Abstract
Aquatic birds are the natural reservoir for most subtypes of influenza A, and a source of novel viruses with the potential to cause human pandemics, fatal zoonotic disease or devastating epizootics in poultry. It is well recognised that waterfowl typically show few clinical signs following influenza A infection, in contrast, terrestrial poultry such as chickens may develop severe disease with rapid death following infection with highly pathogenic avian influenza. This study examined the cellular response to influenza infection in primary cells derived from resistant (duck) and susceptible (chicken) avian hosts. Paradoxically, we observed that duck cells underwent rapid cell death following infection with low pathogenic avian H2N3, classical swine H1N1 and ‘classical' highly pathogenic H5N1 viruses. Dying cells showed morphological features of apoptosis, increased DNA fragmentation and activation of caspase 3/7. Following infection of chicken cells, cell death occurred less rapidly, accompanied by reduced DNA fragmentation and caspase activation. Duck cells produced similar levels of viral RNA but less infectious virus, in comparison with chicken cells. Such rapid cell death was not observed in duck cells infected with a contemporary Eurasian lineage H5N1 fatal to ducks. The induction of rapid death in duck cells may be part of a mechanism of host resistance to influenza A, with the loss of this response leading to increased susceptibility to emergent strains of H5N1. These studies provide novel insights that should help resolve the long-standing enigma of host–pathogen relationships for highly pathogenic and zoonotic avian influenza.
Keywords: apoptosis, avian influenza, cell death, chickens, ducks, host resistance
Influenza A viruses are important pathogens in a wide range of, both mammalian and avian, hosts. The virus is responsible for seasonal epidemics in humans, which cause significant morbidity and mortality each year, most notably in young children and the elderly.1 Emergence of influenza strains, to which the human population has little or no pre-existing immunity, can be associated with widespread and potentially severe disease pandemics. Aquatic birds are the natural reservoir for most subtypes of the virus and a source of novel viruses that can infect mammals or terrestrial poultry.2 Such emergent viruses have the potential to cause pandemic infection in humans or cause sporadic cases of potentially severe zoonotic disease.3 Ducks have had a major role in the generation and maintenance of H5N1 in eastern Asia,4 which has caused over 250 confirmed human deaths since its re-emergence in 2003.5 Influenza A is also responsible for economically important disease outbreaks that have lead to the culling of millions of farmed chickens.3, 6
Influenza A viruses are found throughout bird populations worldwide, including Anseriformes (ducks, geese and swans), Charadriiformes (shorebirds), domestic poultry and passerines. Typically, aquatic birds, such as ducks, exhibit few clinical signs following infection with influenza viruses, yet shed virus, which serves as a source of infection for other hosts. In contrast, terrestrial poultry, such as chickens or turkeys, show clinical signs varying from mild, following infection with low pathogenic avian influenza (LPAI), to severe with rapid death in 1–2 days, following infection with highly pathogenic avian influenza (HPAI) viruses. Such a marked difference in the typical host response to infection within such a short time period post infection suggests that there are underlying differences in the innate host response to influenza A infection, between resistant hosts, exemplified by waterfowl, especially ducks, and susceptible hosts such as chickens. This is supported by experimental studies showing that naïve ducks, with no pre-existing acquired immunity to influenza A, display few clinical signs following challenge with ‘classical' HPAI viruses, whereas chickens show high levels of mortality.7, 8 Rarely, infection with recently emerged strains of HPAI, such as Eurasian lineage H5N1, may cause significant disease and mortality in wild bird populations, including ducks.9, 10
The marked difference in susceptibility to influenza infection, between ducks and chickens, provides a unique opportunity to dissect the molecular mechanisms contributing towards disease resistance. To gain an insight into potential differences in innate immune responses that could explain the contrasting outcome following influenza infection in different avian hosts, we infected primary cells, derived from chicken and duck embryos or lungs, with LPAI, HPAI and classical swine H1N1. Our data suggest that the rapid death of duck cells following influenza infection, associated with induction of cellular caspases, may be a potential mechanism underlying the long held, but hitherto unexplained, observation that ducks remain healthy in the face of influenza infection unlike terrestrial poultry, and is a potential reason why waterfowl, such as ducks, are such an important reservoir for the virus.
Results
Both human and avian type sialic acid (SA) receptors are expressed in primary lung and embryo cells from chickens and ducks
Influenza viruses enter susceptible cells following binding of the viral haemagglutinin to cellular SAs associated with membrane glycoproteins and glycolipids. Avian influenza viruses have been shown to preferentially bind to SA receptors that are linked to galactose by an α2,3 linkage (SAα2,3-Gal), whereas mammalian viruses show preference for receptors with an α2,6 linkage (SAα2,6-Gal).11, 12 Studies on tissues derived from chickens and ducks have shown some differences in the distribution of SA receptors between species.13 To determine whether the SA receptor expression of primary cells might account for species differences following influenza infection in vitro, detection of SA receptors was performed on cultured cells by histochemistry using linkage-specific lectins. There was no significant difference in receptor expression between chicken and duck cells that might account for any difference in the cellular response to infection; both SAα2,3-Gal and SAα2,6-Gal receptors were expressed by chicken and duck, lung and embryo cells (Supplementary Figure S1).
