Abstract
Podocytes have a significant role in establishing selective permeability of the glomerular filtration barrier. Sustained renin–angiotensin–aldosterone system activation is crucial to the pathogenesis of podocyte injury, but the mechanisms by which angiotensin II modulates podocyte survival due to physiological or injurious stimuli remain unclear. Here, we used proteomic analysis to find new mediators of angiotensin II–induced podocyte injury. Antioxidant protein peroxiredoxin 2 expression was decreased in cultured podocytes stimulated with angiotensin II. Peroxiredoxin 2 was found to be expressed in podocytes in vivo, and its expression was decreased in the glomeruli of rats transgenic for angiotensin II type 1 receptors in a podocyte-specific manner, or in rats infused with angiotensin II. Downregulation of peroxiredoxin 2 in podocytes resulted in increased reactive oxygen species release, protein overoxidation, and inhibition of the Akt pathway. Both treatment with angiotensin II and downregulation of peroxiredoxin 2 expression led to apoptosis of podocytes. Thus, peroxiredoxin 2 is an important modulator of angiotensin II–induced podocyte injury.
Keywords: Akt, angiotensin II, apoptosis, peroxiredoxin 2, podocyte, reactive oxygen species
The filtration barrier of the kidney comprises glomerular endothelial cells, the glomerular basement membrane, and podocytes. The foot processes of neighboring podocytes form the slit diaphragm, which has a major role in maintenance of the glomerular filtration barrier.1 Podocyte injury leads to proteinuria, a hallmark of glomerular diseases, which cause 90% of the end-stage kidney disease in the United States.2 Because terminally differentiated podocytes cannot replicate, podocyte loss is an important event in the progression to glomerulosclerosis and end-stage kidney disease.2, 3
Within the glomerulus, angiotensin II (Ang II) decreases the ultrafiltration coefficient and modulates glomerular capillary permselectivity, leading to proteinuria. Ang II concentrations in the tubular ultrafiltrate are up to 1000-fold greater than those in the systemic blood circulation, indicating intrarenal production of Ang II.4 Podocytes contribute to intrarenal Ang II levels because they possess a local renin–angiotensin system that includes Ang II type 1 receptors (AT1Rs).5, 6, 7 A large body of evidence supports the idea that inhibition of the renin–angiotensin–aldosterone system mitigates proteinuria and progression of chronic kidney disease.4, 8 Because the antiproteinuric and antihypertensive effects of AT1R blockers seem to be independent, it has been suggested that Ang II directly influences podocyte functions.9 Indeed, Ang II increases intracellular calcium activity, causes an Rac-dependent and reactive oxygen species (ROS)-mediated F-actin cytoskeleton rearrangement, and results in podocyte injury.9, 10 In addition, in vivo overexpression of AT1R in podocytes causes podocyte damage, proteinuria, and marked glomerulosclerosis.11 Thus, inhibition of renin–angiotensin–aldosterone system prevents many deleterious processes in podocytes, thereby reducing the extent of injury.10 Because renin–angiotensin–aldosterone system inhibition is so important for renal and podocyte protection, it is vital to elucidate the signaling events and proteins involved in Ang II–mediated injury. In this study, we used proteomic analysis to show that the antioxidant protein peroxiredoxin 2 (Prdx2) participates in AT1R-mediated effects on podocytes.
RESULTS
Two-dimensional difference gel electrophoresis proteomics of podocytes stimulated with Ang II
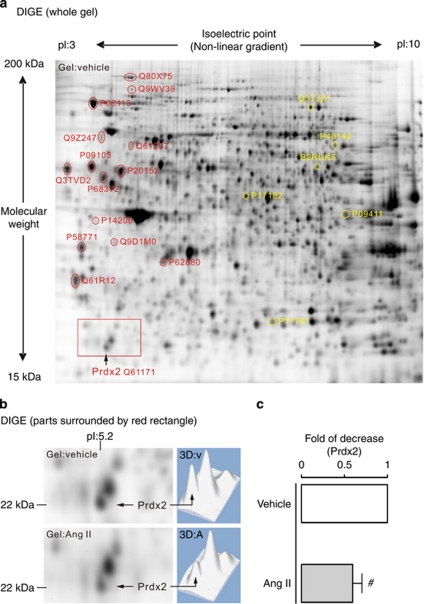
Proteomic analysis of Ang II–stimulated (100 nmol/l, 24 h) cultured AT1R podocytes provided an overview of differential protein expression levels in AT1R signaling. Using the Ettan DIGE system, we identified 21 proteins of interest that showed significant differential expression in podocytes with and without Ang II treatment (Figure 1a). Table 1 presents a summary of these proteins and their general functions. Proteomic results show that both cytosolic enzymes involved in glycolysis (α-enolase, phosphoglycerate kinase 1, transketolase, and triosephosphate isomerase) and a mitochondrial enzyme methylcrotonoyl-coenzyme A carboxylase 2 β are significantly upregulated. Therefore, podocyte metabolic rates and energy consumption may be increased after Ang II stimulation. Ang II is known to induce cytoskeletal rearrangements in differentiated podocytes, which likely explains this increased energy consumption.10 In contrast, Ang II treatment downregulated the expression of proteins involved in protein biosynthesis, the stress-response process, and neo-synthesis of cytoskeleton-related proteins. Reduced synthesis of these possibly vital proteins, especially under conditions of high metabolism and energy consumption, may lead to podocyte injury.
Figure 1.
Two-dimensional fluorescence difference gel electrophoresis (2D DIGE) and proteomic analysis identified that angiotensin II (Ang II) signaling downregulates peroxiredoxin 2 (Prdx2) expression in podocytes. (a) Monitoring of Ang II–induced changes in protein expression pattern of cultured Ang II type 1 receptor podocytes by 2D DIGE. The 15 spots labeled in red were consistently >1.6-fold downregulated (P<0.05), whereas the 6 spots labeled in yellow were consistently >1.6-fold upregulated (P<0.05) after Ang II stimulation (100 nmol/l, 24 h) compared with vehicle-treated control in a representative CyDye 2D gel image (n=4). Each spot analyzed using mass spectrometry is identified with a UniProtKB accession number. (b) Comparative parts (left panel) and three dimensional–computed spot signal intensity reconstruction (right panel; v, vehicle; A, Ang II) of the red rectangle–surrounded areas in a; downregulated Prdx2 (theoretical pI:5.2; molecular weight: 21,179 Da) expression in Ang II–treated cells versus vehicle-treated cells. (c) In quantitative analysis, differential expression of Prdx2 (a 1.69-fold decrease) was confirmed statistically by Student's t-test (#statistically significant with P<0.05). pI, isoelectric point.
