Abstract
Previous studies have demonstrated that patients with borderline personality disorder (BPD) tend to misattribute malevolence to benign social stimuli, including facial expressions. Yet, facial emotion recognition studies examining those with BPD have yielded mixed results, with some studies showing impaired accuracy and others demonstrating enhanced accuracy in the recognition of emotions or mental states. The current study examined the ability to decode mental states from photographs of just the eye region of faces in a nonclinical sample of young adults who exhibited BPD traits (high BPD) compared with those who did not (low BPD). Group differences in mental state decoding ability depended on the valence of the stimuli. The high-BPD group performed better for negative stimuli compared with the low-BPD group, but did not perform significantly different from the low-BPD group for stimuli of neutral or positive valence. The high-BPD group also demonstrated a response bias for attributing negative mental states to facial stimuli. In addition, findings suggested that the group difference in accuracy for negative stimuli could not be explained by response bias, because the group difference in response bias for negative stimuli did not reach significance. These findings suggest that BPD traits may be associated with enhanced ability to detect negative emotions and a bias for attributing negative emotions to nonnegative social stimuli.
Keywords: borderline personality disorder, mental state decoding, emotion recognition, response bias, Reading the Mind in the Eyes Test
Borderline personality disorder (BPD) is a chronic and debilitating disorder characterized by emotional instability, interpersonal dysfunction, identity disturbance, impulsivity, self-injury, and suicidality. BPD is estimated to occur in 1% to 6% of the general population (Grant et al., 2008; Lenzenweger, Lane, Loranger, & Kessler, 2007; Samuels et al., 2002; Taylor & Reeves, 2007; Torgerson, Kringlen, & Cramer, 2001) and is especially prevalent in young adults under the age of 35 (Stone, 1990). Emotional dysregulation, particularly within interpersonal contexts, is one of the most problematic and enduring features of BPD (Linehan, 1993; McGlashan et al., 2005). Both clinicians and investigators have noted that negative affective states among BPD patients are often precipitated by real or imagined events in relationships (Ebner-Priemer et al., 2007 Stiglmayr et al., 2005; Herpertz, 1995; ). Evidence also suggests that BPD features are associated with increased interpersonal stress (Daley, Hammen, Davila, & Burge, 1998; Trull, 1995), intense affective responses to social stimuli (Herpertz et al., 1997), and amygdala hyperactivation in response to socially relevant stimuli (Donegan et al., 2003; Herpertz et al., 2001; Silbersweig et al., 2007).
These clinical and empirical observations have led several theorists to suggest that impaired social cognition is a core mechanism underlying the development and maintenance of BPD (Bateman & Fonagy, 2003; Bender & Skodol, 2007; Levy, 2005; Levy et al., 2006; Westen, 1991). Bateman and Fonagy (2003) have proposed that BPD is characterized by a deficit or inhibition in the capacity for mentalization, a social–cognitive ability that allows one to reflect on one’s own mental experience and the mental experiences of others in terms of underlying emotional states. Mentalization is a concept that is closely related to theory of mind, defined broadly as the ability to ascribe mental states to others and to understand and predict others’ social behavior (e.g., Baron-Cohen, 1989; Langdon, Coltheart, Ward, & Catts, 2002). However, mentalization also involves reflection on the mental states of oneself, in addition to those of others. Linehan (1993) has discussed deficits in a meta-social–cognitive capacity called mindfulness, which involves observing, reflecting, and describing emotional experiences while developing focused attention. Several other theorists have discussed similar social–cognitive deficits as central to BPD (e.g., Blatt, Auerbach, & Levy, 1997; Gunderson, 1996; Kernberg, 1984; Levy & Blatt, 1999; Westen, 1991; Young, Klosko, & Weishaar, 2003). Despite distinctions between many of these theories, they all share a common hypothesis that BPD patients lack the ability to adequately process and appraise emotional information, particularly within social contexts. In addition, a growing body of evidence demonstrates cognitive problems in BPD patients, and several authors have suggested that these difficulties may interact with experiences of childhood maltreatment or neglect, potentially creating vulnerability for problems with mentalization and flexible cognitive processing of social information (Fertuck, Lenzenweger, Clarkin, Hoermann, & Stanley, 2006; Fonagy, Gergely, Jurist, & Target, 2002; Judd, 2005; Judd & McGlashan, 2003; Minzenberg, Poole, & Vinogradov, 2008).
Both clinicians and researchers have noted that one manifestation of social–cognitive dysfunction in BPD might be impairment in the ability to decode mental states or emotions from perceivable social information, such as human facial expressions (for a review, see Domes, Schulze, & Herpertz, 2009). The capacity for mental state reasoning based on facial expressions is a socioperceptual ability that has been described as an important component of theory of mind (Sabbagh, 2004; Tager-Flusberg, 2001). The ability to accurately infer emotional states based on features of the human face allows one to more accurately predict behaviors of others, respond empathically and appropriately in social situations, and regulate one’s own emotional state in social contexts. Thus, impaired facial emotion recognition may trigger extreme emotional reactions and interpersonal conflict. In patients with BPD, such impairment may lead to aggressive, impulsive, and self-destructive behaviors that are potentially life-threatening, functionally debilitating, and damaging to relationships (Domes et al., 2009; Silk, 2000; Yeomans & Levy, 2002).
Empirical studies of facial emotion recognition among individuals with BPD have generally yielded mixed results. There is some evidence that BPD patients may be impaired at recognizing basic emotions in static facial expressions (e.g., Bland, Williams, Scharer, & Manning, 2004; Levine, Marziali, & Hood, 1997). However, other studies suggest that BPD patients are not impaired in the recognition of basic emotions unless the stimuli are complex and multifaceted (Minzenberg, Poole, & Vinogradov, 2006) or the task requires them to rapidly discriminate between negative or neutral expressions (Dyck et al., 2009). A recent study (Lynch et al., 2006) using facial morphing stimuli suggested that BPD patients might have a lower emotion detection threshold than healthy controls, which suggests enhanced sensitivity to detection of emotion in facial expressions; on the other hand, a similar investigation (Domes et al., 2008) found no differences between women with BPD and healthy controls in emotion detection threshold or accuracy. However, the BPD group demonstrated enhanced learning in terms of reduced detection thresholds over the course of the experiment (Domes et al., 2008).
Although it remains unclear whether BPD patients show broad impairments or enhancements in emotion recognition, a consistent and robust finding across a number of studies is that BPD patients tend to imbue benign or neutral facial stimuli with negative emotion (Domes et al., 2008; Donegan et al., 2003; Dyck et al., 2009; Meyer, Pilkonis, & Beevers, 2004; Silbersweig et al., 2007; Wagner & Linehan, 1999). This negativity bias is consistent with evidence that individuals with BPD tend to perceive others as untrustworthy, rejecting, abandoning, and neglectful (Arntz, Dietzel, & Dreessen, 1999; Butler, Brown, Beck, & Grisham, 2002; Jovev & Jackson, 2004; Nordahl, Holthe, & Haugum, 2005). Taken together, these findings suggest that BPD symptoms may be associated with a subtle and specific abnormality in processing of emotions from perceivable social cues. In particular, BPD symptoms might be associated with a tendency to project negative emotions onto ambiguous or even benevolent social stimuli. Whether or not this perceptual bias results in impaired or enhanced emotion recognition accuracy remains an open question.
Most previous studies of facial emotion recognition among those with BPD have used stimuli comprising the entire face and representing only basic emotions, which can be insensitive to the detection of subtle individual differences in mental state decoding. However, the revised Reading the Mind in the Eyes task (RME; Baron-Cohen, Wheelwright, Hill, Raste & Plumb, 2001) is a relatively new method for examining emotion recognition abilities that involves the identification of complex mental states based on pictures of the eye region only (i.e., from the nose to the brow). In the RME task, participants are asked to select the mental state word that best describes the emotion portrayed in the picture from among four word choices. Unlike previously established facial emotion recognition stimuli that were rated based on coding systems (e.g., Ekman & Friesen, 1978), the RME task was developed based on consensus ratings. Because it is considerably more difficult than standard emotion recognition tasks, the RME is exceptionally sensitive to subtle individual differences in facial emotion recognition without being susceptible to floor or ceiling effects.
