continued mouse hematopoietic progenitor (MHP) survival, so positive selection of transduced clones was not necessary; double Hoxa9/Meis1 transductants were selected by FACS (bicistronic Meis1+GFP expression using MigR1 vector; a gift from Dr Warren S. Pear, University of Pennsylvania).
ChIP-sequencing and ChIP-chip
A total of 150 million cells were cross-linked sequentially with disuccinimidylglutarate (45 minutes at room temperature) and 1% formaldehyde (15 minutes at room temperature). Hoxa9 and Meis1 immunoprecipitation was performed with anti-HA Ab (Abcam) preconjugated to Protein G magnetic beads (Dynal/Invitrogen). For C/ebpα, rabbit anti-C/ebpα (Santa Cruz Biotechnology) was compared with preimmune rabbit IgG. Four-hour incubation (4°C with gentle rotation) was followed by washes with Low Salt, High Salt, LiCl, and Tris-EDTA buffers (Upstate/Millipore). Immunoprecipitates were eluted with 0.1% SDS/0.1M NaHCO3, and cross-links were reversed overnight at 65° in 0.2M NaCl. DNA was RNAse-treated and column purified (Qiaquick; QIAGEN). For ChIP-Seq, size selection and sequencing were performed as described previously.24 For ChIP-chip, enriched Hoxa9 and Meis1 regions and 60 randomly selected nonbinding regions were retiled onto Nimblegen 385K/2.1M custom arrays (Mus musculus, February 8, 2006) with 50-mer probes (average spacing of 35 bp). Each region was extended to 4 kb in both directions. In addition, a set of 360 transcription start sites (2 kb upstream and 1 kb downstream) closest to enriched regions was tiled. DNA was amplified before dual hybridization (Nimblegen Systems) of input and immunoprecipitated (see supplemental Data for analysis details, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Plasmids, electroporations, and luciferase reporter assays
Twenty-two Hoxa9/Meis1 binding sites were selected according to their proximity to the nearest transcription start site (TSS) as well as 2 control regions randomly selected in the genome where there is no Hoxa9/Meis1 enrichment. Each ∼ 1000-bp binding region was amplified from mouse genomic DNA with the use of Advantage HD Polymerase (Clontech), and after restriction enzyme digest the fragments were cloned into the multiple cloning site of the pTAL-Luc vector (Clontech) with the use of In-Fusion Cloning PCR System (Clontech). For luciferase assays, 2 μg of pTAL-luc construct and 500 ng of renilla vector were used per electroporation in K562 cells suspended in Gene Pulser Electroporation Buffer (Bio-Rad). Electroporations were performed in 96-well plates with the use of the Gene PulserMXcell Electroporation System (Bio-Rad). After 48 hours on electroporation, luciferase activity was measured with Dual Luciferase Reporter Assay (Promega), normalizing firefly luciferase signal to the Renilla luciferase signal.
ChIP quantitative PCR
ChIP was quantified relative to inputs with the use of Taqman probes (7500 PCR System; Applied Biosystems). Taqman primers were designed with Primer Express Software 3.0 (Applied Biosystems). Probe sequences are available on request.
Quantitative RT-PCR
Primers were designed with DNASTAR. Sequences are available on request. Relative quantification of real-time PCR product was performed with the comparative DDCt method with SYBR green fluorescent labeling (7500 PCR System; Applied Biosystems). At least 2 biologic replicates were performed for each analysis and yielded similar results.
Gene expression profiling analysis
Hoxa9-ER cells were washed 3 times and resuspended in IL-3+ media with/without 100nM 4-OHT (Sigma-Aldrich). At selected intervals, cells were removed for flow cytometric analysis with the use of anti-Gr1 and anti-Mac1 Abs (BD Biosciences). Structure was assessed by cytocentrifugation, followed by staining (Diff-Quick reagents; Intl Med Equip), and RNA extraction (Trizol reagent; Invitrogen), following the manufacturer's instructions. cRNA was synthesized and hybridized to Affymetrix Mouse 430 2.0 arrays.
Identification and verification of interacting proteins
Proteins were extracted from 1 × 109 HA-Hoxa9/Flag-Meis1–transformed BM cells (HM2 cells; M-PER; Pierce Biotechnology). The nuclear pellet was solubilized in 250 U/mL benzonase nuclease (EMD Biosciences). After preclearing with IgG, immunoprecipitation was performed with anti-HA Affinity Matrix (Roche Applied Science) or anti-FLAG M2 Affinity Gel (Sigma-Aldrich). Bound proteins were washed with M-PER+300mM NaCl, eluted in SDS-PAGE loading buffer, and resolved on PAGE gels followed by mass spectroscopy (MS). Details of MS and statistical analysis to identify interacting proteins are provided in supplemental Data. Western blot analysis was performed with rabbit polyclonal anti-Hoxa9 (Millipore), anti-Meis1 (Abcam), and anti-C/ebpα (Cell Signaling Technology). Nuclease-treated extracts were precleared with rabbit IgG-conjugated agarose beads (Santa Cruz Biotechnology), and target proteins were immunoprecipitated with either anti-HA Affinity Matrix (Roche Applied Science) or anti-FLAG M2 Affinity Gel (Sigma-Aldrich). Bound proteins were washed with M-PER+300mM NaCl and eluted in SDS-PAGE sample loading buffer. Western blot detection was performed with rabbit polyclonal anti-HoxA9 (Millipore), anti-MEIS1 (Abcam), anti-Stat5 (C-17; Santa Cruz Biotechnology), anti-C/EBPα (Cell Signaling Technology), and rabbit monoclonal anti-CREB (48H2; Cell Signaling Technology).
Data availability
The ChIP-Sequencing, gene expression, and ChIP-chip data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus25 (GSE33518). Visualization tracks (UCSC) for all data, including motif enrichment analysis results, are available at http://www.pathology.med.umich.edu/faculty/Hess/index.html.