Influenza A infection causes rapid death of duck but not chicken primary cells
To assess the effect of influenza infection on chicken and duck cells, we infected cultured cells, derived from Pekin duck and White Leghorn chicken embryos, with LPAI H2N3 (A/mallard duck/England/7277/06), classical swine H1N1 (A/sw/Iowa/15/30) or with HPAI H5N1 (A/turkey/England/50-92/91; hereafter referred to as H5N1 50-92) viruses. Paradoxically, infection of these primary cells revealed that cell death was induced more rapidly, and to a greater degree, in duck than in chicken cells. This was evident as an increase in the number of cells becoming detached, rounding up and floating in the media (Figures 1a and b). Uninfected cells from both species showed little evidence of such changes (Figures 1c and e). To summarise, duck embryo cells consistently showed more rapid and higher levels of cell death than chicken cells between 24–48 h following infection with LPAI H2N3, classical swine H1N1 or HPAI H5N1 50-92 viruses. To confirm that this response was not cell-type specific, we repeated these experiments using primary cells derived from chicken and duck lungs and again observed the phenomenon of more rapid and extensive cell death in duck cells, 24–48 h post infection, with each of these viruses (Supplementary Figure S2). Experimental infection of ducks with HPAI 50-92,14 LPAI H2N3 or classical swine H1N1, closely related to viruses used in this study,15 does not cause disease in Pekin ducks; however, experimental infection of chickens with H5N1 50-92 results in rapid and high levels of mortality.14 Thus, there is an inverse correlation between the outcome of cell infection in vitro and the effect of virus infection in vivo.
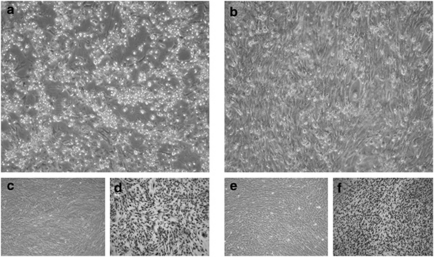
Figure 1.
Cell death in avian embryo fibroblasts following influenza A infection. (a) Duck and (b) chicken embryo fibroblasts infected with classical swine H1N1 virus for 48 h. (c) Uninfected duck and (e) chicken cells are shown for comparison. Immunostaining for viral nucleoprotein antigen in (d) duck and (f) chicken cells 6 h following viral infection at an MOI of 1.0. Similar results were obtained following infection with LPAI H2N3 or with HPAI H5N1 50-92 viruses.
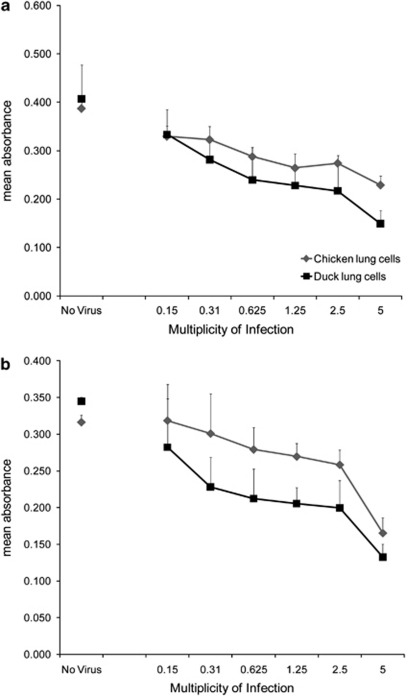
To quantify the observed difference in cell death between duck and chicken cells, we measured cell viability using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide reduction (MTT) assay, 24 h following virus infection. Duck lung cells consistently showed significantly lower metabolic activity than chicken lung cells over a range of multiplicities of infection (MOI) with either LPAI H2N3 (Figure 2a) or classical swine H1N1 (Figure 2b) viruses (P<0.01). As was expected, the effect was dose dependent, with increasing levels of infection inducing greater reductions in cell viability.
Figure 2.
Duck lung cells show lower metabolic activity compared with chicken lung cells following influenza A infection. Measurement of cell viability in duck and chicken lung cells, using an MTT assay, 24 h post infection, with a range of MOI, using (a) LPAI H2N3 or (b) classical swine H1N1 virus. Data points are the mean of quadruplicate wells with error bars showing s.d. There is a significant difference between species (P<0.01; two-way analysis of variance).
To exclude the possibility that the difference in the kinetics of cell death between duck and chicken cells was caused by a difference in the extent of virus infection, we performed immunostaining of infected embryo cells for influenza nucleoprotein, 6–8 h following virus exposure. This showed similar levels of nucleoprotein in cells from each species confirming that the more rapid cell death in duck cells was not due to higher levels of virus entry or early replication (Figures 1d and f). Again, there was no underlying difference in the extent of influenza nucleoprotein expression, between species, following infection of duck and chicken primary lung cells (Supplementary Figure S3). Similar results were found for all viruses used in this study, confirming that both duck and chicken cells supported virus entry, as intimated by the distribution of appropriate SA receptors on the cell surface.