To date, only one published study (Fertuck et al., 2009) has examined RME performance of BPD patients as compared to a healthy control group. In this study, those with BPD actually performed better on the task relative to healthy comparison participants, and these effects were partially mediated by depression. These results are surprising given that Fonagy and Bateman (2008) have cited unpublished findings that BPD patients are impaired on the RME task relative to non-BPD patients. In addition, other empirical evidence suggests that RME performance is also impaired among those with clinically significant depression (Lee, Harkness, Sabbagh, & Jacobsen, 2005) as well as in several other psychiatric populations (e.g., Bora et al., 2005; Craig, Hatton, Craig, & Bentall, 2004; Farzin et al., 2006; Kelemen et al., 2005; Schmidt & Zachariae, 2009), although there is evidence that dysphoria among college students is associated with enhanced RME performance (Harkness, Sabbagh, Jacobson, Chowdrey, & Chen, 2005).
Due to the myriad inconsistencies in previous studies of facial emotion recognition in BPD patients, the association between BPD symptoms and complex mental state decoding abilities deserves further exploration. In addition, few previous studies have examined emotion recognition accuracy, response time, and response bias within the same task. Therefore, the current study examined mental state decoding accuracy, response time, and response biases using the RME task in a sample of college students who endorse a high number of BPD traits as compared to those who endorse few BPD traits. The investigation of BPD traits in a young nonclinical sample is relevant given the prevalence of BPD features in nonclinical populations, particularly among young adults (Lenzenweger, Loranger, Korfine, & Neff, 1997; Taylor & Reeves, 2007; Trull, 1995; Trull, Useda, Conforti, & Doan, 1997).
Based on empirical ratings obtained during pilot testing, RME stimuli without their corresponding mental state terms were categorized by valence in order to examine differences in accuracy for stimuli of different valence categories. Valence ratings were also obtained for all mental state words that appeared in the RME task in order to examine response biases. Considering previous findings of increased sensitivity to negative emotional displays and the misattribution of negativity to benign social stimuli among those with BPD (Domes et al., 2008; Donegan et al., 2003; Dyck et al., 2009; Meyer et al., 2004; Silbersweig et al., 2007; Wagner & Linehan, 1999; see Domes et al., 2009 for a review), we predicted that those with BPD traits would demonstrate enhanced accuracy for negative RME stimuli, as well as a bias toward attributing negative mental states to facial expressions. However, consistent with a negativity bias, we predicted that those with BPD traits would show impaired accuracy for neutral and positive RME stimuli. Response time, present-state affect, and state and trait anxiety were measured in order to control for the influence of speed–accuracy trade-offs and affective experiences on mental state attributions.
Method
Participants
In exchange for credit toward their introductory psychology class research participation requirement, 242 students from the introductory psychology participant pool at a major northeastern university participated in the study. All participants were administered a modified version of the McLean Screening Instrument for BPD (MSI-BPD; Zanarini et al., 2003) in order to derive study groups. Those scoring at least one SD above the mean on the MSI-BPD (n = 40) were identified for the high-BPD group, and those scoring at least one SD below the mean on the MSI-BPD (n = 50) were identified for the low-BPD group. Statistical outliers at p < .01 were identified in each group based on standardized scores more than 2.58 SD below the group mean for RME accuracy or 2.58 SD above or below the group mean for response time (i.e., those with unusually low accuracy or high/low response time). Using these criteria, six outliers were identified (four low-BPD participants and two high-BPD participants). After these participants’ data were excluded from analysis, there were 38 participants in the high-BPD group and 46 participants in the low-BPD group. The demographics characteristics of each sample are presented in Table 1.
Table 1.
Demographics and Clinical Characteristics of Each
| Low-BPD (n = 46) |
High-BPD (n = 38) |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | t | |
| Age | 18.85 | 1.26 | 19.63 | 2.82 | 1.69 |
| Years lived in United States | 17.67 | 4.25 | 19.42 | 3.05 | 2.12* |
| MSI-BPD | 2.00 | 1.28 | 28.37 | 5.96 | 29.25*** |
| STAI-S | 32.75 | 7.38 | 43.16 | 11.52 | 5.01*** |
| STAI-T | 33.02 | 8.06 | 47.26 | 10.31 | 7.11*** |
| PANAS-NA | 12.93 | 2.21 | 19.16 | 8.02 | 5.04*** |
| PANAS-PA | 28.43 | 7.97 | 29.05 | 10.03 | 0.32 |
| Gender | n | % | n | % | χ2 |
| Male | 15 | 33 | 13 | 34 | 0.02 |
| Female | 31 | 67 | 25 | 66 | |
| Race/ethnicity | n | % | n | % | χ2 |
| Caucasian | 36 | 78 | 33 | 87 | 1. 04 |
| Non-Caucasian | 10 | 22 | 5 | 13 | |
Note. BPD = borderline personality disorder; MSI = McLean Screening Instrument for BPD; STAI-S = State-Trait Anxiety Inventory, State Anxiety; STAI-T = State-Trait Anxiety Inventory, Trait Anxiety; PANAS-NA = Positive and Negative Affect Schedule, Negative Affect; PANAS-PA = Positive and Negative Affect Schedule, Positive Affect.
p < .05.
p < .01.
p < .001.
Measures
Demographics questionnaire
This questionnaire contained questions regarding sex, age, ethnicity, race, and years having lived in the United States.
BPD symptom severity
BPD traits were measured using a 21-item modified version of the MSI-BPD (Zanarini et al., 2003). The original MSI-BPD (Zanarini et al., 2003) is a 10-item self-report screener for BPD features with demonstrated test-retest reliability, internal consistency, validity, and diagnostic efficiency for identifying the presence of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) BPD in respondents between the ages of 18 and 59. Items were rewritten in the first-person for self-administration, and some items from the original MSI-BPD were broken up into separate items for more precise assessment. For example, the original MSI-BPD item, “Have you often felt that you had no idea of who you are or that you have no identity?” was presented as two items: “I have often felt that I had no idea who I am” and “I have often felt that I have no identity”. All items were rated on a 4-point scale (0 = False, not at all true; 3 = Very true).The sum of all items was calculated in order to yield a continuous scale score, with internal consistency (coefficient alpha) of α = .93.
State Positive and Negative Affect (PANAS)
The PANAS (Watson, Clark, & Tellegen, 1988) was administered to assess state affective experiences just before engaging in the experimental task. The PANAS consists of two 10-item subscales, one for state positive affect (PANAS-PA) and the other for state negative affect (PANAS-NA). Two additional items, “happy” and “unhappy”, were added to the PANAS for the current study. Each item is rated on a 5-point scale (1 = very slightly; 5 = extremely).Each subscale was calculated based on the sum of the 11 items corresponding to the scale (PANAS-PA, α = .90; PANAS-NA, α = .87).
State and Trait Anxiety
Participants were administered the State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), which is a widely used, 40-item self-report measure of state and trait anxiety. Each item is rated on a 4-point scale (1 = Not at all; 4 = Very much so).Subscales were calculated based on the sum of the 20 items corresponding to state anxiety (STAI-S; α = .92) and trait anxiety (STAI-T; α = .94).
Procedures
Participants arrived at the laboratory either individually or in groups of up to four people. All participants were seated individually at their own desk and computer terminal for the entirety of the study. These work stations were well-separated to ensure that participants had adequate privacy during the experiment. Participants who were run in small groups all began the procedures at the same time. Of the final sample (n = 84), 3 participants completed the task individually, 10 participants completed the task in groups of 2, 18 participants completed the task in groups of 3, and 53 participants completed the task in groups of 4. After a complete description of the study, participants provided written informed consent. Next, participants completed the self-report questionnaires (Demographics Questionnaire, MSI-BPD, PANAS, and STAI) and the RME task, described below. Participants were then debriefed, thanked, and dismissed by the examiner. The protocol for this study was approved by the university’s Office for Research Protections.