Results
Genome-wide identification of Hoxa9 and Meis1 binding sites
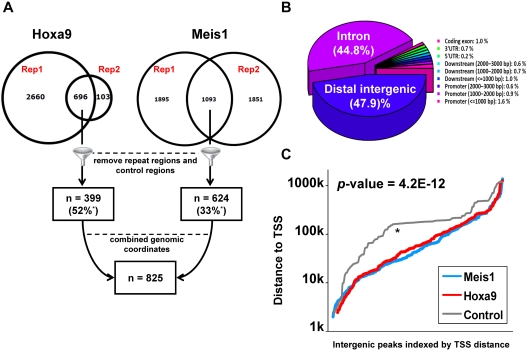
Illumina-based sequencing of ChIP-Seq products was performed to identify binding sites for Hoxa9 and Meis1 on a genome-wide basis. Because commercially available Abs to Hoxa9 and Meis1 did not provide satisfactory ChIP results, we transformed MHPs with HA-Hoxa9+Meis1 or Hoxa9+HA-Meis1 or with untagged Hoxa9+Meis1. The former 2 biologically identical leukemia cell lines allow HA tag-based precipitation and identification of Hoxa9 or Meis1 binding sites, whereas the untagged cell line serves as a control for false-positive binding by the anti-HA Ab. Western blotting experiments showed these cells expressed levels of Hoxa9 and Meis1 that were generally comparable to MLL-AF9 transformed cells (supplemental Figure 1). More than 1.7 Gb of sequence data was obtained on ChIPped chromatin. Enriched regions were required to be identified in both replicates but not in the control ChIP-Seq, consistent with the > 90% overlapping targets standard recommended by the ENCODE consortium.26 A total of 399 Hoxa9 and 624 Meis1 binding regions were identified with the peak-calling algorithm FindPeaks24 at an estimated false discovery rate (FDR) of 0.01 (Figure 1A; supplemental Table 1). Consistent with the known physical association of Hoxa9 and Meis1,22,27 > 50% of the Hoxa9 binding regions overlapped regions bound by Meis1 (Figures 1A and 2). After merging the 2 sets of peaks, 825 genomic regions (H/M peaks) were identified as being bound by either Hoxa9 or Meis1 or both (supplemental Table 1).
Figure 1.
Genome-wide identification of Hoxa9 and Meis1 binding sites in leukemia cells. (A) Schematic diagram of Hoxa9 and Meis1 binding site identification. Two replicate sequencing runs were performed for each factor, and the enriched regions (or peaks) were selected only if they were detected in both biologic replicates, consistent with ENCODE Consortium standard. The peaks from both factors were subsequently merged into one set of peaks (n = 825). Notably, a total of 52% of Hoxa9 peaks overlap with Meis1 peaks and 33% of Meis1 peaks overlap with Hoxa9 peaks. (B) Characterization of genomic localization of Hoxa9 and Meis1 binding sites. (C) Cumulative distribution of genomic localization indicates that Hoxa9 (red) and Meis1 (blue) binding sites are significantly (Kolmogorov-Smirnov test) closer to transcription start sites, compared with control peaks (gray).
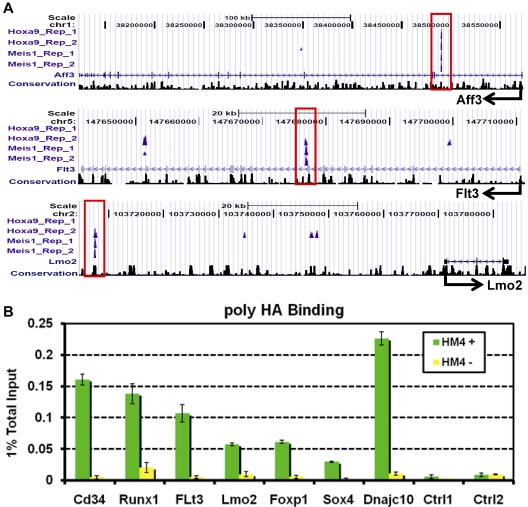
Figure 2.
Validation of Hoxa9 and Meis1 binding sites identified by ChIP-Seq. (A) For each binding site, enrichment profiles are shown for 2 replicates of Hoxa9 and Meis1 ChIP-Seq, with corresponding genomic annotation displayed as UCSC mm8 tracks at the Aff3, Flt3, and Lmo2 loci. A locus is deemed a high-confidence Hoxa9 and Meis1 binding site if it is bound by either Hoxa9 or Meis1in both of the replicate sequencing runs. The sequence tags of nonsignificant peak regions (FDR P < .01) are not displayed. The binding sites are highly conserved as shown by the Phastcon17 conservation track below. No significant binding was detected in the 2 control lanes at any of the regions shown. (B) Confirmation of selected Hoxa9 and Meis1 binding sites by ChIP and quantitative PCR. ChIP experiments were performed with polyclonal anti-HA Abs on HA epitope-tagged Hoxa9-ER/Meis1–transformed myeloblastic cell (HM4) used for ChIP-Seq experiments as described in “Experimental procedures.” Green bars represent PCR signal as a percentage of input for ChIP on cells cultured for 96 hours in the presence of 4-OHT, whereas yellow bars represent ratios for cells cultured for 96 hours in the absence of 4-OHT. These experiments show that Hoxa9 binds at high levels to ChIP-Seq–identified binding sites but not at control peaks and that the Hoxa9 enrichment disappears on 4-OHT withdrawal.
As one test of the binding specificity, the H/M binding sequences were analyzed for enrichment of the experimentally determined Hoxa9/Meis1 consensus motif ATGATTTATGGC (Hoxa9-Meis1-Pbx1)22 and Meis1 consensus TGTC motif.28 Strikingly, 98.5% of the H/M binding sites contain the Hoxa9-Meis1-Pbx1 motif compared with a random sequence background (P < .01). Similarly, the Meis1 motif was found to be significantly enriched in 97.8% of H/M binding sites (P < .01). As another test, a total of 7 enriched regions (Cd34, Runx1, Flt3, Lmo2, Foxp1, Sox4, Dnajc10) and 4 nonbinding regions were tested with conventional ChIP with HA Abs to detect epitope-tagged Hoxa9 and FLAG-tagged Meis1 and followed by quantitative PCR detection. These experiments showed significant binding of Hoxa9 or Meis1 or both only at enriched regions identified by ChIP-Seq (a subset of the results from these experiments is shown in Figure 2B). Finally, ChIP-chip experiments with anti-HA Abs to detect Hoxa9, which is discussed further below (Hoxa9 is organized into enhanceosomes containing lineage-restricted TFs), confirmed that the overall Hoxa9 binding intensity values at the 825 tiled regions are much higher than that of the control regions and peak in the center of the binding sites (Figure 5C).29
Figure 5.