Duck cells show morphological features of apoptosis following influenza A infection
Owing to the rapid induction of cell death following influenza infection in duck cells, we investigated the potential role of apoptosis as a cause of this phenomenon. Examination of dying cells stained by 4′,6-diamino-2-phenylindole dihydrochloride (DAPI) showed characteristic nuclear condensation and fragmentation, supportive of apoptosis as a mechanism of cell death (Supplementary Figure S4).
We initially quantified the degree of apoptosis by measuring nuclear fragmentation, using propidium iodide (PI) staining of ethanol-fixed cells, followed by flow cytometry to detect hypodiploid cells.16 This showed that in duck lung cells, 20–48 h following infection, there was a significantly greater proportion (P<0.01) of hypodiploid cells, evident as a greater sub-G1 peak, than observed in chicken lung cells, after infection with LPAI H2N3 (Figure 3a), classical swine H1N1 (Figure 3b) or HPAI H5N1 50-92 (Figure 3c). The level of apoptosis following influenza A infection was consistently higher in duck compared with chicken cells, at different time points following infection and at MOIs of 1.0 or 0.1. This assay may not be absolutely specific for apoptosis, as a sub-G1 peak indicative of hypodiploid nuclei may sometimes be observed in necrotic cells or due to chromosomal clumping or diminished staining in differentiating cells.16, 17 However, taken together, the data suggest that more rapid cell death, with features of apoptosis, occurs in duck but not chicken cells, following infection with a range of influenza viruses to which ducks would show minimal or no disease following infection in vivo.
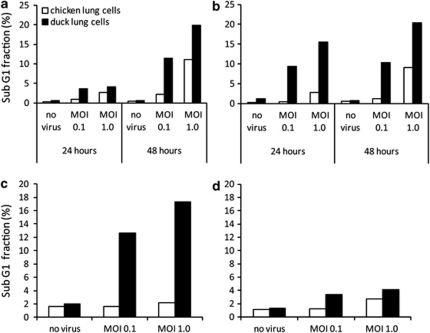
Figure 3.
Duck cells show higher levels of hypodiploid cells than chicken lung cells following influenza A infection. Measurement of cellular levels of DNA fragmentation following influenza A virus infection by propidium iodide staining of ethanol-fixed cells, followed by flow cytometry. Duck lung cells (black bars) and chicken lung cells (white bars) were infected for 24 or 48 h with low pathogenic avian (a) H2N3, (b) classical swine H1N1 or for 20 h with (c) highly pathogenic H5N1 50-92 or (d) H5N1 tyTy05. Analysis of data by three-way analysis of variance for interspecies differences showed a significant difference between species (P<0.01) in the proportion of hypodiploid cells following infection with low pathogenic avian H2N3 (a), classical swine H1N1 (b) or highly pathogenic H5N1 50-92 (c) but not H5N1 tyTy05 (d; P=0.086). Data shown are the mean of duplicate wells.
Contemporary Eurasian H5N1 lineage influenza A does not cause rapid death in duck cells
We next examined the relative effects of infection on chicken and duck cells with a HPAI H5N1 subtype, A/turkey/Turkey/1/2005 (hereafter referred to as H5N1 tyTy05), known to cause severe clinical signs in ducks.18 Significantly, only low levels of hypodiploid cells were detected after infection of duck lung cells at an MOI of 1.0 or 0.1 (less than 5% compared with 12–17% following infection with H5N1 50-92), and there was no significant difference between the levels of hypodiploid cells observed between chicken and duck lung cells following infection at a MOI of 1.0 (Figure 3d). In addition, the characteristic cytopathic effects associated with rapid cell death were not observed in cultures of duck lung cells infected with H5N1 tyTy05, which appeared largely indistinguishable from uninfected cells, 20 h post infection (Supplementary Figure S5). The inverse correlation between the cell response following infection in vitro and the outcome of in vivo infection is quite striking, suggesting that viral factors associated with emergent H5N1 viruses that are pathogenic to ducks are able to subvert the ability of the host cell to initiate cell death.
Caspase activation is associated with rapid cell death in duck cells
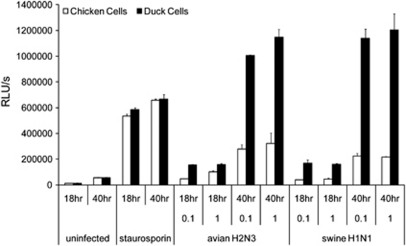
Caspase activation is a key process in both the intrinsic and the extrinsic pathways of apoptosis.19, 20 We measured activation of the effector caspases 3 and 7 in embryo fibroblast cells following infection with H1N1 or H2N3 viruses, using a luminescence assay (Figure 4). Influenza A infection at a MOI of 0.1 or 1.0 led to higher levels of caspase 3/7 activation in duck cells compared with chicken cells (P<0.001). This differential induction of caspase activity in duck cells was greater at 40 h than at 18 h post infection (P<0.001). In both duck and chicken cells, the levels of caspase induction were not significantly increased following infection at an MOI of 1.0 compared with 0.1, with either avian H2N3 or swine H1N1 (P=0.618). Treatment of cells with staurosporin, which induces apoptosis by both caspase-dependent and -independent mechanisms, produced similar levels of caspase 3/7 activation in chicken and duck embryo cells (P=0.074) and rapid cell death in both species. This implies that the differential induction of caspase 3 and 7 in duck compared with chicken cells was an influenza A virus-specific phenomenon. These results suggest that these caspases may be important mediators of the rapid cell death observed in duck cells following influenza A infection.