Table 1. Summary of proteins differentially expressed after stimulation with Ang II.
| UniProtKB accession no. | Annotation | Change (-fold) | P-value |
|---|---|---|---|
| Metabolic enzymes | |||
| P17182 | α-Enolase | +2.10 | 3.0e−006 |
| B2RUK5 | Methylcrotonoyl-coenzyme A carboxylase 2 β | +1.91 | 0.013 |
| P09411 | Phosphoglycerate kinase 1 | +1.73 | 0.0040 |
| P40142 | Transketolase | +1.64 | 0.0071 |
| P17751 | Triosephosphate isomerase | +1.63 | 4.1e−008 |
| Protein biosynthesis | |||
| Q3TJZ1 | Eukaryotic translation elongation factor 2 | +1.96 | 9.0e−005 |
| Q3TVD2 | Calreticulin | −1.92 | 0.0005 |
| Q9D1M0 | Protein SEC13 homolog | −1.82 | 5.3e−006 |
| P09103 | Protein disulfide-isomerase | −1.68 | 3.3e−007 |
| Q9Z247 | Peptidyl-prolyl cis–trans isomerase FKBP9 | −1.66 | 8.1e−005 |
| P14206 | 40S ribosomal protein SA | −1.61 | 4.5e−005 |
| Stress/damage response | |||
| Q61171 | Peroxiredoxin 2 | −1.69 | 0.00017 |
| Q9WV39 | DNA damage-binding protein 1 | −1.65 | 0.00044 |
| Q80X75 | Hypoxia upregulated protein 1 | −1.62 | 0.00019 |
| P08113 | Endoplasmin (heat shock protein 90 kDa β member 1) | −1.62 | 0.00023 |
| Cytoskeleton associated | |||
| P20152 | Vimentin | −1.67 | 6.3e−006 |
| P58771 | Tropomyosin α-1 chain | −1.66 | 8.2e−006 |
| P68372 | Tubulin β-2C chain | −1.65 | 6.6e−006 |
| Q6IRU2 | Tropomyosin α-4 chain | −1.62 | 1.3e−005 |
| Other | |||
| P62880 | Guanine nucleotide-binding protein subunit β-2 | −1.71 | 3.1e−008 |
| Q61207 | Sulfated glycoprotein 1 | −1.60 | 0.0097 |
Downregulation of Prdx2 in Ang II–treated podocytes
Figure 1b and c and the red rectangle-surrounded areas in Figure 1a illustrate a protein spot with a molecular weight of 22 kDa, and the isoelectric point value of 5.2 decreased 1.7-fold in signal intensity after Ang II treatment compared with control cells. This protein was identified by mass spectrometry as Prdx2 (UniProtKB accession no. Q61171), which was initially reported to be a major antioxidant protein.12
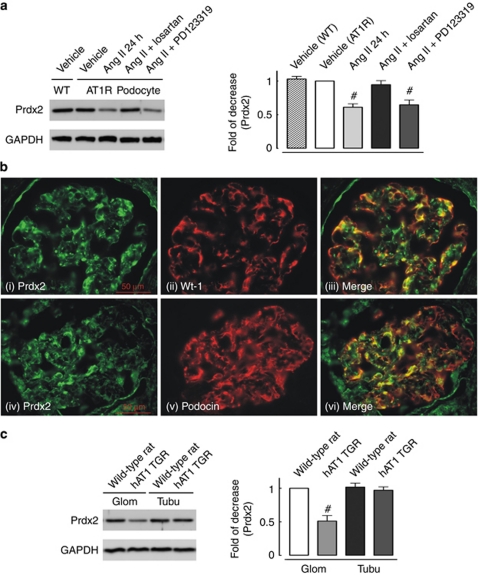
To validate the Ang II–dependent decrease in Prdx2 expression, we performed a western blot analysis of protein lysates from Ang II–stimulated and non-stimulated cultured AT1R podocytes. Ang II downregulated the expression level of Prdx2 in AT1R podocytes to a similar extent (0.61±0.07-fold compared with non-stimulated cells) as by difference gel electrophoresis (DIGE) proteomics (Figure 2a). The AT1R-specific inhibitor losartan but not the AT2R-specific inhibitor PD123319 abolished the Ang II–dependent reduction of Prdx2 expression, indicating an AT1R-specific effect. We performed immunofluorescence microscopic analysis of Prdx2 protein localization in the glomeruli of human kidneys. Double immunostaining with either of the podocyte-specific markers Wt-1 or podocin, and a Prdx2-specific antibody indicated that podocytes abundantly express Prdx2 in vivo (Figure 2b).
Figure 2.
Peroxiredoxin 2 (Prdx2) is downregulated with angiotensin II (Ang II) type 1 receptor (AT1R) signaling both in vitro and in vivo. (a) Representative immunoblotting (left panel) and quantitative analysis (right panel) of Prdx2 expression in cultured AT1R podocytes treated with Ang II (100 nmol/l, 24 h) combined with different inhibitors (losartan, AT1R inhibitor, 200 nmol/l; PD123319, AT2R inhibitor, 200 nmol/l); untreated wild-type podocytes were used as a second control (WT: wild-type cultured podocyte; AT1R podocyte: human AT1R stable expression podocyte cell line; #statistically significant with P<0.05 compared with vehicle-treated AT1R cells). (b) Glomerular expression of Prdx2 is observed in a podocyte pattern in human kidney sections. Colocalization (iii and vi, yellow color in merged images) of Prdx2 (i and iv, in green) and Wilms' tumor-1 protein (ii, in red) or podocin (v, in red) in immunofluorescent staining of human kidney cryosections suggested that in situ podocytes expressed Prdx2 protein. (c) Representative immunoblotting (left panel) and quantitative analysis (right panel) of Prdx2 expression in kidney tissues from 4-week-old Neph-hAT1 transgenic rats (TGRs) and age-matched littermates (WT). Glomerular expression of Prdx2 was significantly lower in Neph-hAT1 TGRs (0.51±0.07-fold; #statistically significant with P<0.05 versus WT Glom, n=3). Tubular Prdx2 expression levels did not differ significantly between Neph-hAT1 TGRs and WT rats. Glom, glomerulus; Tubu, tubule. Immunoblotting of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reveals equal loading of all lanes.
Podocyte-specific AT1R transgenic rats (Neph-hAT1 TGRs) develop proteinuria and glomerulosclerosis.11 Figure 2c shows that Prdx2 expression was 0.51±0.07-fold lower in the glomeruli of 4-week-old Neph-hAT1 TGRs compared with that in glomeruli from age-matched wild-type (WT) littermates. No difference in Prdx2 expression level was detected in the renal tubules (Figure 2c).
