Abstract
Osteoblastic differentiation of vascular smooth muscle cells (VSMCs) is involved in the pathogenesis of vascular calcification. Hydrogen sulfide (H2S) is a gas endogenously produced by cystathionine γ-lyase in VSMC. Here we determined whether H2S plays a role in phosphate-induced osteoblastic transformation and mineralization of VSMC. Hydrogen sulfide was found to inhibit calcium deposition in the extracellular matrix and to suppress the induction of the genes involved in osteoblastic transformation of VSMC: alkaline phosphatase, osteocalcin, and Cbfa1. Moreover, phosphate uptake and phosphate-triggered upregulation of the sodium-dependent phosphate cotransporter (Pit-1) were also prevented by H2S. Reduction of endogenous production of H2S by inhibition of cystathionine γ-lyase activity resulted in increased osteoblastic transformation and mineralization. Low plasma levels of H2S, associated with decreased cystathionine γ-lyase enzyme activity, were found in patients with chronic kidney disease receiving hemodialysis. Thus, H2S is a potent inhibitor of phosphate-induced calcification and osteoblastic differentiation of VSMC. This mechanism might contribute to accelerated vascular calcification in chronic kidney disease.
Keywords: cystathionine γ-lyase, hydrogen sulfide, vascular calcification, vascular smooth muscle cell
Vascular calcification is implicated in the pathogenesis of various vascular diseases and can result in devastating clinical consequences. It is related to an increased risk of cardiovascular morbidity and complications such as atherosclerotic plaque burden,1, 2, 3 myocardial infarction,4, 5 coronary artery disease,6, 7 post-angioplasty dissection,8 and increased ischemic episodes in peripheral vascular disease.9 It has also been shown to be a strong marker of cardiovascular events in patients with diabetes and chronic kidney disease (CKD).6 Studies also indicate that coronary calcification may be predictive of increased propensity for sudden cardiac death.10, 11 Strong associations between arterial calcification and stiffness, pulse pressure, or mortality in dialysis patients have also been shown to contribute to the high rates of cardiac and peripheral ischemic disease and left ventricular hypertrophy in this population.12, 13, 14 Although the precise mechanisms of vascular calcification are not completely understood, abnormalities in mineral metabolism are considered important risk factors. High extracellular phosphate (Pi) has been widely established to induce vascular calcification.15, 16, 17, 18, 19 Pi uptake through a sodium-dependent phosphate cotransporter, Pit-1, is essential for vascular smooth muscle cell (VSMC) calcification and phenotypic modulation in response to elevated Pi.20 Contrary to previous conception, accumulating evidence now suggests that vascular calcification is a delicate and well-regulated cellular process in which VSMC gain an osteoblastic phenotype. This is indicated by the increase in expression of the core-binding factor alpha-1 (Cbfa1), which is an osteoblast-specific transcription factor required for osteoblast differentiation, bone matrix gene expression, and, consequently, bone mineralization.21 Upregulation of alkaline phosphatase (ALP; an important enzyme in early osteogenesis) and osteocalcin (OC; a major non-collagenous protein in bone matrix that regulates mineralization) was also shown to occur.22
Hydrogen sulfide (H2S) has traditionally been considered a toxic gas; however, recently, it has been recognized as the third endogenous gaseous transmitter besides carbon monoxide and nitric oxide.23 In mammals, H2S is produced by two enzymes: cystathionine β-synthase and cystathionine γ-lyase (CSE) via the transsulfuration pathway using homocysteine, cystathionine, and -cysteine as substrates. On the basis of the literature, the concentration of H2S in the peripheral system ranges from 30 to 50 μmol/l,24 although recently it has been suggested that this is an overestimation (reviewed in ref. 25). It was shown that in the aorta, H2S concentration is 20- to 100-fold higher than that in other tissues.26 In the vasculature, H2S is generated mainly by vascular smooth muscle cells by CSE.27 H2S exerts a number of physiological actions in the cardiovascular system: (i) it dilates blood vessels mostly, if not exclusively, by a mechanism that involves opening of adenosine 5′ triphosphate-sensitive K+ channels of smooth muscle cells,28 (ii) it is cardioprotective against ischemic/reperfusion damage and myocardial inflammation,29 and (iii) it preserves both mitochondrial structure and function after injury.30 Accumulating evidence suggests that it has direct inhibitory effects on the development of atherosclerosis. H2S induces apoptosis,31, 32 suppresses endothelin-induced proliferation of VSMC,33 and influences vascular inflammatory reactions.34 It has also been demonstrated that H2S inhibits the oxidation of low-density lipoprotein and lipids from atheromatous plaques.35 In fact, the progression of atherosclerosis was shown to be significantly slower in patients with Down's syndrome, a state of H2S overproduction.36 In the context of CKD, plasma level of H2S was reported to be decreased in stage 5 CKD patients.37 Calciphylaxis can be successfully treated with intravenous administration of sodium thiosulfate,38, 39 a drug that—as discovered recently—increases H2S biogenesis by inducing CSE expression.40 In addition, H2S was recently reported to ameliorate vascular calcification induced by vitamin D3 plus nicotine in rats.41 However, the mechanisms underlying the protective effect of H2S and the role of CSE in vascular calcification in CKD have not been explored. The purpose of this study was to investigate the role that H2S and biogeneration of H2S may have in the process of VSMC mineralization and transition of smooth muscle cells into osteoblast-like cells. We observed that H2S from both extracellular and intracellular origin suppresses high Pi-induced calcification and osteoblastic differentiation of human aortic smooth muscle cells (HAoSMCs) through suppression of Pit-1, and that the enzyme activity of CSE is decreased in stage 5 CKD.