Figure 4.
Caspase 3/7 induction is greater in duck cells compared with chicken cells following influenza A infection. Chicken (white bars) and duck embryo fibroblasts (black bars) were infected with avian H2N3 or classical swine H1N1 viruses for 18 or 40 h at an MOI of either 1.0 or 0.1. Chemical treatment of cells with staurosporin (4 μ) was used as a positive control. Levels of caspase 3/7 induction were quantified using Caspase-Glo 3/7 Assay (Promega). Data points are the mean of triplicate wells with error bars showing s.d. Analysis of data by three-way analysis of variance showed a significant difference between species (P<0.001) following influenza infection with either virus at both MOI; no significant difference was observed between species following staurosporin treatment (P=0.074). RLU/s, relative light units per second.
Rapid cell death in duck cells is accompanied by a reduced output of infectious virus but not viral RNA
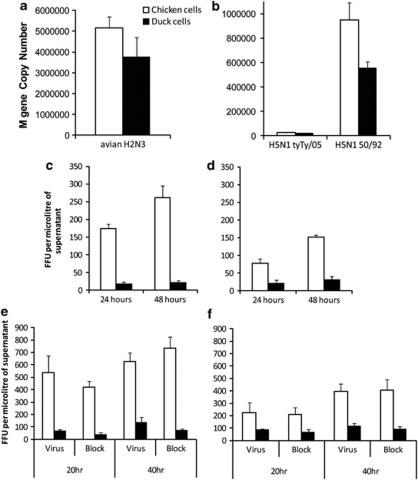
To determine whether the more rapid cell death observed in duck cells was associated with a decreased viral output from infected cells, we measured matrix (M) gene copy number using quantitative real-time PCR 21, 22 on culture supernatants collected following influenza infection. Virus output from infected cell cultures, between 20–48 h following infection, was comparable between chicken and duck lung cells (Figures 5a and b). Although differences in viral RNA production were occasionally observed, in the course of repeated experiments with lung and embryo cells, using different viruses, these were not consistently higher for either species, and it seems unlikely that the relatively small differences seen were biologically significant. For HPAI infection of cells, levels of viral H5 RNA were also measured by real-time PCR,23 with gene expression levels correlating with those for M gene expression (data not shown).
Figure 5.
Replication of influenza A in duck and chicken cell cultures. Chicken cells (white bars) and duck cells (black bars) were infected with a range of viruses for 20–48 h and virus production measured by real-time PCR and titration of virus in culture supernatants. (a) Duck and chicken lung cells infected with LPAI H2N3 at an MOI of 1.0 for 24 h and virus output measured by detection of viral M gene RNA in supernatant by quantitative reverse transcription PCR. (b) Duck and chicken embryo cells infected with HPAI H5N1 50-92 or H5N1 tyTy05 for 20 h at an MOI of 1.0 and virus production quantified by viral M gene RNA PCR. Significant difference in M gene copy number between species following H5N1 50-92 (P<0.05) but not H2N3 infection or H5N1 tyTy05 infection. (c, d) Infectious virus in supernatants from duck and chicken lung cells following infection for 24 or 48 h, with (c) LPAI avian H2N3 and (d) classical swine H1N1 measured by titration on Madin-Darby canine kidney cells. (e, f) Levels of infectious virus in supernatants following infection of chicken embryo cells and duck embryo cells at an MOI of 1.0 for 20 or 40 h with (e) HPAI H5N1 50-92 or (f) H5N1 tyTy05. Pretreatment with a pan-caspase inhibitor Q-VD-OPh (10 μ) before HPAI H5N1 infections (Block). Analysis of data by three-way analysis of variance, for interspecies differences in the production of infectious virus, showed significant differences (P<0.001) following influenza infection with H5N1 50-92 or tyTy05. No significant difference on the level of infectious virus production was observed following treatment with Q-VD-OPh (P>0.05). Data show the mean of triplicate wells with error bars showing s.d. FFU, focus-forming units.
