SUMMARY
Semen serves as a vehicle for HIV and promotes sexual transmission of the virus, which accounts for the majority of new HIV cases. The major component of semen is the coagulum, a viscous structure composed predominantly of spermatozoa and semenogelin proteins. Due to the activity of the semen protease PSA, the coagulum is liquefied and semenogelins are cleaved into smaller fragments. Here, we report that a subset of these semenogelin fragments form amyloid fibrils that greatly enhance HIV infection. Like SEVI, another amyloid fibril previously identified in semen, the semenogelin fibrils exhibit a cationic surface and enhance HIV virion attachment and entry. Whereas semen samples from healthy individuals greatly enhance HIV infection, semenogelin-deficient semen samples from patients with ejaculatory duct obstruction are completely deficient in enhancing activity. Semen thus harbors distinct amyloidogenic peptides derived from different precursor proteins that commonly enhance HIV infection and likely contribute to HIV transmission.
INTRODUCTION
Sexual transmission accounts for the vast majority of new HIV infections, and semen is the vehicle fueling the global spread of this retrovirus. We and others have demonstrated that semen enhances in vitro HIV infection of multiple cell types, including primary macrophages and CD4+ T cells, two biologically relevant targets of HIV in vivo (Kim et al., 2010; Munch et al., 2007; Olsen et al., 2010; Roan et al., 2009). Fractionation of a peptide library generated from semen led to the identification of peptides derived from prostatic acid phosphatase (PAP) that self-assembled into amyloid fibrils that greatly enhanced HIV infection under conditions of limiting viral innocula (Munch et al., 2007). The amyloids were termed SEVI, for Semen-derived Enhancer of Viral Infection.
We previously showed that SEVI enhances HIV infection by promoting viral attachment to target cells (Munch et al., 2007; Roan et al., 2009). SEVI is highly cationic (eight of its 39 residues are positively charged at neutral pH), and the positive charges of SEVI interact directly with the negatively charged surfaces of target cells and HIV virions to promote attachment and fusion. When the cationic property of SEVI is abrogated by site-directed mutagenesis or by adding anionic polymers, SEVI loses its ability to augment HIV infection (Roan et al., 2009). Importantly, anionic polymers that inhibit the infection enhancing activity of chemically synthesized SEVI also inhibit the enhancing activity of semen (Roan et al., 2009), suggesting that endogenous SEVI or other positively charged factors in semen play a role in its ability to enhance viral infection.
We sought to confirm the role of positively charged factors for the ability of semen to enhance HIV infection by depleting these factors. In line with our previous data using anionic polymers (Roan et al., 2009), semen depleted of positively charged factors lost the ability to enhance HIV infection. Surprisingly, when we identified the cationic factors that were specifically depleted, we detected not SEVI but rather fragments from the semenogelins (SEMs), abundant proteins in semen that together with fibronectin constitute the semen coagulum, a gelatinous structure crucial for fertilization (de Lamirande, 2007; Robert and Gagnon, 1999). We further pursued this finding and describe in this manuscript the identification of peptides from SEM that form amyloids that enhance HIV infection. We further demonstrate that variations in SEM levels directly correlate with enhancement of HIV infection and that semen samples naturally lacking SEMs are completely deficient in this effect. All together, our data suggest that SEMs may play a previously unrecognized role in promoting HIV infection in semen, and indicate that factors whose physiological purpose is to promote fertilization may unfortunately also promote HIV transmission.
RESULTS
Seminal Fluid Depleted of Cationic Factors is Deficient in Enhancing HIV Infection
We previously demonstrated that the anionic polymers heparin and dextran sulfate, but not less negatively charged polymers like chondroitin sulfate, inhibit the ability of semen to enhance HIV infection. Over-sulfation of chondroitin sulfate converted this polymer into a potent inhibitor, suggesting that anionic polymers with higher concentrations of negative charges are more effective in inhibiting semen’s ability to enhance HIV infection (Roan et al., 2009). These results suggest that positively charged factors are responsible for the viral infection enhancing activity of semen.
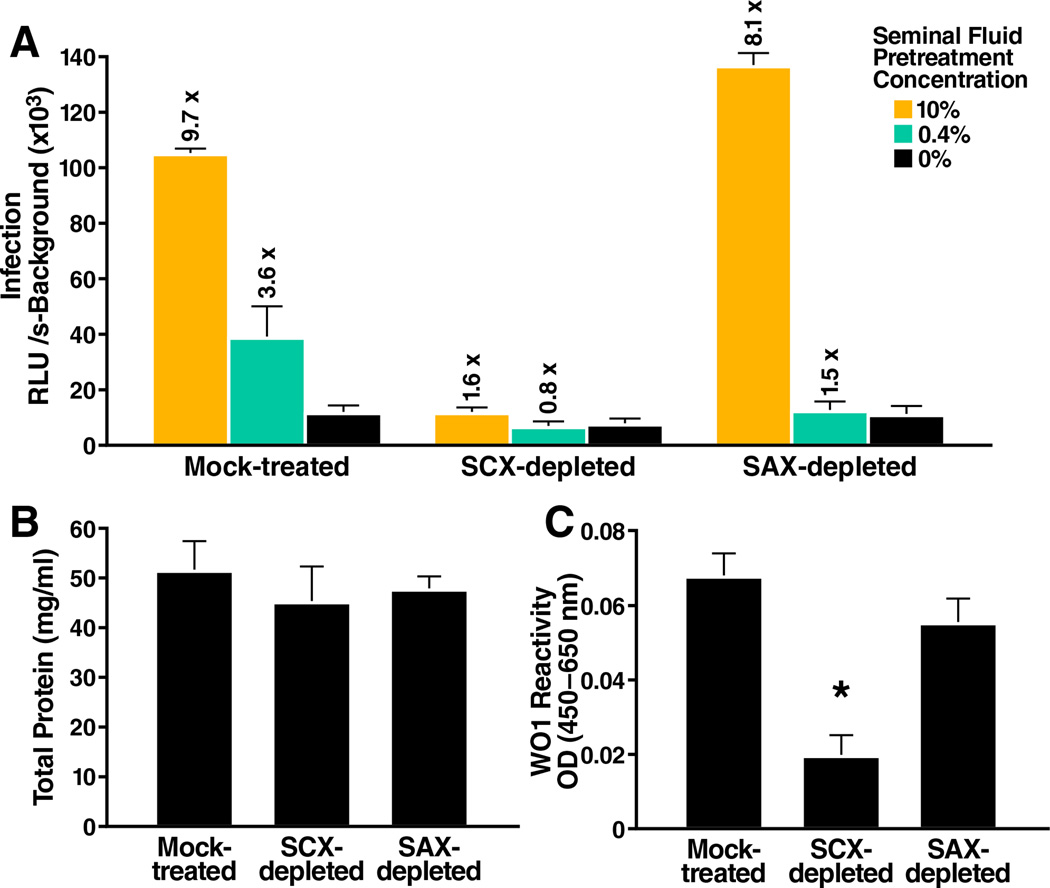
To further test this hypothesis, we depleted cationic (positively charged) factors from seminal fluid (pooled from 20 donors) using strong cation exchange (SCX) beads. As negative controls, we mock-depleted seminal fluid or depleted anionic (negatively charged) factors with strong anion exchange (SAX) beads. Depletion of positively charged factors, but not negatively charged factors, abrogated the ability of semen to enhance HIV infection of TZM-bl reporter cells (Figure 1A). Total protein content was similar in the SCX- and SAX-depleted seminal fluid (Figure 1B), suggesting that the absence of viral enhancing activity in SCX-depleted seminal fluid was not due to the absence of protein.
Figure 1. Seminal Fluid Depleted of Cationic Factors Lacks Viral Enhancement Activity.
(A) Seminal fluid that was mock-treated, depleted of cationic factors (SCX-depleted), or depleted of anionic factors (SAX-depleted) was tested for the ability to enhance HIV infection. 81A virions were pre-treated for 5 min with the indicated concentrations of seminal fluid and then diluted 15-fold and added to TZM-bl cells. Medium was replaced after 2 hours, and cells were assayed for Tat-inducible β-galactosidase activity 3 days later. The numbers above the bars indicate the n-fold infectivity enhancement relative to infection measured in the absence of seminal fluid. RLU/s: relative light units / second. Shown are average values (± standard deviations) of triplicate measurements from one of three independent experiments that yielded similar results. See also Figure S1.