RESULTS
H2S decreases HAoSMC mineralization in a dose–responsive manner
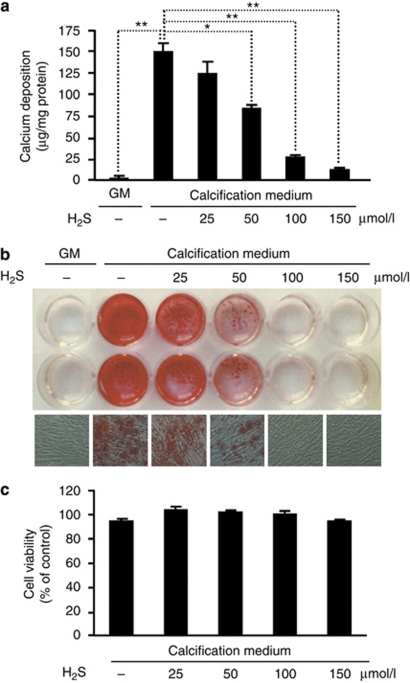
To establish an in vitro model of human vascular calcification, we cultured HAoSMC in calcification medium, which was prepared by addition of 3 mmol/l Pi to the growth medium (GM). HAoSMC was cultured in calcification medium in the presence or absence of H2S for 7 days, followed by calcium measurement (Figure 1a). As expected, Pi provoked calcification, whereas in the control culture no deposits were formed during this period. Importantly, H2S inhibited calcium deposition in a dose–responsive manner, providing a significant inhibition at concentrations of ⩾50 μmol/l. To confirm the effect of H2S on calcium deposition, we also performed Alizarin red staining of HAoSMC (Figure 1b). HAoSMC maintained in calcification medium showed development of granular calcium deposits throughout the cell culture. Supplementation of the calcification medium with H2S prevented the accumulation of calcium in the extracellular matrix. To test the viability of cells exposed to H2S, we carried out MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Figure 1c). We did not observe a decline in viability of HAoSMC challenged with H2S in the concentration range of 25–150 μmol/l.
Figure 1.
H2S decreases HAoSMC mineralization in a dose–responsive manner. (a–c) HAoSMCs were cultured in GM or in calcification medium alone or supplemented with 25, 50, 100, and 150 μmol/l of H2S for 7 days. (a) Calcium content is shown as mean±standard deviation of three independent experiments conducted in duplicates; *P<0.05, **P<0.01. (b) Representative images of Alizarin red staining of plates (upper) and microscopic views ( × 100, lower) from three independent experiments are shown. (c) MTT assay is shown as mean±standard deviation of two independent experiments conducted in triplicates. GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
H2S inhibits osteoblastic differentiation of HAoSMC
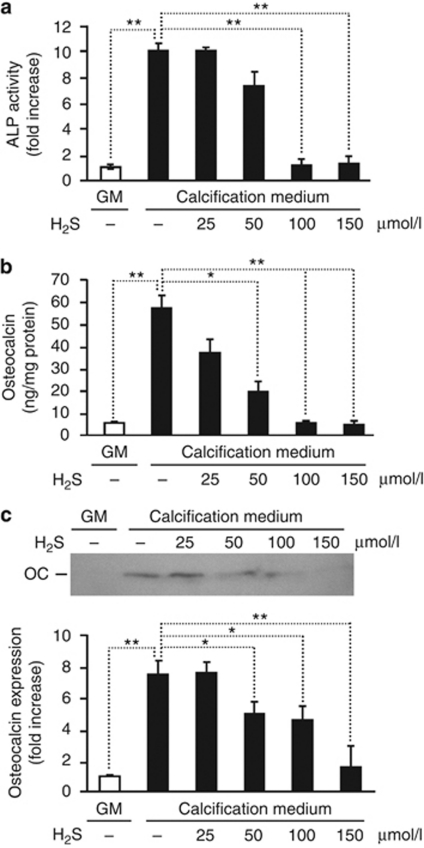
It has been shown that vascular calcification in vivo resembles bone mineralization; therefore, we examined whether H2S suppresses the phenotype transition of HAoSMC into osteoblast-like cells. Because upregulation of ALP, an important enzyme in osteogenesis, and OC, a major non-collagenous protein found in bone matrix, are implicated in the pathogenesis of vascular calcification, we measured the level of their expression in HAoSMC treated with H2S. Although HAoSMC maintained in calcification medium for 7 days exhibited around a 10-fold increase in ALP activity compared with controls, addition of H2S to the calcification medium resulted in a dose-dependent suppression providing a complete attenuation at a dose of 100 μmol/l (Figure 2a). Similar to ALP activity, the induction of OC was also abolished by H2S. Maintaining HAoSMC in calcification medium for 7 days led to a >10-fold increase in OC content in the extracellular matrix compared with control. H2S decreased the expression of OC to the basal level, observed in HAoSMC (Figure 2b and c) cultured in GM.
Figure 2.
H2S inhibits Pi-mediated upregulation of osteoblast-specific proteins in HAoSMC. (a–c) HAoSMCs were cultured in GM or in calcification medium in the absence or presence of different concentrations of H2S for 7 days. (a) ALP activity is presented as means±standard deviation of three independent experiments each conducted in duplicates; **P<0.01. (b) Osteocalcin level in ethylenediaminetetraacetate (EDTA)-solubilized extracellular matrix was determined by enzyme-linked immunosorbent assay and shown as means±standard deviation of three independent experiments each conducted in duplicates; *P<0.05, **P<0.01. (c) Osteocalcin detected by western blot from EDTA-solubilized extracellular matrix. Blot is representative of three independent experiments. A graph of band intensity means±standard deviation of three experiments is shown; *P<0.05, **P<0.01. ALP, alkaline phosphatase; GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide.