RME task
The RME task (Baron-Cohen et al., 2001) consists of 36 black-and-white photographs (15 cm × 6 cm) of the eye region of faces from just above the eyebrows to halfway down the bridge of the nose. The following instructions were presented to participants on the computer screen prior to the task:
You will see a series of photographs of faces. Your task is to decide what each person is thinking or feeling. For each face, enter the number on the keyboard that corresponds with the number of the word that best describes what the person in the photograph is thinking or feeling. You may feel that more than one word is applicable, but please just choose one word which you consider to be the most suitable. Before making your choice, make sure that you have read all 4 words.
The stimuli were presented in the center of the computer screen, preceded by a fixation cross, with a white background. The four descriptive mental state terms (one target and three distracters) appeared at the four corners of each photograph, equally spaced from the center of the screen, and participants were asked to select the term that best described the mental state portrayed in the photograph. Participants responded by pressing one of four keys (1, 2, 3, 4) corresponding to the four terms. The 36 trials for the RME task were presented in randomized order. Participants’ responses and response times (in milliseconds) were digitally recorded.
Classification of stimuli
To examine mental state decoding accuracy for stimuli of particular emotional valence, the 36 RME stimuli were classified into positive, neutral, and negative valence categories based on ratings of the stimuli that we obtained from a separate sample of 40 undergraduate students. Whereas previous classifications of RME stimuli by valence were obtained by presenting each eyes photograph alongside its target mental state term (Harkness et al., 2005), this method potentially confounds photograph valence ratings with implied valence of the attached mental state word (e.g., eyes stimuli may be rated more negative if presented next to the target word “upset” than if the stimuli are presented alone). For this reason, we obtained stimulus ratings without target mental state words attached to the photographic stimuli, which provides valence categorizations of the RME stimuli that are based solely on the valence of the photographs.
In the stimulus valence rating procedure, we presented the 36 stimuli (photographs only) one at a time in random order in the center of the computer screen on a white background, and asked participants to rate each of the photographs on a 7-point scale (1 = very negative; 7 = very positive). Following the stimulus classification procedures employed by Harkness et al. (2005), those stimuli with mean ratings significantly below neutral (one-sample t(39) = 2.02, µ = 4, p < .05, uncorrected) were classified as negative, those with mean ratings significantly above neutral were classified as positive, and those that did not differ from neutral were classified as neutral. This procedure resulted in the classification of 10 stimuli as negative, 17 stimuli as neutral, and 9 stimuli as positive. The classification results for the 36 RME stimuli are presented in Table 2. An interrater reliability analysis using the Kappa (κ) statistic demonstrated very low concordance between our RME stimulus classifications and those from Harkness et al. (2005), κ = 0.14, p = .26, 95% confidence interval (CI) = −0.128, 0.398. This suggests that our classification of the RME stimuli is not redundant with the classification produced by Harkness and colleagues.
Table 2.
Reading the Mind in the Eyes Task (RME) Stimulus Classification Results From Single-Sample T-Tests (N = 40)
| Valence | Item no. | Target | t | Item no. | Target | t |
|---|---|---|---|---|---|---|
| Negative | 2 | Upset | −7.82 | 14 | Accusing | −4.28 |
| 4 | Insisting | −4.72 | 26 | Hostile | −12.89 | |
| 5 | Worried | −3.98 | 32 | Serious | − 17.64 | |
| 8 | Despondent | −5.35 | 33 | Concerned | −3.10 | |
| 12 | Skeptical | −3.12 | 36 | Suspicious | −3.37 | |
| Neutral | 3 | Desire | 1.66 | 22 | Preoccupied | −1.71 |
| 6 | Fantasizing | −0.10 | 23 | Defiant | −1.18 | |
| 7 | Uneasy | 0.85 | 24 | Pensive | 0.48 | |
| 9 | Preoccupied | −0.84 | 27 | Cautious | 0.56 | |
| 10 | Cautious | 0.11 | 29 | Reflective | 0.90 | |
| 11 | Regretful | −0.25 | 30 | Flirtatious | 1.70 | |
| 16 | Thoughtful | 1.83 | 31 | Confident | 1.16 | |
| 17 | Doubtful | −0.24 | 35 | Nervous | −1.11 | |
| 18 | Decisive | −0.19 | ||||
| Positive | 1 | Playful | 5.81 | 21 | Fantasizing | 6.15 |
| 13 | Anticipating | 2.37 | 25 | Interested | 4.33 | |
| 15 | Contemplative | 4.60 | 28 | Interested | 3.40 | |
| 19 | Tentative | 5.54 | 34 | Distrustful | 2.99 | |
| 20 | Friendly | 9.16 |
RME accuracy scores for negative, neutral, and positive stimuli were calculated as the percentage of items of a particular valence on which participants selected the correct (target) mental state word (Baron-Cohen et al., 2001). Response times for negative, neutral, and positive stimuli were calculated as the mean of response times for stimuli in each valence category.
Calculation of response valence
To characterize the valence of responses on the RME task, an additional sample of 40 undergraduate students rated each of the 99 mental state terms (targets and distracters) that appeared during the RME task on a 7-point valence scale (1 = very negative; 7 = very positive). Each term was presented one at a time in random order on a white computer screen. These ratings were used to calculate a mean valence level for each mental state term. Due to space considerations, the mean valence ratings for the 99 mental state terms are not presented here, but are available upon request from the first author. For our analysis, response valence corresponds to the mean valence of the mental state term that was chosen by a participant as a response for any RME item. The means of these response valence values for positive, neutral, and negative stimuli were then calculated for each participant, with higher values indicating the choice of more positive mental states and lower values indicating the choice of more negative mental states.
Results
Item Analysis
To confirm the validity of each item in the RME task, we conducted a series of Bonferonni-corrected binomial tests comparing the proportion of participants who selected the correct response to the proportion that would be expected by chance (p = .25). More participants selected the target than would be expected by chance for all items (at least 34 of 84 participants, binomial test, p < .0013). Thus, all 36 items were retained for calculation of RME accuracy scores. Mean overall RME task accuracy rates for our low-BPD and high-BPD groups were 73.85% and 74.71% correct (SDs = 8.50 and 8.54), respectively. Based on single-sample t tests (95% confidence interval), these accuracy rates were not significantly different from the average mean accuracy rate (73.1% according to Fertuck et al., 2009) of healthy control groups across six studies (Baron-Cohen et al., 2001; Domes, Heinrichs, Michel, Berger, & Herpertz, 2007; Harkness et al., 2005; Kelemen, Kéri, Must, Benedek, & Janka, 2004; Lee et al., 2005; Richell et al., 2003), t(45) = 0.60, p = .55 and t(37) = 1.16, p = .25, respectively.
Preliminary Data Analysis
Data analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago). Statistical significance was set at p < .05, and Bonferroni corrections were applied where required for multiple comparisons. In the case of inhomogeneity of variance, the Greenhouse-Geisser (G-G) correction was applied.
χ2 analyses demonstrated that the two groups did not differ in distributions of men and women or ethnicity/race (Table 1). After checking the Gaussian distribution of the data by Kolmogorov-Smirnov tests, the groups were compared in continuous demographic and clinical parameters with two-tailed t tests. Groups did not differ in the number of participants who completed the task in the room at the same time (low-BPD M = 3.35, SD = 0.88; high-BPD M = 3.55, SD = 0.80; t(82) = 1.11, ns). As shown in Table 1, the high-BPD group scored significantly higher than the low-BPD group in BPD symptom severity (MSI-BPD), state negative affect (PANAS-NA), state anxiety (STAI-S), and trait anxiety (STAI-T). In addition, the high-BPD group was significantly higher in number of years having lived in the United States. There were no significant differences between groups in age or state positive affect (PANAS-PA).