De novo motif discovery of TF motifs in Hoxa9 and Meis1 binding sites and comparison to previously characterized macrophage enhancer sequences. (A) Six de novo DNA sequence motifs and their STAMP logos29 and enrichment statistics, including observed frequencies and similarity measures. A complete list of de novo motifs (n = 15) is given in supplemental Figure 5. (B) Spatial distributions of motifs listed in A with respect to centers of H/M binding sites. (C) Comparison of normalized ChIP-chip signal of 6 TFs at H/M binding sites that are cooccupied by C/EBPα (red), PU.1 (blue), or both C/EBPα+PU.1 factors.42 In most cases (except HOXA9 and STAT5), H/M peaks that are cobound by C/EBPα showed highest expression intensity (red line), followed by C/EBPα+PU.1 (black line) and PU.1 (blue line). Similarly, the ChIP-chip signal at 60 randomly selected control regions is depicted by the gray line. (D) A large proportion of H/M peaks were found to overlap with enhancer sequences bound by C/EBPα (red), PU.1 (blue), or both C/EBPα+PU.1 (black) in lipopolysaccharide-stimulated macrophages.43 (E) Comparative motif enrichment analysis showed increased level enrichment of HOXA, PBX/MEIS1, and STAT motifs in the set of enhancers described by Heinz et al43 that are bound by Hoxa9 (green) and Meis1 (blue), but not in those that are not associated with Hoxa9 and Meis1 (red).
H/M peaks were then annotated with information on promoters and TSSs.30 Surprisingly, only 5.1% (n = 41) of the identified binding sites are located within promoter regions (−2/+1 kb relative to TSSs). Instead, most H/M binding regions are located in intronic (44.8%) and distal intergenic (47.9%) regions (Figure 1B). Although these regions, on average, are > 10 kb from the nearest TSS, this is significantly closer to TSSs than the 229 peaks seen in ChIP of no epitope tag controls (Figure 1C).
Hoxa9 and Meis1 specifically associate with enhancers
Increasing evidence shows that highly conserved noncoding elements (HCNEs), which are often located far from TSSs, play important roles in transcriptional regulation.31 With the use of 17-vertebrate PhastCons evolutionary conservation scores,32 > 98% of H/M peaks showed > 50% overlap with highly conserved noncoding elements (P = 5.1 ×10−8 compared with sequences one peak-width adjacent to enriched regions). Conservation decreased precipitously to a baseline outside of the H/M peak boundaries (supplemental Figure 2). This highly circumscribed evolutionary conservation of H/M sequences, which are highly enriched for the Hoxa9-Meis1-Pbx1 consensus motif, suggests these sites probably play a role in transcriptional regulation. These sites were further evaluated for their regulatory potential (RP), which is determined from a comparative genetics study of 7 mammalian species and has been shown to identify regulatory elements with > 94% accuracy.33 The RP scores associated with H/M binding regions show a remarkable coincidence with the sequence tags measured by ChIP-Seq (ρ = 0.81, P < .0001), with average RP score > 0.065 (score values > 0 are indicative of regulatory elements), which drops sharply when extended beyond the boundaries of conserved H/M binding regions (Figure 3A).
Figure 3.
Hoxa9 and Meis1 binding sites show high regulatory potential and carry the epigenetic signature of enhancer sequences. (A) Regulatory potential scores are high at the center of Hoxa9 and Meis1 binding with correlation of 0.81 (P < .0001). The lines depict average of regulatory potential scores (blue) and sequencing reads in a ± 4-kb region centered at Hoxa9 and Meis1 binding sites. (B) Spatial distribution of epigenetic modifications surrounding high-confidence Hoxa9 and Meis1 binding sites. Epigenetic modification status was examined in ± 4-kb regions centered on Hoxa9 and Meis1 binding loci with the use of a custom Nimblegen tiling array. The normalized log2 ratios of a modification marker over input are shown relative to the center of the binding sites. (C) Spatial distribution of epigenetic modifications at the promoter region (+ 1 kb upstream and − 2 kb downstream) of a selected set of 360 genes that are closest to Hoxa9 and Meis1 binding sites. The normalized log2 ratio of a modification marker over input are shown for each nucleotide with respect to their distance to the transcription start sites. (D) The 3-dimensional projection of 7 epigenetic modification markers at the Hoxa9 and Meis1 binding sites with the use of principal component analysis. The first 3 principal components account for 82.1% of the total variance. The figure shows the loadings of each epigenetic modification on these components. The p300, CBP, H3K4me1 epigenetic signature that is characteristic of enhancer sequences includes > 65% of all Hoxa9 and Meis1 binding sites.
Enhancer sequences have characteristic epigenetic signatures that differ significantly from promoters, showing high-level histone H3 and H4 acetylation, H3K4 monomethylation (H3K4me1), low-level histone H3K4 trimethylation (H3K4me3), as well as high-level binding of the histone acetyltransferases p300 and CBP.34,35 To assess whether H/M binding sites have this signature, we performed epigenetic profiling with the use of Abs specific for histone H3K4me1, H3K4me3, H3K27me3, H3ac, H4ac, CBP, and P300, followed by ChIP-chip with the use of Nimblegen custom tiling arrays. All H/M sequences and a selected set of the nearest 360 TSSs were tiled, along with 60 negative control sequences that were selected randomly from the mouse genome. ChIP-chip data were analyzed with MA2C36 and a customized R package.
These studies showed that H3 and H4 acetylation and p300/CBP binding is centered on the H/M peaks and is flanked by regions of histone H3K4me1 in a bimodal distribution characteristic of enhancer sequences (Figure 3B).34,35 H/M peaks are unlikely to be promoters, because they differ significantly from the signature of the promoters at adjacent genes. In particular, they lack the signature of nucleosomal eviction that is observed at the TSS (Figure 3C). Principal component analysis and heatmaps indicate that, although most H/M sites appear to be enhancer sequences, there is some heterogeneity. We noted minor subsets of H3K27 trimethylated regions, sequences with high-level H3K4me3 and H3 and H4 acetylation and a small subset of other H/M peaks that lack significant levels of histone acetylation, methylation, and p300/CBP binding raising the possibility that some H/M sites do not function as enhancers (Figure 3D).
To test for potential enhancer (or repressor activity), 22 Hoxa9/Meis1 binding sites as well as 2 control regions randomly selected in the genome where there is no Hoxa9/Meis1 binding were cloned as ∼ 1000-bp fragments. These were inserted into the multiple cloning site of the pTAL-Luc vector, electroporated into myeloblastic K562 cells and were analyzed by Dual Luciferase Reporter Assays (Promega; supplemental Figure 3). These experiments showed that 8 of the 22 H/M binding regions had significant (> 150% of normalized activity for empty vector) enhancer activity, 4 had repressive activity (≤ 50% of empty vector activity), whereas the remainder as well as 2 control regions did not have significant effects on activity. Although all regions showed variable levels of p300 binding and histone acetylation, only sequences that scored positively for enhancer activity in Luciferase showed significantly increased levels of these marks when Hoxa9 is active (in presence of 4-OHT). These data tend to support a role for Hoxa9 in modulating activity of a subset of enhancers (supplemental Figure 3).