(B) The total protein content of mock-treated, SCX-depleted, or SAX-depleted seminal fluid was assessed by the BCA assay. Shown are average values (± standard deviations) of triplicate well measurements from one of two independent experiments that yielded similar results.
(C) Mock-treated, SCX-depleted, or SAX-depleted seminal fluid was tested for relative reactivity to the amyloid-specific antibody WO1 by ELISA. The reactivity of WO1 to PBS was subtracted from all values. The OD(450–650 nm) indicates the relative levels of WO1-reactive amyloids in each sample. *P < 0.005 vs. mock-treated or SAX-depleted seminal fluid (two-tailed t test). Shown are average values (± standard deviations) of triplicate well measurements from one of two independent experiments that yielded similar results.
Lack of SEM Fragments in Seminal Fluid Depleted of Cationic Factors
To identify the factors that were enriched in the SAX-depleted seminal fluid (which enhanced HIV infection) relative to the SCX-depleted seminal fluid (which did not enhance HIV infection), we employed mass spectrometry. In two independent experiments, Matrix-Assisted Laser Desorption Ionization / tandem mass spectrometry (MALDI MS/MS) detected exclusively fragments from SEM1 and SEM2 (Table S2A), highly homologous proteins in semen that interact with fibronectin to form the semen coagulum. This coagulum is progressively cleaved by the protease prostate specific antigen (PSA) at specific sites within the SEMs during semen liquefaction (Robert et al., 1997). The SEM peptides identified by MALDI MS-MS included PSA-generated proteolytic products (Robert et al., 1997) (Table S2A). Although PAP248–286 or related fragments known to form SEVI were not detected by the MALDI MS/MS, when assessed by ELISA, PAP248–286 was partially depleted by the SCX beads (Figure S1). The depletion of SEM1 and SEM2 fragments by SCX is not surprising, as both of these basic proteins are positively charged at neutral pH. Because the SEMs were deficient in seminal fluid depleted of viral enhancing activity, we pursued the possibility of whether the SEMs, like SEVI, might correspond to natural enhancers of HIV infection.
SEM Fragments Form Amyloid Fibrils
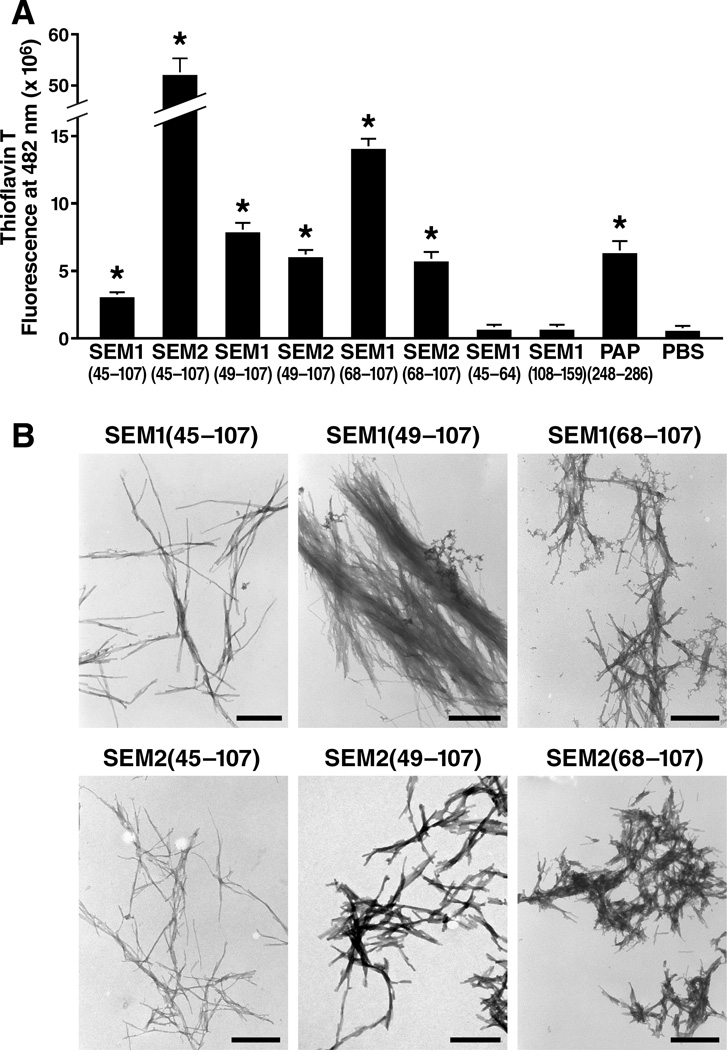
We first investigated whether the SEMs were capable of forming amyloid fibrils, with the rationale that if SEMs enhance HIV infection like SEVI, they may also do so as an amyloid fibril. Although to our knowledge no peptides from SEM have been directly demonstrated to form amyloid fibrils, antibodies raised against an N-terminal region of SEM1 (SEM1(45–64)) recognize fibrillar structures isolated from the seminal vesicles of patients with senile seminal vesicle amyloid (SSVA), one of the most common forms of localized amyloidosis (Linke et al., 2005). As full-length SEMs are rare in semen because of the efficiency of PSA cleavage (Koistinen et al., 2002; Robert et al., 1997), an amyloid form of the SEMs in semen would likely be composed of SEM fragments. To determine if we could identify amyloidogenic fragments from semen, we took advantage of WO1, an amyloid-specific antibody that recognizes the general amyloid fold in a sequence-independent manner (O'Nuallain and Wetzel, 2002). WO1 had less reactivity to SCX-depleted seminal fluid than to mock-treated or SAX-depleted seminal fluid (Figure 1C), suggesting a relative deficiency of amyloids in seminal fluid depleted of cationic factors. Another amyloid-specific antibody, OC (Kayed et al., 2007), also showed higher reactivity to SAX-depleted seminal fluid than to SCX-depleted seminal fluid (data not shown). To identify the amyloid species, we conjugated WO1 to magnetic beads and incubated them with seminal fluid. Bead-bound material was digested with trypsin and then identified by liquid chromatography (LC) MALDI MS/MS. In three independent experiments, we identified fragments from the N-terminus of the SEM proteins in WO1 samples and not control samples, suggesting that SEM amyloids might be present in semen. Interestingly, the identified peptides were immediately C-terminal to SEM1(45–64), the region that was suggested to be amyloidogenic in SSVA patients (Linke et al., 2005). We chemically synthesized a peptide corresponding to SEM1(45–64) but never found that it formed amyloid fibrils when tested under multiple conditions (data not shown). We reasoned that as we had detected tryptic peptides immediately downstream of SEM1(45–64), the region C-terminal of SEM1(45–64) might be important for amyloid fibril formation. We therefore chemically synthesized three peptides harboring sequences C-terminal of SEM1(45–64): SEM1(45–107), SEM1(49–107), and SEM1(68–107). The junctions following residues 49 and 68 were chosen because they correspond to natural PSA cleavage sites within SEM1 (Robert et al., 1997). As such, peptides cleaved at these sites are likely to be present in liquefied semen. The corresponding highly homologous SEM2 peptides were also synthesized (Table S2B).