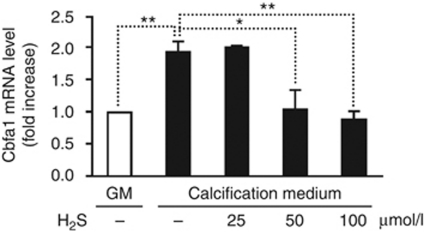
Next, we examined the level of Cbfa1, a transcription factor required for osteoblast differentiation, in our in vitro model. Pi increased Cbfa1 mRNA level by ∼1.8-fold compared with cells grown in control medium. As shown in Figure 3, H2S completely suppressed the induction of Cbfa1 mRNA provoked by elevated Pi.
Figure 3.
H2S prevents Pi-mediated upregulation of osteoblast-specific transcription factor Cbfa1 in HAoSMC. HAoSMCs were cultured in GM or in calcification medium alone or in the presence of H2S (25, 50 and 100 μmol/l) for 24 h. Cbfa1 messenger RNA (mRNA) levels were determined by quantitative reverse transcriptase-polymerase chain reaction. Results are shown as means±standard deviation of five independent experiments conducted in triplicates; *P<0.05, **P<0.01. Cbfa1, core-binding factor alpha-1; GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide.
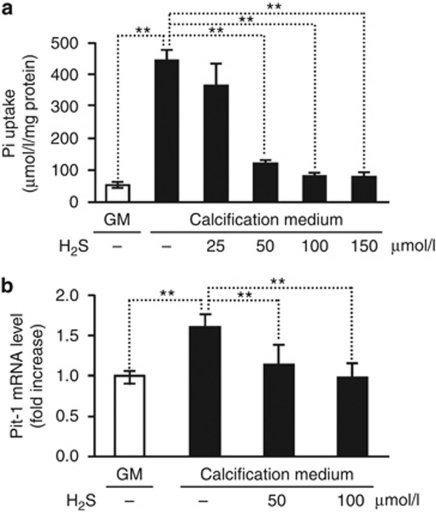
Evidence suggests that the effects of hyperphosphatemia are mediated via Pit-1, which facilitates entry of Pi into vascular cells. To further explore the mechanism by which H2S inhibits vascular calcification, we measured Pi uptake of HAoSMC in the presence or absence of H2S. Intriguingly, addition of H2S inhibited Pi uptake in a dose–responsive manner, providing a significant and complete suppressions at concentrations of 50 μmol/l and 100 μmol/l, respectively (Figure 4a). To explore the mechanism underlying the inhibition of Pi uptake, we examined the expression of Pit-1. Maintaining HAoSMC in calcification medium, we observed a 1.6-fold elevation in Pit-1 mRNA level. Addition of H2S into the calcification medium prevented the increase in Pit-1 expression (Figure 4b).
Figure 4.
H2S inhibits phosphate uptake by HAoSMCs. (a) HAoSMCs were cultured in GM and calcification medium alone, or supplemented with H2S (25, 50, 100, 150 μmol/l) for 4 days. Pi levels were determined from cell lysates and shown as means±standard deviation of three independent experiments conducted in duplicates; **P<0.01. (b) HAoSMCs were cultured in GM and calcification medium alone, or supplemented with H2S (25, 50, 100, 150 μmol/l) for 48 h and Pit-1 messenger RNA level was determined by quantitative reverse transcriptase-polymerase chain reaction. Results are presented as means±standard deviation of five independent experiments conducted in triplicates; **P<0.01. GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide.
It was recently reported that induction of ferritin prevents Pi-mediated calcification and osteoblastic differentiation of HAoSMC.42 We therefore tested whether H2S modulates its expression in HAoSMC. As shown in Figure 5, the expressions of H- and L-ferritin were not altered, suggesting that the mechanism by which H2S prevents calcification is independent of ferritin.
Figure 5.
H2S does not alter the expression of H- and L-ferritin in HAoSMCs. (a–c) HAoSMCs were cultured in growth medium or in calcification medium in the absence or presence of H2S (25, 50, 100, 150 μmol/l) for 24 h. (a) H- and L-ferritin detected by western blot from cell lysates. Membranes were reprobed with GAPDH for normalization. Blots are representatives of three experiments. (b, c) Bar graphs show densitometric measurement of band intensities as means±standard deviation of three experiments. Ft, ferritin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide.