Increased cellular oxidative stress is associated with Prdx2 downregulation in podocytes
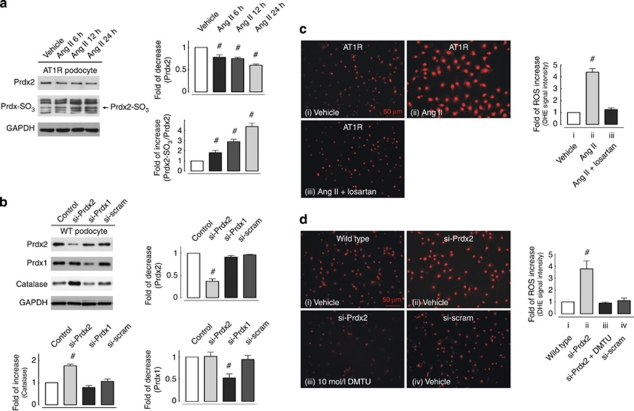
Recently, we showed that Ang II increases ROS levels in podocytes.10 The physiological significance of the antioxidant Prdx2 is unclear and little is known about whether Prdx2 downregulation affects the cellular redox balance in podocytes. Therefore, we examined the expression over time of the overoxidized form of Prdx, Prdx-SO3, in which the peroxidatic cysteine of Prdx is oxidized from thiol to sulfonic acid.13, 14 Figure 3a shows that the Ang II–dependent downregulation of Prdx2 correlates with increased expression of overoxidized Prdx-SO3 and Prdx2-SO3, suggesting that ROS increase oxidative stress in podocytes.
Figure 3.
Angiotensin II (Ang II) stimulation and downregulated peroxiredoxin 2 (Prdx2) expression increases intracellular reactive oxygen species (ROS) level and protein overoxidation in podocytes. (a) The left plot illustrates time-dependent Prdx2 expression downregulation and the associated increase of overoxidized Prdx-SO3 in cultured Ang II type 1 receptor (AT1R) podocytes with Ang II (100 nmol/l) treatment, whereas the right plot summarizes the time-dependent relative change in Prdx2 expression and the percentage of overoxidized Prdx2 (Prdx2-SO3) in Ang II–treated cells (n=3; #statistically significant with P<0.05). (b) (Left panel) Using a short hairpin sequence targeting mouse Prdx2 nucleotide sequence positions 233–251 and a retroviral gene delivery method, Prdx2 expression level could be knocked down to 0.39±0.05-fold in cultured podocytes (si-Prdx2 cells) versus control cells. To exclude off-target effect, a second short hairpin sequence targeting mouse Prdx1 nucleotide sequence positions 158–177 (producing 0.53±0.13-fold knockdown) and a scrambled short hairpin sequence were stably transfected to cultured podocytes. Upregulation of catalase, an oxidative stress-responsive protein, was additionally observed in Prdx2 knockdown podocytes. Immunoblotting of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reveals equal loading of all lanes. Right and left lower plots revealed quantitative analysis (n=3; #statistically significant with P<0.05). (c, d) Intracellular ROS level in adherent cultured podocytes was measured with a dihydroethidium (DHE) assay. Ang II (100 nmol/l, 24 h) treatment induced a 4.38±0.06-fold increase in ROS level compared with vehicle-treated cells (c), and this response could be effectively suppressed by preincubation with an AT1R-specific inhibitor (losartan, 200 nmol/l). In d, specifically knocking down Prdx2 expression in cultured podocytes (si-Prdx2) also resulted in a 3.77±0.66-fold increase of ROS level compared with scrambled sequence small interfering RNA-transfected (si-scram) or control (wild type) cells. In addition, the ROS-increasing effects of Prdx2 knockdown could be eliminated by treatment with the free-radical scavenger dimethylthiourea (iii, DMTU, 10 mmol/l, 2 h; n=3; #statistically significant with P<0.05).
To elucidate the effects of Prdx2 downregulation in podocytes, small interfering RNAs (siRNAs) targeting Prdx2, Prdx1, and a scrambled short hairpin sequence (control) were stably expressed in cultured podocytes by a retroviral-based gene delivery method. Sustained suppression of Prdx2 expression (downregulated ∼60% compared with WT or scrambled siRNA-transfected podocytes) was verified by immunoblotting (Figure 3b). Downregulation of Prdx2 but not Prdx1 increased the expression of the oxidative stress-responsive protein catalase. Both Ang II treatment and Prdx2 (si-Prdx2) knockdown induced significant increases of intracellular ROS in an AT1R-dependent manner (Ang II: 4.38±0.06-fold compared with non-stimulated cells; see Figure 3c; Prdx2: 3.77±0.66-fold compared with scrambled siRNA-transfected or WT podocytes; see Figure 3d). The oxidative effects of Prdx2 knockdown could be eliminated by treating the cells with the free-radical scavenger dimethylthiourea (Figure 3d, panel iii). Interestingly, knockdown of Prdx1, which has 91% amino-acid sequence identity to Prdx2,15 increases ROS levels only slightly (data not shown).
Downregulation of Prdx2 and associated ROS increase mediates Ang II–induced apoptosis in podocytes
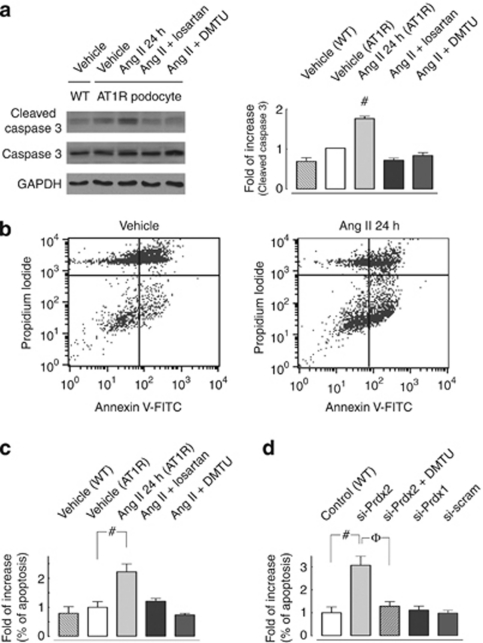
Ang II is known to mediate apoptosis.16, 17 However, the role of Ang II–induced ROS formation and its molecular mechanisms in podocyte survival and apoptosis remain unknown. Figure 4a shows that Ang II treatment increased cleaved caspase 3 expression in cultured AT1R podocytes compared with non-stimulated WT and AT1R cells. Losartan or dimethylthiourea significantly attenuated Ang II–induced cleaved caspase 3 expression, indicating the presence of an AT1R-specific and ROS-mediated signaling mechanism in podocytes.
