Abstract
Although plasma neutrophil gelatinase-associated lipocalin (NGAL) is a promising biomarker for early detection of acute kidney injury, its ability to predict recovery is unknown. Using RIFLE criteria to define kidney injury, we tested whether higher plasma NGAL concentrations on the first day of RIFLE-F would predict failure to recover in a post hoc analysis of a multicenter, prospective, cohort study of patients with community-acquired pneumonia. Recovery was defined as alive and not requiring renal replacement therapy during hospitalization or having a persistent RIFLE-F classification at hospital discharge. Median plasma NGAL concentrations were significantly lower among the 93 of 181 patients who recovered. Plasma NGAL alone predicted failure to recover with an area under the receiver operating characteristic curve of 0.74. A clinical model using age, serum creatinine, pneumonia severity, and nonrenal organ failure predicted failure to recover with area under the curve of 0.78. Combining this clinical model with plasma NGAL concentrations did not improve prediction. The reclassification of risk of renal recovery, however, significantly improved by 17% when plasma NGAL was combined with the clinical model. Thus, in this cohort of patients with pneumonia-induced severe acute kidney injury, plasma NGAL appears to be a useful biomarker for predicting renal recovery.
Keywords: acute kidney injury, neutrophil gelatinase-associated lipocalin, RIFLE criteria, renal recovery
Sepsis is a leading cause of acute kidney injury (AKI) in hospitalized patients,1, 2, 3, 4, 5 and development of AKI is associated with increased risk of death.3, 5 Recent studies of patients with AKI in which sepsis was the main contributing factor for AKI demonstrated that a large number of survivors failed to recover renal function. First, the Beginning and Ending Supportive Therapy for the kidney study showed that 13.8% of patients with severe AKI require renal replacement therapy at hospital discharge.1 Second, the Acute Renal Failure Trial Network study revealed that only 16% of participants were alive and free of renal replacement therapy by hospital discharge, and only 25% by day 60.6 These findings emphasize the need for exploring interventions to improve outcomes following AKI.
However, in order to design interventions for AKI, it is important to distinguish patients at risk for failure to recover kidney function from those who are likely to spontaneously recover renal function. Such early risk stratification has important therapeutic implications such as to enroll a homogenous group of patients in clinical trials of AKI, to determine the timing of initiation of renal replacement therapy in those with severe AKI, and withdraw care in those who are likely to have poor prognosis. However, existing clinical severity scores for AKI7, 8, 9, 10, 11, 12 have neither high levels of discrimination nor calibration to predict renal recovery.13 On the other hand, emerging newer biomarkers of AKI could aid in risk stratification analogous to biomarkers of cardiovascular disease.14, 15
Neutrophil gelatinase-associated lipocalin (NGAL) is emerging as an important biomarker for early detection of AKI in various settings,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 including sepsis-induced AKI,24 and would be a logical choice to evaluate for prediction of recovery. In addition, our previous work has suggested that plasma interleukin (IL)-6, a proinflammatory molecule, was strongly associated with sepsis-induced AKI.32 However, neither plasma NGAL (pNGAL) nor IL-6 has been evaluated as a marker of outcome following AKI. In the present analysis, we examined whether pNGAL or IL-6 are useful biomarkers for predicting recovery from AKI in a multicenter, prospective study of patients with community-acquired pneumonia. We chose community-acquired pneumonia because it is a leading cause of AKI and a common infectious cause of hospitalization.33
Using the widely validated Risk, Injury, Failure, Loss, and End-stage Kidney Disease (RIFLE) criteria to define AKI, we performed the following analyses. First, among patients with severe AKI (RIFLE-F), we performed an outcome-stratified analysis of baseline characteristics of patients who recovered renal function from those who did not. Recovery was defined as alive and neither requiring renal replacement therapy during hospitalization nor having persistent RIFLE-F at hospital discharge. Second, we examined differences in pNGAL and IL-6 concentration by recovery status. Third, we examined whether pNGAL predicted failure to recover after adjusting for differences in demographics, severity of illness, process of care, and within the strata of risk categories for nonrecovery. Fourth, we assessed the contribution of pNGAL to a clinical model in reclassifying recovery using net reclassification index (NRI) and integrated discrimination improvement (IDI).