Pearson correlations were used to examine relationships between all dependent variables (i.e., RME response time [RT], RME accuracy, and response valence) and demographic variables, number of participants who completed the task in the room at the same time, and state and trait affect scales. Participant age was negatively correlated with average RME accuracy, r(84) = −.31, p = .005. Hence, age was entered as a covariate in the data analysis for accuracy. However, none of the other variables were correlated with any of the dependent variables at significant or trend levels (all ps > .10). RT was also uncorrelated with task accuracy. Therefore, results are reported without controlling for these factors.
Experimental Task Analyses
RME RT
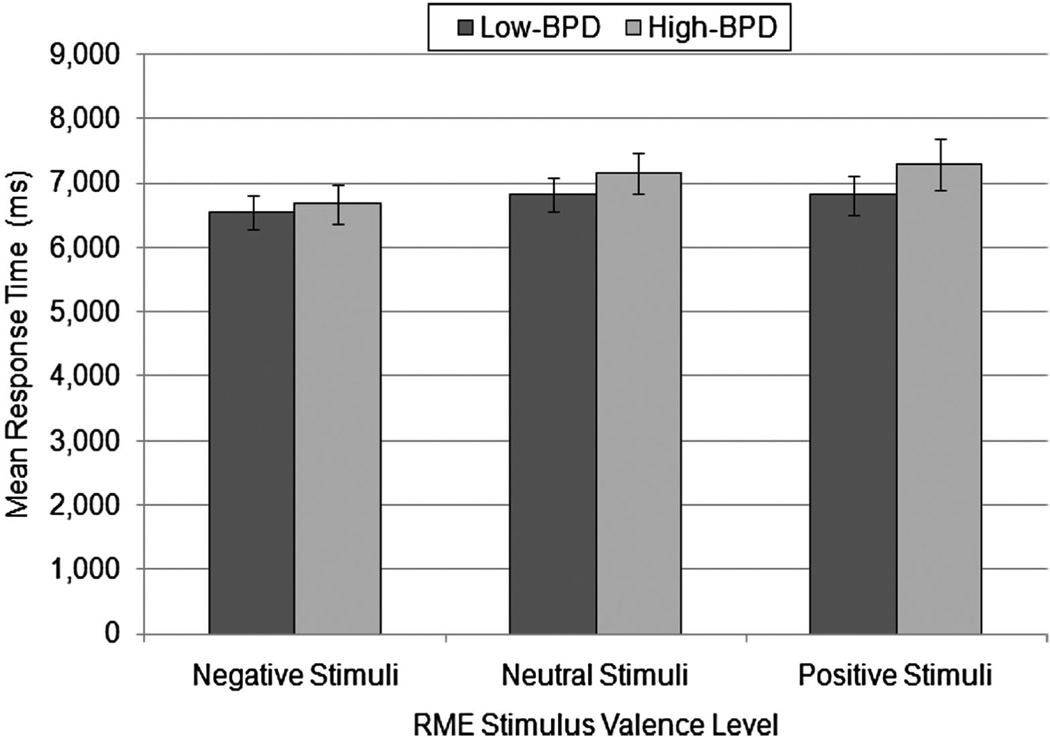
To examine valence and group differences in RT, we conducted a mixed model analysis of variance (ANOVA) with valence (negative, neutral, and positive) as the within-participants factor, group (high and low BPD) as the between-participants factor, and RT as the dependent measure. There was no significant main effect of group, F(1, 82) = 0.71, p = .40, , demonstrating that the two groups did not differ in RT overall when collapsed across valence. However, there was a within-participants main effect of valence on RT, F(1.83, 146.36) = 3.99, p = .02, G-G corrected, . Bonferroni-corrected paired comparison tests demonstrated that participants responded significantly faster for negative (M = 6603.35, SE = 196.75) than for neutral stimuli (M = 6975.99, SE = 200.25), p < .05; RTs did not differ significantly between negative (M = 6603.35, SE = 196.75) and positive stimuli (M = 7029.02, SE = 242.71), p = .08. There was no significant valence by group interaction, F(1.83, 146.36) = 0.50, p = .59, G-G corrected, . Hence, there were no differences between groups in RT whether collapsed across valence or at specific valence levels, suggesting that any group differences in task accuracy cannot be explained by a speed-accuracy trade-off. Figure 1 illustrates group means and standard errors for response times at each valence level.
Figure 1.
Mean response times (ms) in high-borderline personality disorder (high-BPD; n = 38) and low-BPD (n = 46) groups for Reading the Mind in the Eyes task (RME) stimuli in each valence category. No significant group differences in response times were found. SEs are represented in the figure by the error bars attached to each column.
RME accuracy
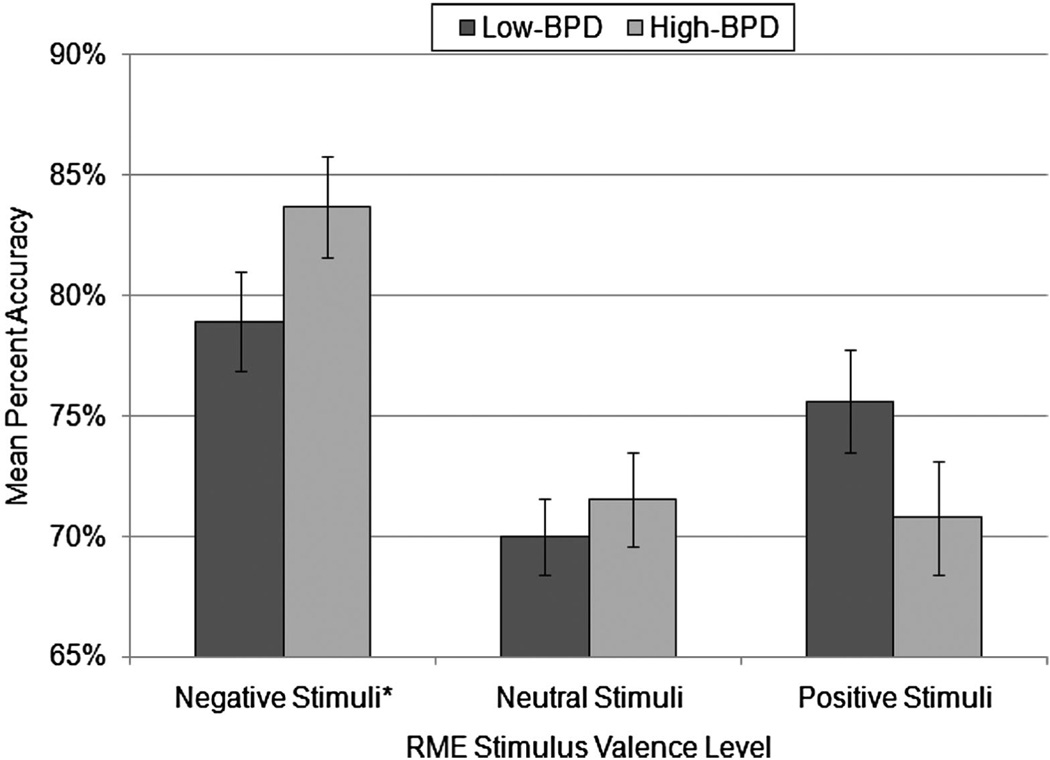
To examine valence and group differences in RME task performance, we conducted a mixed model analysis of covariance (ANCOVA) with stimulus valence as the within-participants factor, group as the between-participants factor, and percent accuracy on the RME task as the dependent measure. As previously mentioned, participants’ age was significantly correlated with task accuracy, and therefore, was retained as a covariate in the analysis. However, as recommended for repeated-measures ANCOVA (e.g., Annaz, Karmiloff-Smith, Johnson, & Thomas, 2009), because the within-participants main effects of valence are independent of the effects of a between-participants covariate such as age, the analysis was first conducted excluding the covariate in order to examine pure within-participants main effects. The main effect of valence on accuracy was significant, F(2, 164) = 17.30, p < .001, . Bonferroni-corrected paired comparison tests demonstrated that, collapsed across groups, accuracy was higher for negative stimuli (M = 81.07%, SE = 1.48) than for both neutral (M = 70.66%, SE = 1.23) and positive (M = 73.41%, SE = 1.60) stimuli, ps < .001, but accuracy for neutral stimuli did not differ from accuracy for positive stimuli, p = .55. After controlling for the between-participants effect of age, F(1, 81) = 11.32, p = .001, , the main effect of group on accuracy was not significant, F(1, 81) = 0.80, p = .38, , indicating that the high-BPD and low-BPD groups did not differ in overall task accuracy when collapsed across valence.
