Abstract
BACKGROUND
Preeclampsia (PE) is more common at high than low altitude and contributes to the altitude-related decline in birth weight. Since inflammatory markers are implicated in PE, we asked if such markers differed in PE vs. normotensive pregnant (NORM) women residing at high altitude (3600-4100 m), and were related to uterine artery blood flow (UA BF) or fetal growth.
METHODS
Subjects were 33 Andean pregnant residents of Bolivia, comprising six with early-onset PE (≤ 34 wk), 12 with late-onset PE (> 34 wk), and 15 gestational-age matched NORM. Maternal pro- and anti-inflammatory cytokines were measured using a multiplex bead-based assay and UA BF by Doppler ultrasound.
RESULTS
PE compared to NORM women had higher levels of the pro-inflammatory cytokines IL-6 and IL-8 as well as higher levels of the anti-inflammatory cytokine IL-1ra, but only IL-6 levels were higher when gestational age was controlled. Women with early- vs. late-onset PE had higher TNFα levels, and higher IL-6 was negatively correlated with birth weight in all women at ≤ 34 wk. We suggest that pro-inflammatory factors influence both the timing and severity of PE at high altitude.
Keywords: Andean, birth weight, fetal growth, hypoxia
INTRODUCTION
Bolivia has one of the highest maternal and infant mortality rates in Latin America [1], which is likely due in part to the increased incidence of preeclampsia (PE) and small-for-gestational age (SGA) infants seen at high altitude [2-4] where nearly two-thirds of its population resides. PE is characterized by impaired trophoblast invasion and spiral artery remodeling [5-7] that, in turn, raises uteroplacental vascular resistance, reduces uterine artery blood flow (UA BF) [8, 9], and leads to symptoms that collectively define the condition. At low altitude, an excessive inflammatory response is thought to contribute to the vascular dysfunction and endothelial damage characteristic of the disease [10-16]. While ambient hypoxia likely contributes to the increased frequency of PE at high altitude, the roles of pro- and anti-inflammatory cytokines in such an increase is unknown.
Given that cytokines are integral to the immunological imbalance characteristic of PE, we asked if systemic inflammatory markers differed in high-altitude residents with PE compared to normotensive controls (NORM) and, if so, whether these factors related to UA BF and fetal growth. Since early-onset PE is particularly severe at high altitude [17], comparisons were also conducted between women with early- (≤34 wk) vs. late- (> 34 wk) onset disease in an effort to develop criteria for identifying women at greatest risk, allocating scarce health-care resources, and alleviating the heavy toll exerted by PE in high-altitude regions.
Material and methods
Subjects
A total of 33 women were studied, comprised of six with early-onset PE (≤ 34 wk), 12 with late-onset (> 34 wk) disease, and 15 gestational-age matched NORM living in La Paz or El Alto, Bolivia (3600-4000 m). NORM women were recruited during their prenatal visits and PE women contacted through high-risk clinics at the several hospitals serving this region. Studies were conducted at wk 28.3±1.6 of pregnancy in early-onset PE women and at wk 29.2 ± 1.5 in their NORM controls (p=NS). Women with late-onset PE were studied at wk 37.4±0.6 of pregnancy and at wk 36.0 ± 0.8 for their NORM controls (p=NS).
Subjects were excluded if they had lived at low altitude for more than three months, smoked, consumed more than two alcoholic beverages weekly, or had any known risk factor for developing PE (i.e., a current multiple-gestation pregnancy, chronic hypertension, gestational diabetes or any cardiovascular or renal disease). Each woman gave written informed consent approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Denver and its Bolivian counterpart, Colegio Médico.