To determine the level of infectious virus produced following infection, we titrated the viral supernatants on Madin-Darby canine kidney cells. Interestingly, there was a significant decrease in the number of infectious virions produced following infection of duck lung cells compared with chicken lung cells (Figures 5c–f). When infected with avian H2N3, chicken cells produced between 10- to 12-fold more infectious virus, 24–48 h post infection, than duck cells. Following infection with swine H1N1, chicken cells produced three- to five-fold more infectious virus than duck cells (Figure 5d). With H5N1 50-92 infection, chicken cells produced about four- to eightfold more infectious virus, 20–40 h post infection, than duck cells (Figure 5e). Following H5N1 tyTy05 infection, the interspecies difference in infectious virus produced was less (approximately two- to fourfold more virus produced from chicken cells; Figure 5f). This data suggests that the rapid induction of cell death in duck cells following influenza A infection may lead to containment of viral replication, although replication of viral RNA is unaffected. To investigate whether caspase blockade would lead to enhanced production of infectious virus from duck cells, we pre-treated cells with Q-VD-OPh, a pan-caspase inhibitor, which we determined effectively blocked caspases in avian cells (confirmed by assaying cells for caspase 3/7 after treatment with Q-VD-OPh). However, virus levels in the supernatants of infected cells pre-treated with Q-VD-OPh were not significantly different from untreated cells (Figures 5e and f). However, although pre-treatment of duck cells with a caspase inhibitor did reduce cell death following influenza infection, it did not reduce levels to that of uninfected cells; for example, 24 h after H5N1 50-92 infection, a reduction in hypodiploid cells from 11 to 7% was observed, compared with 2% for uninfected cells (data not shown).
Cells derived from mallard ducks also exhibit rapid cell death following influenza A infection
Aquatic birds are the major reservoir for influenza A, with wild ducks harbouring the majority of virus subtypes and typically showing very few clinical signs.2, 10 We therefore investigated whether cells derived from mallard embryos showed similar levels of rapid cell death in vitro in comparison with Pekin duck embryo cells. In fact, the level of hypodiploid cells, measured using PI staining of ethanol-fixed cells, was much greater in mallard (34%) than observed for either duck (10.5%) or chicken (1.3%) embryo cells following infection with H1N1 at an MOI of 1.0 (Supplementary Figure S6). This was supported by the morphological appearance of the cells, with mallard cells showing more severe cytopathic effects than Pekin duck cells.
Discussion
In conclusion, contrary to our previous expectations, we found that influenza infection elicited more rapid and greater levels of cell death in primary cells derived from inherently resistant Pekin or mallard ducks than cells from susceptible White Leghorn chickens. This cell death was mediated, at least in part, by apoptosis, as evident by clearly elevated levels of nuclear condensation and fragmentation, and caspase activation in duck cells. We further demonstrated that this accelerated cell death in infected duck cells was accompanied by reduced output of infectious virus relative to chicken cells. Significantly, in the exceptional situation where a HPAI subtype causes lethal infection in juvenile ducks, as in the case of H5N1 tyTy05,18 duck cells infected by this subtype did not display accelerated cell death but appeared deceptively healthy, much like infected chicken cells. It could be relevant to note that ducks infected with virulent subtypes of H5N1 viruses often display neurological signs before death and, at postmortem, together with other vital organs, pathological lesions and virus infiltrates are found in the brain and heart,18, 24 two organs in which apoptosis and subsequent regeneration may occur less readily, without detriment to the host. The potential for apoptosis to cause such tissue damage has been recognised for other viral infections of the heart and nervous system, where the degree of apoptosis and cellular destruction are correlated with disease severity.25
There are a number of potential benefits to the avian host in containing virus replication by rapid induction of cell death following infection of target cells. We have demonstrated that this phenomenon is associated with reduced production of infectious virus in vitro in resistant duck cells, but no alteration in viral RNA released from infected cells, suggesting that virus assembly or processing is affected rather than replication of the viral genome. Should this phenomenon occur in vivo, it could lead to early containment of infection with reduced spread of virus to secondary sites of infection, such as the heart or brain. Indeed, following non-lethal H5N1 infection, virus dissemination is less widespread, and lower viral antigen levels are present in duck organs in comparison with chickens.24
It is well established that infection with many types of virus, including influenza A, can cause apoptosis of infected cells, both in vitro26, 27 and in vivo.28 However, the role of apoptosis in influenza A infection is complex. Apoptosis appears to be necessary for efficient virus replication; for example, blocking caspase 3 activation29 or reducing Bax, directly via gene knockout30 or via overexpression of Bcl-2,31 causes diminished virus production. Activation of apoptosis is specifically required for the release of viral ribonucleoprotein from the nucleus29 and for correct processing of the viral haemagglutinin.31 However, influenza viruses appear to prevent early apoptotic cell death by upregulation of PI3K-Akt signalling, mediated, at least in part, by the viral NS1 protein.32, 33 Although some studies have shown that p53 is essential for the induction of cell death in influenza infected cells,34 others have shown that it may not be required.32 Intriguingly, Shen et al.35 demonstrated that p53 accumulates in a biphasic pattern following influenza infection. It seems plausible that early apoptosis, associated with accumulation of p53, may be a protective response favoured by host cells, but is blocked by interaction of influenza A with the cell, whereas later accumulation, leading to cell death, favours virus replication. This argument is strengthened by the observation that early induction of cell death by p53 appears to limit viral titres produced by infected cells.34 The sum of this evidence suggests that the rapid induction of apoptosis may be beneficial to the host, whereas delayed apoptosis may be advantageous to the virus. As a consequence, influenza A has evolved a number of mechanisms to delay apoptosis in infected cells.