RESULTS
Participant characteristics and hospital course by recovery
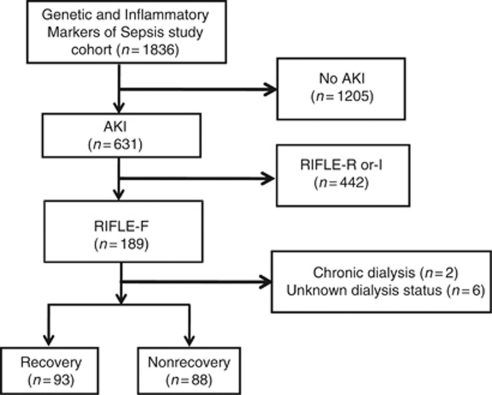
Of the 1836 subjects with community-acquired pneumonia, 631 developed AKI.32 We selected a subset of 189 patients (30%) who met the RIFLE-F criteria. Two patients who received chronic dialysis before study enrollment and 6 patients in whom renal replacement therapy status during hospitalization was unknown were excluded. Of the 181 patients with severe AKI who formed the analysis cohort, 93 subjects (51.4%) had renal recovery (Figure 1). Table 1 shows the baseline characteristics stratified by recovery status. Subjects who did not recover were likely to be older, had higher serum creatinine and blood urea nitrogen, and had more severe pneumonia. Patients who did not recover developed more severe nonrenal organ dysfunction during hospitalization.
Figure 1.
Subject disposition for the Genetic and Inflammatory Markers of Sepsis study cohort. AKI, acute kidney injury; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease.
Table 1. Patient characteristics by renal recovery.
|
No. (%) |
|||
|---|---|---|---|
| Characteristic | Recovery (n=93) | Nonrecovery (n=88) | P-value |
| Age, mean (s.d.), years | 67.9 (15.6) | 72.9 (14.9) | 0.009 |
| Male gender | 48 (51.6) | 53 (60.2) | 0.24 |
| White race | 75 (80.7) | 74 (84.1) | 0.54 |
| Baseline creatinine, mean (s.d.), mg/dl | 0.93 (0.19) | 1.03 (0.71) | 0.65 |
| Serum creatinine on the first day of RIFLE-F,a mean (s.d.), mg/dl | 1.75 (1.87) | 3.14 (2.8) | <0.001 |
| BUN on day 1, mean (s.d.), mg/dl | 29.26 (17.87) | 40.27 (23.66) | 0.001 |
| BUN on the first day of RIFLE-F, mean (s.d.), mg/dl | 29.09 (19.17) | 44.53 (26.91) | <0.001 |
| Previous antibiotic use | 19 (20.43) | 12 (13.64) | 0.23 |
| Severe sepsis on day 1b | 57 (61.29) | 60 (68.18) | 0.33 |
| Pneumonia severity index on day 1,c mean (s.d.) | 117.12 (37.94) | 133.27 (39.86) | 0.005 |
| Pneumonia severity index class | |||
| I and II | 16 (17.2) | 8 (9.1) | 0.43 |
| III | 14 (15.1) | 15 (17) | |
| IV | 43 (46.2) | 42 (47.7) | |
| V | 20 (21.5) | 23 (26.1) | |
| APACHE III score on day 1,d mean (s.d.) | 48.4 (16.4) | 52.4 (18) | 0.22 |
| Charlson comorbidity index,e mean (s.d.) | 1.67 (1.96) | 1.93 (1.96) | 0.35 |
| Maximum nonrenal SOFA score,f mean (s.d.) | 4.35 (2.91) | 5.45 (3.56) | 0.04 |
| Nonrenal SOFA score on day 1,g mean (s.d.) | 2.68 (2.18) | 2.83 (2.09) | 0.49 |
| Leukocyte count of the day of RIFLE-F, mean (s.d.) | 15,480 (9410) | 18,240 (11,860) | 0.58 |
Abbreviations: APACHE, acute physiology and chronic health evaluation; BUN, blood urea nitrogen; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease; SOFA, sequential organ failure assessment.