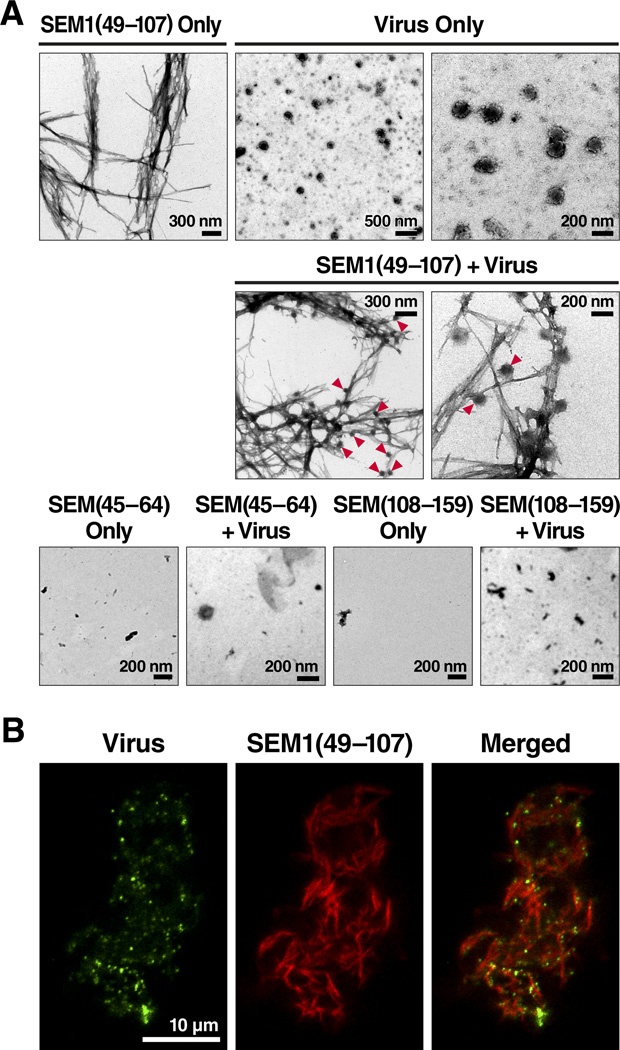
After overnight agitation at 37°C to promote fibril formation, SEM1(45–107), SEM2(45–107), SEM1(49–107), SEM2(49–107), SEM1(68–107), and SEM2(68–107) all formed amyloid fibrils, while SEM1(45–64) and a further downstream peptide SEM1(108–159) did not. Evidence for fibril formation included an increase in the fluorescence intensity of Thioflavin T (Figure 2A), binding of Congo Red dye (data not shown), and detection of typical thread-like fibrillar structures by electron microscopy analyses (Figure 2B). Electron micrographs of the SEM fibrils together with HIV virions demonstrated extensive interactions between the two components (Figure 3A), and these results were further verified by fluorescence microscopy (Figure 3B). Three-color imaging of the fibrils, virions, and cells suggested that the fibrils promoted attachment of the virions to target cells (Figure S3). Thus, peptides harboring sequences immediately C-terminal of SEM1(45–64), and the SEM2 peptide homologs, are capable of forming amyloid fibrils and interacting with virions and cells.
Figure 2. Peptides from SEM1 and SEM2 Form Amyloid Fibrils.
(A) The indicated agitated peptides were mixed with 5 µM Thioflavin T and the emissions at 482 nm were recorded. PAP248–286 is an amyloidogenic peptide that polymerizes into SEVI (Munch et al., 2007). *P < 0.0005 vs. PBS control (two-tailed t test). Shown are average values (± standard deviations) of triplicate well measurements from one of seven independent experiments that yielded similar results. See Table S2 for peptide sequences.
(B) Electron micrographs of the fibrillar SEM peptides. Scale bars indicate 500 nm. Shown are results from one of two independent experiments that yielded similar results.
Figure 3. Visualization of Viral Particles Bound to SEM Fibrils.
(A) Electron micrographs of SEM1(49–107) fibrils, HIV viral particles, and SEM1(49–107) fibrils incubated together with the viral particles. Arrowheads indicate examples of the sites of contact of virions with fibrils. Non-fibrillar SEM1(45–64) and SEM1(108–159) were used as negative controls. Shown are results from one of two independent experiments that yielded similar results.
(B) Fluorescence microscopy of SEM1(49–107) fibrils (red) and MLV-gag-YFP viral particles (green). Shown are results from one of two independent experiments that yielded similar results. See also Figure S3.
SEM Fibrils Enhance HIV Infection
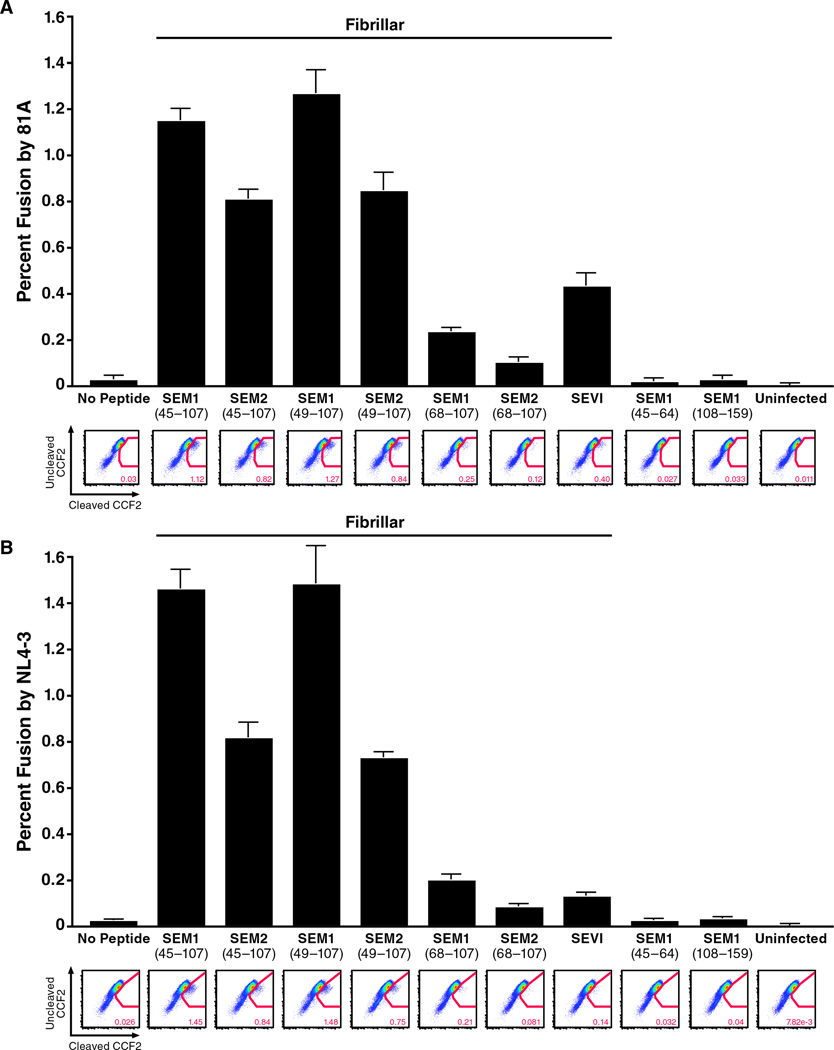
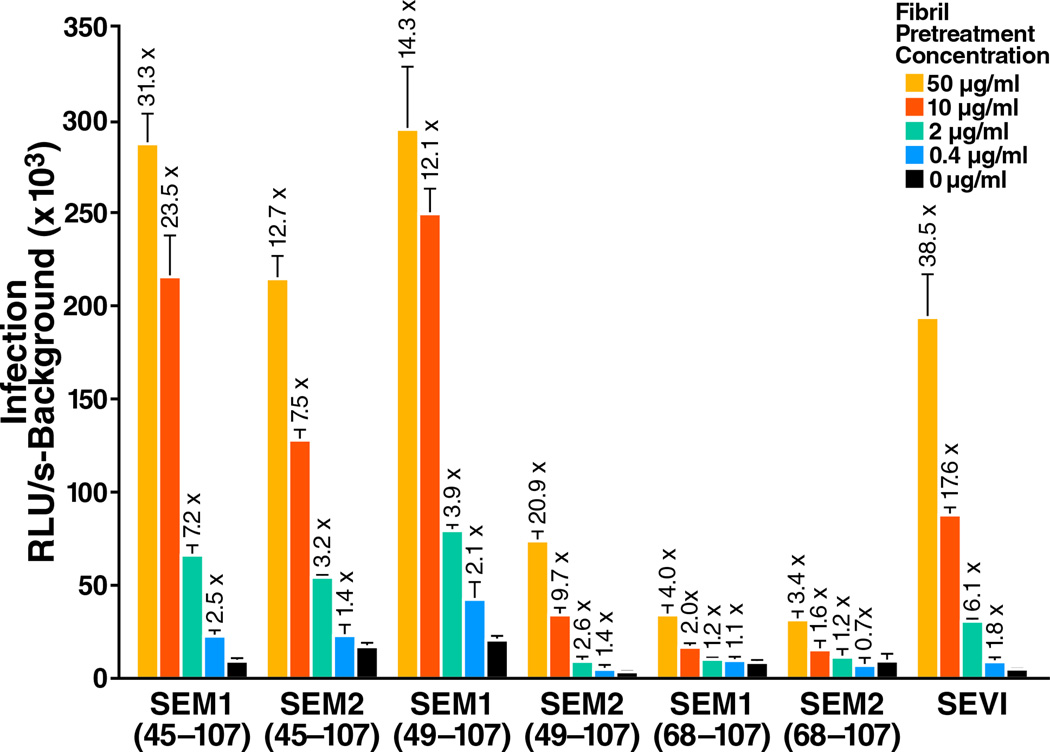
We next tested whether these amyloid fibrils enhance HIV infection. Like SEVI, the SEM fibrils are rich in basic residues and thus exhibit a net positive charge at neutral pH (isoelectric points of the SEM peptides used in this study range from 8.16 – 10.12, as indicated in Table S2B). As SEVI enhances HIV fusion by promoting attachment of HIV virions to target cells (Munch et al., 2007; Roan et al., 2009), we tested whether SEM fibrils act in a similar manner. The six SEM peptides that form fibrils enhanced fusion of CCR5-tropic HIV-1 81A virions to primary CD4+ T cells (Figure 4A). In contrast, SEM1(45–64) and SEM1(108–159), which fail to form fibrils (Figure 2A), did not enhance viral fusion. Similar to SEVI, the enhancing effect of the SEM fibrils was not co-receptor-specific, as fusion of CXCR4-tropic HIV-1 NL4-3 to CD4+ T cells was similarly enhanced (Figure 4B). Interestingly, the fibrils made up of the shorter peptides SEM1(68–107) and SEM2(68–107) enhanced fusion to a lesser extent than the fibrils formed with the longer peptides SEM1(45–107), SEM2(45–107), SEM1(49–107), and SEM2(49–107).