Endogenous production of H2S inhibits calcification and osteoblastic differentiation of HAoSMC
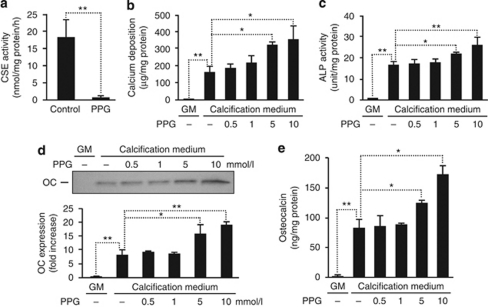
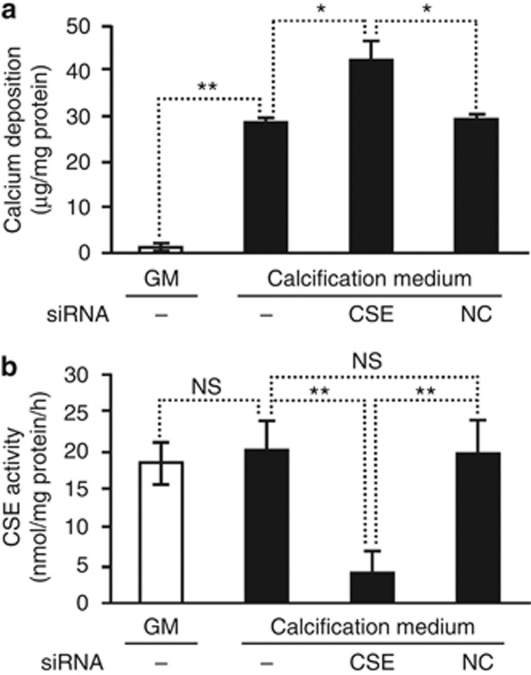
In the vasculature, H2S is produced by VSMC expressing the pyridoxal-5′-phosphate-dependent enzyme CSE. On the basis of our observations, we thereby hypothesized that inhibition of CSE enzyme activity would lead to increased mineralization. Therefore, first we inhibited CSE using dl-propargylglycine (PPG), a well-known inhibitor of CSE activity. Cells treated with PPG showed a gradual decrease in CSE enzyme activity (Figure 6a). Suppression of CSE by PPG almost doubled the deposition of calcium in the extracellular matrix of HAoSMC maintained in calcification medium for 7 days (Figure 6b), as compared with cells cultured in calcification medium without PPG. Accordingly, PPG provoked a significant additional increase in the activity of ALP (Figure 6c) and expression of OC (Figure 6d and e) by 73% and 120%, respectively. As a second approach to decrease CSE activity, we transfected HAoSMC with CSE-specific small interfering RNA (siRNA). Transfection provided an ∼70% reduction in CSE enzyme activity, which was accompanied by increased calcium deposition in HAoSMC (Figure 7).
Figure 6.
Inhibition of endogenous production of H2S by dl-propargylglycine (PPG) promotes calcification and osteoblastic differentiation of HAoSMCs. (a) CSE activity determined from HAoSMC in the absence or presence of PPG (10 mmol/l) is shown as means±standard deviation of three independent experiments; **P<0.01. (b–e) HAoSMCs were cultured in GM or in calcification medium alone or in the presence of PPG (0.5, 1, 5 and 10 mmol/l) for 7 days. Extracellular calcium deposition (b), ALP activity (c), and osteocalcin (OC) expression (d, e) are shown. Data are expressed as means±standard deviation of three independent experiments each conducted in duplicates; *P<0.05, **P<0.01. ALP, alkaline phosphatase; CSE, cystathionine γ-lyase; GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide.
Figure 7.
Silencing of cystathionine γ-lyase (CSE) provokes mineralization of HAoSMCs. (a) Calcium deposition of HAoSMC transfected with small interfering RNA (siRNA) for CSE or negative control siRNA (NC) after culturing cells in calcification medium for 4 days is shown as means±standard deviation of three independent experiments; *P<0.05, **P<0.01. (b) CSE activity of transfected HAoSMC 48 h post transfection is shown as means±standard deviation of three independent experiments; **P<0.01. GM, growth medium; HAoSMC, human aortic smooth muscle cell; H2S, hydrogen sulfide; NS, non-significant.
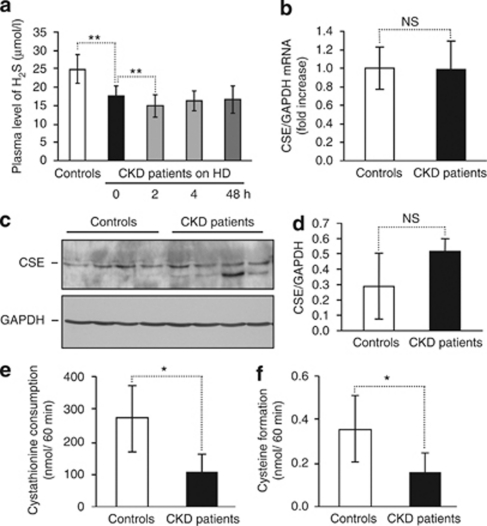
Decreased plasma H2S levels are associated with reduced CSE activity in stage 5 CKD patients
In agreement with previous observations,37 we found that plasma concentration of H2S was decreased in stage 5 CKD patients, and that H2S level was further lowered by hemodialysis (Figure 8a). As the main enzyme responsible for H2S biogenesis in the vasculature is CSE, we compared its expression and activity in mononuclear cells derived from stage 5 CKD patients treated with hemodialysis and healthy controls. Levels of CSE mRNA and protein expression were similar in CKD patients and controls (Figure 8b–d). Importantly, CSE enzyme activity—which was measured by cystathionine consumption and cysteine production—was markedly decreased in mononuclear cells derived from stage 5 CKD patients treated with hemodialysis compared with healthy individuals (Figure 8e and f).
Figure 8.