For severity of AKI, patients were classified according to the maximum RIFLE stage (risk, injury, or failure) reached during the entire hospitalization as proposed by the Acute Dialysis Quality Initiative.43
Defined as sepsis plus acute organ dysfunction according to 2001 international consensus criteria for severe sepsis.46
Pneumonia Severity Index was measured according to criteria by Fine et al.42
APACHE III score assessed on first hospital day regardless of whether subject was admitted to intensive care unit (ICU) or not.7
According to the method of Charlson et al.45
Cumulative SOFA score, excluding the renal part, assessed on the first 7 days of hospital admission according to the method of Vincent et al.8
SOFA score, excluding the renal part, assessed on the first day of hospital admission according to the method of Vincent et al.8
Table 2 shows the hospital course and outcomes by recovery status. The proportions of patients who were admitted to intensive care unit, received mechanical ventilation, and intensive care unit length of stay were similar. However, subjects who did not recover from AKI were more likely to stay in the hospital shorter than recovery group. This apparently shorter duration of stay among those who did not recover was mainly because of higher hospital mortality among those who did not recover. Patients who did not recover, however, also incurred higher 30-, 60-, and 90-day mortality compared with those who did recover.
Table 2. Hospital course and outcomes by renal recovery.
|
No (%) |
|||
|---|---|---|---|
| Characteristic | Recovery (n=93) | Nonrecovery (n=88) | P-value |
| ICU admission | 18 (19.4) | 13 (14.8) | 0.41 |
| Mechanical ventilation | 35 (37.6) | 46 (52.3) | 0.05 |
| Length of stay in ICU, mean (s.d.) | 5.6 (5.5) | 5.4 (6.0) | 0.38 |
| Length of stay in hospital, mean (s.d.) | 14.7 (7.6) | 11.6 (7.1) | 0.003 |
| Received renal replacement therapy in the hospital | 0 | 14 (15.9) | <0.001 |
| Persistent RIFLE-F at hospital discharge | 0 | 60 (68.2) | <0.001 |
| Mortality | |||
| Hospital | 0 | 43 (48.9) | <0.001 |
| 30-day | 4 (4.3) | 42 (47.7) | <0.001 |
| 60-day | 9 (9.7) | 49 (55.7) | <0.001 |
| 90-day | 12 (12.9) | 49 (55.7) | <0.001 |
Abbreviations: ICU, intensive care unit; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease.
Biomarker concentration by recovery
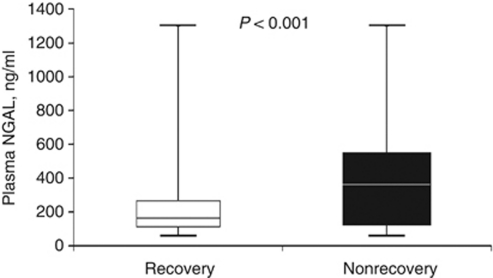
Median pNGAL concentration on the first day of RIFLE-F was significantly lower in the recovery group: recovery vs nonrecovery 165 ng/ml (interquartile range (IQR) 113–266) vs 371 ng/ml (IQR 201–519), P<0.001 (Figure 2). Nonsurvivors of AKI had higher median pNGAL concentration compared with survivors: nonsurvivor vs survivor, 409.5 ng/ml (IQR 201–588) vs 207 ng/ml (IQR 127–381), P<0.001. Similarly, we also found a higher median pNGAL concentration among those with persistent RIFLE-F (308 ng/ml, IQR 185.5–490) compared with those without (203.5 ng/ml, IQR 122.5–377) (P=0.005). However, pNGAL concentration among those with and without renal replacement therapy was not different (404 ng/ml (IQR 202–522) vs 210 ng/ml (IQR 127–381), P=0.137). Finally, we restricted our analysis only to survivors (n=138) and pNGAL concentration was again significantly lower in those who recovered: recovery vs nonrecovery (165 ng/ml (IQR 113–266) vs 302 ng/ml (IQR 200–466), P< 0.001).