Figure 4. SEM Fibrils Enhance Fusion of Both CCR5- and CXCR4-Tropic HIV-1 to Primary CD4+ T Cells.
CD14-CD4+ T cells were infected with BlaM-Vpr-containing 81A (A) or NL4-3 (B) virions pretreated with 31.25 µg/ml of the indicated agitated peptides. Peptides that formed fibrils (Figure 2) are indicated. Shown are the averages of triplicate samples with a representative FACS plot presented directly below. Similar results were obtained using cells from three other donors. See also Figure S4.
Similar to SEVI (Roan et al., 2009), the enhancing effect of the SEM fibrils was dependent on their cationic property: shielding the positive charges of the fibrils with the anionic polymer heparin abrogated their viral enhancing activities (Figure S4). Furthermore, when surface-exposed positive charges of the fibrils were measured by zeta potential, we found that the SEM1(45–107), SEM2(45–107), SEM1(49–107), and SEM2(49–107) fibrils exhibited high potentials, whereas the two fibrils made up of the shorter peptides SEM1(68–107) and SEM2(68–107) exhibited low potentials (Table S2B). This decreased zeta potential may account for why the SEM1(68–107) and SEM2(68–107) fibrils enhanced HIV fusion less potently than the other four. In support of this possibility, the zeta potentials of the fibrils directly correlated with the relative ability to enhance HIV fusion to target cells (Figure S6A).
To determine if the SEM fibrils enhance productive infection, we infected TZM-bl cells with an R5-tropic HIV-1 (Papkalla et al., 2002) in the presence of increasing concentrations of the fibrils. Each of the six SEM fibrils enhanced HIV infection (Figure 5). Although the relative levels of viral enhancement between the different SEM fibrils varied between experiments, in general we observed that the fibrils derived from the shorter peptides SEM1(68–107) and SEM2(68–107) were less effective at enhancing HIV infection. Finally, we demonstrated that SEM-derived fibrils were capable of rescuing the viral enhancing activity of SCX-depleted seminal fluid (Figure S5).
Figure 5. SEM Fibrils Enhance Productive Infection of Target Cells by HIV-1.
Virions were pre-treated for 5 min with the indicated concentrations of SEM fibrils and used to infect TZM-bl cells. Cells were assayed for Tat-inducible β-galactosidase activity 2 days later. The numbers above the bars indicate the n-fold infectivity enhancement relative to infection measured in the absence of peptide. RLU/s: relative light units / second. Shown are average values (± standard deviations) of triplicate measurements from one of three independent experiments that yielded similar results. See also Figure S5.
Levels of SEM Correlate with HIV Enhancing Activity in Semen Samples from Different Donors
To develop tools to assess endogenous levels of SEM fragments, we generated polyclonal antibodies against fibrils formed by the two fragments SEM1(49–107) and SEM2(49–107). Anti-SEM1(49–107) (hereafter called anti-S1) recognized SEM1(49–107) in a dose-dependent manner, and anti-SEM2(49–107) (hereafter called anti-S2) recognized SEM2(49–107) in a dose-dependent manner (Figure S6B). The antibodies were not fibril-specific, as they recognized the monomeric and fibrillar forms of the peptides to similar extents (data not shown). When we compared the reactivities of anti-S1 and anti-S2 to a standard curve, we found that the levels of reactivity against the antisera in semen samples from 20 individuals corresponded to concentrations of antigen ranging from 90 – 3,000 µg/ml, well above the range necessary for enhancing HIV infection.
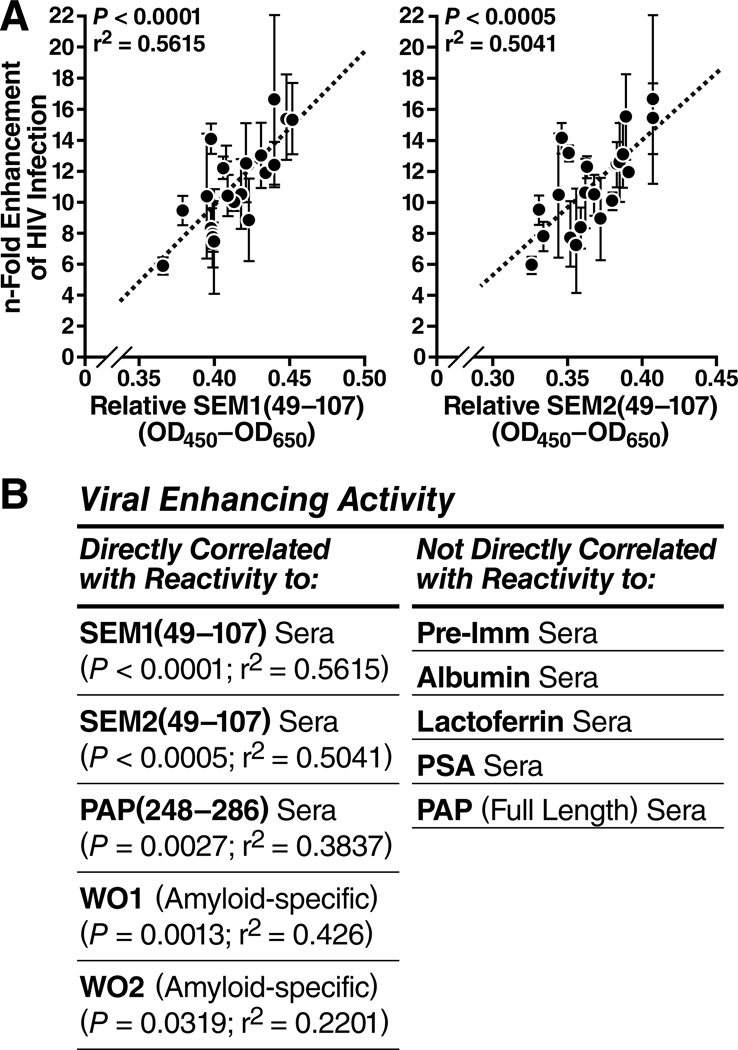
We tested the relative ability of semen samples from 20 individuals to enhance HIV-1 infection of TZM-bl cells, and plotted this as a function of the relative levels of SEM1- and SEM2-containing fragments as assessed using anti-S1 and anti-S2. The relative abilities to enhance HIV infection correlated directly with levels of SEM1 and SEM2 (Figure 6A). Consistent with published results (Kim et al., 2010), we also observed a direct correlation between the levels of PAP248–286 / SEVI and enhancing activity (Figure 6B, Figure S6C). In contrast, the levels of the seminal proteins albumin, lactoferrin, PSA, and full-length PAP did not correlate with viral enhancing activity. Finally, a statistically significant direct correlation existed between viral enhancing activity and reactivity to the antibodies WO1 and WO2, which recognize the general amyloid fold (O'Nuallain and Wetzel, 2002) (Figure 6B, Figure S6C). All together, these results support roles for both SEM and SEVI amyloids in semen-mediated enhancement of viral infection.