Protocol, Variables and Instrumentation
We employed a cross-sectional study design in which women were studied once between 28.8 ± 1.0 and 36.3 ± 1.5 wks of pregnancy. Information was obtained from each woman by self-report concerning her altitude of birth, age, duration of residence at high altitude, years of education, monthly household income, and reproductive and health history. All women were of predominantly Andean descent as is reported for the larger group of subjects of which these women were a part [17]. Gestational age was calculated from the date of the last menstrual period. A physical exam was conducted for measuring height by stadiometer, weight by balance scale, and blood pressure by arm cuff sphygmomanometer with values expressed as an average of left and right arm measurements. Diagnosis of PE required elevated systolic (>140 mmHg) and diastolic (>90 mmHg) blood pressures after the 20th week of pregnancy in a woman who was normotensive prior to pregnancy, and the presence of ≥1+ proteinuria at presentation and confirmed by protein levels ≥300 mg in a 24-h collection. Women were classified as having early-onset PE if symptoms developed ≤ 34 wk of gestation or late-onset PE if they began > 34 wk of pregnancy [18]. NORM women were studied at similar time points during pregnancy to serve as controls.
Peripheral blood samples were obtained from the antecubital vein at the time of study. Blood was withdrawn into collection tubes containing citrate, centrifuged and plasma stored in 1 mL aliquots at -80°C. The cytokines IL-1β, IL6, IL-8, IL-10, TNFα, and IL-1ra were assayed simultaneously and in duplicate using a human cytokine 6-plex antibody bead kit (Bio Rad Laboratories, Richmond, CA) and a Luminex-100 array assay reader (Luminex Corp, Austin, TX). Analyte levels were reported if values exceeded the minimum standard concentrations as calculated from standard curves using 5-PL logistic regression as specified by the manufacturer.
UA characteristics were measured percutaneously using a Micro Maxx (Sonosite, Inc. Bothell, WA) equipped with C60e and L38e transducers as previously described [17]. Briefly, the UA was imaged where the vessel appears to cross the external iliac artery [19]. UA blood flow was calculated as the sum of left- and right-sided flows using the equation Q = πr2 × TAM × 60 where r is the mean vessel radius (cm), TAM the time-averaged mean flow velocity (cm/s), and 60 to convert values to mL/min. To eliminate inter-operator error, a single individual performed all measurements. The UA resistance index (UA RI) and fetal biometry data were also obtained using the Micro Maxx, and birth weight, length, gestational age and infant sex recorded by hospital personnel at delivery.
Statistics
Data are reported as the mean ± standard error of the mean (SEM) or 95% confidence interval (CI) for proportions. Group means for continuous variables were compared using one-way analysis of variance (ANOVA) with multiple comparisons and χ2- tests for nominal or ordinal variables. Birth weights were corrected for gestational age at the time of delivery among early- or late-onset between PE women and their respective controls. Relationships between cytokine levels and UA RI, UA BF, or birth weight were assessed using linear regression with the actual gestational age at time of study included as a covariate. All analyses were conducted using SPSS v.12 (Chicago, IL). Significance was reported when p-values were < 0.05 and trends considered when 0.05 < p < 0.10.
RESULTS
Maternal characteristics
Women who developed PE vs. NORM women were similar in height and parity but older, heavier, had higher monthly income and, of course, had higher blood pressures (Table 1A). Preeclamptic women more frequently delivered by Caesarean section (c-section) compared to controls (65% vs. 15% csection, respectively; p<0.01). Women with early vs. late-onset PE did not differ in any characteristic (Table 1B), including the frequency of c-section compared to vaginal delivery (33% vs. 75% c-section, respectively; p=0.08).
Table 1.