Most importantly, there was a significant valence by group interaction effect, F(2, 162) = 3.18, p = .04, , suggesting that groups differed in accuracy depending on valence. Examination of the task accuracy means (shown in Figure 2) and the significant linear contrast effect for the interaction term, F(1,81) = 5.16, p = .03, , suggested that for negative stimuli only, the high-BPD group appears to show enhanced accuracy relative to the low-BPD group. Examination of the ANOVA model parameters for group differences in accuracy at each level of valence demonstrated that the low-BPD group was less accurate than the high-BPD group at detecting mental states from negative stimuli, t(81) = 2.13, p = .04, d = .47, but the group differences in accuracy were not significant for neutral, t(81) = 0.86, p = .39, d = .19, or positive stimuli, t(81) = 1.10, p = .28, d = .24.
Figure 2.
Mean percent task accuracy in high-borderline personality disorder (high-BPD; n = 38) and low-BPD (n = 46) groups for Reading the Mind in the Eyes task (RME) stimuli in each valence category. Groups differed significantly in accuracy for negative stimuli (*p < .05), but not for neutral or positive stimuli (ps > .05). SEs are represented in the figure by the error bars attached to each column.
Response valence
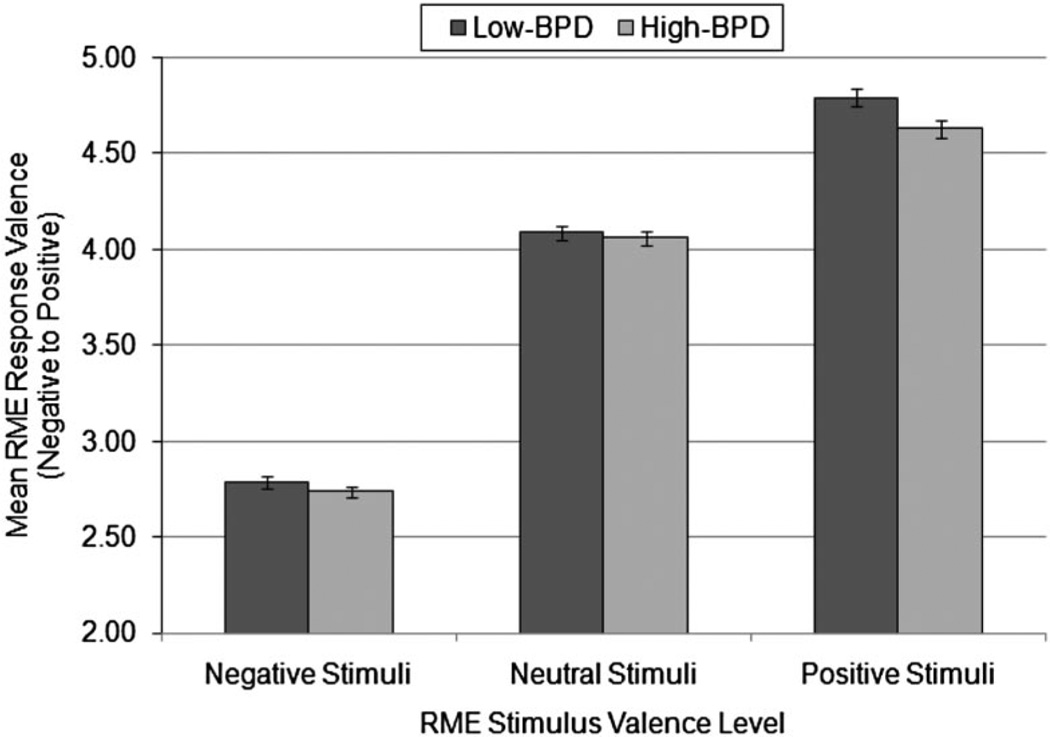
To determine whether there were group differences in the valence of responses (i.e., a response bias) on the RME task across the three types of stimuli, we conducted a mixed model ANOVA with stimulus valence as the within-participants factor, group as the between-participants factor, and valence of responses (“response valence”) as the dependent measure (Figure 3). There was a significant main effect of group on response valence, F(1,82) = 4.73, p = .03, . Averaged across stimuli of all valence categories, the high-BPD group’s response choices were more negative in valence (M = 3.81, SE = 0.03) compared to the low-BPD group’s response choices (M = 3.89, SE 0.03). These results suggest that the high-BPD group showed a response bias characterized by the tendency to choose more negatively valenced mental state terms. Furthermore, the main effect of stimulus valence on response valence was highly significant, F(2, 164) = 1592.42, p < .001, . Bonferroni-corrected pairwise comparisons demonstrated that, collapsed across groups, the mean valence of responses for each set of stimuli were significantly different from each other, all ps < .001, with responses being more negative for negative stimuli (M = 2.76, SE = 0.02), more positive for positive stimuli (M = .71, SE = 0.03), and in the middle for neutral stimuli (M = 4.07, SE = 0.03). This pattern was expected given that response choices on the RME should be similar in valence to corresponding stimuli. The valence by group interaction effect was not significant, F(2, 164) = 2.16, p = .12, .
Figure 3.
Mean response valence in high-borderline personality disorder (high-BPD; n = 38) and low-BPD (n = 46) groups for Reading the Mind in the Eyes task (RME) stimuli in each valence category. The main effect of group was significant, with the high-BPD group showing a negative response bias (p = .03). There was no significant interaction between valence and group (p > .05). SEs are represented in the figure by the error bars attached to each column.
This finding, combined with the significant main effect of group, suggests that the high-BPD group tended to show a negative bias for all stimuli, regardless of the valence of the stimuli.
The analyses so far suggest that the group difference in accuracy for negative stimuli could be driven by a negative response bias, even when looking at negative stimuli. Therefore, we examined the group differences in response valence for negative stimuli. However, the group effect for response valence at the level of negative stimuli was not significant, t(82) = 1.20, p = .23, d = .27. Hence, the group difference in accuracy for negative stimuli could not be explained by any group difference in response bias at the level of negative stimuli in particular.
Discussion
We examined mental state decoding abilities as measured by the RME task in young adults who were high in BPD traits as compared to those who were low in BPD traits. Based on previous studies, we predicted that those with BPD traits would demonstrate enhanced accuracy for negative RME stimuli and impaired accuracy for neutral and positive RME stimuli, as well as a bias toward attributing negative mental states to facial expressions. Our results partially supported these hypotheses. Specifically, the high-BPD group was more accurate than the low-BPD group in decoding mental states from negative stimuli on the RME, but there was no significant difference between groups in task accuracy for neutral or positive stimuli. Consistent with our hypotheses, analysis of the valence of RME task responses showed that those who were high in BPD traits tended to ascribe more negative mental states to social stimuli than those who were low in BPD traits. Further analysis suggested that the relationship between BPD traits and enhanced accuracy for negative stimuli was not attributable to a group difference in response bias when looking specifically at negative stimuli. Preliminary correlational analyses suggested that task performance was not attributable to RT or affective states, and we controlled for any influence of age on accuracy.