Figure 2.
Plasma neutrophil gelatinase-associated lipocalin (NGAL) concentration stratified by recovery. Values are displayed as box-plot summaries with P<0.05 denoting statistical significance. The vertical box represents the 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values, whereas the whiskers represent the maximum and minimum values.
We found no difference in plasma IL-6 concentration on the first day of RIFLE-F between the two groups: recovery vs nonrecovery 3.6 (IQR 2.2–4.5) vs 3.9 (IQR 2.5–5.1) log ng/ml, P=0.20. There was also a poor correlation between pNGAL and IL-6 (correlation coefficient=0.31).
Prediction of renal recovery
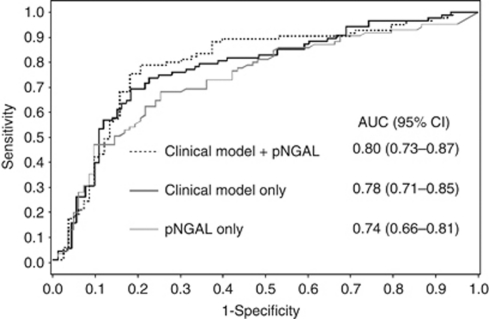
Table 3 lists the derived sensitivities, specificities, and predictive values for pNGAL at the cutoff concentrations that provided the maximum summation of sensitivity and specificity. The sensitivity and specificity were optimal at the 257 ng/ml cutoff, and area under the curve (AUC) was 0.74 (95% confidence interval (CI) 0.66–0.81; Figure 3). Plasma NGAL only modestly predicted death (AUC 0.71, 95% CI 0.61–0.80), requirement of renal replacement therapy (AUC 0.62, 95% CI 0.45–0.81), and persistent RIFLE-F (AUC 0.63, 95% CI 0.54–0.72). Of the survivors (n=138), pNGAL predicted failure to recover renal function with AUC of 0.71 (95% CI 0.61–0.81).
Table 3. Plasma NGAL concentration at various cutoff values to predict failure to recover renal function.
| pNGAL cutoff (ng/ml) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 125 | 0.91 | 0.33 | 0.58 | 0.77 |
| 257 | 0.68 | 0.75 | 0.73 | 0.70 |
| 393 | 0.47 | 0.90 | 0.83 | 0.63 |
Abbreviations: NPV, negative predictive value; pNGAL, plasma neutrophil gelatinase-associated lipocalin; PPV, positive predictive value.
Figure 3.
The area under the curve (AUC) for prediction of failure to recover renal function: plasma neutrophil gelatinase-associated lipocalin (pNGAL) alone (gray solid line), a clinical prediction model (black solid line), and a combined model (dotted line). Clinical predictors included age, serum creatinine, pneumonia severity index, and maximum nonrenal Sequential Organ Failure Assessment (SOFA) score.
Table 4 shows the univariable and multivariable analyses to predict renal recovery. When adjusted for differences in age, serum creatinine, pneumonia severity index and nonrenal SOFA (Sequential Organ Failure Assessment) score, pNGAL independently predicted failure to recover renal function (odds ratio=2.02, 95% CI 1.03–3.31, P=0.016). These results remained unchanged when pneumonia severity index score was excluded from the model (data not shown).
Table 4. Analysis of predictors of failure to recover renal function.
| Characteristic | Odds ratio (95% CI) (unadjusted) | P-value | Odds ratio (95% CI) (adjusted) | P-value |
|---|---|---|---|---|
| Age (per 5 years) | 1.11 (1.01–1.23) | 0.03 | 1.09 (0.96–1.24) | 0.20 |
| Creatinine on the first day of RIFLE-F | 1.47 (1.18–1.84) | <0.001 | 1.32 (1.07–1.64) | 0.011 |
| PSI on day 1 (per 10 points) | 1.12 (1.03–1.21) | 0.007 | 0.99 (0.90–1.10) | 0.9039 |
| Maximum nonrenal SOFA score (per 2 points) | 1.23 (1.03–1.48) | 0.025 | 1.37 (1.09–1.74) | 0.0078 |
| pNGAL (per 300 ng/ml) | 3.31 (1.82–4.46) | <0.001 | 2.02 (1.03–3.31) | 0.0155 |
Abbreviations: CI, confidence interval; pNGAL, plasma neutrophil gelatinase-associated lipocalin; PSI, pneumonia severity index; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease; SOFA, sequential organ failure assessment.