Figure 4.
Downregulation of peroxiredoxin 2 (Prdx2) mediates angiotensin II (Ang II)–induced apoptosis in podocytes. (a) Representative immunoblotting (left panel) and quantitative analysis (right panel) show that Ang II (100 nmol/l, 24 h) triggers caspase 3 activation in cultured podocytes. Ang II–induced cleaved caspase 3 increase (1.76±0.06-fold; n=3; #statistically significant with P<0.05, Ang II– versus vehicle-treated Ang II type 1 receptor (AT1R) cells) could be suppressed either by pretreatment with an AT1R-specific inhibitor (losartan, 200 nmol/l) or by coincubation with a free-radical scavenger (dimethylthiourea (DMTU), 10 mmol/l; n=3; #statistically significant at P<0.05 compared with the vehicle-treated AT1R cell group). (b–d) Fluorescence-activated cell sorting analysis via Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining was used to observe the induction of apoptosis. Cells in the lower right quadrant indicate Annexin V-positive/PI-negative early apoptotic cells. Representative plots in b revealed that Ang II stimulation (100 nmol/l, 24 h) induced a greater degree of apoptosis than that seen in untreated AT1R cells. (c) Quantitative analysis of flow cytometric Annexin V-FITC/PI staining measurements illustrate that Ang II induced a 2.22±0.27-fold increase in apoptosis in cultured AT1R podocytes compared with vehicle-treated cells. This effect could also be eliminated either by pretreatment with an AT1R-specific inhibitor (losartan; 200 nmol/l) or by coincubation with a free-radical scavenger (DMTU, 10 mmol/l; n=3; #statistically significant at P<0.05 compared with the vehicle-treated AT1R cell group). (d) Quantitative analysis of flow cytometric Annexin V-FITC/PI staining measurements showed that small interfering RNA (siRNA) knockdown of Prdx2 protein expression in cultured podocytes (si-Prdx2 cells) caused a 3.07±0.36-fold increase in apoptosis compared with control cells (#statistically significant at P<0.05), and the effect could be eliminated by coincubation with a free-radical scavenger (DMTU, 10 mmol/l; φ: statistically significant at P<0.05 versus si-Prdx2 cells without DMTU). Neither Prdx1-specific knockdown (si-Prdx1) podocytes nor scramble siRNA-transfected (si-scram) cells exerted significant apoptotic effects. A minimum of three independent experiments were conducted (n=3). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Moreover, quantitative analysis of flow cytometric Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining (Figure 4b) showed that Ang II induced a significant increase of apoptosis in cultured AT1R podocytes (2.22±0.27-fold increase versus vehicle-treated AT1R cells, Figure 4c). This effect could be inhibited by either pretreatment with losartan or coincubation with dimethylthiourea, suggesting that Ang II induces apoptosis in podocytes via an AT1R-specific and ROS-mediated signaling pathway.
The crucial role of Prdx2 downregulation and associated increased ROS levels in Ang II–induced podocyte apoptosis is illustrated in Figure 4d. In flow cytometric Annexin V-FITC/PI staining, knockdown of Prdx2 protein expression in cultured podocytes (si-Prdx2 cells) was observed to increase apoptosis significantly compared with control cells (3.07±0.36-fold). Neither Prdx1-specific knockdown (si-Prdx1) podocytes nor scramble siRNA-transfected (si-scram) cells exerted these significant apoptotic effects.
Ang II–driven Akt dephosphorylation is involved in Prdx2 downregulation-mediated apoptosis in podocytes
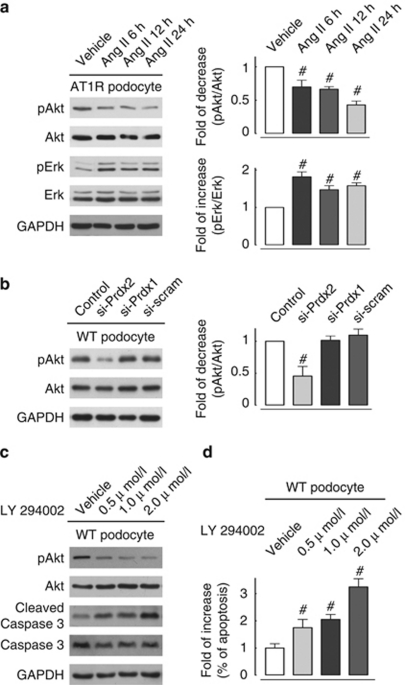
Akt, or protein kinase B, is a serine/threonine kinase that is critical in controlling mammalian cell survival and apoptosis.18 Failure to phosphorylate Akt has been recently reported to promote podocyte/kidney injury in a diabetic mouse model19 and in a renin–angiotensin system overactivating transgenic Ren2 rat model.20 In this study, we examined phosphorylation at the Ser-473 position of Akt in Ang II–stimulated (100 nmol/l) AT1R podocytes. Figure 5a illustrates that Ang II treatment decreased Akt phosphorylation in podocytes in a time-dependent manner. In contrast, the activity of extracellular signal-regulated kinase (Erk), another proposed regulator of mammalian cell lifespan,21 is significantly upregulated in Ang II–treated podocytes compared with vehicle-treated cells.
Figure 5.