We have shown that caspase activation and nuclear fragmentation are increased during rapid death of infected duck cells. However, although caspase blockade did reduce cell death in duck cells infected with influenza A, it did not lead to increased production of infectious virus from duck cells infected with H5N1, suggesting that other pathways, for example, mitochondrial outer membrane permeabilisation, may also be responsible for cell death observed and the associated decrease in virus production from infected duck cells.36 Alternatively, it is possible that increased cell death in influenza-infected duck cells, although correlated to reductions in viral titres, may not be mechanistically responsible. Further work is therefore required to dissect the molecular pathways in avian cells following influenza infection and to determine the mechanism for induction of apoptosis, and the relative roles of intrinsic or extrinsic pathways. Alternative pathways of ‘programmed cell death' are autophagy, oncosis and pyroptosis.37 It is possible that some of the cell death observed in this study may have been caused by such alternate pathways; however, although DNA cleavage may be observed with both apoptosis and pyroptosis, nuclear fragmentation and caspase 3 induction are hallmarks of apoptosis but not pyroptosis. In contrast to apoptosis, pyroptosis is associated with cleavage of caspase 1, cell swelling and lysis.37 It is conceivable that in addition to the different kinetics of cell death between avian hosts, the mechanism of influenza virus-induced cell death may also differ and, as a consequence, directly influence host immune and inflammatory responses, and the degree of tissue damage observed in vivo. Apoptosis is associated with a minimal inflammatory responses, whereas pyroptosis is inherently inflammatory.37 Human studies and animal models have implicated the induction of an aberrant pro-inflammatory cytokine response, or so called cytokine storm, as a potential cause of severe disease in mammals.38, 39 Although the pathophysiology of influenza infection in avian species is less well understood, it has recently been shown that pro-inflammatory cytokine expression is upregulated in the lungs of chickens infected with HPAI H5N1, accompanied by high levels of virus replication.40 Studies in mice support the role of apoptosis followed by removal of apoptotic bodies by phagocytosis as a crucial mechanism for host survival following influenza A infection; inhibition of phagocytosis leads to increased host mortality.41 We propose that the rapid induction of cell death in ducks mediated, at least in part, by apoptosis accounts for the natural resistance of such hosts to influenza A infection. In contrast, the lack of such a response in highly susceptible chickens is associated with the development a pro-inflammatory cytokine response and more fulminant disease. Similarly, the loss of a rapid host cell-death response in duck cells, following infection with emergent strains of HPAI H5N1 such as Eurasian lineage H5N1 clade 2.2 (tyTy05), is also associated with a more severe clinical outcome. Recent work by Ueda et al.36 supports our observation of rapid apoptosis in duck cells following influenza A infection, with an H5N1 non-lethal to ducks, Cw/Kyoto causing increased cell death compared with LPAI viruses, essentially as we have observed for H5N1 50-92. There is some difference in their findings with a clade 2.2 H5N1, where they observed increased cell death in duck cells post infection, in contrast to the reduced cell death in cells infected with clade 2.2 H5N1 tyTy05. This difference may have been due to differences in the MOI used, origin of cells, virus-specific characteristics or other differences in methodology; however, there is a clear need to further characterise the mechanisms leading to rapid death in duck cells and the virus factors that are responsible. The recent observation of differences in the RIG-I signalling pathway between ducks and chickens42 suggests that this may also have a role in the resistance of ducks to influenza; the relative importance and degree of complementarity or interaction between these mechanisms should be determined.
To conclude, the data from our in vitro studies correlates entirely with the observed pathogenesis in relevant hosts arising from natural infection with the same viruses. This offers an insight into a potential mechanism that might account for the long-held observation that aquatic birds are inherently more resistant to influenza A infection than terrestrial poultry. Further experiments using resistant and susceptible avian hosts should help to determine the relative importance of this mechanism in vivo.
Methods
Viruses
A LPAI H2N3 (A/mallard duck/England/7277/06), a classical swine H1N1 (A/sw/Iowa/15/30), and two highly pathogenic avian H5N1 viruses (A/turkey/England/50-92/91 and A/turkey/Turkey/1/05) were used in this study. The former derives from the ‘classical' Eurasian lineage of H5 viruses and causes non-lethal infection in ducks. In contrast, the latter strain is a contemporary Eurasian H5N1 virus (clade 2.2) associated with the global panzootic since 2003. Some of these viruses can cause severe disease with mortality in ducks. All viruses were grown by allantoic inoculation of 10-day-old embryonated chicken eggs.
Virus titration
Serial 10-fold dilutions of a known volume of virus or culture supernatant were used to infect Madin-Darby canine kidney cells in 24-well cell culture plates (Corning Life Sciences, Amsterdam, Netherlands). Cells were washed after a 2-h incubation with virus, followed by a further 4-h incubation and then fixed with 1:1 acetone:methanol. Cells were subjected to viral nucleoprotein detection by a primary mouse monoclonal antibody (Abcam, Cambridge, UK) followed by visualization with Envision+system-HRP (DAB; Dako, Ely, UK). Cells expressing viral nucleoprotein were counted and the mean number of positive cells in five fields used to calculate focus-forming units of virus per microlitre of inoculum.