Decreased plasma level of H2S and reduced CSE activity in stage 5 CKD patients. (a) Plasma levels of hydrogen sulfide in healthy individuals (n=23) and in stage 5 CKD patients (n=21) on hemodialysis (HD) treatment at different time points is shown as means±standard deviation; **P<0.01. (b) CSE gene expression was measured by quantitative reverse transcriptase-polymerase chain reaction from PBMC messenger RNA (mRNA) isolated from controls (n=16) and stage 5 CKD patients (n=14). (c, d) CSE protein expression determined by western blot in PBMC isolated from controls (n=4) and stage 5 CKD patients (n=4). Levels of CSE were normalized to GAPDH. (e, f) CSE enzyme activities assessed by cystathionine consumption (e) and cysteine production (f) in PBMC isolated from controls (n=10) and stage 5 CKD patients (n=10) are shown as means±standard deviation; *P<0.05. CKD, chronic kidney disease; CSE, cystathionine γ-lyase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; H2S, hydrogen sulfide; NS, non-significant; PBMC, peripheral blood mononuclear cell.
DISCUSSION
Elevated Pi level has long been recognized as a significant predictor of soft tissue mineralization. Such elevated Pi levels are commonly seen in patients with end-stage kidney disease in which the Pi homeostasis is deranged because of inability of the kidneys to excrete phosphate. Evidence shows that vascular calcification results in increased cardiovascular mortality in hemodialysis patients.43, 44, 45 Vascular calcification follows two distinct patterns: (i) intimal calcification, which occurs with atherosclerotic plaques, and (ii) medial calcification, which is characterized by diffuse calcification of the media, particularly at the level of the internal elastic lamina that is commonly seen in hemodialysis patients and is not always accompanied by atherosclerosis. Furthermore, development of calciphylaxis, which is a syndrome of diffuse arteriolar calcification and skin necrosis, is almost exclusively seen in patients with stage 5 CKD and correlates with extremely high fatal rates.
Previous studies indicated that elevated phosphate could induce VSMC calcification as well as an osteochondrogenic phenotypic change.46, 47 Evidence suggests that this transition is a highly regulated cellular process18, 20, 21, 22 involving upregulation of Cbfa1, a key regulatory transcription factor critical for the differentiation of osteoblasts, and its downstream transcript proteins such as ALP, a crucial enzyme in the context of bone and teeth formation, and OC, which is a very specific protein indicative of osteoblast activity.
H2S is now recognized as a gas with important functions in the cardiovascular system.23 In the vasculature, it is produced by VSMC by CSE enzyme,27 and is involved in the regulation of vascular tone28 and myocardial contractility.48 H2S deficiency was observed in various animal models of arterial49 and pulmonary hypertension.50 Exogenous H2S ameliorates myocardial dysfunction associated with the ischemia/reperfusion injury.51, 52 More recently, it was reported that H2S reduces vascular calcification induced by vitamin D3 plus nicotine in rats.41 The potential inhibitory effect of H2S on vascular calcification has recently emerged in calcific uremic arteriolopathy/calciphylaxis, a life-threatening complication of renal failure. Calciphylaxis can be successfully treated with intravenous administration of sodium thiosulfate.38, 39 Sodium thiosulfate was shown to enhance H2S generation by inducing CSE expression.40 However, the effector function(s) of H2S in the treatment of calciphylaxis remained to be elucidated.
Drawing upon these previous observations, we examined the role that H2S may have in VSMC calcification and transition of VSMC into osteoblast-like cells. We observed that H2S suppressed deposition of calcium in the extracellular matrix of HAoSMC induced by Pi in a dose–responsive manner. More importantly, the inhibitory effect of H2S was not limited to calcium deposition. In fact, H2S suppressed the induction of genes involved in osteoblastic transformation of HAoSMC. H2S inhibited Pi-mediated upregulation of ALP and OC in a dose-dependent manner. Cbfa1 is required for osteoblast differentiation, bone matrix gene expression, and, consequently, mineralization;21 therefore, we examined the effect of H2S on Cbfa1 expression in HAoSMC. Similar to ALP and OC, the Pi-provoked upregulation of Cbfa1 was attenuated by H2S.
Emerging evidence suggests that the effect of hyperphosphatemia on VSMC calcification is mediated through Pit-1, which facilitates entry of Pi into vascular cells.20 We therefore tested whether H2S alters intracellular Pi level in HAoSMC. Importantly, the marked increase in the level of intracellular Pi due to phosphate exposure was substantially reversed by H2S. We demonstrate that inhibition of entry of Pi into vascular cells provided by H2S occurs through suppression of Pit-1 expression, thus decreasing the intracellular level of Pi that is fundamental to osteoblastic transformation of HAoSMC. Apoptosis of VSMC is also implicated in the pathogenesis of calcification in vessels, which is seen both in the intima in advanced plaques and in the media in CKD. Apoptotic smooth muscle cells may function as both a nidus for calcification and actively concentrate both calcium and phosphate to generate hydroxyapatite.53, 54, 55 H2S was shown to induce apoptosis of HAoSMC. The concentration at which H2S exhibits proapoptotic effect is ⩾200 μmol/l. In our study, we did not observe alterations in viability of HAoSMC challenged with Pi or H2S at concentrations studied.
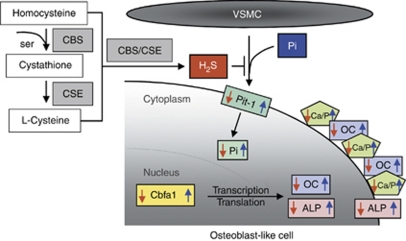
H2S is generated as an alternative product of the transsulfuration pathway (Figure 9), and in the vasculature it is produced mainly by VSMC via CSE-catalyzed reaction.27 Recently, it has been shown that deletion of CSE in mice results in hypertension.56 Our data confirmed that the plasma concentration of H2S was decreased in stage 5 CKD patients. Moreover, the level of H2S was further lowered by hemodialysis. We revealed that enzyme activity of CSE in monocytes derived from stage 5 CKD patients treated with hemodialysis was markedly reduced compared with healthy individuals, without changes in mRNA or protein expression. These results suggest potential post-translational modifications in CSE in CKD that remain to be determined.