Hoxa9 regulates genes controlling proliferation, inflammation and differentiation
We examined the relation between H/M binding sites and expression of neighboring genes by generating a conditional form of Hoxa9 by fusing a modified ER in-frame to the C-terminus of Hoxa9. This construct was retrovirally packaged and transduced into 5-fluorouracil–primed BM to generate a myeloblastic cell line (a similar approach fusing ER to either the N- or C-terminal of Meis1 did not result in immortalization). These Hoxa9-ER–transformed cells grow in the presence of 4-OHT, but growth arrest and differentiate into macrophages when 4-OHT is withdrawn (supplemental Figure 4). For gene expression profiling, RNA from cells harvested at 48, 72, 96, and 120 hours after 4-OHT withdrawal was analyzed in triplicate by Affymetrix microarray hybridization.
No statistically significant gene expression changes (FDR P < .05) were observed until 72 hours after 4-OHT withdrawal. Overall, 6991 genes show significant changes in expression after 4-OHT withdrawal (composite significance criterion, FDR P < .05; median fold change > 1.5; supplemental Table 2). On the basis of their temporal expression patterns, these genes were clustered into 4 subgroups (supplemental Figure 4E). The first group represents a large number of Hoxa9 up-regulated genes (n = 4253) whose expression started decreasing, beginning 72 hours after 4-OHT withdrawal and remained decreased at 120 hours. Gene ontology analysis of this group showed extremely high association with RNA processing (6.63 × 10−68), DNA metabolic processes (1.73 × 10−51), and cell-cycle regulatory genes (1.79 × 10−44). The group includes Camk2d, Cdk6, Erg, Etv6, Flt3, Foxp1, Gfi1, Kit, Lck, Lmo2, Myb, and Sox4, which are strongly implicated in either murine or human leukemias (Figure 4).37,38 Overall, of the 52 targets showing > 5-fold altered expression between Hoxa9- and Hoxa9+Meis1-immortalized MHPs published by Wang et al,39 14% showed association with the H/M ChIP-Seq peaks identified here. The other main group represents Hoxa9 down-regulated genes (n = 2502) whose expression levels increased over time (Figure 4; supplemental Figure 4E). Gene ontology analysis shows their strong associations with immune response (Fisher exact P = 1.82 ×10−26), inflammatory response (1.82 × 10−22), and cell activation (5.57 × 10−14). Many of these genes are located near the H/M binding sites and are related to inflammation and myeloid differentiation, including Ifit1, Tlr4, Ccl3, Ccl4, Csf2rb, Ifngr1, Runx1, Cd28, and Cd33.
Figure 4.
Heatmap showing temporal expression of subset of Hoxa9-regulated genes closely associated with Hoxa9 and Meis1 binding sites. A subset of genes are shown that are significantly differentially expressed over the 120-hour period after 4-OHT withdrawal (FDR P < .05 and median fold change > 1.5) compared with controls. Data are normalized across samples such that the expression value of each individual gene has zero mean and SD of 1. Many of the up-regulated genes shown have been directly implicated in leukemogenesis in both human studies and animal models (see text). Many of the down-regulated genes are involved in myeloid differentiation. Heatmaps of the entire set of differentially regulated genes are provided in supplemental Figure 4, and additional information on statistical analysis is provided in supplemental Data.
Two other clusters showed more complex expression dynamics. One group consists of 100 genes whose expression was increased at 72 and 96 hours but decreased by 120 hours. Many of these genes are involved in cholesterol biosynthesis (6.13 × 10−5) or sterol biosynthesis (1.38 × 10−4). Another cluster includes 136 genes with decreased expression at 72 and 120 hours that showed increased expression at 120 hours. These genes were associated with pattern specification (1.99 × 10−3) or regulation of nervous system development (2.34 × 10−3).
Hoxa9 is organized into enhanceosomes containing lineage-restricted TFs
Previous studies of homeobox proteins in both Drosophila and mammals suggest that Hox proteins function in concert with other TFs, which either function as “cofactors” that physically interact and bind cooperatively with the Hox proteins (examples for Hoxa9 being the 3-amino acid loop extension cofactors Pbx2/3 and Meis1) or as “collaborators” that modulate binding or transcriptional output via closely opposed binding sites.21 We applied both de novo motif discovery and motif enrichment analysis on H/M sequences to search for potential binding loci for such Hoxa9/Meis1 cofactors or collaborators. Three independent runs of de novo motif discovery analysis were performed with Gadem to search for motifs of lengths between 6 and 20 nucleotides,40 which showed that Hoxa9 and Meis1 binding motifs are the most significantly enriched, occurring in 53% and 34% of H/M sequences, respectively (Figure 5A; supplemental Table 3). The second-most frequently occurring motif, which was found in 51% of peaks, is for the ETS family of TFs. In addition, a total of 204 sequences (36%) contain the CCAAT-enhancer binding protein C/EBP motif, with more than one-half of them occurring in combination with the Hoxa9/Meis1/Pbx1 motif. This was followed in frequency by motifs for RUNX, which was present in 14% of peaks and the STAT motif, present in 10% of peaks. Other enriched motifs include putative binding sites for a number of other TF deregulated in hematologic malignancies, including MAF and E2A. An additional 5 consensus novel motifs were identified that do not match known TFs (supplemental Figure 5).
Similar results were obtained in motif enrichment analysis in which the H/M sequences were searched for known TF consensus motifs from Genomatix TF databases. The position weight matrices of 727 factors were analyzed with Genomatix Region Miner and showed high level of enrichment for the canonical Hoxa9 motif and the canonical Meis1 motif (93.1% and 67.5%, respectively). Compared with de novo motif discovery results, this higher enrichment level of Hoxa9 and Meis1 motifs is probably because of the increased sensitivity and detection power of motif enrichment analysis that leverages existing knowledge of TF binding characteristics.41 Significant enrichment was also seen for other factors, including C/EBP, CREB, STAT, RUNX1, and ETS (especially PU.1) binding sites (supplemental Tables 4-5). The Hoxa9 and Meis1 motifs are strikingly concentrated at the center of the binding regions, whereas RUNX1, STAT, CREB, and C/EBP motifs are more broadly distributed across the locus of Hoxa9 and Meis1 binding (Figure 5B; supplemental Figure 5). The motif enrichment analysis also identified a number of other significantly enriched motifs for bZIP, MEIS, caudal MYB, and MYC TF family members (supplemental Tables 4,6).