Cell cultures
Primary cell cultures were obtained from lungs of 4-week-old broiler chickens and 6-week-old Pekin ducks. The lungs from euthanased birds were aseptically collected into Dulbecco's modified Eagle's medium (DMEM, Invitrogen Ltd., Paisley, UK) with antibiotics and washed three times in phosphate-buffered saline (PBS) to remove red blood cells. The lungs were then sliced into fine pieces with a scalpel blade and digested at 4 °C overnight in dissociation medium containing equal volumes of DMEM and Ham's F12 (Invitrogen Ltd.) supplemented with pronase (1.4 mg ml−1; Sigma-Aldrich Ltd., Poole, UK) and antibiotics. Large undigested tissue pieces were removed using a cell strainer and the cells were plated onto collagen-coated cell culture flasks (Corning Life Sciences) in DMEM and Ham's F12 (1:1) supplemented with 2% chicken embryo extract (Biosera, Ringer, UK), 5% fetal calf serum, 1% insulin-transferrin selenium (Invitrogen) and antibiotics.
Fibroblasts were extracted from White Leghorn chicken and Pekin (or mallard) duck embryos after 8- or 10.5-day incubation, respectively. Embryos were digested in 0.25% trypsin in dissociation medium at 37 °C for 1 h. Cells were plated into cell culture flasks (Corning Life Sciences) and maintained in DMEM with 10% fetal calf serum and antibiotics.
Madin-Darby canine kidney cells were grown in DMEM supplemented with 10% fetal calf serum and antibiotics.
Influenza receptor expression on cultured cells
Influenza receptor expression on primary cells was determined by a method previously described,13 using Sambucus nigra agglutinin lectin specific for ‘mammalian type' sialic acid α2,6-galactose (SAα2,6-Gal)-linked receptors, and Maackia amurensis agglutinin (MAA II) lectin specific for α2,3-galactose (SAα2,3-Gal)-linked ‘avian type' receptors. Briefly, cells were grown on cover slips in 24-well culture plates and fixed with 4% paraformaldehyde. After blocking endogenous biotin with the use of a streptavidin blocking kit (Vector Laboratories, Peterborough, UK), the cells were incubated overnight at 4 °C in the dark with fluorescein isothiocyanate-labelled S. nigra agglutinin lectin, and biotinylated M. amurensis agglutinin (MAA II) lectin (Vector Laboratories), each at a concentration of 10 μg ml−1. After three washes with Tris-buffered saline, the cells were incubated with 1:500 dilution of streptavidin-AlexaFluor594 conjugate (Invitrogen Ltd.) in Tris-buffered saline for 2 h at room temperature. The cells were washed three times with Tris-buffered saline and then mounted with ProLong Gold antifade reagent with DAPI (Invitrogen Ltd.). Negative controls were processed without the primary reagents and the cells were imaged using a fluorescent microscope (Leica Microsystems Ltd., Milton Keynes, UK).
Virus infection of cells
Monolayers of primary cells in 12- or 24-well plates were infected with avian or swine influenza viruses at MOI of 0.1 or 1.0. Cells were rinsed with PBS and infected with virus in serum-free infection medium comprising 2% Ultroser G (Pall Biosepra, Portsmouth, UK), 500 ng ml−1 TPCK Trypsin (Sigma-Aldrich Ltd.) and antibiotics in Ham's F12. After 2-h incubation, infection medium was removed, the cells were washed three times with PBS and fresh medium was added. Cells were further incubated for up to 48 h. Cells for immunostaining were fixed in 1:1 acetone:methanol for 10–15 min. Cells for PI flow cytometry assays were trypsinized and washed twice in PBS with glucose (1 mg ml−1) before fixing in 70% ethanol. To assess the effect of caspase blockade, 10 μ of the pan-caspase inhibitor Q-VD-OPh (Cambridge Bioscience Ltd., Cambridge, UK) was added to cells 1 h before virus infection, and replenished following washing at 2 h post infection.
Measurement of cell metabolic activity (MTT Assay)
Measurement of metabolic activity of primary cells following influenza virus infection was carried out by MTT assay. Primary chicken and duck lung cells were grown in 96-well culture plates (approximately 5000 cells per well) and infected with influenza viruses at a range of MOI from 0.15 to 2.5. The cells were analyzed for metabolic activity at 24 h post infection using CellTiter 96, a non-radioactive cell proliferation assay (Promega, Madison, WI, USA) according to manufacturer's instructions.
Propidium iodide flow cytometry assay
A PI flow cytometry assay to measure hypodiploid cells was carried using a previously described method16. Ethanol-fixed cells were vortexed briefly and centrifuged at high speed (∼3000 r.p.m.) for 5 min. The cell pellet was resuspended before staining in 1 ml of propidium iodide staining solution containing 50 μg of propidium iodide, 100 kunitz units of ribonuclease A (Sigma) and 1 mg glucose in 1 ml of PBS. Cells were then incubated at room temperature on a rocking platform for 30–90 min in the dark and analyzed by flow cytometer using a BD FACS CANTO II (BD Biosciences, Oxford, UK).
Caspase 3/7 assay
Levels of activated caspase 3 and 7 in primary cells following influenza virus infection were quantified using Caspase-Glo 3/7 Assay (Promega), according to manufacturer's instructions. Staurosporin (Sigma-Aldrich Ltd.) was used as a positive control at a concentration of 4 μ.