Figure 9.
Scheme of H2S biogenesis and its involvement in Pi-induced osteoblastic transformation of VSMC. H2S is generated as an alternative product of the transsulfuration pathway. H2S inhibits all the steps of osteoblast transition of VSMC. Pi-induced phosphate uptake, Pit-1 upregulation, Cbfa1, ALP, osetocalcin (OC) expression, and Ca deposition are all inhibited by H2S. Blue arrows represent responses to elevated Pi, whereas red arrows represent the effect of H2S. ALP, alkaline phosphatase; Cbfa1, core-binding factor alpha-1; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; H2S, hydrogen sulfide; VSMC, vascular smooth muscle cell.
To prove the role of CSE in inhibiting Pi-induced HAoSMC mineralization, we used strategies to decrease endogenous production of H2S via both pharmacological inhibition of CSE enzyme activity and silencing of CSE gene expression. By reducing CSE-mediated endogenous H2S biogeneration, we observed a significant increase in both HAoSMC calcification and the expression of ALP and OC.
Our findings further corroborate the imperative role of H2S and CSE in the vasculature and suggest that reduced activity of CSE and subsequent decrease in the level of H2S in stage 5 CKD patients could exacerbate the cardiovascular complications that commonly accompany this particular group of patients.
In conclusion, our study demonstrates a novel role of H2S in the process of Pi-provoked mineralization and transition of HAoSMC into osteoblast-like cells (Figure 9). We provide evidence that H2S, regardless of its exogenous or endogenous origin, inhibits the upregulation of osteoblast-specific genes such as ALP, OC, and Cbfa1. The inhibition of Pi uptake through Pit-1 is essential for providing beneficial effects against calcification and phenotypic modulation of HAoSMC by H2S. Reduced CSE activity leading to decreased H2S levels in stage 5 CKD patients might facilitate calcification of vasculature. These results offer a new strategy to prevent vascular calcification.
MATERIALS AND METHODS
Cell culture and reagents
HAoSMCs were obtained from Cambrex Bioscience (Wokingham, UK) and fetal bovine serum from Invitrogen/GIBCO (Carlsbad, CA). Unless otherwise mentioned, all other reagents were obtained from Sigma (St Louis, MO). Cell cultures were maintained in GM DMEM (high glucose) containing 15% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and neomycin, and 1 mmol/l sodium pyruvate. Cells were grown to confluence and used from passages 3–7. H2S was introduced as sodium hydrosulfide dissolved in deionized water.
Patients
Controls were healthy subjects recruited among hospital staff without any known diseases including hypertension, dyslipidemia, and liver and kidney malfunctions (n=23, mean age 53 years, F/M 9/14). Stage 5 CKD patients treated with hemodialysis were selected (n=21, mean age 61 years, F/M 13/8) from our dialysis unit. The patients were on hemodiafiltration therapy of three 4-h sessions weekly, using High-Flux Dialysers FX 60 and FX 600 (Fresenius, Fresenius AG, Homburg, Germany). The Kt/V ratio was 1.25±0.3. Folate (3 mg/day) and intravenous iron (Ferrlecit, Aventis Pharma, Dagenham, UK) (62.5 mg every other week) were given to patients from the beginning of hemodialysis therapy. They also received rHu-EPO depending on their hemoglobin value. Blood was drawn in Vacutainers (BD, Franklin Lakes, NJ) using ethylenediaminetetraacetate immediately before the hemodialysis session from CKD patients by venipuncture. Participants gave their informed consent to the study, which was approved by the Regional and Institutional Ethics Committee of the University of Debrecen, Medical and Health Science Center (Nr. DEOEC RKEB/IKEB 3287A-2010).
Separation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were separated from blood by density gradient centrifugation using Histopaque 1077 (Sigma). Cells were disrupted by ultrasonication in 100 mmol/l phosphate buffer, pH 7.4, for immediate determination of CSE activity or in 100 mmol/l phosphate buffer containing 1% Triton X-100 and a mixture of protease inhibitors (Sigmafast Protease Inhibitor Tablets, Sigma). Cell lysate was maintained at −70 °C until use.
Measurement of plasma H2S concentration
Immediately after drawing, blood was centrifuged (3 min, 3000 g) and 40 μl of plasma was rapidly added to the assay mixture that contained 50 μl of 1% zinc acetate, 40 μl of FeCl3 (30 mmol/l in 1.2 mol/l HCl), and 50 μl of N,N-dimethyl-p-phenylenediamine dihydrochloride (20 mmol/l in 7.2 mol/l HCl). To deprotonize samples, 70 μl of 10% trichloracetic acid was added, and then the mixture was centrifuged at 3000 g for 30 min at room temperature. The absorbance of the supernatant was read at 670 nm, and concentration was calculated using a calibration curve.
Induction of calcification
At confluence, HAoSMCs were switched to calcification medium, which was prepared by adding 3 mmol/l of inorganic phosphate to the GM. Both GM and calcification medium were changed every 2 days. For time-course experiments, the first day of culture in calcification medium was defined as day 0.