Our results are partially consistent with those of Fertuck et al. (2009), who found that BPD patients performed better on the RME task than healthy comparisons regardless of stimulus valence. Our findings are also partially consistent with those of Harkness et al. (2005), who found enhanced RME task accuracy among dysphoric college students regardless of stimulus valence. However, our accuracy results suggest that the advantage in mental state decoding abilities among college students with BPD traits is specific to negative facial stimuli and does not generalize to neutral or positive stimuli. Among studies using whole-face and static picture methods, our results are at least partially consistent with those of Wagner and Linehan (1999), who found enhanced accuracy in the detection of fearful expressions among BPD patients. Our results are also partially consistent with those of Lynch et al. (2006) who found evidence of enhanced sensitivity in emotion detection among BPD patients.
Many of the discrepancies between our results and those of other researchers may be attributable to methodological differences between the studies and/or heterogeneity in research samples. Although our RME accuracy findings are discrepant from those of some researchers (e.g., Bland et al., 2004; Levine et al., 1997) who have found impairment among BPD patients in facial emotion recognition, those studies have used very different methods for assessing emotion recognition that involve the identification of basic emotions in whole-face, static stimuli that are based on a different scientific framework than the RME task. In comparison to the only published study on RME performance in patients with BPD versus healthy comparisons (Fertuck et al., 2009), our study differed in sample type (i.e., nonclinical vs. clinical), presentation of stimuli (i.e., computer vs. card administration), and classification of stimuli according to valence (i.e., our own classification system vs. Harkness et al.’s (2005) system). Moreover, previous studies suggest that BPD patients’ emotion recognition performance might be impaired on speeded tasks and enhanced on tasks that allow unlimited time or the ability to change answers (for a review, see Domes et al., 2009). Although we did not tell participants to emphasize either speed or accuracy in our task instructions, participants were not given the option to return to previous items in order to change a response after their response was given, which may have attenuated mental state decoding strengths in the high-BPD group relative to the low-BPD group.
Our finding of a bias toward the attribution of negative mental states to RME stimuli in the high-BPD group is consistent with numerous studies demonstrating an association between BPD symptoms and the tendency to misattribute negative emotional states to benign social information (Domes et al., 2008; Donegan et al., 2003; Dyck et al., 2009; Meyer et al., 2004; Silbersweig et al., 2007; Wagner & Linehan, 1999; see Domes et al., 2009, for a review). Of note, the high- and low-BPD groups did not differ in the valence of their responses for negative stimuli, nor did they not differ in RT for negative stimuli. These findings suggest that the enhanced accuracy for negative stimuli observed in the high-BPD group is unlikely to be driven by either response bias or RT. Hence, other variables should be explored in future research as potential mechanisms of enhanced negative emotion recognition accuracy in those with BPD features.
Our finding of enhanced negative emotion detection and a response bias toward the perception of negative emotions among those with BPD traits is consistent with conceptualizations of the phenomenological affective experiences of BPD patients as tending to be dominated by negative affect with relatively few experiences of positive affect (Depue & Lenzenweger, 2001; Ebner-Premier et al., 2007; Kernberg, 1984). These results could also be understood in the context of response expectancy theory (Kirsch, 1997). From this perspective, heightened sensitivity to negative or threatening social cues may represent an overlearned response set resulting from an accumulation of negative interpersonal experiences. Consistent with this interpretation, a high percentage of those with BPD have histories of childhood maltreatment (Brodsky, Cloitre, & Dulit, 1995; Zanarini, 2000). Furthermore, there is evidence that maltreated children are more sensitive to the detection of threat in facial stimuli, even when threat is not present (Pollak, Cicchetti, Hornung, & Reed, 2000; Pollak & Sinha, 2002). In addition, Wagner and Linehan (1999) found that patients with a history of childhood sexual abuse (both with and without BPD) were less accurate in their identification of neutral emotion in facial expressions and more likely to misattribute negative emotions to neutral expressions. It is possible that increased vigilance to threat cues in human facial expressions represents a type of adaptation for survival in an abusive or emotionally challenging environment. A child who is able to quickly detect negative emotion in a volatile parent may be more equipped to predict, cope with, or avoid abuse or neglect. As suggested by Harkness et al. (2005), hyper-vigilance to emotion in facial expressions may result from attempts to regain a sense of control over the environment (Weary & Edwards, 1994). However, this adaptation may become maladaptive when even neutral or positive social cues are imbued with malevolence without flexibility or the recognition that one’s interpretations may be erroneous.
Our study has several strengths and limitations that deserve mention. First, we have measured mental state decoding abilities using a task that is sensitive to subtle individual differences in this capacity. Second, we have measured RT, state and trait anxiety, and state negative and positive affect, and we found that these results were not attributable to these variables. Third, our groups did not differ significantly in distributions of gender or ethnicity/ race, and we controlled for the effect of age on accuracy. Although education and intelligence were not measured in this study, our groups can also be assumed to have similar education and intelligence levels given that they were drawn from the same introductory psychology subject pool and they were approximately the same age.
In addition, we developed and utilized a classification of RME stimuli according to valence that is independent of the valence of the target mental state terms that correspond to the eyes photographs. Our classification of stimuli also allows for the investigation of response bias during the task. Based on our classification of stimuli, we found that RTs were faster and accuracy was higher for negative stimuli in comparison to other stimuli. This was consistent with findings by Harkness et al. (2005), even though our own classifications of stimuli were generally not concordant with those of Harkness and colleagues. The strong within-participants effect of valence on accuracy and RT suggests that researchers using the RME to investigate mental state decoding should not only examine overall RME accuracy, but should also examine accuracy at particular valence levels.
Perhaps the most notable limitation of the current study is the use of a homogeneous and nonclinical sample derived from a self-report method of assessing BPD traits. Although studies have shown that young adults with BPD traits experience significant amounts of distress and dysfunction (Lenzenweger et al., 1997; Taylor & Reeves, 2007; Trull, 1995; Trull et al., 1997), these results have limited generalizability to heterogeneous clinical populations of treatment-seeking patients. In addition, our sample size for group comparisons was relatively small, and did not provide significant power to detect small effects. Moreover, given the evidence that depressive symptoms may enhance RME accuracy in some samples (e.g., Harkness et al., 2005; Fertuck et al., 2009), our lack of measurement of depression is a significant limitation. Furthermore, we did not assess childhood maltreatment in the current study, and evidence suggests that experiences of maltreatment could be an important potential mechanism of emotion detection from social information. There are multiple other variables that were not measured in this study and that may influence social perception in those with BPD features, such as other psychopathological symptoms on both Axis I and II, educational and intelligence level, treatment history, and medication status.
An additional potential limitation to the interpretation of these results is that we did not provide participants with a glossary so that they could check the meaning of words in the task (as incorporated in the original RME) because of the possibility that checking a glossary might influence RT. However, the lack of a glossary did not appear to invalidate any of the items on the task, as evidenced by our preliminary item analysis, and both groups demonstrated accuracy rates that were commensurate with those found in nonclinical samples both with and without the use of a glossary. One might also expect that RTs would be much longer and accuracy rates much lower for participants who had difficulty understanding words in the task, and we excluded any participants who had unusually long RTs or extremely low accuracy rates from data analysis. Nonetheless, the possibility that some participants’ performance might have suffered due to poor understanding of the words in the task cannot be ruled out.