We confirmed binding of a subset of these TFs by performing ChIP-chip experiments in myeloblastic cells. These experiments showed that the peaks associated with Hoxa9 and Meis1 are extensively bound by C/EBPα, Pu.1, and Stat5a/b and overlap the sites of Hoxa9 binding (Figure 5C). In addition, these peaks show higher levels of CBP and p300 binding than peaks lacking C/EBPα and Pu.1 motifs. This coincidence of TF binding in Hoxa9 and Meis1 peaks is noteworthy, given recent studies showing that C/EBPα, Pu.1, and Runx1 physically interact42 and collectively define myeloid enhancer sequences in murine macrophages.43 Remarkably, > 30% of the 825 H/M peaks identified here overlap with these previously identified myeloid enhancers (Figure 3D). To identify the sequence determinants for Hoxa9 association with enhancers, we compared the motif enrichment patterns in enhancers bound by H/M, C/EBPα, and H/M+C/EBPα. As expected, myeloid enhancers bound by Hoxa9 were highly enriched for HOX consensus motifs compared with the statistically significantly lower presence of the same motifs in sequences bound by C/EBPα alone or along with MEIS-PBX heterodimers (Figure 5E). Of note, Hoxa9 bound enhancers were also enriched for STAT (Figure 5E), MYB, estrogen response elements, BRN5, PDX1, CDX, and peroxisome proliferator activated protein motifs (supplemental Figure 7B). Collectively, these findings suggest that Hoxa9 is organized into specialized enhanceosomes composed of lineage-specific TFs.21
Our experiments show that Hox consensus sequences are present in most Hoxa9-bound enhancers (Figure 5) and are probably a major determinant of Hoxa9 targeting to these enhancers. However, this sequence is present at numerous sites in the genome, making it probable that interaction with Hox sequences beyond the homeodomain and interaction with cooperatively binding cofactors, such as the Meis and Pbx families further increases DNA binding affinity and specificity.21 Our experiments suggest that a third tier of Hoxa9 specificity is achieved through combinatorial interactions with TFs such as C/ebpα, Stat5, and Creb1, each expressed at variable levels in a specific cell type that collectively account for lineage-specific Hoxa9 recruitment43 (Figure 6). To identify such interactions, we performed immunoaffinity purification and MS of Hoxa9 and Meis1 complexes isolated from the epitope-tagged Hoxa9- and Meis1-transformed cells used for ChIP-Seq and ChIP-chip experiments. Immunoprecipitates were analyzed on polyacrylamide gels, which were then sliced and analyzed by MS. As a control, extracts were also prepared from Hoxa9 and Meis1 cells lacking epitope tags. After subtraction of proteins identified in the controls, proteins identified with high probability were then correlated with the list of motifs enriched in H/M binding sites.
Figure 6.
Examples of motifs enriched in Hoxa9-associated enhancers. Enriched motifs, each depicted as a colored rectangular box, are plotted for the central 200 bp of 8 representative Hoxa9-bound enhancers. These Hoxa9 enhancers are highly enriched for HOX, HOX-MEIS-PBX, CREB, MYB, CAUDAL, ETS, MYC, and STAT sites, among others. All motifs shown are significantly enriched in these Hoxa9 enhancers compared with random genome background (Motif Enrichment Analysis; supplemental Data). An enrichment statistic is computed with z-test comparing the observed frequencies (in H/M peaks) versus the expected frequencies (in random genomic background; P < .001). A complete compendium of motifs is provided in supplemental Data.
Remarkably, in multiple experiments that isolated either HA-tagged Hoxa9 or FLAG-tagged Meis1, we identified not only Hoxa9 as well as Pbx2 and Pbx3 and Meis1, but also C/EBPα, Creb1, and Stat5, whose motifs are enriched in Hoxa9 and Meis1 binding sites. These interactions were further confirmed by immunoprecipitations with nuclease-treated nuclear extracts to rule out the possibility of DNA tethering (Figure 7A) and are in keeping with reports that Hox proteins of other paralog groups coimmunoprecipitate with ETS TFs, including PU.1, which interacts with C/EBPα.42,44,45 Together, this suggests that Hoxa9 associates with enhanceosomes through homeodomain-mediated interactions, interactions with DNA binding affinity cofactors such as Meis1, and interactions with TFs that include C/EBPα, CREB1, and STAT5.
Figure 7.
Hoxa9 association with enhanceosomes is associated with coactivator recruitment and histone acetylation. (A) Coimmunoprecipitations performed on Hoxa9/Meis1 transformed cells show that Meis1, Stat5, C/ebpα, and Creb1 immunoprecipitate with Hoxa9. Nuclease-treated extracts from BM cells stably transduced with either TAPTAG-Hoxa9/Meis1 (HM1) or HA-Hoxa9/FLAG-Meis1 (HM2) were immunoprecipitated (IP) with anti-HA Affinity Matrix (Roche Applied Science) or anti-FLAG M2 Agarose (Sigma-Aldrich). Proteins were separated by SDS-PAGE and detected by Western blot (WB) analysis. Meis1, Stat5, C/ebpα, and Creb1 coelute with Hoxa9, whereas Hoxa9, C/ebpα, and Creb1, but not Stat5, coimmunoprecipitate with Meis1. (B) Hoxa9 association is closely correlated with association of other enhanceosome components and histone acetylation at representative sites in Cd34, Flt3, and Dnajc10 loci. ChIP experiments were performed with anti-HA Meis1, C/ebpα, Stat5, P300, and histone H3 pan acetyl Abs on a HA epitope-tagged Hoxa9-ER/Meis1-transformed myeloblastic cell line (HM4) as described in “Experimental procedures.” Green bars represent the PCR signal as a percent of input for ChIP on cells cultured for 96 hours in the presence of 4-OHT, and yellow bars represent ratio for cells cultured for 96 hours in the absence of 4-OHT with the exception of C/ebpα, which was cultured for 168 hours. Two control regions not determined to be bound by ChIP-Seq are shown. Additional data are shown in supplemental Figure 6. (C) Model for Hoxa9 regulation of enhanceosome activity. Hoxa9 interacts with enhanceosomes containing as a result of homeodomain domain–DNA interactions, association with Meis1 (Pbx proteins are not shown but may further enhance binding) and direct or indirect interactions with C/ebpα, Creb1 and Stat5a/b. Binding of Hoxa9 promotes P300/CBP recruitment through the Meis1 C terminal domain and potentially other interactions with enhanceosome-associated TFs. A variety of oncogenic alterations, including MLL fusion proteins, CDX2 or CDX4 overexpression, NPMc or NUP98 fusion proteins enforce high level Hoxa9 expression, making enhanceosome coactivator activity refractory to physiologic differentiation signals. The resulting persistent expression of proliferative target genes such as Flt3, Sox4, Lmo2 and Myb leads to leukemic transformation.