In order to assess the discriminatory characteristics of pNGAL along with the clinical variables on nonrecovery, we performed a stepwise analysis combining pNGAL with clinical variables (Table 5). The clinical model comprising age, serum creatinine, pneumonia severity index score, and maximum nonrenal SOFA score (Figure 3) only modestly predicted failure to recover from AKI with AUC of 0.78 (0.71–0.85). However, addition of pNGAL to the clinical model did not significantly improve discrimination (combined AUC=0.80, 95% CI 0.73–0.87; P=0.22).
Table 5. Stepwise analysis for prediction of failure to recover renal function.
| Characteristic | Model | AUC (95% CI) |
|---|---|---|
| Age | A | 0.61 (0.53–0.70) |
| Serum creatinine on first day of RIFLE F | B | 0.72 (0.64–0.79) |
| PSI score on day 1 | C | 0.62 (0.54–0.70) |
| Maximum nonrenal SOFA score | D | 0.59 (0.50–0.67) |
| Plasma NGAL alone | 0.74 (0.66–0.81) | |
| A+B | 0.73 (0.65–0.81) | |
| A+B+C | 0.73 (0.66–0.81) | |
| A+B+C+D | 0.78 (0.71–0.85) | |
| A+pNGAL | 0.74 (0.66–0.82) | |
| B+pNGAL | 0.76 (0.68–0.83) | |
| C+pNGAL | 0.73 (0.65–0.81) | |
| D+pNGAL | 0.77 (0.70–0.87) | |
| A+B+C+D+pNGAL | 0.80 (0.73–0.87) |
Abbreviations: AUC, area under the curve; CI, confidence interval; pNGAL, plasma neutrophil gelatinase-associated lipocalin; PSI, pneumonia severity index; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease; SOFA, sequential organ failure assessment.
Reclassification of recovery by NGAL
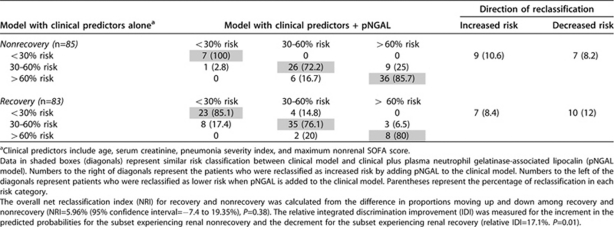
We also assessed the capability of pNGAL to ‘reclassify' the degree of risk of recovery and nonrecovery. Based on the clinical prediction model (Table 5), subjects were categorized into pre-specified ‘low', ‘intermediate', and ‘high' risk of nonrecovery (Table 6) using cutoffs of <30%, 30 to 60%, and >60%, respectively.34 We then compared the proportions of reclassified subjects across each of these three risk groups when pNGAL was added to the clinical prediction model. Among 85 patients who did not recover from AKI, 10.6% were reclassified as increased risk of nonrecovery when pNGAL was added to the clinical prediction model and 8.2% were reclassified as lower risk. Among 83 patients who recovered from AKI, 8.4% were reclassified as increased risk for recovery whereas 12% were reclassified as lower risk for recovery. There was no overall significant improvement in reclassification among recovery and non recovery when pNGAL was combined with clinical model (NRI=5.96%, P=0.38). However, using the relative IDI, the reclassification of risk of recovery improved by 17.1% when pNGAL was combined with clinical model (P=0.01).
Table 6. Risk reclassification using pNGAL and clinical predictors compared with clinical predictors alone.