Attenuated activity of the Akt pathway associated with peroxiredoxin 2 (Prdx2) downregulation is involved in angiotensin II (Ang II)–induced apoptotic signaling. (a) Representative immunoblotting (left plot) and quantitative analysis (right plot) of Akt and Erk pathway activity in cultured Ang II type 1 receptor (AT1R) podocytes stimulated with Ang II (100 nmol/l) in a time-course manner. Akt phosphorylation at Ser-473 (pAkt) decreased significantly after Ang II treatment at 6 h (0.70±0.06-fold), 12 h (0.67±0.04-fold), and 24 h (0.43±0.06-fold) compared with the vehicle-treated group (n=3; #all values were P<0.05 versus vehicle-treated cells). Erk phosphorylation (pErk) at Thr202/Tyr204 of Erk1 (Thr185/Tyr187 of Erk2) in Ang II–treated podocytes was significantly upregulated in each time point compared with vehicle-treated cells (n=3; #all statistically significant at P<0.05 versus vehicle-treated cells). (b) Representative immunoblotting (left plot) and quantitative analysis (right plot) of Akt and Erk pathway activity in cultured Prdx knockdown podocytes. Small interfering RNA (siRNA) knockdown of Prdx2 protein expression (si-Prdx2) in cultured podocytes caused a 0.46±0.12-fold decrease of Akt phosphorylation compared with control cells (n=3; #statistically significant at P<0.05), whereas neither Prdx1-specific knockdown (si-Prdx1) podocytes nor scramble siRNA-transfected (si-scram) cells exerted significant effects. No difference in Erk phosphorylation level was observed in Prdx2 knockdown podocytes compared with control cells. (c) Representative immunoblotting showed that concentration-dependent Akt pathway inhibition by treating cultured podocytes with a phosphatidylinositol 3-kinase inhibitor (LY294002, 24 h) causes significant increase in cleaved caspase 3 versus that in vehicle-treated cells. Immunoblotting of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reveals equal loadings for all lanes (n=3). (d) Quantitative analysis of flow cytometric Annexin V-fluorescein isothiocyanate/propidium iodide staining measurements showed that concentration-dependent inhibition of the Akt pathway with LY294002 (24 h) in cultured podocytes caused a 1.75±0.12-fold (0.5 μmol/l), 2.05±0.19-fold (1.0 μmol/l), and 3.25±0.30-fold (2.0 μmol/l) increase in apoptosis compared with vehicle-treated cells (n=3; #statistically significant at P<0.05). WT, wild type.
To further explore the mechanisms linking Prdx2 downregulation and Akt dephosphorylation to Ang II–induced apoptosis, we examined phosphorylation of Akt and Erk in cultured Prdx2 knockdown podocytes. Figure 5b illustrates that Prdx2 knockdown reduces Akt phosphorylation in Ang II–treated podocytes compared with control cells. However, no change in Erk phosphorylation was observed in Prdx2 knockdown podocytes. This indicates that Akt but not Erk signaling is modulated by Prdx2. In agreement with these experiments, treatment of podocytes with a phosphatidylinositol 3-kinase inhibitor (LY294002) significantly increased cleaved caspase 3 (Figure 5c) and Annexin V-FITC/PI staining (Figure 5d, measured by flow cytometry) in cultured podocytes.
Downregulation of glomerular Prdx2 correlates with oxidative stress and apoptosis in Ang II–infused rats
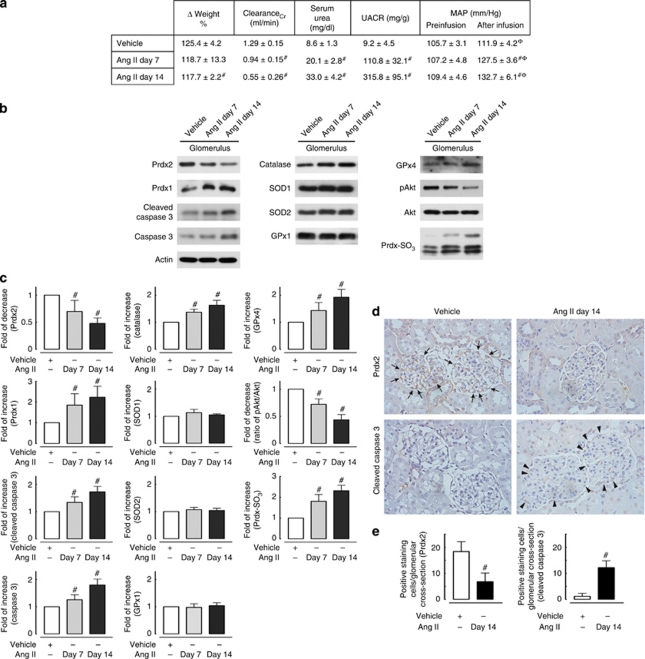
To further confirm the biological role of Ang II in regulating Prdx2 expression, we examined its expression in glomeruli of rats that have been infused with Ang II. Sustained low-dose Ang II infusion (150 ng/kg/min) increased urinary albumin excretion and reduced creatinine clearance at 7 and 14 days (Figure 6a). Figure 6b and c shows that in comparison with vehicle Ang II treatment resulted in glomerular Prdx2 downregulation, decreased Akt phosphorylation, upregulation of Prdx-SO3, caspase 3, and cleaved caspase 3 expression. After Ang II infusion, the expression level of other oxidative-defense proteins was either increased (Prdx1, catalase, and GPx4) or had no significant change (SOD1, SOD2, and GPx1). The association between reduction of the expression of Prdx2 in podocytes and apoptosis is demonstrated by immunohistostaining of Prdx2 and cleaved caspase 3 in glomeruli from Ang II–infused and vehicle-treated rats, respectively (Figure 6d and e).
Figure 6.
Downregulation of glomerular peroxiredoxin 2 (Prdx2) correlates with oxidative stress and apoptosis in angiotensin II (Ang II)–infused rats. The control group (Sprague–Dawley rat, n=6) was administered only saline, and the experimental group was subcutaneously infused with Ang II via an osmotic minipump from 6 weeks of age for 7 days (n=6) and 14 days (n=6). (a) Body weight changes (shown as a percentage of the initial weight), creatinine clearance (ClearanceCr), urine albumin creatinine excretion ratio (UACR), serum urea nitrogen levels, and preinfusion and after infusion mean arterial pressures (MAP, #statistically significant with P<0.05 compared with the vehicle-treated group; φ: statistically significant with P<0.05 compared with preinfusion blood pressure level in each group). (b) Representative immunoblotting of Prdx2, Prdx1, Prdx-SO3, cleaved caspase 3 expression, Akt phosphorylation, and expression level of other reactive oxygen species-protective enzymes (catalase, SOD1, SOD2, GPx1 and GPx4) in glomerular extract of rats treated with Ang II at different time points. SOD, superoxide dismutase; GPx, glutathione peroxidase. (c) Quantitative analysis of immunoblotting showed that glomerular Prdx2 expression decreased significantly after Ang II infusion at 7 (0.69±0.20-fold) and 14 days (0.56±0.11-fold) compared with the vehicle-treated group. Ang II–dependent downregulation of Prdx2 in rat glomeruli correlates with increased expression of Prdx-SO3 (overoxidized form of Prdx), suggesting that Ang II increases the oxidative stress status in vivo. Ang II also increased both cleaved caspase 3 (1.34±0.20-fold at day 7 and 1.73±0.11-fold at day 14) and caspase 3 expression levels (1.26±0.18-fold at day 7 and 1.59±0.11-fold at day 14) in rat glomerular extracts (n=3; #statistically significant with P<0.05 compared with vehicle-treated rats). (d) Representative immunohistochemical staining for glomerular Prdx2 and cleaved caspase 3 expression in Ang II– and vehicle-infused rat kidneys obtained on day 14. Prdx2 staining in a podocyte cytoplasmic pattern was observed to decrease following Ang II treatment and to correlate with increased glomerular cleaved caspase 3 expression. Arrows indicate the podocytes expressing Prdx2 and arrowheads mark the cleaved caspase 3 positive-staining podocytes after Ang II administration. (e) Quantitative analysis of immunohistochemical staining. Expressions of Prdx2 and cleaved caspase 3 were evaluated by counting glomerular positive-staining podocytes in Ang II– and vehicle-infused rat kidneys. At least six kidneys were used for one experimental group, and at least 50 glomeruli were counted in each kidney. The numbers of positive-staining cells per glomerular section were calculated and represented as means±s.e.m. (#statistically significant with P<0.05 compared with vehicle-treated rats).