Quantification of calcium deposition
Cells grown on 48-well plates were washed twice with phosphate buffered saline and decalcified with 0.6 mol/l HCl for 24 h. Calcium content of the supernatants was determined by the QuantiChrome Calcium Assay Kit (Gentaur, Hayward, CA). After decalcification, cells were solubilized with a solution of 0.1 mol/l NaOH and 0.1% sodium dodecyl sulfate, and protein content of the samples were measured with the BCA protein assay kit (Thermo Scientific, Rockford, IL). Calcium content of the cells was normalized to protein content and expressed as μg/mg protein. Mineralization was also determined by Alizarin red staining.
Alkaline phosphatase activity assay
HAoSMCs grown on six-well plates were washed with phosphate-buffered saline twice, solubilized with 1% Triton X-100 in 0.9% NaCl, and assayed for ALP activity. Briefly, 130 μl of Alkaline Phosphatase Yellow Liquid Substrate (Sigma) was combined with 50 μg of protein samples, and then the kinetics of p-nitrophenol formation was followed for 30 min at 405 nm at 37 °C. Maximum slope of the kinetic curves was used for calculation.
Phosphate measurement
Pi content of the cell lysate was determined by the QuantiChrome Phosphate Assay Kit (Gentaur). After 4 days of culture, cells were washed twice with phosphate-buffered saline and solubilized with 1% Triton, and the cell lysates were assayed for Pi. Phosphate content of the cells was normalized to protein content and expressed as μmol/l/mg cell protein.
Quantification of OC
Osteocalcin was quantified as described previously.42
Detection of OC, CSE, ferritin H- and L-chain by western blot
To determine the protein expression level of OC, extracellular matrix was dissolved in ethylenediaminetetraacetate (0.5 mol/l, pH 6.9) and was electrophoresed in 16.5% Tris-Tricin/Peptide-PAGE (Bio-Rad Laboratories, Hercules, CA). Ferritin western blot was performed as described previously.42 For CSE determination, cell lysate of PBMC was electrophoresed in 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was performed with a polyclonal anti-OC antibody at 1:200 dilution (Santa Cruz Biotechnology, Santa Cruz, CA) and with anti-CSE antibody (Sigma) at 1:100 dilution, followed by horseradish peroxidase-labeled anti-mouse immunoglobulin-G antibody. Antigen–antibody complexes were visualized with the horseradish peroxidase chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK). Chemiluminescence was quantified by using Alpha DigiDoc RT quantification software (Alpha Innotech, San Leandro, CA).
Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated, reverse transcribed, and polymerase chain reactions were performed using iQ SYBR Green Supermix (Bio-Rad) and the following primers: Cbfa1 forward 5′-ATGGCGGGTAACGATGAAAAT-3′ and reverse 5′-ACGGCGGGGAAGACTGTG-3′ Pit-1 forward 5′-GCCAAAGTGAGCGAAACCATCC-3′ and reverse 5′-CCACACAGCAGAACCAAACATAGC-3′. To measure CSE mRNA levels in CKD patients and controls, blood mononuclear cell mRNA was isolated from whole blood with the QIAamp RNA Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Primers for CSE were the following: forward: 5′-GCCTTCATAATAGACTTCGTTTC-3′ and reverse: 5′-GCAGCCCAGGATAAATAACC-3′. Polymerase chain reactions were carried out using the iCycler iQ Real-Time PCR System (Bio-Rad). Results were normalized by glyceraldehyde-3-phosphate dehydrogenase mRNA levels.
Cystathionine γ-lyase siRNA transfection
Small interfering RNA specific to CSE and negative control siRNA were obtained from Ambion (Austin, TX). Transfection of siRNA into HAoSMC was achieved using the Oligofectamine Reagent (Invitrogen). Briefly, the cells were plated overnight to form 60–70% confluent monolayers. CSE siRNA at 30 nmol/l and transfection reagent complex were added to the cells in serum-free medium for 4 h. Fresh normal GM was added further and the cells were incubated for another 20 h.
Cystathionine γ-lyase activity measurement
CSE activity was measured according to the Stipanuk method.57 CSE activity from PBMC was assessed by cystathionine consumption and cysteine production. Lysed PBMC was incubated with cystathionine (2 mmol/l), pyridoxal 5′-phosphate (0.25 mmol/l) in Tris-HCl buffer (100 mmol/l, pH 8.3) for 60 min at 37 °C. Thereafter, acid ninhydrine reagent was added, and samples were boiled for 5 min. After cooling the samples, optical densities at 455 and 560 nm were determined and used for calculation of cystathionine and cysteine concentrations, respectively.
MTT assay
MTT assay was performed as described previously.42
Statistical analysis
Data are shown as mean±standard deviation. Statistical analysis was performed by analysis of variance test, followed by post hoc, Newmann–Keuls test for multiple comparisons. A value of P<0.05 was considered significant and marked with *, and P<0.01 was considered highly significant and marked with **.
Acknowledgments
We thank Erika Barna for technical assistance. This work was supported by Hungarian Government grants OTKA-K75883, OTKA-K83478, OTKA-PD83435, ETT-147/2009, MTA-DE-11003, European Reintegration Grant, FP7-PEOPLE-2010-268332, by the TÁMOP 4.2.1./B-09/1/KONV-2010-0007 project. VJ is partly supported by the Sectoral Operational Programme Human Resource Development (POSDRU), financed by the European Social Fund and the Romanian Government under the contract number POSDRU 60782. The project is implemented through the New Hungary Development Plan, cofinanced by the European Social Fund.
All the authors declared no competing interests.