DISCUSSION
In this study, we found that patients who did not recover from an episode of AKI in the setting of community-acquired pneumonia were older, and had higher serum creatinine and severity of illness during hospitalization. Plasma NGAL concentration was significantly higher in those who failed to recover and had a fair predictive value. A pNGAL level of 257 ng/ml predicted failure to recover with a sensitivity of 68% and specificity of 75% with a positive and negative predictive value of 73% and 70%, respectively. When pNGAL was combined with clinical variables, there was a 17% improvement in reclassification of risk of not recovering renal function. Most previous studies have tested pNGAL as a marker for early diagnosis of AKI.16, 18, 20, 24, 26, 28, 30, 31, 35 However, to our knowledge, this is the first study to examine the predictive value of pNGAL in recovery from pneumonia-induced severe AKI.
Advanced age and higher clinical severity score were associated with nonrecovery. These findings are not unexpected and corresponded with the previous studies.1, 9, 36 However, for a given severity of AKI, those who did not recover had higher serum creatinine on the first day of RIFLE-F compared with those who recovered. This suggests that faster rate of increase of serum creatinine in the nonrecovery group might be because of higher severity of illness or varying degrees of severity within the subgroup of RIFLE-F.
We defined failure to recover as a composite outcome of either death or dialysis or persistent RIFLE-F at hospital discharge because of face validity, as recovery is not just for renal outcome alone but for patient outcome as a whole. Moreover, for early prognostication of patients with severe AKI, we used first day of RIFLE-F to measure pNGAL concentration and built prediction models around nonrecovery rather than recovery. This has therapeutic implications such as to identify patients who are at risk for failure to recover early and enroll a uniform cohort of patients who are unlikely to recover in a biomarker-guided clinical trial of severe AKI.
We found that a higher concentration of pNGAL was associated with failure to recover following severe AKI. This negative association with recovery could be related to increased production, decreased elimination, or both. Experimental and human studies indicate that NGAL is present within the two distinct pools: systemic and renal.16 Higher pNGAL concentration in the systemic pool following AKI may be because of three factors. First, increased NGAL produced in the distant organs, especially a liver and lung and its release into the circulation following AKI.37 Second, NGAL production from the neutrophils, macrophages, and other immune-competent cells.37 Third, the decreased elimination of pNGAL because of a decrease in glomerular filtration rate after AKI might have resulted in higher plasma concentrations in our study.38
Currently, there is no definitive cutoff value for pNGAL in the prediction nonrecovery. However, based on our result, the cutoff for prediction of renal nonrecovery tends to be higher than the usual cutoff value for prediction of AKI.20, 26, 29, 39, 40 The higher pNGAL concentration might reflect the higher severity of kidney injury and is a real-time indicator of kidney dysfunction.38 This seems to be confirmed by the correlation of pNGAL level with the severity of illness and serum creatinine. In contrast, we found no difference in IL-6 concentration by recovery, as well as poor correlation between IL-6 and pNGAL, suggesting that pNGAL is a unique marker for persistent kidney injury than IL-6.
Overall, in our study the predictive value of pNGAL did not improve when it was combined with the clinical model. However, using IDI, the reclassification of risk of not recovering improved by 17% when pNGAL was combined with the clinical model.
There are several limitations to our study. First, we have tested pNGAL at only single time point (on the first day of RIFLE-F) and could not answer the predictive value of pNGAL at other time points. Nevertheless, risk stratification and prognostication using a biomarker is likely to be useful only when biomarker concentrations are measured early. Second, we were unable to measure process of care variables such as the effect of cointerventions on biomarkers and its influence on recovery. For instance, we were unable to assess the influence of fluid resuscitation on pNGAL concentration and recovery. Third, we found that nearly one-half of patients with nonrecovery had died before hospital discharge. We defined recovery to include survival, and hence death is not a competing risk. However, we were unable to separate recovery and death because of lack of cause-specific mortality data. Nevertheless, differences in plasma NGAL concentrations between recovery and nonrecovery groups persisted when the analysis was restricted to survivors. Fourth, as a post hoc analysis, our findings should be interpreted with caution, tested, and validated in prospective studies. For instance, the first day of RIFLE-F in order to measure pNGAL may not be apparent clinically, unless baseline renal function of the patient is known and serum creatinine is measured on a daily basis.