DISCUSSION
Both Ang II enhancement of NADPH-dependent oxidase activity and the role of ROS in regulating renal function during Ang II infusion have been well described;22, 23 however, little is known about the functions of other oxidant and antioxidant proteins involved in the effects of Ang II in podocytes.24
In this study, we used proteomic analysis and showed that in addition to altering the expression of other metabolic, cytoskeletal, and stress-responsive proteins, Ang II treatment downregulates the expression of the antioxidant protein Prdx2 in podocytes. The Prdx family of peroxidases is currently known to comprise six mammalian isoforms that catalyze the peroxide reduction of H2O2, organic hydroperoxides, and peroxynitrite, thus protecting cells against oxidative damage.13, 25 Experimental and clinical studies have shown that Prdx family members modulate many cellular processes by switching over the oxidative–reductive environment within cells.13, 26, 27, 28 The functional roles of Prdx2 in erythrocyte lifespan,29 vascular remodeling,30 and cardiovascular diseases31 have been recently described.
Little is known about the cellular distribution and function of Prdx2 in the kidney. We showed that Prdx2 is expressed in vivo in podocytes and that glomerular Prdx2 expression was reduced in AT1R transgenic rats. As a better approach to the in vivo situation, we conducted experiments in rats that were treated with Ang II. In this animal model, a minimal increase of blood pressure and doubling of the NADH oxidase activity in vessels has been described by others.32, 33, 34 Ang II treatment resulted in glomerular Prdx2 downregulation, decreased Akt phosphorylation, upregulation of Prdx-SO3, caspase 3, and cleaved caspase 3 expression, suggesting that the effect of Ang II on Prdx2 has a biological in vivo significance.
These data suggest that Prdx2 is involved in AT1R-mediated glomerular functions. The peroxidatic catalytic cysteine of Prdx is highly susceptible to overoxidation to sulfonic acid (Prdx-SO3) as a result of excess ROS/oxidative status in podocytes, which results in loss of activity.35 To determine whether Prdx2 is also a sensor and transmitter of redox signals, we examined the level of overoxidized Prdx (Prdx-SO3) in Ang II–treated podocytes and found that increases in Prdx-SO3 and Prdx2-SO3 were associated with Ang II–induced Prdx2 downregulation, indicating a switch to an excess of ROS and oxidative stress status. In addition, Ang II treatment or knockdown of Prdx2 increases ROS levels in podocytes.
A growing body of evidence supports the hypothesis that podocyte apoptosis is a major cause of reduced podocyte numbers, which leads to proteinuria and/or glomerulosclerosis. In vitro and in vivo studies have shown that Ang II induces podocyte apoptosis.36 In this study, we showed that both Ang II treatment and Prdx2 knockdown leads to apoptosis of podocytes, and that this apoptosis could be prevented by a free-radical scavenger. Prdx2-induced protection from H2O2-induced apoptosis has been described in other cell types, such as thyroid cells.37 In addition, a correlation between loss of Prdx2 and podocyte apoptosis and proteinuria was also demonstrated in the Ang II–infused rats.
Akt and Erk 1/2 seem to have important roles in the signaling of the slit diaphragm and in podocyte function. The slit diaphragm proteins nephrin and CD2AP interact with phosphatidylinositol 3-kinase p85, increasing Akt activity and decreasing apoptosis.38 Galectin-1 (Gal-1), a newly described slit diaphragm protein, triggers increased tyrosine phosphorylation of nephrin, which activates Erk 1/2.39 In contrast, Neph1 has been shown to negatively regulate Erk 1/2 signaling in podocytes.40 Although the role of these kinases in glomerular diseases is not fully understood, it has been suggested in a diabetic mouse model that failure to phosphorylate Akt causes podocyte apoptosis.19 Podocyte injury mediated by p38 MAP kinase and Erk 1/2 has been observed in several experimental models of glomerular disease.41 In this study, we showed that Ang II decreases Akt phosphorylation and increases Erk 1/2 phosphorylation in podocytes. Knockdown of Prdx2 reduced Akt phosphorylation but did not affect Erk phosphorylation, suggesting that Prdx2 inhibits the Akt but not the Erk pathway in podocytes. Interestingly, activation of Akt has been reported recently in Prdx1-deficient fibroblasts and epithelial cells.42 It has been shown that transient overexpression of Prdx2 inhibits epidemal growth factor-induced phosphorylation of Akt in HeLa cells,43 suggesting a different role of Prdx2 in regulation of the Akt pathway in these cells.
AT1R signaling in podocytes thus activates Rac-1 and NADPH oxidase to produce additional ROS and may downregulate the antioxidant protein Prdx2 to maintain an oxidative environment that favors signal propagation. In a ‘floodgate' mechanism,13 Prdx2 consumes low levels of ROS through its peroxidase activities, but is inactivated by higher ROS concentrations and, thus, provides a localized zone in which ROS are available to react with other targets and propagate the signal. In conclusion, our findings indicate that Prdx2 mediates podocyte signaling and regulates AT1R-mediated injury. In the future, activation of Prdx2 may supplement renin–angiotensin–aldosterone system blockade as a strategy for treating chronic kidney diseases.