Maternal characteristics
| A. Comparison of normotensive (NORM) and preeclamptic (PE) pregnant women
| |||
|---|---|---|---|
| Variables | NORM | PE | p-value |
| Age, yr | 26.7 ± 1.5 (15) | 31.0 ± 1.4 (18) | p<0.05 |
| Height, cm | 150.3 ± 0.9 (15) | 152.3 ± 1.3 (18) | NS |
| Weight, kg | 65.0 ± 1.9 (15) | 75.5 ± 2.9 (18) | p<0.01 |
| Parity, no. of live births | 2.0 ± 0.0 (15) | 2.0 ± 0.0 (18) | NS |
| Monthly Income, US dollars | 94.3 ± 13.1 (15) | 164.7 ± 22.1 (18) | p<0.05 |
| Systolic blood pressure, mmHg | 94.7 ± 2.0 (15) | 131.1 ± 4.0 (18) | p<0.001 |
| Diastolic blood pressure, mmHg | 64.7 ± 2.3 (15) | 89.4 ± 3.3 (18) | p<0.001 |
| Mean arterial pressure, mmHg | 85.7 ± 2.0 (15) | 117.4 ± 3.7 (18) | p<0.001 |
|
| |||
|
B. Comparison between women with early- and late-onset PE
| |||
| Variables | Early-onset PE | Late-onset PE | p-value |
|
| |||
| Age, yr | 30.3 ± 3.0 (6) | 31.4 ± 1.7 (12) | NS |
| Height, cm | 154.2 ± 2.0 (6) | 151.3 ± 1.7 (12) | NS |
| Weight, kg | 70.0± 6.3 (6) | 78.3 ± 2.9 (12) | NS |
| Parity, no. of live births | 2.0 ± 0.0 (6) | 2.0 ± 0.0 (12) | NS |
| Monthly Income, US dollars | 188.1 ± 50.76 (6) | 153.0 ± 22.5 (12) | NS |
| Systolic blood pressure, mmHg | 131.7 ± 7.0 (6) | 130.8 ± 5.0 (12) | NS |
| Diastolic blood pressure, mmHg | 90.0 ± 5.8 (6) | 89.2 ± 4.2 (12) | NS |
| Mean arterial pressure, mmHg | 117.5 ± 6.5 (6) | 117.3 ± 4.7 (12) | NS |
Values are shown as mean ± SEM, with sample sizes in parentheses.
Pro-and anti-inflammatory cytokines
Comparing all subjects, PE compared to NORM women had higher levels of the pro-inflammatory IL-6 and IL-8 as well as higher anti-inflammatory IL-1ra cytokines but only IL-6 was higher after controlling for gestational age (Table 2). Women with early- vs. late-onset PE had higher levels of TNFα only.
Table 2.
Pro- and anti-inflammatory cytokines in all normal vs. preeclamptic (PE) pregnant women, and in early- (≤ 34 wk) vs. late- (>34 wk) onset PE women and their respective normotensive (Normal) controls.
| Proinflammatory cytokines | Anti-inflamatory cytokines | ||||||
|---|---|---|---|---|---|---|---|
| Pregnancy status | IL-1ra, pg/ml | IL-6, pg/ml | TNFα, pg/ml | IL-8, pg/ml | IL-10, pg/ml | IL-1ra, pg/ml | |
| Normal | 0.39 ± 0.03 (15) | 2.50 ± 1.22 (13) | 1.74 ± 0.35 (9) | 2.90 ± 0.31 (15) | 1.49 ± 0.13 (15) | 26.69 ± 3.14 (11) | |
| Preeclampsia | 0.42 ± 0.03 (15) | 9.29 ± 1.22 (13) | 1.13 ± 0.37 (8) | 4.05 ± 0.31 (15) | 1.67 ± 0.13 (16) | 37.58 ± 3.94 (7) | |
| p-value | NS | p<0.001 | NS | p<0.05 | NS | p<0.05 | |
| Preeclampsia | |||||||
| Early onset | 0.42 ± 0.13 (4) | 12.53 ± 3.30 (4) | 2.14 ± 0.69 (3) | 3.08 ± 1.34 (4) | 1.53 ± 0.59 (5) | 36.59 ± 10.21 (3) | |
| Late onset | 0.42 ± 0.05 (11) | 7.58 ± 2.05 (9) | 0.54 ± 0.45 (5) | 4.39 ± 0.60 (11) | 1.74 ± 0.31 (11) | 33.16 ± 15.61 (4) | |
| p-value | NS | NS | p<0.05 | NS | NS | NS | |
| Early-onset PE and controls | |||||||
| Preeclampsia | 0.42 ± 0.13 (4) | 12.53 ± 3.30 (4) | 2.14 ± 0.69 (3) | 3.08 ± 1.34 (4) | 1.53 ± 0.59 (5) | 36.59 ± 10.21 (3) | |
| Normal | 0.39 ± 0.06 (6) | 3.47 ± 1.90 (4) | 3.33 ± 0.65 (3) | 2.53 ± 0.68 (6) | 1.53 ± 0.29 (6) | 27.59 ± 5.48 (6) | |
| p-value | NS | p<0.001 | NS | NS | NS | NS | |
| Late-onset PE and controls | |||||||
| Preeclampsia | 0.42 ± 0.05 (11) | 7.58 ± 2.05 (9) | 0.54 ± 0.45 (5) | 4.39 ± 0.60 (11) | 1.74 ± 0.31 (11) | 33.16 ± 15.61 (4) | |
| Normal | 0.40 ± 0.04 (9) | 2.02 ± 1.90 (7) | 1.01 ± 0.40 (6) | 3.08 ± 0.45 (9) | 1.47 ± 0.20 (9) | 27.59 ± 5.48 (5) | |
| p-value | NS | p<0.05 | NS | NS | NS | NS | |
Values are mean ± SEM, with sample sizes in parentheses.