To further examine the role Hoxa9 plays at enhanceosomes, we performed ChIP to detect binding of other cofactors and collaborating TFs on the inducible Hoxa9-ER/Meis1–transformed cells in the presence and absence of 4-OHT. These studies show that Hoxa9 dissociates from enhanceosomes on 4-OHT withdrawal (Figure 7B). Dissociation of Hoxa9 is accompanied by a concordant fall in association of Meis1 and a global reduction in binding of Pu.1, C/ebpα, and Stat5a/b (Figure 7B; supplemental Figure 8). This in turn is associated with a dramatic decrease of P300 and CBP, as well as of histone H3 and H4 acetylation at most binding sites (Figure 7B; supplemental Figure 8). Together, these results suggest that Hoxa9 interaction with enhanceosomes is dynamic and is associated with increased acetylation and coactivator recruitment.
Discussion
Despite their central role in development, hematopoiesis, and leukemia, the mechanisms through which HOX proteins regulate transcription remain poorly understood. Our gene expression profiling data suggest that Hoxa9 can have either positive or negative effects on target gene expression, depending on the cell type and chromatin context. Consistent with its role as a proto-oncogene, Hoxa9 generally up-regulates proliferative genes, while suppressing expression of myeloid differentiation and inflammatory genes. Many Hoxa9 up-regulated direct targets have been shown to be leukemogenic in retroviral insertional mutagenesis assays, including Camk2d, Cdk6, Erg, Etv6, Flt3, Foxp1, Gfi1, Kit, Lck, Lmo2, Myb, and Sox4.37,38 Several of these, such as Flt3, Lmo2, and Myb, have been previously implicated as Hox9 or Meis1 targets39,46–48 and are highly characteristic of MLL-rearranged leukemias with high-level HOX expression.5,8,49,50 Some of the genes identified here, including Lmo2, Erg, Sox4, and Cd34, have also been reported to be regulated by Hoxb4, which, like Hoxa9, promotes HSC expansion.51 In contrast to previous studies,49,51,52 our data indicate that most Hoxa9 and Meis1 binding sites are not located at promoters of target genes. We speculate that the promoter binding previously reported could be the result of transient looping interactions with promoters (see below) or possibly the result of intronic Hoxa9 and Meis1 binding sites adjacent to the TSS being detected in ChIP assays.
Our data suggest that Hoxa9 mediates its transcriptional effects by interacting with enhanceosomes, which in myeloid leukemia cells are composed of combinatorial arrangements of Hox cofactors (Meis1) and collaborators including C/ebpα, Pu.1, Myb, and Stat5 among others (Figure 7C). Several of the Hoxa9-bound sequences identified here overlap with well-characterized tissue-specific enhancers, including the Lmo2 upstream regulatory region,53 the −35-kb enhancer for Gfi1, which is located in the neighboring Evi5 locus,54 and the intronic Erg stem cell enhancer.48 In addition, many of the sequences identified here overlap with previously identified lipopolysaccharide-responsive myeloid-specific enhancers.43 Our MS and coimmunoprecipitation experiments show that Hoxa9/Meis1 complexes physically interact with several TFs that are associated with enhanceosomes bound by Hoxa9, including Stat5, C/ebpα, and Creb1 (Figure 7A). This suggests Hox proteins achieve their specificity through a combination of direct homeodomain interactions, interactions with Hox cofactors such as Meis1, and a third tier of interactions with other TFs such as Stat5, C/ebpα, and Creb1. This also raises the possibility that Hoxa9 and Meis1 target genes depend on the cell type in both the normal and leukemic state. Hoxa9 promotes stem cell expansion as well B and T-cell development, and Hoxa9 overexpression is associated with mixed lineage, pro-B cell, and T lymphoblastic leukemias.1 Heinz et al found that enhancers marked by H3K4me1 and bound by Pu.1 in B cells are associated with a different set of TFs than in myeloid cells, including RUNX1, E2A, OCT, and NF.43 Given these findings, it will be important to assess whether the different lineages seen in MLL-rearranged leukemias result from differential association of Hoxa9/Meis1 with enhancers that depends on the TF milieu in the progenitor in which the translocation occurs.
Several Hox proteins (Hoxb1, Hoxb7) have been shown to recruit CBP/P300.55,56 In addition, recruitment of CREB1/CBP by the Meis1 C-terminal region appears to be crucial for Hox protein transcriptional activity as well as for transformation.57 In our experiments, inactivation of Hoxa9-ER by 4-OHT withdrawal resulted in dissociation of Meis1, Pu.1 C/ebpα, and Stat5 from a number of loci, including Flt3, Dnajc10, Cd34, and Igfr2r. This was accompanied by a sharp drop in P300 and CBP association along with decreased H3 and H4 acetylation (Figure 7B; supplemental Figures 8-9). This decrease in P300/CBP association could be because of the dissociation of Meis1 from the enhanceosome or to the dissociation of other TFs associated with P300/CBP, including Creb1, C/ebpα, or Stat5. ChIP for RNA Pol II shows increased association of Pol II with distal enhanceosome, for example, at the Foxp1 locus when Hoxa9 is active (supplemental Figure 9). This raises the possibility that H/M sites make looping interactions with adjacent promoters in a manner that is mediated either directly or indirectly by Hoxa9. Together, this suggests that high levels of Hoxa9 and Meis1 result in increased P300/CBP recruitment at enhanceosomes and ultimately up-regulation of leukemogenic target genes.
It is remarkable that, of the > 350 TFs expressed in myeloid cells,43 only a small number are enriched in enhanceosomes bound by Hoxa9 and, of these, most are strongly implicated in human leukemias. Among the proliferative TFs whose motifs are enriched at these sites, HOXA9 is commonly overexpressed in AML.1,58 Overexpression and phosphorylation of STAT5b is common in human AML cases with high-level HOXA9 expression.59 STAT5 appears to be the preferred second messenger of FLT3 in AML, which is very commonly mutated in cases with high-level HOXA9 expression.60 Furthermore, Stat5b knockout markedly decreases leukemia-initiating cells frequency in Hoxa9/MN1-induced leukemias, highlighting the importance of the functional synergy of Hoxa9 and STAT5 in leukemogenesis.59 The data provided here should greatly expedite studies on how Hoxa9 modulates target gene expression during normal hematopoiesis and how this mechanism is disrupted by mutation or abnormal expression of enhanceosome components such as C/EBPα and STAT5 in acute leukemia.
Acknowledgments
The authors thank Joshua Jacques for assistance with flow cytometry, Venkatesha Basrur for assistance with mass spectrometry, Mark Deming for assistance with graphics preparation, and Hugh Brock for critical review of the manuscript.