References
- Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–1237. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- Beadenkopf WG, Daoud AS, Love BM. Calcification in the coronary arteries and its relationship to arteriosclerosis and myocardial infarction. AJR Am J Roentgenol. 1964;92:865–871. [PubMed] [Google Scholar]
- Loecker TH, Schwartz RS, Cotta CW, et al. Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol. 1992;19:1167–1172. doi: 10.1016/0735-1097(92)90319-i. [DOI] [PubMed] [Google Scholar]
- Lehto S, Niskanen L, Suhonen M, et al. Medial artery calcification: a neglected harbinger of cardiovascular complications in non–insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- Olson JC, Edmundowicz D, Becker DJ, et al. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49:1571–1578. doi: 10.2337/diabetes.49.9.1571. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:322–324. doi: 10.1161/01.cir.86.1.64. [DOI] [PubMed] [Google Scholar]
- Niskanen LK, Suhonen M, Siitonen O, et al. Aortic and lower limb artery calcification in type 2 (noninsulin-dependent) diabetic patients and non-diabetic controlsubjects: a five year follow-up study. Atherosclerosis. 1990;84:61–71. doi: 10.1016/0021-9150(90)90009-8. [DOI] [PubMed] [Google Scholar]
- Burke AP, Taylor A, Farb A, et al. Coronary calcification: insights from sudden coronary death victims. Z Kardiol. 2000;89:49–53. doi: 10.1007/s003920070099. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Burke AP, O'Malley PG, et al. A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death. Circulation. 2000;101:1243–1248. doi: 10.1161/01.cir.101.11.1243. [DOI] [PubMed] [Google Scholar]
- Guérin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- Blacher J, Guérin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Jono S, Shioi A, et al. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:34–37. doi: 10.1053/ajkd.2001.27394. [DOI] [PubMed] [Google Scholar]
- Giachelli CM. Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol. 2003;43:300–304. doi: 10.1097/01.asn.0000081663.52165.66. [DOI] [PubMed] [Google Scholar]
- Shioi A, Nishizawa Y, Jono S, et al. Beta-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–2009. doi: 10.1161/01.atv.15.11.2003. [DOI] [PubMed] [Google Scholar]
- Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- Lomashvili KA, Cobbs S, Hennigar RA, et al. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Steitz S, Jono S. Potential roles of bone matrix proteins in vascular calcification. Clin Calcium. 1999;9:20–27. [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Doeller JE, Isbell TS, Benavides G, et al. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Olson KR. Is hydrogen sulfide a circulating ‘gasotransmitter' in vertebrate blood. Biochim Biophys Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011;15:373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- Erickson PF, Maxwell IH, Su LJ, et al. Sequence of cDNA for rat cystathionine γ-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990;269:335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, et al. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- Du J, Hui Y, Cheung Y, et al. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels. 2004;19:75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- Jeong SO, Pae HO, Oh GS, et al. Hydrogen sulphide potentiates interleukin-1-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;345:938–944. doi: 10.1016/j.bbrc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Jeney V, Komódi E, Nagy E, et al. Supression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H(2)S) Free Radic Biol Med. 2009;46:616–623. doi: 10.1016/j.freeradbiomed.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P, Belardinelli MC, Chabli A, et al. Endogenous hydrogen sulphide overproduction in Down syndrome. Am J Med Genet. 2003;116:310–311. doi: 10.1002/ajmg.a.10847. [DOI] [PubMed] [Google Scholar]
- Perna AF, Luciano MG, Ingrosso D, et al. Hydrogen sulphide-generating pathways in haemodialysis patients: a study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol Dial Transplant. 2009;24:3756–3763. doi: 10.1093/ndt/gfp378. [DOI] [PubMed] [Google Scholar]
- Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104–1108. doi: 10.1053/j.ajkd.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258–262. doi: 10.1111/j.1525-139X.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- Sen U, Vacek TP, Hughes WM, et al. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology. 2008;82:201–213. doi: 10.1159/000156486. [DOI] [PubMed] [Google Scholar]
- Wu SY, Pan CS, Geng B, et al. Hydrogen sulphide ameliorates vascular calcification induced by vitamin D3 plus nicotine in rats. Acta Pharmacol Sin. 2006;27:299–306. doi: 10.1111/j.1745-7254.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- Zarjou A, Jeney V, Arosio P, et al. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J Am Soc Nephrol. 2009;20:1254–1263. doi: 10.1681/ASN.2008070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GA. Prevalence and clinical consequences of elevated Ca × P product in hemodialysis patients. Clin Nephrol. 2000;54:318–324. [PubMed] [Google Scholar]
- Burke SK. Phosphate is a uremic toxin. J Ren Nutr. 2008;18:27–32. doi: 10.1053/j.jrn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Giachelli CM. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am J Pathol. 1999;154:671–675. doi: 10.1016/S0002-9440(10)65313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- Geng B, Chang L, Pan C, et al. Endogenous hydrogen sulphide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- Chunyu Z, Junbao D, Dingfang B, et al. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun. 2003;302:810–816. doi: 10.1016/s0006-291x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- Bian JS, Yong QC, Pan TT, et al. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- Zhu YZ, Wang ZJ, Ho P, et al. Hydrogen sulfide and its cardioprotective effects in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- Clarke M, Bennett M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am J Nephrol. 2006;26:531–535. doi: 10.1159/000097815. [DOI] [PubMed] [Google Scholar]
- Proudfoot D, Skepper JN, Hegyi L, et al. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- Proudfoot D, Davies JD, Skepper JN, et al. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106:3044–3050. doi: 10.1161/01.cir.0000041429.83465.41. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]