Our study has several strengths. First, as our study is the first multicenter study of pNGAL concentration in the adult population with pneumonia, our findings are highly generalizable. Second, in the clinical practice, it is very uncommon to know the onset of AKI except in some specific situations such as post cardiac surgery or post radiocontrast administration where the injury is predictable. However, in our study, we tested pNGAL on the first day of RIFLE-F in a homogenous cohort of patients with severe AKI: a population at highest risk for nonrecovery. Third, we have chosen to test blood, instead of urine, by the fact that (1) severe oliguria is common in patients with severe sepsis and may preclude the availability of urine, and (2) potentially confounding alterations in urine biomarker concentrations can be induced by volume status and diuretic therapy.
In summary, pNGAL appears to be a useful marker for predicting renal nonrecovery following community-acquired pneumonia. Our data suggest that pNGAL when used in conjunction with clinical variables improves reclassification of risk of renal nonrecovery, and augments clinical risk prediction. Further research is warranted in a larger cohort of patients with pneumonia-induced severe AKI to validate our findings.
MATERIALS AND METHODS
Study design and participant selection
This study was a post hoc analysis performed as part of the GenIMS (Genetic and Inflammatory Markers of Sepsis) study,41 which was a large, multicenter, prospective, cohort study of subjects with community-acquired pneumonia presenting to the emergency departments of 28 teaching and nonteaching hospitals in the United States between November 2001 and November 2003. Eligible criteria were age ⩾18 years and a clinical and radiological diagnosis of community-acquired pneumonia using criteria by Fine et al.42 Exclusion criteria included transfer from another hospital, discharge from an acute care hospital within the previous 10 days, pneumonia within the previous 30 days, chronic dependency on mechanical ventilation, cystic fibrosis, active pulmonary tuberculosis, admission for palliative care, previous enrollment in the study, incarceration, and pregnancy. The institutional review boards at all participating sites approved the study, and we obtained written informed consent from all participants or their proxies.
Definition of AKI and nonrecovery
AKI was classified according to the RIFLE criteria as proposed by the Acute Dialysis Quality Initiative at any time during hospitalization.43 The RIFLE stage was determined based on the worse of either serial serum creatinine or urine output. Baseline creatinine was defined by premorbid creatinine. For patients with no known premorbid creatinine and no known medical history of chronic kidney disease, we estimated premorbid creatinine using the Modification of Diet in Renal Disease equation.44 Patients were classified as stage Risk, if serum creatinine was 1.5 times the baseline creatinine, or urine output <0.5 ml/kg/h for 6 h; stage Injury, if serum creatinine was twice the baseline, or urine output <0.5 ml/kg/h for 12 h; stage Failure, if serum creatinine was thrice the baseline, or creatinine ⩾4 mg/dl with an acute rise >0.5 mg/dl, or urine output <0.3 ml/kg/h for 24 h, or anuria for 12 h.43 Of patients who developed RIFLE-F during hospitalization, recovery was defined as a composite end point of being alive at discharge and neither requiring renal replacement therapy during hospitalization nor persistent RIFLE-F at hospital discharge.
The presence or absence of underlying disease before the onset of pneumonia was defined by Charlson comorbidity index.45 Severe sepsis was defined as pneumonia plus acute organ dysfunction following the 2001 International Sepsis Definitions Conference Criteria.46 Acute organ dysfunction was defined as a new SOFA score following the European Society of Intensive Care Medicine sepsis occurrence in the acutely ill patient study criteria.8 Pneumonia Severity Index was measured according to criteria by Fine et al.42 APACHE III score was assessed on first hospital day regardless of whether subject was admitted to intensive care unit or not.7
Biomarker assay
Plasma NGAL was tested only in subjects who developed severe AKI and only on the first day of RIFLE-F using the Triage NGAL kit (Alere, San Diego, CA), a point-of-care, fluorescence immunoassay, or quantitative measurement of pNGAL and expressed as ng/ml.18 We measured IL-6 concentrations by chemiluminescent immunoassay using an automated analyzer (IMMULITE; Diagnostic Products, Los Angeles, CA).41
Statistical analyses
We compared the baseline characteristics between patients who recovered from AKI and those who failed to recover. Continuous data were expressed as mean±s.d. and compared using the Student's t-test or Wilcoxon rank-sum test. Categorical data were expressed as proportions and compared using the χ2 test or Fisher's exact test. We fitted logistic regression models to evaluate the association between pNGAL and failure to recover and generated a receiver operating characteristic curve and calculated the AUC to assess model discrimination. The optimal cutoff points were determined by the largest sum of sensitivity and specificity. To assess the additive prediction ability of pNGAL to the traditional clinical predictors, we first identified the clinical prediction model based on the AUC analysis and then added each marker individually to this clinical model. The AUCs from the combined models were compared with the AUC of the clinical model.