Figure 6. The Magnitude of Enhancement of HIV-1 Infection by Individual Semen Samples Correlates Directly with the Relative Levels of SEM peptides and PAP248–286.
(A) Direct correlation between viral enhancement activity and the quantity of SEM1 (left) and SEM2 (right) fragments measured using the anti-S1 and anti-S2 sera, respectively. The n-fold enhancement on the y-axis was determined using a TZM-bl infectivity assay. Error bars reflect standard deviations of triplicate well measurements. The relative SEM levels on the x-axis were determined by ELISA. See also Figure S6B.
(B) Summary of all correlation analysis of viral enhancing activity vs. reactivity to the indicated antibodies. Semen samples from 20 donors were tested for relative viral enhancing activity and for reactivity to the indicated antibodies. The left column displays cases where there was a direct correlation (P-values all < 0.05, as shown) between viral enhancing activity and reactivity to the indicated antibody. The right column displays cases where there was no direct correlation. See also Figure S6C.
Semen Samples Naturally Deficient in SEM Do Not Enhance HIV Infection
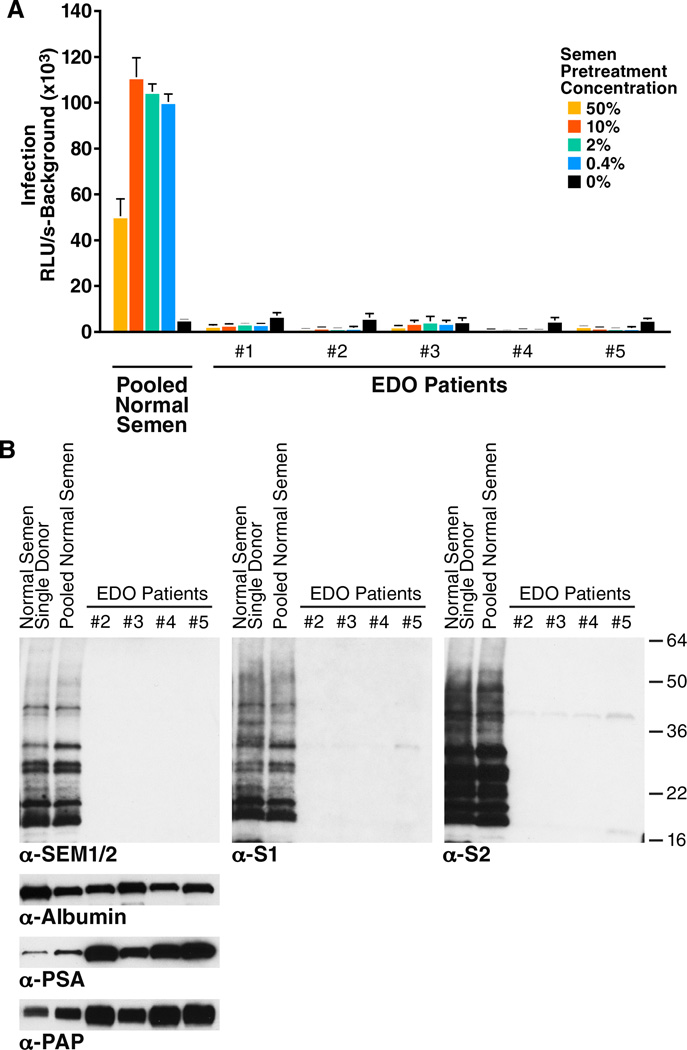
Semen samples from patients with ejaculatory duct obstruction (Van Damme et al.) have low levels of SEMs because secretions from the seminal vesicles do not effectively reach the ejaculate (Edstrom et al., 2008; Pryor and Hendry, 1991). If SEMs are important for the viral enhancing activity of semen, then semen from EDO patients should be deficient in this activity. Five semen samples from EDO patients were tested for the ability to enhance HIV-1 81A infection of TZM-bl cells. All five samples from EDO patients completely lacked HIV enhancement activity, in contrast to semen pooled from 20 normal donors (Figure 7A).
Figure 7. Semen Samples from EDO Patients Lack Viral Enhancing Activity and are Deficient in SEMs.
(A) Pooled semen from normal donors or individual semen samples from patients with EDO were pre-treated at the indicated concentrations with 81A virions for 5 min. Samples were then diluted 15-fold and added to TZM-bl cells. Medium was replaced after 2 hours, and cells were assayed for Tat-inducible β-galactosidase activity 3 days later. RLU/s: relative light units / second. Shown are average values (± standard deviations) of triplicate measurements. The decrease in infectivity enhancement with a semen pretreatment concentration of 50% is due to semen-mediated toxicity to the cells (Kim et al., 2010).
(B) Semen samples from normal donors or from EDO patients were analyzed by Western blot using commercial antibodies recognizing both SEM1 and SEM2 (α-SEM1/2), or the anti-S1 and anti-S2 antibodies generated for this study. Samples were also blotted for albumin, PSA, and PAP as indicated. Note that EDO1 was not analyzed by Western blot analysis because of limited material. See also Figure S7.
We used Western blots to confirm the lack of SEM in the EDO samples. Commercially available antibodies against SEM1/2, and our anti-S1 and anti-S2, all detected SEM fragments in semen from healthy donors (Figure 7B). The predominance of multiple SEM fragments in semen is consistent with prior reports that PSA cleaves SEMs at multiple sites immediately post-ejaculation (Koistinen et al., 2002; Robert et al., 1997). In contrast to semen from healthy donors, samples from EDO patients had very little reactivity to the SEM antibodies (Figure 7B). Albumin levels were uniform across the samples, suggesting the EDO samples were not simply deficient in all proteins. Interestingly, the levels of PSA and PAP were higher in EDO samples, possibly from over-representation of prostate-derived proteins in semen from these patients (Figure 7B). These Western blot results were confirmed by ELISA (data not shown). When we assessed the levels of the SEVI precursor peptide PAP248–286, we found that whereas the levels of full-length PAP were increased in EDO samples (Figure 7B), the levels of PAP248–286 were decreased (Figure S7). These data suggest that the proteolytic activity necessary to generate SEVI from PAP may be decreased in semen from EDO patients. In conclusion, semen samples naturally deficient in secretions from the seminal vesicles lack the ability to enhance HIV infection.
DISCUSSION
In this paper, we identify a set of amyloidogenic peptides derived from SEMs, the major components of the semen coagulum. These SEM-derived amyloid fibrils join SEVI as semen factors that enhance virion attachment and entry into target cells. We further demonstrate that endogenous SEMs contribute greatly towards the viral enhancing activity of semen by showing that: (1) The relative enhancing activity of semen samples from different donors correlates with SEM levels; (2) SEM-deficient semen from EDO patients lack viral enhancing activity.
EDO patients, whose semen lacks secretions from the seminal vesicles, represent a small subset of infertility cases. We found that semen from these patients are deficient not only in SEMs but also in SEVI. Because semen from EDO patients harbor higher levels of full-length PAP (the precursor for SEVI), we suspect that the protease responsible for cleaving full-length PAP into the SEVI peptide may reside in the seminal vesicles. The identity of this protease remains to be determined. Because EDO semen samples are deficient in both SEM and SEVI amyloids, it is unclear which species represents the dominant enhancing activity of semen. Although it is unknown whether SEVI or SEM amyloids contribute more towards the viral enhancing activity of semen, it is noteworthy that SEMs are more abundant than PAP, the precursor for SEVI. While the concentration of full-length SEM proteins is estimated to be 10–20 mg/ml in semen (Yoshida et al., 2003), PAP is only 1–2 mg/ml (Ronnberg et al., 1981). Failure of the amyloid-specific WO1 antibody to pull out SEVI from seminal fluid may be due to a higher abundance of the SEM proteins. We have confirmed that WO1 recognizes synthetic SEVI amyloid fibrils but not the monomeric PAP(248–286) peptide (data not shown). However, it is also possible that WO1 may not detect endogenous SEVI in semen, particularly if natural SEVI exists mainly as small protofibrils, since amyloid-specific antibodies often recognize epitopes found in mature fibrils but not prefibrillar oligomers (Kayed et al., 2007). Furthermore, because amyloids can adopt a wide diversity of structures [reviewed by (Toyama and Weissman)], WO1 may not recognize all the different forms of SEVI that may exist in semen. To directly assess the relative contribution of SEVI and SEM fibrils for the enhancing activity of semen, we are developing conformation-specific monoclonal antibodies that can selectively immunodeplete these fibrils from semen. Regardless of which factor contributes more, because semen from EDO patients are completely deficient in viral enhancing activity, a key prediction is that HIV-infected patients with EDO will be less likely to sexually transmit HIV. Testing this hypothesis would be interesting albeit technically challenging, as EDO patients are estimated to represent only 1 – 5% of all male infertility cases (Pryor and Hendry, 1991).