This work was supported by the National Institutes of Health (grant R01 CA116570-01A1, J.L.H.) and by a pilot grant from the University of Michigan Center for Computational Medicine and Bioinformatics (CCMB, J.L.H. and A.H.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.H. performed bioinformatic analysis and wrote the paper; K.S., J.B., D.S., M.D., and C.C. performed experiments; G.R. supervised and performed bioinformatic analysis; J.M., T.C., M.B., N.T., Y.Z., T.Z., and M.H. performed bioinformatic analysis; A.H. and S.J. supervised bioinformatic analysis; and J.L.H. designed experiments, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jay L. Hess, Department of Pathology, University of Michigan Medical School, M5240 Medical Science Bldg 1, 1301 Catherine Ave, Ann Arbor, MI 48109-0602; e-mail: jayhess@umich.edu.
References
- 1.Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26(47):6766–6776. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- 2.Pineault N, Helgason CD, Lawrence HJ, Humphries RK. Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Exp Hematol. 2002;30(1):49–57. doi: 10.1016/s0301-472x(01)00757-3. [DOI] [PubMed] [Google Scholar]
- 3.Thorsteinsdottir U, Mamo A, Kroon E, et al. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood. 2002;99(1):121–129. doi: 10.1182/blood.v99.1.121. [DOI] [PubMed] [Google Scholar]
- 4.Izon DJ, Rozenfeld S, Fong ST, Komuves L, Largman C, Lawrence HJ. Loss of function of the homeobox gene Hoxa-9 perturbs early T-cell development and induces apoptosis in primitive thymocytes. Blood. 1998;92(2):383–393. [PubMed] [Google Scholar]
- 5.Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 6.Casas S, Nagy B, Elonen E, et al. Aberrant expression of HOXA9, DEK, CBL and CSF1R in acute myeloid leukemia. Leuk Lymphoma. 2003;44(11):1935–1941. doi: 10.1080/1042819031000119299. [DOI] [PubMed] [Google Scholar]
- 7.Rozovskaia T, Feinstein E, Mor O, et al. Upregulation of Meis1 and HoxA9 in acute lymphocytic leukemias with the t(4:11) abnormality. Oncogene. 2001;20(7):874–878. doi: 10.1038/sj.onc.1204174. [DOI] [PubMed] [Google Scholar]
- 8.Ferrando AA, Armstrong SA, Neuberg DS, et al. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003;102(1):262–268. doi: 10.1182/blood-2002-10-3221. [DOI] [PubMed] [Google Scholar]
- 9.Moskow JJ, Bullrich F, Huebner K, Daar IO, Buchberg AM. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol Cell Biol. 1995;15(10):5434–5443. doi: 10.1128/mcb.15.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, Largaespada DA, Lee MP, et al. Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat Genet. 1996;12(2):154–158. doi: 10.1038/ng0296-154. [DOI] [PubMed] [Google Scholar]
- 11.Golub TR, Slonim DK, Tamayo P, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286(5439):531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 12.Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17(18):2298–2307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisig BB, Milne T, Garcia-Cuellar MP, et al. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol Cell Biol. 2004;24(2):617–628. doi: 10.1128/MCB.24.2.617-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borrow J, Shearman AM, Stanton VP, Jr, et al. The t(7;11)(p15;p15) translocation in acute myeloid leukaemia fuses the genes for nucleoporin NUP98 and class I homeoprotein HOXA9. Nat Genet. 1996;12(2):159–167. doi: 10.1038/ng0296-159. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Largaespada DA, Shaughnessy JD, Jr, Jenkins NA, Copeland NG. Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukaemias. Nat Genet. 1996;12(2):149–153. doi: 10.1038/ng0296-149. [DOI] [PubMed] [Google Scholar]
- 16.Bansal D, Scholl C, Frohling S, et al. Cdx4 dysregulates Hox gene expression and generates acute myeloid leukemia alone and in cooperation with Meis1a in a murine model. Proc Natl Acad Sci U S A. 2006;103(45):16924–16929. doi: 10.1073/pnas.0604579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholl C, Bansal D, Dohner K, et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Invest. 2007;117(4):1037–1048. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawat VP, Thoene S, Naidu VM, et al. Overexpression of CDX2 perturbs HOX gene expression in murine progenitors depending on its N-terminal domain and is closely correlated with deregulated HOX gene expression in human acute myeloid leukemia. Blood. 2008;111(1):309–319. doi: 10.1182/blood-2007-04-085407. [DOI] [PubMed] [Google Scholar]
- 19.Soulier J, Clappier E, Cayuela JM, et al. HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL). Blood. 2005;106(1):274–286. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- 20.Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 1998;17(13):3714–3725. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen WF, Montgomery JC, Rozenfeld S, et al. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol. 1997;17(11):6448–6458. doi: 10.1128/mcb.17.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitwala KV, Dandekar MN, Hess JL. HOX proteins and leukemia. Int J Clin Exp Pathol. 2008;1(6):461–474. [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson G, Hirst M, Bainbridge M, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4(8):651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 25.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozowsky J, Euskirchen G, Auerbach RK, et al. PeakSeq enables systematic scoring of ChIP-seq experiments relative to controls. Nat Biotechnol. 2009;27(1):66–75. doi: 10.1038/nbt.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnabel CA, Jacobs Y, Cleary ML. HoxA9-mediated immortalization of myeloid progenitors requires functional interactions with TALE cofactors Pbx and Meis. Oncogene. 2000;19(5):608–616. doi: 10.1038/sj.onc.1203371. [DOI] [PubMed] [Google Scholar]
- 28.Chang CP, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol Cell Biol. 1997;17(10):5679–5687. doi: 10.1128/mcb.17.10.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahony S, Benos PV. STAMP: a web tool for exploring DNA-binding motif similarities. Nucleic Acids Res. 2007;35(Web Server issue):W253–W258. doi: 10.1093/nar/gkm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin H, Liu T, Manrai AK, Liu XS. CEAS: cis-regulatory element annotation system. Bioinformatics. 2009;25(19):2605–2606. doi: 10.1093/bioinformatics/btp479. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein BE, Mikkelsen TS, Xie X, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125(2):315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 32.King DC, Taylor J, Elnitski L, Chiaromonte F, Miller W, Hardison RC. Evaluation of regulatory potential and conservation scores for detecting cis-regulatory modules in aligned mammalian genome sequences. Genome Res. 2005;15(8):1051–1060. doi: 10.1101/gr.3642605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor J, Tyekucheva S, King DC, Hardison RC, Miller W, Chiaromonte F. ESPERR: learning strong and weak signals in genomic sequence alignments to identify functional elements. Genome Res. 2006;16(12):1596–1604. doi: 10.1101/gr.4537706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heintzman ND, Stuart RK, Hon G, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39(3):311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 35.Visel A, Blow MJ, Li Z, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457(7231):854–858. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song JS, Johnson WE, Zhu X, et al. Model-based analysis of two-color arrays (MA2C). Genome Biol. 2007;8(8):R178. doi: 10.1186/gb-2007-8-8-r178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Shen H, Himmel KL, et al. Leukaemia disease genes: large-scale cloning and pathway predictions. Nat Genet. 1999;23(3):348–353. doi: 10.1038/15531. [DOI] [PubMed] [Google Scholar]