The results of the current study suggest that young adults with significant BPD traits may be particularly sensitive to detecting negative emotions in social situations based on perceivable social cues. However, our findings also imply that BPD features are related to negatively biased appraisals of social information, including neutral or positive social information. Our results add to the emerging body of evidence that those with significant BPD features may demonstrate strengths rather than deficits in certain aspects of social perception (e.g., Fertuck et al., 2009; Lynch et al., 2006). Considering the multiple theories and treatments for BPD that focus on social–cognitive impairment as a core characteristic of the disorder, it appears to be important for researchers to investigate domains of social cognition other than emotion recognition in order to better understand these processes as putative mechanisms underlying BPD. Studies that investigate social– cognitive abilities among BPD patients under conditions that resemble real-life experiences may be particularly informative, such as under time pressure, with multimodal stimuli, with personally relevant stimuli, or under conditions of heightened emotional intensity. Potential relationships between neurocognitive problems and difficulties with social cognition should also be explored. Additionally, given the mixed evidence with regard to emotion recognition abilities in those with BPD, the heterogeneity of the disorder might be examined as a potential moderator of these abilities. It is possible that certain BPD features predict better or worse performance in emotion recognition, and this may have implications for understanding the heterogeneity of interpersonal functioning in patients with BPD.
Acknowledgments
The authors would like to thank Stevie Grassetti, Zach Infantolino, Justin Meyer, Lauren Testa, and Rachel Tomko for their assistance with data collection and data entry.
References
- Annaz D, Karmiloff-Smith A, Johnson MH, Thomas MSC. A cross-syndrome study of the development of holistic face recognition in children with autism, Down syndrome, and Williams syndrome. Journal of Experimental Child Psychology. 2009;102:456–486. doi: 10.1016/j.jecp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Arntz A, Dietzel R, Dreessen L. Assumptions in borderline personality disorder: Specificity, stability and relationship with etiological factors. Behavior Research and Therapy. 1999;37:545–557. doi: 10.1016/s0005-7967(98)00152-1. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The autistic child’s theory of mind: A case of specific developmental delay. Journal of Child Psychology and Psychiatry. 1989;30:285–297. doi: 10.1111/j.1469-7610.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Bateman A, Fonagy P. The development of an attachment-based treatment program for borderline personality disorder. Bulletin of the Menninger Clinic. 2003;67:187–211. doi: 10.1521/bumc.67.3.187.23439. [DOI] [PubMed] [Google Scholar]
- Bender DS, Skodol AE. Borderline personality as self-other representational disturbance. Journal of Personality Disorders. 2007;21:500–517. doi: 10.1521/pedi.2007.21.5.500. [DOI] [PubMed] [Google Scholar]
- Bland AR, Williams CA, Scharer K, Manning S. Emotion processing in borderline personality disorders. Issues in Mental Health Nursing. 2004;25:655–672. doi: 10.1080/01612840490486692. [DOI] [PubMed] [Google Scholar]
- Blatt SJ, Auerbach JS, Levy KN. Mental representations in personality development, psychopathology, and the therapeutic process. General Psychology Review. 1997;1:351–374. [Google Scholar]
- Bora E, Vahip S, Gonul AS, Akdeniz F, Alkan M, Ogut M, et al. Evidence for theory of mind deficits in euthymic patients with bipolar disorder. Acta Psychiatrica Scandinavica. 2005;112:110–116. doi: 10.1111/j.1600-0447.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Cloitre M, Dulit RA. Relationship of dissociation to self-mutilation and childhood abuse in borderline personality disorder. American Journal of Psychiatry. 1995;152:1788–1792. doi: 10.1176/ajp.152.12.1788. [DOI] [PubMed] [Google Scholar]
- Butler AC, Brown GK, Beck AT, Grisham J. Assessment of dysfunctional beliefs in borderline personality disorder. Behaviour Research and Therapy. 2002;40:1231–1240. doi: 10.1016/s0005-7967(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: Comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophrenia Research. 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- Daley SE, Hammen C, Davila J, Burge D. Axis II symptomatology, depression, and life stress during the transition from adolescence to adulthood. Journal of Consulting and Clinical Psychology. 1998;66:595–603. doi: 10.1037//0022-006x.66.4.595. [DOI] [PubMed] [Google Scholar]
- Depue RA, Lenzenweger MF. A neurobehavioral dimensional model of personality disorders. In: Livesley WJ, editor. Handbook of personality disorders. New York: Guilford Press; 2001. pp. 136–176. [Google Scholar]
- Domes G, Czieschnek D, Weidler F, Berger C, Fast K, Herpertz SC. Recognition of facial affect in borderline personality disorder. Journal of Personality Disorders. 2008;22:135–147. doi: 10.1521/pedi.2008.22.2.135. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves ‘mind-reading’ in humans. Biological Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Herpertz SC. Emotion recognition in borderline personality disorder: A review of the literature. Journal of Personality Disorders. 2009;23:6–19. doi: 10.1521/pedi.2009.23.1.6. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: Implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Dyck M, Habel U, Slodezyk J, Schummer J, Backes V, Schneider F, et al. Negative bias in fast emotion discrimination in borderline personality disorder. Psychological Medicine. 2009;39:855–864. doi: 10.1017/S0033291708004273. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Kuo J, Kleindienst N, Welch SS, Reisch T, Reinhard I, et al. State affective instability in borderline personality disorder assessed by ambulatory monitoring. Psychological Medicine. 2007;37:961–970. doi: 10.1017/S0033291706009706. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Facial action coding system. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- Farzin I, Platek SM, Panyavin IS, Calkins ME, Kohler C, Siegel SJ, et al. Self-face recognition and theory of mind in patients with schizophrenia and first-degree relatives. Schizophrenia Research. 2006;88:151–160. doi: 10.1016/j.schres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Fertuck EA, Jekal A, Song I, Wyman B, Morris MC, Wilson ST, et al. Enhanced “Reading the Mind in the Eyes” in borderline personality disorder compared to healthy controls. Psychological Medicine. 2009;39:1979–1988. doi: 10.1017/S003329170900600X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck EA, Lenzenweger MF, Clarkin JF, Hoermann S, Stanley B. Executive neurocognition, memory systems, and borderline personality disorder. Clinical Psychology Review. 2006;26:346–375. doi: 10.1016/j.cpr.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Fonagy P, Bateman A. The development of borderline personality disorder: A mentalizing model. Journal of Personality Disorders. 2008;22:4–21. doi: 10.1521/pedi.2008.22.1.4. [DOI] [PubMed] [Google Scholar]
- Fonagy P, Gergely G, Jurist E, Target M. Affect regulation mentalization, and the development of the self. New York: Other Press; 2002. [Google Scholar]
- Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: Results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2008;69:533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson JG. Borderline patient’s intolerance of aloneness: Insecure attachments and therapist availability. American Journal of Psychiatry. 1996;153:752–758. doi: 10.1176/ajp.153.6.752. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Sabbagh MA, Jacobson JA, Chowdrey NK, Chen T. Enhanced accuracy of mental state decoding in dysphoric college students. Cognition and Emotion. 2005;19:999–1025. [Google Scholar]
- Herpertz S. Self-injurious behaviour: Psychopathological and nosological characteristics in subtypes of self-injurers. Acta Psychiatrica Scan-dinavica. 1995;91:57–68. doi: 10.1111/j.1600-0447.1995.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Herpertz S, Gretzer A, Steinmeyer EM, Muehlbauer V, Schuerkens A, Sass H. Affective instability and impulsivity in personality disorder: Results of an experimental study. Journal of Affective Disorders. 1997;44:31–37. doi: 10.1016/s0165-0327(97)01444-4. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Jovev M, Jackson H. Early maladaptive schemas in personality disordered individuals. Journal of Personality Disorders. 2004;18:467–478. doi: 10.1521/pedi.18.5.467.51325. [DOI] [PubMed] [Google Scholar]