We and others observed that semen enhances HIV infection in vitro (Bouhlal et al., 2002; Hauber et al., 2009; Kim et al., 2010; Munch et al., 2007; Olsen et al., 2010; Roan et al., 2009; Roan et al., 2010), supporting a potential role for SEM fibrils and SEVI in enhancing HIV infection during sexual transmission. In contrast, others have reported that HIV infection is inhibited by semen (Balandya et al., 2010; Martellini et al., 2009; Sabatte et al., 2007). These contrasting results may reflect multiple factors, including the presence of both enhancing and inhibitory factors for HIV infection, inhibitory effects due to semen-mediated toxicity on target cells, and different experimental conditions among laboratories. Of note, the inhibitory activity reported by Sabatte et al. was specific for DC-SIGN-dependent enhancement of trans-infection to T cells (Sabatte et al., 2007), and indeed we previously confirmed the presence of this inhibitory activity (Kim et al., 2010). Conversely, our observation that semen enhances direct HIV infection of multiple cell types stands in sharp contrast with reports that semen inhibits HIV infection in vitro (Balandya et al., 2010; Martellini et al., 2009). We have carefully reproduced the conditions used by Martellini and coworkers, who suggested that semen may inhibit HIV infection. Our analysis reveals a direct correlation between semen-induced cytotoxicity and the observed viral inhibitory activity of semen, suggesting that the observed inhibition was likely due to low viability of target cells (Kim et al., 2010). More recently, Balandya et al. reported that seminal fluid inhibits HIV infection by down-modulating a whole panel of cell-surface receptors (Balandya et al., 2010). As they were working with a concentration of seminal fluid that is just at the threshold of killing target cells, we believe that cytopathic effects of the seminal fluid likely accounted for the observed inhibition of HIV infection. Indeed, their observation of a general decrease in all cell-surface receptors except for CCR5, whose expression is increased on late apoptotic cells (Ariel et al., 2006), suggests that the target cells they were using may have been undergoing apoptosis at the time of infection. Furthermore, we have found that inhibitory semen concentrations utilized by Balandya et al. are markedly cytotoxic for PBMCs (unpublished data). Recognizing that the in vitro cytotoxic effects of semen can complicate its evaluation, we have worked out and described methods for examining the effect of semen on HIV infection under conditions that preserve the viability of target cells (Kim et al., 2010; Munch et al., 2007). We believe it is paramount to carry out semen analysis under such non-toxic conditions, since viral gene expression is dependent on the “fitness” of cells and cytotoxic effects may result in misleading conclusions.
Ultimately, the effect of semen on HIV infection (whether inhibitory or enhancing) must be carefully assessed in in vivo models of mucosal HIV infection. Two studies have examined the effect of semen on vaginal infection of macaques with SIV. Miller et al. observed that at low but not high levels of SIV innocula, human seminal plasma increased the rate of transmission (Miller et al., 1994). A second study reported increased vaginal SIVmac251 infection of macaques in the presence of semen (Neildez et al., 1998), again only under conditions of low viral innocula. Interestingly, the enhancing effects of SEVI and semen on HIV infection in vitro are also most prominent at low viral innocula (Munch et al., 2007). Unfortunately, in both of the in vivo studies mentioned, the small number of animals examined precluded meaningful statistical analysis. As such, the effect of semen in a low-dose vaginal challenge study powered with a sufficiently large number of animals to yield statistically significant results is urgently needed.
Previous studies support the notion that SEM peptides may form amyloids in vivo. Amyloid deposits reactive to SEM-specific antibodies have been observed in the seminal vesicles of patients with localized amyloidosis (Linke et al., 2005). Furthermore, localized amyloidosis of the seminal vesicles has been detected at a frequency of 17% in men over age 50 at autopsy (Bursell, 1942) and is thought to be a common condition (Pitkanen et al., 1983). The pathogenesis of such amyloids, if any, is unknown, but our identification of SEM peptides that spontaneously form fibrils in vitro may provide insights into this condition.
Although the natural function of amyloid fibrils in semen, if any, is unknown, SEMs and their fragments have numerous roles in fertilization (de Lamirande, 2007; Robert and Gagnon, 1999). SEMs block sperm motility (Robert 1996, Iwamoto 1988) and prevent sperm capacitation (de Lamirande et al., 2001) thereby inhibiting premature release of active sperm. SEMs also activate sperm hyaluronidase, which helps degrade the egg envelope during fertilization (Mandal and Bhattacharyya, 1995). Cleavage of the SEMs by PSA during liquefaction is important for fertilization, as men with semen with a slow liquefaction rate are infertile (Robert and Gagnon, 1995). In addition, several lines of evidence suggest that SEM fragments are associated with spermatozoa. SEM fragments have been reported to bind to the posterior head, midpiece, and tail of spermatozoa (Bjartell et al., 1996; de Lamirande, 2007), and antibodies raised against whole spermatozoa were found to recognize SEM1 (Herr et al., 1986). Interestingly, a SEM1 fragment was found within sperm nuclei, suggesting that SEM fragments may gain entry into these cells (Zalensky et al., 1993). Whether any of these reported activities of SEM or SEM fragments involve SEM-derived amyloid fibrils remains to be explored. Of note, spermatozoa are stainable with the amyloid-binding dye Congo Red (Liu and Foote, 1998), suggesting the presence of one or more bound amyloid fibrils. It is certainly possible SEM amyloids coat the surface of spermatozoa. This may in turn promote HIV binding to the spermatozoa and faciliate transfer of virions to susceptible target cells in both the lower and upper reproductive tracts (Ceballos et al., 2009).
Notably, semen is thus far the only biological fluid that has been reported to contain multiple amyloidogenic peptides derived from abundant proteins. PAP and SEMs, the precursors for these amyloidogenic peptides, are proteins whose expression is for the most part limited to male reproductive organs. The existence of naturally occurring amyloidogenic peptides in semen may have an evolutionary role in promoting fertilization. Since SEM fibrils can increase HIV fusion, might they also have a role in fertilization by enhancing sperm-egg fusion? Many parallels exist between the fusion of HIV to cells and the fusion of sperm to egg [reviewed in (Doncel, 2006)]. Both processes require lipid rafts and glycoproteins, and the molecular basis of the fusion processes share signaling pathways and involve integrins and tetraspanins. Furthermore, spermicidal compounds inhibit HIV infection while virucidal compounds such as polyanions and antimicrobial peptides block sperm-egg fusion (Doncel, 2006). As we obtain a broader understanding of natural factors in semen that enhance HIV infection, we may gain additional insights into the molecular events promoting fertilization and the potential involvement of these same factors. Perhaps agents that block HIV infection by targeting viral enhancing factors such as semen-derived amyloid fibrils might also serve as contraceptives.
EXPERIMENTAL PROCEDURES
Reagents
Custom peptides (synthesized by Celtek Peptides, Nashville, TN and CPC Scientific, Sunnyvale, CA) were dissolved in PBS at concentrations of 2.5 – 10 mg/ml, and amyloid fibril formation was promoted by overnight agitation at 37°C at 1,400 rpm with an Eppendorf Thermomixer. De-identified semen samples from healthy donors (obtained from the UCSF Fertility Clinic, San Francisco, CA) were allowed to liquefy for 2 hours at room temperature and then frozen at −20°C. Seminal fluid was generated by centrifuging semen pooled from 20 donors for 30 minutes at 4°C and collecting the supernatant. De-identified semen samples from patients with EDO (diagnosed by low seminal levels of fructose, as fructose in semen originates from the seminal vesicles) were kindly provided by Olë Sorensen (Lund University, Sweden). Most infectivity experiments used seminal fluid instead of semen; the exception was the analysis of individual semen samples, as there was not enough material to generate seminal fluid for the assays. Of note, the viral enhancing activity of semen and seminal fluid are similar (Munch et al., 2007). Use of semen samples was conducted under guidelines approved by the UCSF Committee on Human Research.