- 38.Mikkers H, Berns A. Retroviral insertional mutagenesis: tagging cancer pathways. Adv Cancer Res. 2003;88:53–99. doi: 10.1016/s0065-230x(03)88304-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang GG, Pasillas MP, Kamps MP. Meis1 programs transcription of FLT3 and cancer stem cell character, using a mechanism that requires interaction with Pbx and a novel function of the Meis1 C-terminus. Blood. 2005;106(1):254–264. doi: 10.1182/blood-2004-12-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L. GADEM: a genetic algorithm guided formation of spaced dyads coupled with an EM algorithm for motif discovery. J Comput Biol. 2009;16(2):317–329. doi: 10.1089/cmb.2008.16TT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLeay RC, Bailey TL. Motif Enrichment Analysis: a unified framework and an evaluation on ChIP data. BMC Bioinformatics. 2010;11:165. doi: 10.1186/1471-2105-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovick MS, Hiebert SW, Friedman AD, Hetherington CJ, Tenen DG, Zhang DE. Multiple functional domains of AML1: PU.1 and C/EBPalpha synergize with different regions of AML1. Mol Cell Biol. 1998;18(7):3915–3925. doi: 10.1128/mcb.18.7.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada T, Shimizu T, Suzuki M, et al. Interaction between the homeodomain protein HOXC13 and ETS family transcription factor PU.1 and its implication in the differentiation of murine erythroleukemia cells. Exp Cell Res. 2008;314(4):847–858. doi: 10.1016/j.yexcr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Yamada T, Shimizu T, Sakurai T, et al. Physical and functional interactions between hematopoietic cell-specific ETS transcription factors and homeodomain proteins. Leuk Res. 2009;33(3):483–489. doi: 10.1016/j.leukres.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Dorsam ST, Ferrell CM, Dorsam GP, et al. The transcriptome of the leukemogenic homeoprotein HOXA9 in human hematopoietic cells. Blood. 2004;103(5):1676–1684. doi: 10.1182/blood-2003-07-2202. [DOI] [PubMed] [Google Scholar]
- 47.Argiropoulos B, Palmqvist L, Yung E, et al. Linkage of Meis1 leukemogenic activity to multiple downstream effectors including Trib2 and Ccl3. Exp Hematol. 2008;36(7):845–859. doi: 10.1016/j.exphem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Thoms JA, Birger Y, Foster S, et al. ERG promotes T-acute lymphoblastic leukemia and is transcriptionally regulated in leukemic cells by a stem cell enhancer. Blood. 2011;117(26):7079–7089. doi: 10.1182/blood-2010-12-317990. [DOI] [PubMed] [Google Scholar]
- 49.Rozovskaia T, Ravid-Amir O, Tillib S, et al. Expression profiles of acute lymphoblastic and myeloblastic leukemias with ALL-1 rearrangements. Proc Natl Acad Sci U S A. 2003;100(13):7853–7858. doi: 10.1073/pnas.1132115100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hess JL, Bittner CB, Zeisig DT, et al. c-Myb is an essential downstream target for homeobox-mediated transformation of hematopoietic cells. Blood. 2006;108(1):297–304. doi: 10.1182/blood-2005-12-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oshima M, Endoh M, Endo TA, et al. Genome-wide analysis of target genes regulated by HoxB4 in hematopoietic stem and progenitor cells developing from embryonic stem cells. Blood. 2011;117(15):e142–150. doi: 10.1182/blood-2010-12-323212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang GG, Pasillas MP, Kamps MP. Persistent transactivation by meis1 replaces hox function in myeloid leukemogenesis models: evidence for co-occupancy of meis1-pbx and hox-pbx complexes on promoters of leukemia-associated genes. Mol Cell Biol. 2006;26(10):3902–3916. doi: 10.1128/MCB.26.10.3902-3916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landry JR, Bonadies N, Kinston S, et al. Expression of the leukemia oncogene Lmo2 is controlled by an array of tissue-specific elements dispersed over 100 kb and bound by Tal1/Lmo2, Ets, and Gata factors. Blood. 2009;113(23):5783–5792. doi: 10.1182/blood-2008-11-187757. [DOI] [PubMed] [Google Scholar]
- 54.Wilson NK, Timms RT, Kinston SJ, et al. Gfi1 expression is controlled by five distinct regulatory regions spread over 100 kilobases, with Scl/Tal1, Gata2, PU. 1, Erg, Meis1, and Runx1 acting as upstream regulators in early hematopoietic cells. Mol Cell Biol. 2010;30(15):3853–3863. doi: 10.1128/MCB.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleh M, Rambaldi I, Yang XJ, Featherstone MS. Cell signaling switches HOX-PBX complexes from repressors to activators of transcription mediated by histone deacetylases and histone acetyltransferases. Mol Cell Biol. 2000;20(22):8623–8633. doi: 10.1128/mcb.20.22.8623-8633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chariot A, van Lint C, Chapelier M, Gielen J, Merville MP, Bours V. CBP and histone deacetylase inhibition enhance the transactivation potential of the HOXB7 homeodomain-containing protein. Oncogene. 1999;18(27):4007–4014. doi: 10.1038/sj.onc.1202776. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Iwasaki M, Ficara F, et al. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell. 2010;17(6):597–608. doi: 10.1016/j.ccr.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278(5340):1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 59.Heuser M, Sly LM, Argiropoulos B, et al. Modeling the functional heterogeneity of leukemia stem cells: role of STAT5 in leukemia stem cell self-renewal. Blood. 2009;114(19):3983–3993. doi: 10.1182/blood-2009-06-227603. [DOI] [PubMed] [Google Scholar]
- 60.Obermann EC, Arber C, Jotterand M, Tichelli A, Hirschmann P, Tzankov A. Expression of pSTAT5 predicts FLT3 internal tandem duplications in acute myeloid leukemia. Ann Hematol. 2010;89(7):663–669. doi: 10.1007/s00277-009-0890-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ChIP-Sequencing, gene expression, and ChIP-chip data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus25 (GSE33518). Visualization tracks (UCSC) for all data, including motif enrichment analysis results, are available at http://www.pathology.med.umich.edu/faculty/Hess/index.html.