- Judd PH. Neurocognitive impairment as a moderator in the development of borderline personality disorder. Development and Psychopathology. 2005;17:1173–1196. doi: 10.1017/s0954579405050558. [DOI] [PubMed] [Google Scholar]
- Judd PH, McGlashan TH. A developmental model of borderline personality disorder: Understanding variations in course and outcome. Washington, DC: American Psychiatric Association; 2003. [Google Scholar]
- Kelemen O, Erdelyi R, Pataki I, Benedek G, Janka Z, Keri S. Theory of mind and motion perception in schizophrenia. Neuropsychology. 2005;19:494–500. doi: 10.1037/0894-4105.19.4.494. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Kéri S, Must A, Benedek G, Janka Z. No evidence for impaired ‘theory of mind’ in unaffected first-degree relatives of schizophrenia patients. Acta Psychiatrica Scandinavica. 2004;110:146–149. doi: 10.1111/j.1600-0047.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- Kernberg OF. Severe personality disorders: Psychotherapeutic strategies. New Haven, CT: Yale University Press; 1984. [Google Scholar]
- Kirsch I. Response expectancy theory and application: A decennial review. Applied and Preventative Psychology. 1997;6:69–79. [Google Scholar]
- Langdon R, Coltheart M, Ward PB, Catts SV. Disturbed communication in schizophrenia: The role of poor pragmatics and poor mind-reading. Psychological Medicine. 2002;32:1273–1284. doi: 10.1017/s0033291702006396. [DOI] [PubMed] [Google Scholar]
- Lee L, Harkness KL, Sabbagh MA, Jacobson JA. Mental state decoding abilities in clinical depression. Journal of Affective Disorders. 2005;86:247–258. doi: 10.1016/j.jad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV Personality disorders in national comorbidity survey replication. Biological Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, Loranger AW, Korfine L, Neff C. Detecting personality disorders in a non-clinical population: Application of a 2-stage case identification. Archives of General Psychiatry. 1997;54:345–351. doi: 10.1001/archpsyc.1997.01830160073010. [DOI] [PubMed] [Google Scholar]
- Levine D, Marziali E, Hood J. Emotion processing in borderline personality disorders. Journal of Nervous and Mental Disease. 1997;185:240–246. doi: 10.1097/00005053-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Levy KN. The implications of attachment theory and research for understanding borderline personality disorder. Development and Psychopathology. 2005;17:959–986. doi: 10.1017/s0954579405050455. [DOI] [PubMed] [Google Scholar]
- Levy KN, Blatt SJ. Attachment theory and psychoanalysis: Further differentiation within insecure attachment patterns. Psychoanalytic Inquiry. 1999;19:541–575. [Google Scholar]
- Levy KN, Clarkin JF, Yeomans FE, Scott LN, Wasserman RH, Kernberg OF. The mechanisms of change in the treatment of borderline personality disorder with transference focused psychotherapy. Journal of Clinical Psychology. 2006;62:481–501. doi: 10.1002/jclp.20239. [DOI] [PubMed] [Google Scholar]
- Linehan MM. New York: Guilford Press; 1993. Cognitive-behavioral treatment of borderline personality disorder. [Google Scholar]
- Lynch TR, Rosenthal MZ, Kosson DS, Cheavens JS, Lejuez CW, Blair RJR. Heightened sensitivity to facial expressions of emotion in borderline personality disorder. Emotion. 2006;6:647–655. doi: 10.1037/1528-3542.6.4.647. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Grilo CM, Sanislow E, Ralevski LC, Morey LC, Gunderson JG, et al. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders: Toward a hybrid model of axis II disorders. American Journal of Psychiatry. 2005;162:883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Pilkonis PA, Beevers CG. What’s in a (neutral) face? Personality disorders, attachment styles, and the appraisal of ambiguous social cues. Journal of Personality Disorders. 2004;18:320–336. doi: 10.1521/pedi.2004.18.4.320. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Vinogradov S. Social-emotion recognition in borderline personality disorder. Comprehensive Psychiatry. 2006;47:468–474. doi: 10.1016/j.comppsych.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Vinogradov S. A neurocognitive model of borderline personality disorder: Effects of childhood sexual abuse and relationship to adult social attachment disturbance. Development and Psychopathology. 2008;20:341–368. doi: 10.1017/S0954579408000163. [DOI] [PubMed] [Google Scholar]
- Nordahl HM, Holthe H, Haugum JA. Early maladaptive schemas in patients with or without personality disorders: Does schema modification predict symptomatic relief? Clinical Psychology and Psychotherapy. 2005;12:142–149. [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology. 2000;36:679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38:784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Richell RA, Mitchell DGV, Newman C, Leonard A, Baron-Cohen S, Blair RJR. Theory of mind and psychopathy: Can psychopathic individuals read the “language of the eyes”? Neuropsychologia. 2003;41:523–526. doi: 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: Implications for autism. Brain and Cognition. 2004;55:209–219. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Samuels J, Eaton WW, Bienvenu OJIII, Brown CH, Costa PT, Jr, Nestadt G. Prevalence and correlates of personality disorders in a community sample. British Journal of Psychiatry. 2002;180:536–542. doi: 10.1192/bjp.180.6.536. [DOI] [PubMed] [Google Scholar]
- Schmidt JZ, Zachariae R. PTSD and impaired eye expression recognition: A preliminary study. Journal of Loss & Trauma. 2009;14:46–56. [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. American Journal of Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Silk KR. Borderline personality disorder: Overview of biological factors. Psychiatric Clinics of North America. 2000;23:61–75. doi: 10.1016/s0193-953x(05)70143-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stiglmayr CE, Grathwol T, Linehan MM, Ihorst G, Fahrenberg J, Bohus M. Aversive tension in patients with borderline personality disorder: A computer-based controlled field study. Acta Psychiatrica Scandinavica. 2005;111:372–379. doi: 10.1111/j.1600-0447.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- Stone MH. The fate of borderline patients: Successful outcome and psychiatric practice. New York: Guilford Press; 1990. [Google Scholar]
- Tager-Flusberg H. A re-examination of the theory of mind hypothesis of autism. In: Burack J, Charman T, Yirmiya N, Zelazo PR, editors. Development in autism: Perspectives from theory and research. Hillsdale, NJ: Erlbaum; 2001. pp. 173–193. [Google Scholar]
- Taylor J, Reeves M. Structure of borderline personality disorder symptoms in a non-clinical sample. Journal of Clinical Psychology. 2007;63:805–816. doi: 10.1002/jclp.20398. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Archives of General Psychiatry. 2001;58:590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- Trull TJ. Borderline personality disorder features in nonclinical young adults: 1. Identification and validation. Psychological Assessment. 1995;7:33–41. [Google Scholar]
- Trull TJ, Useda JD, Conforti K, Doan B. Borderline personality disorder features in nonclinical young adults: 2. Two-year outcome. Journal of Abnormal Psychology. 1997;106:307–314. doi: 10.1037//0021-843x.106.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AW, Linehan MM. Facial expression recognition ability among women with borderline personality disorder: Implications for emotion regulation. Journal of Personality Disorders. 1999;13:329–344. doi: 10.1521/pedi.1999.13.4.329. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weary G, Edwards JA. Social cognition and clinical psychology: Anxiety, depression, and the processing of social information. In: Wyer RS Jr, Srull TK, editors. Handbook of social cognition. Hillsdale, NJ: Erlbaum; 1994. pp. 289–338. [Google Scholar]
- Westen D. Social cognition and object relations. Psychological Bulletin. 1991;109:429–445. [Google Scholar]
- Yeomans F, Levy KN. An object relations perspective on borderline personality disorder. Acta Neuropsychiatrica. 2002;14:76–80. doi: 10.1034/j.1601-5215.2002.140205.x. [DOI] [PubMed] [Google Scholar]
- Young JE, Klosko JS, Weishaar ME. Schema therapy: A practitioner’s guide. New York: Guilford Press; 2003. [Google Scholar]
- Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatric Clinics of North America Special Issue: Borderline Personality Disorder. 2000;23:89–101. doi: 10.1016/s0193-953x(05)70145-3. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. A screening measure for BPD: The Mclean Screening Instrument for Borderline Personality Disorder (MSI-BPD) Journal of Personality Disorders. 2003;17:568–573. doi: 10.1521/pedi.17.6.568.25355. [DOI] [PubMed] [Google Scholar]