METHODS
Antibodies and reagents
Losartan potassium was gifted by Merck (Whitehouse Station, NJ). Ang II, PD123319, and dimethylthiourea were purchased from Sigma-Aldrich (Munich, Germany). LY294002 was purchased from Merck-Calbiochem (Darmstadt, Germany). Antibodies specific for Prdx2, Prdx1, catalase, superoxide dismutase 1/2, glutathione peroxidase 1/4, podocin, and peroxiredoxin-SO3 were purchased from AbFRONTIER (Seoul, South Korea). Antibodies for phospho-Akt (Ser 473), Akt, caspase 3, cleaved caspase 3, and phospho-Erk were obtained from Cell Signaling Technology (Danvers, MA). Antibodies for Erk 1 and Erk 2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody to Wilms' tumor-1 was obtained from DAKO (Glostrup, Denmark). Alexa Fluor 488-conjugated goat anti-rabbit antibody, Alexa Fluor 594-conjugated goat anti-mouse antibodies, propidium iodide, and dihydroethidium (DHE) were obtained from Molecular Probes (Darmstadt, Germany). FITC-conjugated Annexin V antibody was purchased from BD Biosciences (Erembodegem, Belgium).
Cell culture, animals, and experimental protocol
The two conditionally immortalized mouse podocyte cell lines used in the current study, WT podocytes and AT1R podocytes (cells stably expressing functional human AT1Rs), have previously been described in detail.10, 44 In the in vivo Ang II model, rats were infused with Ang II (150 ng/kg/min) for 7 or 14 days using mini-osmotic pumps (ALZET, 2002, Cupertino, CA) implanted subcutaneously as described in the literature.33, 34 Serum and urine creatinine were measured by a picric acid-based colorimetric autoanalyzer. Urine albumin was detected by the immunoturbidimetric method. Podocyte-specific human AT1R transgenic rats (Neph-hAT1 TGRs) and age-matched WT littermates were previously described in detail and develop significant albuminuria and concurrent glomerular structural changes from 8 to 15 weeks of age.11 Glomeruli were isolated from rat kidneys via sequential sieving as described previously.10 All studies were approved by the institutional animal care review committee and conducted according to government regulations.
Two-dimensional fluorescence DIGE system and proteomics
For two-dimensional (2D) polyacrylamide gel-based analysis, podocytes were harvested and homogenized in 2D electrophoresis lysis buffer. Protein samples from vehicle- and Ang II–treated podocytes were labeled with Cy3- and Cy5-CyDye DIGE Fluor dyes (GE Healthcare, Munich, Germany), respectively, for 30 min at 4 °C. A pooled sample of vehicle- and Ang II–treated podocyte lysates was similarly labeled with Cy2-CyDye DIGE Fluor dye for use as an internal control.
Cy3-, Cy5-, and Cy2-labeled samples (50 μg each) were isoelectrically focused on an immobilized pH gradient strip (24 cm; pH 3–10, non-linear) using a PROTEAN IEF cell (Bio-Rad, Munich, Germany) to reach ∼80,000 total voltage hours. After focusing, strips were equilibrated and applied to a 12% polyacrylamide gel for electrophoresis under a constant current. Samples were prepared and then electrophoresed in triplicate.
A Typhoon scanner in fluorescence mode (GE Healthcare) was used to obtain Cy3, Cy5, and Cy2 images of analytical gels. Images were analyzed using DeCyder software (GE Healthcare) to identify protein spots exhibiting statistically significant changes between groups using Student's t-test and analysis of variance. Preparative gels (loaded to ∼1 mg protein of lysate per gel) were then fixed in 30% ethanol and 7.5% acetic acid for 2 h, followed by Coomassie staining overnight for total protein visualization. Identified proteins of interest were picked from preparative gels as gel plugs.
Gel plugs were digested with trypsin (Promega, Mannheim, Germany), and peptides were extracted and analyzed with an UltraflexIII matrix-assisted laser desorption ionization time-of-flight mass spectrometer (Bruker Daltonics, Bremen, Germany) operating in reflection positive-ion mode. The Swiss-Prot database was searched with GPS Explorer software (Applied Biosystems, Carlsbad, CA) and the MASCOT search engine (Matrix Sciences, London, UK) to identify peptide mass data acquired using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. A peptide group was considered a positive match for a database protein when the confidence interval for the match was at least 95% as determined by GPS Explorer, and when the spot position on the 2D gel reflected approximately the theoretical isoelectric point and molecular weight of the specified protein. MASCOT scores >61 were considered significant (P<0.05).
Western blotting
Cultured podocytes or rat kidney tissues were washed with ice-cold phosphate-buffered saline (PBS) and homogenized in ice-cold lysis buffer (12 mmol/l Tris-base, 8 mmol/l HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 50 mmol/l NaCl, 15 mmol/l KCl, 1.5 mmol/l MgCl2, 1 mmol/l ethylene glycol tetraacetic acid, 10 mmol/l Na2H2P2O7, 1 mmol/l ATP, 20 mmol/l NaF, 1 mmol/l Na3VO4, 1% Triton X-100, and complete protease inhibitor) on ice. The lysates were centrifuged for 1 h at 16,000g. Supernatant protein concentrations were measured using Advanced Protein Assay agents. Equal amounts of protein extract were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted. Chemiluminescent signals (LumiLight, Roche Diagnostics) were detected with a Lumi-Imager or X-ray films and quantified using the LumiAnalyst program (Roche Diagnostics, Mannheim, Germany).
Immunofluorescent staining, immunohistochemistry, and transmission electron microscopy
Kidney tissue was placed on glass slides, air dried, and then washed three times with PBS. Bovine albumin solution (4%) in PBS was applied to block nonspecific endogenous antigens. The unlabeled primary antibodies (detecting Prdx2, podocin, or Wilms' tumor-1) were applied in volumes of 50–100 μl using Pasteur pipettes. The glass slides were placed in the staining box and incubated for 1 h at room temperature or overnight at 4 °C. After three more 5-min PBS washes, fluorophore-labeled secondary antibodies were applied and reacted in the same manner as the primary antibodies. After three more 5-min PBS washes, the specimen was mounted and glass coverslips were attached for visualization under a fluorescent microscope (Axiovert 100, Zeiss, Oberkochen, Germany).
Paraffin-embedded formalin-fixed 3-μm-thick kidney sections from Ang II–infused and control rats were deparaffinized, treated with 0.3% H2O2 to block endogenous peroxidase activity, and microwaved for two consecutive 10-min periods at 500 W and 250 W, respectively. The sections were then incubated with antibody to Prdx2 or cleaved caspase 3. Immunoperoxidase staining was performed using the Vecstain ABC kit (Vector laboratories, Burlingame, CA) according to the manufacturer's instructions. The immunoperoxidase staining was performed using diaminobenzidine (Sigma-Aldrich).