Uterine artery characteristics
Women with early-onset PE had higher UA resistance indices (UA RI) and markedly lower UA BF when compared to early NORM despite similar vessel diameters, indicating that differences in flow velocity accounted for the lower blood flows (Table 3). Women who developed late-onset PE had greater UA RI than their NORM controls but UA BF and diameter were similar.
Table 3.
Uterine artery characteristics and fetal outcomes
| Early Pregnancy | Late Pregnancy | |||||
|---|---|---|---|---|---|---|
| Variables | Preeclampsia | Normal | p-value | Preeclampsia | Normal | p-value |
| UA resistance index | 0.7 ± 0.0 (6) | 0.5 ± 0.0 (6) | p<0.001 | 0.5 ± 0.0 (12) | 0.4 ± 0.0 (9) | p<0.01 |
| UA blood flow, mL/min | 595 ± 76 (6) | 1405 ± 172 (6) | p<0.01 | 1496 ± 241 (12) | 1883 ± 241 (9) | NS |
| UA diameter, cm | 0.61 ± 0.02 (6) | 0.58 ± 0.03 (6) | NS | 0.64 ± 0.05 (12) | 0.69 ± 0.03 (9) | NS |
| Biparietal diameter, cm | 6.9 ± 0.4 (6) | 7.3 ± 0.4 (6) | NS | 8.9 ± 0.1 (12) | 8.8 ± 0.1 (9) | NS |
| Head circumference, cm | 25.7 ± 1.6 (6) | 27.0 ± 1.3 (6) | NS | 32.0 ± 0.4 (12) | 31.8 ± 0.4 (9) | NS |
| Abdominal circumference, cm | 23.6 ± 1.9 (6) | 25.6 ± 1.7 (6) | NS | 32.2 ± 0.9 (12) | 32.8 ± 1.3 (9) | NS |
| Femur length, cm | 4.9 ± 0.4 (6) | 5.6 ± 0.3 (6) | NS | 7.0 ± 0.2 (12) | 7.3 ± 0.1 (9) | NS |
| GA at birth, wks | 29.3 ± 1.7 (6) | 39.8 ± 0.5 (6) | p<0.01 | 37.9 ± 0.6 (11) | 38.4 ± 0.4 (9) | NS |
| Birth weight, gr | 1350 ± 252 (6) | 3278 ± 213 (6) | p<0.001 | 2859 ± 154 (12) | 3271 ± 97 (9) | p<0.05 |
Values are shown as mean ± SEM, with sample sizes in parentheses
Abbreviations: UA: uterine artery; GA: gestational age
Fetal growth characteristics
Fetal biometry values at the time of study were similar in the early- or late-onset PE groups compared to their respective controls but infants born to women with early-onset PE weighed less, and were delivered on average nine weeks earlier (Table 3). Earlier delivery was not due to differences in mode of delivery, given that the frequency of c-sections was equivalent between groups. Infants born to women with late-onset PE also weighed less than those born to NORM women but their gestational ages were similar.