The increase in AUC due to the addition of a new significant marker may be very small if the current clinical model already includes important risk factors and has a reasonably good discrimination.34, 47, 48 Therefore, we also calculated the IDI and NRI using the methods suggested by Pencina et al.34 IDI is based on the new model's ability to improve integrated (average) sensitivity without sacrificing average specificity. As the absolute IDI depends on the event rate of the data, we used the relative IDI to reflect the relative improvement. NRI focuses on reclassification tables constructed separately for subjects with and without events based on predefined risk categories. We determined tertiles of risk categories of <30, 30 to 60, and >60% risk a priori to reflect low, intermediate, and high probability of nonrecovery.34 The NRI was calculated as a measure to estimate any overall improvement in reclassification with clinical variables and pNGAL instead of clinical variable alone.34 All analyses were performed using SAS 9.0 (SAS Institute, Cary, NC) at a significance level of 0.05.
Acknowledgments
GenIMS was funded by the National Institute of General Medical Science (NIGMS) R01GM61992 with additional support from GlaxoSmithKline for enrollment and clinical data collection, and Diagnostic Products Corporation for the interleukin-6 assay. NGAL kits were provided by Alere. Analyses pertaining to this study were also supported in part by a career development grant KL2RR024154 from the National Center for Research Resources (NCRR) and by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK070910. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIGMS, NCRR, NIDDK, or the National Institutes of Health.
JAK has received grant support and consulting fees from Alere. All the other authors declared no competing interests. Alere provided pNGAL kits for use in this study. The company had no influence on the study design or analysis or on the content of this article.
References
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Uchino S, Bellomo R, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24:129–140. doi: 10.1016/j.jcrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- Neveu H, Kleinknecht D, Brivet F, et al. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Pascual MT, Gruta CG, et al. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- Liano F, Gallego A, Pascual J, et al. Prognosis of acute tubular necrosis: an extended prospectively contrasted study. Nephron. 1993;63:21–31. doi: 10.1159/000187139. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Soroko SH, Paganini EP, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- Paganini EP, Halstenberg WK, Goormastic M. Risk modeling in acute renal failure requiring dialysis: the introduction of a new model. Clin Nephrol. 1996;46:206–211. [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Morimatsu H, et al. External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med. 2005;33:1961–1967. doi: 10.1097/01.ccm.0000172279.66229.07. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- Wagener G, Gubitosa G, Wang S, et al. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52:425–433. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Zhaohui N, Ben H, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–c181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin C, Yulong X, Yu C, et al. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30:904–913. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- Constantin JM, Futier E, Perbet S, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010;25:176.e1–176.e6. doi: 10.1016/j.jcrc.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36:444–451. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit Care Med. 2009;37:553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- Tuladhar SM, Puntmann VO, Soni M, et al. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53:261–266. doi: 10.1097/FJC.0b013e31819d6139. [DOI] [PubMed] [Google Scholar]
- Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D′Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond Stat Med 200827157–172.discussion 207–112. [DOI] [PubMed] [Google Scholar]
- Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- Lins RL, Elseviers MM, Daelemans R, et al. Re-evaluation and modification of the Stuivenberg Hospital Acute Renal Failure (SHARF) scoring system for the prognosis of acute renal failure: an independent multicentre, prospective study. Nephrol Dial Transplant. 2004;19:2282–2288. doi: 10.1093/ndt/gfh364. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009;88:124–130. doi: 10.1016/j.athoracsur.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]