Depletion of Cationic and Anionic Components from Semen
SCX and SAX Dynabeads (Invitrogen, Carlsbad, CA) were used according to the manufacturer’s protocol. Briefly, after washing and equilibration, Dynabeads were incubated with 20% pooled seminal fluid (diluted in PBS) for 1 hour at 4°C. A total of four rounds of depletion was carried out for each condition. For mock-depleted samples, 20% pooled seminal fluid samples were carried through the four rounds of 4°C incubations in the absence of added beads. Total protein content in the samples was determined by the BCA (bicinchoninic acid) assay (Thermo Scientific, Waltham, MA).
ELISA
Immunolon II plates (Fisher, Waltham, MA) were coated for 12–18 hours at 4°C with 1% semen or the indicated concentration of antigen diluted in PBS. Next, wells were blocked for 2 hours at room temperature with 1% BSA diluted in PBS. Wells were then incubated with sera (diluted in 1% BSA / PBS) for 1 hour at room temperature. After washing, wells were incubated with HRP-conjugated secondary antibody (GE Healthcare, South San Francisco, CA) diluted in PBS / 1% BSA. After a second wash, TMB substrate (Sigma-Aldrich, St. Louis MO) was added and the reaction was stopped with 2M H2SO4. OD at 450/650nm was monitored using a spectrophotometer. Commercial monoclonal antibodies recognizing PSA (O14) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Commercial polyclonal antibodies recognizing PAP, lactoferrin, and albumin were purchased from Sigma-Aldrich. Amyloid-specific antibodies WO1 and WO2 were kindly provided by Ron Wetzel (University of Pittsburgh). Antibodies against PAP248–286, SEM1(49–107), and SEM2(49–107) were custom-produced by Pocono Rabbit Farms (Canadensis, PA).
Thioflavin T
125 µg/ml of agitated peptides were incubated with 5 µM Thioflavin T (Sigma-Aldrich), and the increase in fluorescence (excitation 440 nm, emission 482 nm) in triplicate wells was assayed with an Ls-5B luminescence spectrometer (Perkin-Elmer, Waltham, MA).
Electron Microscopy
Agitated peptides were adsorbed on glow-discharged carbon coated grids and negatively stained with 2% uranyl acetate. For imaging of virions with fibrils, env-defective HIV particles were centrifuged for 70 minutes at 14,000 rpm, and the pellet was resuspended in PBS and incubated at 4°C for 1 hour. Viral particles were then incubated with 500 µg/ml SEM1(49–107) fibrils for 10 minutes and fibrils were pelleted at 14,000 rpm for 10 minutes. Pellets were resuspended and adjusted to a peptide concentration of 5 mg/ml and prepared for electron microscopy. Samples were adsorbed on glow discharged coated grids, fixed with 2.5% glutaraldehyde in phosphate buffer (pH 7.3, 0.2 M), and negatively stained with 0.5% uranyl acetate. All samples were imaged with a Zeiss EM 10 transmission electron microscope (Zeiss, Oberkochen) at an accelerating voltage of 80 kV.
Fluorescence Microscopy
200 µg/ml SEM1(49–107) fibrils was added to the ProteoStat® Amyloid Plaque Detection Kit (Enzo Life Sciences, Plymouth Meeting, PA). Fibrils were then incubated 1:1 with MLV-gag-YFP viral particles and where indicated added to 50,000 TZMbl cells stained with CellTrace™ Violet Cell Proliferation Kit (Invitrogen). Samples were transferred onto μ-slides VI0.4 (Ibidi, Munich, Germany) and imaged with a Zeiss LSM confocal microscope using the LSM 710 Release version 5.5SP1 software.
Cell Culture
Primary CD4+ T cells were isolated from buffy coats by Ficoll-Hypaque density gradients, followed by negative selection with CD14+ microbeads and positive selection with CD4+ microbeads (Miltenyi, Bergisch Gladbach, Germany). The purified CD14−CD4+ cells were cultured in RPMI medium supplemented with 10% FBS, L-glutamine (2 mM), penicillin (50 U/ml), and streptomycin (50 µg/ml). 293T and TZM-bl cells were cultured in DMEM medium supplemented with 10% FBS, L-glutamine (2 mM), penicillin (50 U/ml), and streptomycin (50 µg/ml).
Viral Production
CCR5-tropic 81A viruses used in TZM-bl infectivity assays were produced by Fugene-mediated transfection (Roche, Switzerland) of 293T cells with a proviral DNA expression plasmid. CCR5-tropic NL4-3 92th014.12 viruses (Papkalla et al., 2002) used in TZM-bl infectivity assays were produced by transient calcium phosphate transfection of 293T cells with the proviral pBRHIV-1NL4-3_92Th014 expression plasmid. BlaM-Vpr chimeric CCR5-tropic 81A and NL43 viruses used in the fusion assays were produced by Fugene-mediated transfection of 293T cells with proviral DNA expression plasmids as previously described (Cavrois et al., 2002). Two days after transfection, supernatants were clarified by sedimentation and titered for p24 content by anti-p24Gag ELISA (Perkin-Elmer).
Virion Fusion Assay
Virion fusion was performed similar to previously described methods (Cavrois et al., 2002). HIV-1 virions (200 ng/ml) were pretreated with SEM or SEVI fibrils (31.25 µg/ml) for 1 hour at 37°C and then added to 5 × 105 CD14−CD4+ target cells. To examine the effect of heparin on the viral enhancing activity of the SEM fibrils, target cells instead of virions were pretreated, as heparin has direct inhibitory effects on HIV (Roan et al., 2009). For target cell pre-treatment, 5 × 105 CD14−CD4+ cells were incubated with the indicated SEM fibrils (31.25 µg/ml) in the absence or presence of heparin (31.25 µg/ml, Sigma-Aldrich) for 1 hour. After pre-treatment, cells were washed three times with medium and added to virions. Viral fusion was allowed to proceed for 4 hours at 37°C, after which cells were loaded overnight with CCF2. Cells were stained with surface antibodies against CD3 and CD4, fixed with 2% paraformaldehyde, and then analyzed on a BD LSRII. Flow cytometric data were processed with FlowJo software (Treestar, Ashland, OR).
TZM-bl Infectivity Assays
Infectivity assays were essentially performed as described (Kim et al., 2010; Roan et al., 2009). HIV-1 from the supernatant of transfected 293T cells was diluted to 0.1 µg/ml p24 and pretreated for 5 min with peptide or semen/seminal fluid at the indicated concentrations. 20 µl of the pretreated virions were added to TZM-bl cells (104/well in 96-well flat-bottom plates) in 180 µl medium (for peptide treatment) or 280 µl medium (for semen / seminal fluid treatment). To minimize toxic effects mediated by prolonged exposure of semen / seminal fluid to target cells, the medium was replaced after 2 hours, and infection was assayed 2 – 3 days later by monitoring β-galactosidase activity using the Gal-Screen kit (Life Technologies, Carlsbad, CA). Background signals obtained from uninfected cells were subtracted from values obtained with infected cells.
Western Blotting
The equivalent of 0.2 – 1 µl of seminal fluid was loaded onto 12.5% Criterion Tris-HCl polyacrylamide gels (Bio-Rad, Hercules, CA). Proteins were transferred onto nitrocellulose iBlot membranes (Invitrogen) and blocked with PBS in the presence of 5% milk and 0.1% Tween-20. Commercial antibodies against SEM1/2 and PSA were purchased from Santa Cruz Biotechnology (sc33819 and sc80304), commercial antibodies against albumin and PAP were purchased from Sigma (A0433 and P5664), and custom antibodies against PAP248–286, SEM1(49–107), and SEM2(49–107) were custom-produced by Pcocono Rabbit Farms. Primary antibodies were used at a dilution of 1:1,000 – 1:40,000, and HRP-conjugated secondary antibodies were used at a final dilution of 1:5,000. Blots were developed using the Western Lightning ECL kit (Perkin-Elmer).
HIGHLIGHTS.
Peptides from semenogelins, major semen coagulum components, form amyloid fibrils
Semenogelin-derived amyloid fibrils enhance HIV infection
Ability of semen to enhance HIV infection correlates with semenogelin levels
Semen samples naturally deficient in semenogelins fail to enhance HIV infection
Supplementary Material
ACKNOWLEDGEMENTS
We thank R. Wetzel for providing the WO1 and WO2 antibodies; O. Sörensen for semen samples from EDO patients; members of the Greene laboratory for helpful discussions; G. Howard for editorial assistance; J. Carroll, G. Maki, C. Goodfellow, and T. Roberts for assistance in preparing the figures; and S. Cammack and R. Givens for administrative assistance.