Relationship between pro-and anti-inflammatory cytokines and UA and fetal characteristics
Although sample sizes were very small due to the inability to measure cytokine levels below the assay’s limit of detection, higher pro-inflammatory cytokine IL-1β levels were closely correlated with higher UA BF among women with early-onset PE (Figure 1). This resulted from a strong inverse relationship with UA RI (R2=0.56, p<0.05), indicating that variation in velocity rather than UA diameter was responsible.
Figure 1.
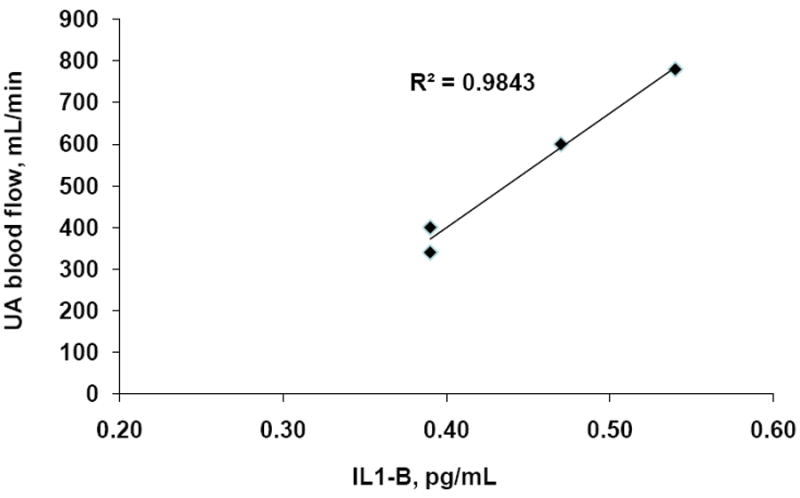
Pro-inflammatory cytokine IL-1β levels were positively associated with uterine artery (UA) blood flow among women with early-onset PE.
Higher pro-inflammatory IL-6 cytokine levels were negatively associated with infant birth weight in all women studied ≤ 34 wk of pregnancy (Figure 2). The relationships between IL-6 levels and birth weight were present within both subgroups as well (early-onset PE: R2=0.93, p<0.05; NORM: R2=0.81, p<0.05).
Figure 2.
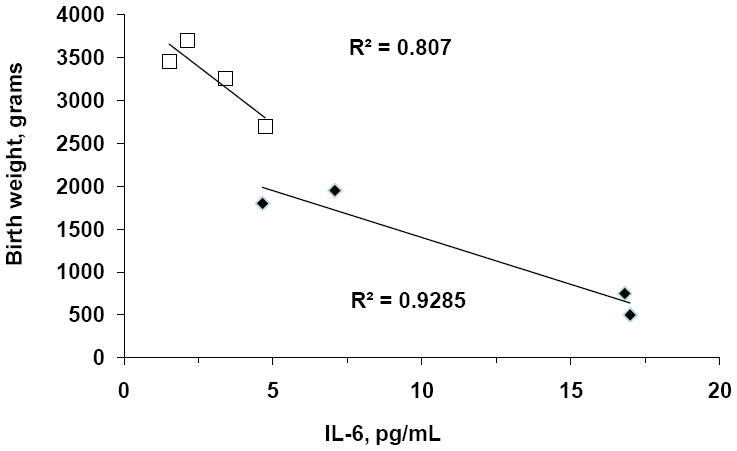
IL-6 cytokine levels were negatively associated with infant birth weight in all women studied ≤ 34 wk of pregnancy. The relationships between IL-6 levels and birth weight were present within both subgroups as well [early-onset preeclampsia (open square symbols: R2=0.93, p<0.05); early normal pregnancy (solid black diamond symbols: R2=0.81, p<0.05)].
DISCUSSION
To our knowledge this is the first study to compare pro- and anti-inflammatory cytokines between women with early- and late-onset PE at high altitude, and to explore the relationship between these variables and compromised UA BF and fetal growth. Our principal findings were that levels of the pro-inflammatory cytokine IL-6 were greater in PE than gestational age-matched NORM women and related closely to a diminution in the birth weight of babies born to all women who were studied ≤ 34 wk or just those with early-onset PE. Together with the higher levels of the pro-inflammatory cytokine TNFα in women with early- vs. late-onset PE, we concluded that pro-inflammatory cytokines adversely influence disease severity and contribute to the heavy toll exerted by PE in high-altitude regions.