Generation of Prdx2 knockdown podocytes
The RNA interference method (siRNA) was based on the GenScript retroviral siRNA expression vector (pRNA-H1.4/retro) and performed as directed by technical manual no. 0187 (GenScript, Piscataway, NJ). A small DNA insert (roughly 70–80 base pairs, designed husing GenScript's siRNA Target Finder and siRNA Construct Builder) encoding a short hairpin RNA targeting the gene of interest was cloned into this vector between the MluI and XhoI sites. The sense oligonucleotide sequences of the short hairpin RNA insert targeting mouse Prdx2 (NM_011563; GeneID:21672) and peroxiredoxin 1 (NM_011034; GeneID:18477) were 5′-ACTCTCAGTTCACCCACCT-3′ and 5′-CCACGGAGATCATTGCTTT-3′, respectively. The RNA short hairpin gene-containing viruses were produced by co-transfecting HEK293 cells with three plasmids (2.5 μg pMD.G, 7.5 μg pMD.gag/pol, and 10 μg pRNA-TH1.4/Retro-mPrdx) using the calcium phosphate method. The remaining retroviral gene delivery procedures were performed exactly as described previously.10 Podocytes infected with the virus soup were selected with hygromycin (25 μg/ml) beginning 4 days after viral transduction to obtain 100% positive cells.
ROS measurement by DHE assay
ROS (mainly superoxide, O2·−) generation in adherent cultured podocytes was estimated by DHE staining as described previously.45 Briefly, podocytes cultured on glass coverslips were loaded with 10 μmol/l DHE in HEPES buffer for 30 min at 38 °C under 5% CO2. After staining, the cultures were washed briefly with PBS and mounted. The stained cells were examined at excitation/emission wavelengths of 560/660 nm using an inverted fluorescence microscope (Axiovert 100, Zeiss). Image analysis was performed using ImageJ software (NIH, Bethesda, MD). Total cell numbers and DHE fluorescence intensity of each cell were counted and quantified in six separate fields (75–100 cells per field) for each of the conditions. The relative fluorescence intensity was calculated by dividing the total integrated optical density by the total number of cells in each field. Mean fluorescence intensity measurements were obtained from three separate experiments in each group.
Quantification of apoptosis and necrosis by flow cytometry
Apoptosis was determined by detecting Annexin V binding using flow cytometry as described previously.46 After 24-h incubation with the indicated stimuli, cultured podocytes were harvested using cold PBS on ice, scraped, and collected by centrifugation. Cells were washed with staining buffer and incubated with 5 μl Annexin V-FITC and 5 μg/ml PI in 100 μl of staining buffer for 30 min at 4 °C in the dark. Cells were washed again, resuspended in 250 μl of staining buffer, and analyzed by flow cytometry on a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA). A total of 10,000 events were triggered by forward-scatter and side-scatter light and the samples were analyzed using CellQuest software (Becton-Dickinson). Cells that stained positive for Annexin V-FITC and negative for PI were considered apoptotic.
Statistical analyses
All data are represented as means±s.e.m. unless otherwise specified. Group differences were tested by Student's t-test. Comparisons involving more than two groups were performed using two-way analysis of variance (SPSS, Chicago, IL). The level of statistical significance was set to P<0.05.
Acknowledgments
This work was supported by grant from the Deutsche Forschungsgemeinschaft (DFG), Pa 483/16-1.
All the authors declared no competing interests.
References
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit. Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294:F830–F839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- Liebau MC, Lang D, Bohm J, et al. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol. 2006;290:F710–F719. doi: 10.1152/ajprenal.00475.2004. [DOI] [PubMed] [Google Scholar]
- Gloy J, Henger A, Fischer KG, et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the rat. J Clin Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Hsu HH, Hoffmann S, Endlich N, et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med. 2008;86:1379–1394. doi: 10.1007/s00109-008-0399-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Podlich D, Hahnel B, et al. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–1487. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- Shau H, Kim A. Identification of natural killer enhancing factor as a major antioxidant in human red blood cells. Biochem Biophys Res Commun. 1994;199:83–88. doi: 10.1006/bbrc.1994.1197. [DOI] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen TJ, Weirather K, Deming PB, et al. Oxidation state governs structural transitions in peroxiredoxin II that correlate with cell cycle arrest and recovery. J Cell Biol. 2006;175:779–789. doi: 10.1083/jcb.200606005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Choi KS, Riddell J, et al. Human peroxiredoxin 1 and 2 are not duplicate proteins: the unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J Biol Chem. 2007;282:22011–22022. doi: 10.1074/jbc.M610330200. [DOI] [PubMed] [Google Scholar]
- Godin N, Liu F, Lau GJ, et al. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int. 2010;77:1086–1097. doi: 10.1038/ki.2010.63. [DOI] [PubMed] [Google Scholar]
- Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A, Habibi J, Wei Y, et al. Mineralocorticoid receptor antagonism attenuates glomerular filtration barrier remodeling in the transgenic Ren2 rat. Am J Physiol Renal Physiol. 2009;296:F1013–F1022. doi: 10.1152/ajprenal.90646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(alpha)-dependent NADPH oxidase activation. J Biol Chem. 2010;285:21323–21328. doi: 10.1074/jbc.M110.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley TD, Verwiebe E, Zhong W, et al. Localization of the thioredoxin system in normal rat kidney. Free Radic Biol Med. 2001;30:412–424. doi: 10.1016/s0891-5849(00)00486-x. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Barranco-Medina S, Lazaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583:1809–1816. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Kang SW, Rhee SG, Chang TS, et al. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Kim SU, Yu SL, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- Choi MH, Lee IK, Kim GW, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- Zhao W, Fan GC, Zhang ZG, et al. Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis. Basic Res Cardiol. 2009;104:377–389. doi: 10.1007/s00395-008-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, et al. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang L, Zhu Q, et al. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011;79:300–310. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8:4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Ding G, Zhu J, et al. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28:500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee TH, Park ES, et al. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J Biol Chem. 2000;275:18266–18270. doi: 10.1074/jbc.275.24.18266. [DOI] [PubMed] [Google Scholar]
- Huber TB, Hartleben B, Kim J, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Khoshnoodi J, Akimoto Y, et al. Expression of galectin-1, a new component of slit diaphragm, is altered in minimal change nephrotic syndrome. Lab Invest. 2009;89:178–195. doi: 10.1038/labinvest.2008.125. [DOI] [PubMed] [Google Scholar]
- Harita Y, Kurihara H, Kosako H, et al. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J Biol Chem. 2008;283:9177–9186. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa M, Mukoyama M, Mori K, et al. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol. 2006;16:2690–2701. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- Cao J, Schulte J, Knight A, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiwek D, Endlich N, Holzman L, et al. Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int. 2004;66:91–101. doi: 10.1111/j.1523-1755.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, et al. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Dohle F, Terstesse M, et al. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol. 2002;168:6152–6158. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]