This work was supported, in whole or in part, by 1PO1 AI083050-01 PPG and grant number W81XWH-11-1-0562 from the Department of Defense (to W. C. G.), the DFG and the Ministry of Science (to J.M.), and the Giannini Foundation fellowship (to N. R. R.). The UCSF Sandler-Moore Mass Spectrometry Core Facility acknowledges support from the Sandler Family Foundation, the Gordon and Betty Moore Foundation, and NIH/NCI Cancer Center Support Grant P30 CA082103.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Non-Endorsement Disclaimer: The views, opinions and/or findings contained in this publication are those of the authors and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
REFERENCES
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nature immunology. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandya E, Sheth S, Sanders K, Wieland-Alter W, Lahey T. Semen protects CD4+ target cells from HIV infection but promotes the preferential transmission of R5 tropic HIV. J Immunol. 2010;185:7596–7604. doi: 10.4049/jimmunol.1002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartell A, Malm J, Moller C, Gunnarsson M, Lundwall A, Lilja H. Distribution and tissue expression of semenogelin I and II in man as demonstrated by in situ hybridization and immunocytochemistry. Journal of andrology. 1996;17:17–26. [PubMed] [Google Scholar]
- Bouhlal H, Chomont N, Haeffner-Cavaillon N, Kazatchkine MD, Belec L, Hocini H. Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells. J Immunol. 2002;169:3301–3306. doi: 10.4049/jimmunol.169.6.3301. [DOI] [PubMed] [Google Scholar]
- Bursell S. Upsala Lakaref Forh. 1942;47:313–331. [Google Scholar]
- Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- Ceballos A, Remes Lenicov F, Sabatte J, Rodriguez Rodrigues C, Cabrini M, Jancic C, Raiden S, Donaldson M, Agustin Pasqualini R, Jr, Marin-Briggiler C, et al. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. The Journal of experimental medicine. 2009;206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lamirande E. Semenogelin, the main protein of the human semen coagulum, regulates sperm function. Seminars in thrombosis and hemostasis. 2007;33:60–68. doi: 10.1055/s-2006-958463. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Yoshida K, Yoshiike TM, Iwamoto T, Gagnon C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. Journal of andrology. 2001;22:672–679. [PubMed] [Google Scholar]
- Doncel GF. Exploiting common targets in human fertilization and HIV infection: development of novel contraceptive microbicides. Human reproduction update. 2006;12:103–117. doi: 10.1093/humupd/dmi040. [DOI] [PubMed] [Google Scholar]
- Edstrom AM, Malm J, Frohm B, Martellini JA, Giwercman A, Morgelin M, Cole AM, Sorensen OE. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol. 2008;181:3413–3421. doi: 10.4049/jimmunol.181.5.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr JC, Summers TA, McGee RS, Sutherland WM, Sigman M, Evans RJ. Characterization of a monoclonal antibody to a conserved epitope on human seminal vesicle-specific peptides: a novel probe/marker system for semen identification. Biology of reproduction. 1986;35:773–784. doi: 10.1095/biolreprod35.3.773. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Molecular neurodegeneration. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen H, Soini T, Leinonen J, Hyden-Granskog C, Salo J, Halttunen M, Stenman UH, Seppala M, Koistinen R. Monoclonal antibodies, immunofluorometric assay, and detection of human semenogelin in male reproductive tract: no association with in vitro fertilizing capacity of sperm. Biology of reproduction. 2002;66:624–628. doi: 10.1095/biolreprod66.3.624. [DOI] [PubMed] [Google Scholar]
- Linke RP, Joswig R, Murphy CL, Wang S, Zhou H, Gross U, Rocken C, Westermark P, Weiss DT, Solomon A. Senile seminal vesicle amyloid is derived from semenogelin I. J Lab Clin Med. 2005;145:187–193. doi: 10.1016/j.lab.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Liu Z, Foote RH. Bull sperm motility and membrane integrity in media varying in osmolality. Journal of dairy science. 1998;81:1868–1873. doi: 10.3168/jds.S0022-0302(98)75757-1. [DOI] [PubMed] [Google Scholar]
- Mandal A, Bhattacharyya AK. Sperm hyaluronidase activation by purified predominant and major basic human seminal coagulum proteins. Human reproduction (Oxford, England) 1995;10:1745–1750. doi: 10.1093/oxfordjournals.humrep.a136167. [DOI] [PubMed] [Google Scholar]
- Martellini JA, Cole AL, Venkataraman N, Quinn GA, Svoboda P, Gangrade BK, Pohl J, Sorensen OE, Cole AM. Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. Faseb J. 2009;23:3609–3618. doi: 10.1096/fj.09-131961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, Hendrickx AG. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Neildez O, Le Grand R, Cheret A, Caufour P, Vaslin B, Matheux F, Theodoro F, Roques P, Dormont D. Variation in virological parameters and antibody responses in macaques after atraumatic vaginal exposure to a pathogenic primary isolate of SIVmac251. Research in virology. 1998;149:53–68. doi: 10.1016/s0923-2516(97)86900-2. [DOI] [PubMed] [Google Scholar]
- O'Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1485–1490. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JS, Brown C, Capule CC, Rubinshtein M, Doran TM, Srivastava RK, Feng C, Nilsson BL, Yang J, Dewhurst S. Amyloid-binding small molecules efficiently block SEVI (semen-derived enhancer of virus infection)- and semen-mediated enhancement of HIV-1 infection. J Biol Chem. 2010;285:35488–35496. doi: 10.1074/jbc.M110.163659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkalla A, Munch J, Otto C, Kirchhoff F. Nef enhances human immunodeficiency virus type 1 infectivity and replication independently of viral coreceptor tropism. J Virol. 2002;76:8455–8459. doi: 10.1128/JVI.76.16.8455-8459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen P, Westermark P, Cornwell GG, 3rd, Murdoch W. Amyloid of the seminal vesicles. A distinctive and common localized form of senile amyloidosis. The American journal of pathology. 1983;110:64–69. [PMC free article] [PubMed] [Google Scholar]
- Pryor JP, Hendry WF. Ejaculatory duct obstruction in subfertile males: analysis of 87 patients. Fertility and sterility. 1991;56:725–730. doi: 10.1016/s0015-0282(16)54606-8. [DOI] [PubMed] [Google Scholar]
- Roan NR, Munch J, Arhel N, Mothes W, Neidleman J, Kobayashi A, Smith-McCune K, Kirchhoff F, Greene WC. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol. 2009;83:73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Sowinski S, Munch J, Kirchhoff F, Greene WC. Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection) J Biol Chem. 2010;285:1861–1869. doi: 10.1074/jbc.M109.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M, Gagnon C. Sperm motility inhibitor from human seminal plasma: association with semen coagulum. Human reproduction (Oxford, England) 1995;10:2192–2197. doi: 10.1093/oxfordjournals.humrep.a136267. [DOI] [PubMed] [Google Scholar]
- Robert M, Gagnon C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944–960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–3819. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- Ronnberg L, Vihko P, Sajanti E, Vihko R. Clomiphene citrate administration to normogonadotropic subfertile men: blood hormone changes and activation of acid phosphatase in seminal fluid. International journal of andrology. 1981;4:372–378. doi: 10.1111/j.1365-2605.1981.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Sabatte J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J, Salamone G, Salomon H, Amigorena S, Geffner J. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J Virol. 2007;81:13723–13734. doi: 10.1128/JVI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Weissman JS. Amyloid structure: conformational diversity and consequences. Annual review of biochemistry. 80:557–585. doi: 10.1146/annurev-biochem-090908-120656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. The New England journal of medicine. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Yamasaki T, Yoshiike M, Takano S, Sato I, Iwamoto T. Quantification of seminal plasma motility inhibitor/semenogelin in human seminal plasma. Journal of andrology. 2003;24:878–884. doi: 10.1002/j.1939-4640.2003.tb03139.x. [DOI] [PubMed] [Google Scholar]
- Zalensky AO, Yau P, Breneman JW, Bradbury EM. The abundant 19-kilodalton protein associated with human sperm nuclei that is related to seminal plasma alpha-inhibins. Molecular reproduction and development. 1993;36:164–173. doi: 10.1002/mrd.1080360207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.