Previous studies of PE at low altitude have described increases in pro- as well as anti-inflammatory cytokine levels. These findings have been interpreted as suggesting that an excessive inflammatory response causes vascular endothelial damage that leads, in turn, to the maternal symptoms that define the disease [20-22]. Our high-altitude observations agree with this literature insofar as PE compared to NORM women had higher levels of the pro-inflammatory IL-6 [11, 23] and IL-8 [24] cytokines. In line with reports that IL-6 is high in PE compared to gestational-age matched controls [23], only IL-6 levels were higher in the PE than NORM women after controlling for gestational age. Together with the negative association between IL-6 and birth weight, this pro-inflammatory cytokine appears to play a role in the fetal growth restriction present at high altitude and especially when high altitude and PE are combined.
Early-onset PE is associated with higher rates of intrauterine growth restriction [25] and thus considered more severe than late-onset disease [26]. This is particularly true at high altitude where there is a more frequent occurrence of stillbirths in early- vs. late-onset PE [2]. Our findings are consistent with these observations insofar as women with early-onset PE more frequently delivered prematurely and had lower birth-weight babies compared with NORM controls or those with late-onset PE. This was true whether absolute or gestational-age adjusted birth weights were considered (data not shown), indicating that fetal growth restriction rather than shortened gestation was chiefly responsible for the lower birth weights observed. Given previous reports that elevated TNFα levels can cause vascular dysfunction [22, 27] and raise blood pressure in pregnant rats [28], our finding of higher TNFα levels in early- than late-onset PE suggests that higher levels of this pro-inflammatory cytokine may be one factor influencing the earlier timing and greater severity of early-onset PE.
A prominent model for the etiology of PE involves impaired trophoblast invasion and spiralartery remodeling that collectively raise UA RI and lower UA BF [6] [29]. Our data are consistent with this insofar as women with early-onset PE had higher UA RI and lower UA BF than NORM women or women with late-onset PE. Supporting the involvement of cytokines were the higher levels of the pro-inflammatory IL-6 seen in all PE vs. NORM women or just the gestational-age matched NORM women, as well as the negative relationship between IL-6 and the birth weight of babies born to women with early-onset disease. However, higher cytokines could not account for the reduction in UA BF seen in the PE women. Rather IL-1β levels were positively correlated with UA BF in healthy Andean high-altitude residents studied at week 20 [30] and in the small numbers of women with early-onset PE reported here. While our present observations are limited by small sample size, IL-1β has been described as a positive regulator of trophoblast differentiation [31] and to have angiogenic as well as vasodilatory effects [32, 33]. Thus, perhaps in additions to its pro-inflammatory actions, IL-1β may also contribute to raising UA blood flow at mid- pregnancy and helping to defend UA blood flow in severe pregnancy complications.
We concluded that pro- and anti-inflammatory cytokines are likely involved in the timing of the onset of PE at high altitude, its severity, and consequently reduced fetal growth. Additional studies are needed to confirm the present observations and to better define the contribution of cytokines to pregnancy- and altitude-associated alterations in UA BF and fetal growth. Since numerous other factors such as angiogenic substances, antioxidants, and glucose as well as other nutrient availability are also important for fetal growth [34-36], the influence of cytokines for preeclampsia and fetal growth we report here are likely not acting alone.
Acknowledgments
We thank all the women who kindly agreed to participate in this study, as well as to the many technicians for their invaluable technical support. In particular, we thank Martha Aguilar, Ana-María Alarcón, Dolly Condori, Cristina Gonzáles, Jennifer Hageman, Freddy Limachi, Lourdes Mabrich, Zaida Martínez, Gene and Rosann McCullough, Armando Rodriguez, and Wilmar Velásquez. Financial support was received from grants from the National Institutes of Health (HL60131, HL07171, and HL079647).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conde-Agudelo A, Belizan JM, Diaz-Rossello JL. Epidemiology of fetal death in Latin America. Acta Obstet Gynecol Scand. 2000;79(5):371–8. [PubMed] [Google Scholar]
- 2.Keyes LE, et al. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54(1):20–5. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 3.Mahfouz AA, et al. Altitude and socio-biological determinants of pregnancy-associated hypertension. Int J Gynaecol Obstet. 1994;44(2):135–8. doi: 10.1016/0020-7292(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SK, et al. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180(5):1161–8. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 5.Meekins JW, et al. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101(8):669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 6.Pijnenborg R, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98(7):648–55. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin S, et al. Uterine artery Doppler velocimetry in relation to trophoblast migration into the myometrium of the placental bed. Obstet Gynecol. 1995;85(5 Pt 1):760–5. doi: 10.1016/0029-7844(95)00020-r. [DOI] [PubMed] [Google Scholar]
- 8.Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci. 2007;12:2967–77. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamudio S, et al. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol. 1995;79(1):15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Freeman DJ, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–14. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 11.Greer IA, et al. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84(6):937–40. [PubMed] [Google Scholar]
- 12.Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2006;13(5):680–6. doi: 10.1016/s1472-6483(10)60659-1. [DOI] [PubMed] [Google Scholar]
- 13.Raghupathy R, Kalinka J. Cytokine imbalance in pregnancy complications and its modulation. Front Biosci. 2008;13:985–94. doi: 10.2741/2737. [DOI] [PubMed] [Google Scholar]
- 14.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59(2):161–73. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 15.Omu AE, et al. Differential levels of T helper cytokines in preeclampsia: pregnancy, labor and puerperium. Acta Obstet Gynecol Scand. 1999;78(8):675–80. [PubMed] [Google Scholar]
- 16.Teran E, et al. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001;75(3):243–9. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 17.Browne VA, et al. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol. 2011;300(5):R1221–9. doi: 10.1152/ajpregu.91046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 19.Palmer SK, et al. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstetrics and Gynecology. 1992;80:1000–1006. [PubMed] [Google Scholar]
- 20.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106(2):c72–81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 21.Gadonski G, et al. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48(4):711–6. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 22.Granger JP. Inflammatory cytokines, vascular function, and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R989–90. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lockwood CJ, et al. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172(6):1571–9. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowen RS, et al. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12(6):428–32. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Xiong X, et al. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155(3):203–9. doi: 10.1093/aje/155.3.203. [DOI] [PubMed] [Google Scholar]
- 26.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 27.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37(3):240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 28.Davis JR, et al. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R390–9. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 29.Prefumo F, Sebire NJ, Thilaganathan B. Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum Reprod. 2004;19(1):206–9. doi: 10.1093/humrep/deh037. [DOI] [PubMed] [Google Scholar]
- 30.Davila RD, et al. Do Cytokines Contribute to the Andean-Associated Protection From Reduced Fetal Growth at High Altitude? Reprod Sci. 2010 doi: 10.1177/1933719110380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Librach CL, et al. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269(25):17125–31. [PubMed] [Google Scholar]
- 32.Takizawa S, Ozaki H, Karaki H. Interleukin-1beta-induced, nitric oxide-dependent and - independent inhibition of vascular smooth muscle contraction. Eur J Pharmacol. 1997;330(2-3):143–50. doi: 10.1016/s0014-2999(97)00164-7. [DOI] [PubMed] [Google Scholar]
- 33.Voronov E, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100(5):2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maynard S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davila RD, et al. Do anti-angiogenic or angiogenic factors contribute to the protection of birth weight at high altitude afforded by Andean ancestry? Reprod Sci. 2010;17(9):861–70. doi: 10.1177/1933719110372418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Julian CG, V E, Browne VA, Wilson MJ, Bigham AW, Rodriguez C, McCord JM, Moore LG. Potential role for elevated maternal enzymatic antioxidant status in Andean protection against altitude-associated SGA. The Journal of Maternal-Fetal & Neonatal Medicine. 2011 doi: 10.3109/14767058.2011.636102. In Progress. [DOI] [PMC free article] [PubMed] [Google Scholar]