Quantification of virus production using quantitative real-time reverse transcription PCR
Viral RNA from culture supernatants was extracted using Hipure viral RNA kit (Roche Diagnostics Ltd., Burgess Hill, UK) or QIAamp Viral RNA Mini Kit (Qiagen, Crawley, UK). A one-step reverse transcription PCR assay using influenza virus matrix gene-specific PCR primers and hydrolysis probe was performed as previously described.21, 22 Threshold cycle (Ct) values were converted to viral gene copy number by a standard curve generated using in vitro-transcribed viral RNA.
Statistical analysis
Data derived from PI, caspase and viral quantification assays were analyzed using SigmaStat software (Version 3.5, Systat Software, Richmond, CA, USA). Data were compared between groups using a two- or three-way analysis of variance, as appropriate, followed by pairwise comparisons using the Holm-Sidak method.
Acknowledgments
We acknowledge the support of the many staff at the VLA who have helped during our work in containment, and Dr Karen Blyth, University of Glasgow, for helpful discussions and assistance in assay development. SVK was funded by a National Overseas Scholarship from the Government of India and is grateful to S.V. Veterinary University, Tirupati, India for allowing him study leave.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- World Health Organisation Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO2010
- Alexander DJ, Brown IH. History of highly pathogenic avian influenza. Rev Sci Tech. 2009;28:19–38. doi: 10.20506/rst.28.1.1856. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Parsons G, Manvell RJ. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol. 1986;15:647–662. doi: 10.1080/03079458608436328. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Allan WH, Parsons DG, Parsons G. The pathogenicity of four avian influenza viruses for fowls, turkeys and ducks. Res Vet Sci. 1978;24:242–247. [PubMed] [Google Scholar]
- Kim JK, Negovetich NJ, Forrest HL, Webster RG. Ducks: the ‘Trojan horses' of H5N1 influenza. Influenza Other Respi Viruses. 2009;3:121–128. doi: 10.1111/j.1750-2659.2009.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA. 2004;101:4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux P, Cheriyan M, Hurtado-Ziola N, van der Linden EC, Anderson D, McClure H, et al. Human-specific regulation of alpha 2-6-linked sialic acids. J Biol Chem. 2003;278:48245–48250. doi: 10.1074/jbc.M309813200. [DOI] [PubMed] [Google Scholar]
- Kuchipudi SV, Nelli R, White GA, Bain M, Chang KC, Dunham S. Differences in influenza virus receptors in chickens and ducks: Implications for interspecies transmission. J Mol Genet Med. 2009;3:143–151. doi: 10.4172/1747-0862.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GW, Parsons G, Alexander DJ. Replication of influenza A viruses of high and low pathogenicity for chickens at different sites in chickens and ducks following intranasal inoculation. Avian Pathol. 1995;24:545–551. doi: 10.1080/03079459508419093. [DOI] [PubMed] [Google Scholar]
- Kida H, Yanagawa R, Matsuoka Y. Duck influenza lacking evidence of disease signs and immune response. Infect Immun. 1980;30:547–553. doi: 10.1128/iai.30.2.547-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Bedner E, Smolewski P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001;38:179–193. doi: 10.1016/s0037-1963(01)90051-4. [DOI] [PubMed] [Google Scholar]
- Londt BZ, Nunez A, Banks J, Nili H, Johnson LK, Alexander DJ. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathol. 2008;37:619–627. doi: 10.1080/03079450802499126. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Slomka MJ, Pavlidis T, Coward VJ, Voermans J, Koch G, Hanna A, et al. Validated RealTime reverse transcriptase PCR methods for the diagnosis and pathotyping of Eurasian H7 avian influenza viruses. Influenza Other Respi Viruses. 2009;3:151–164. doi: 10.1111/j.1750-2659.2009.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka MJ, Pavlidis T, Banks J, Shell W, McNally A, Essen S, et al. Validated H5 Eurasian real-time reverse transcriptase-polymerase chain reaction and its application in H5N1 outbreaks in 2005–2006. Avian Dis. 2007;51:373–377. doi: 10.1637/7664-060906R1.1. [DOI] [PubMed] [Google Scholar]
- Antarasena C, Sirimujalin R, Prommuang P, Blacksell SD, Promkuntod N, Prommuang P. Tissue tropism of a Thailand strain of high-pathogenicity avian influenza virus (H5N1) in tissues of naturally infected native chickens (Gallus gallus), Japanese quail (Coturnix coturnix japonica) and ducks (Anas spp.) Avian Pathol. 2006;35:250–253. doi: 10.1080/03079450600714510. [DOI] [PubMed] [Google Scholar]
- Clarke P, Tyler KL. Apoptosis in animal models of virus-induced disease. Nat Rev Microbiol. 2009;7:144–155. doi: 10.1038/nrmicro2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw VS, Olsen CW, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74 (Part 11:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76 (Part 11:2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- Wurzer WJ, Planz O, Ehrhardt C, Giner M, Silberzahn T, Pleschka S, et al. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22:2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JE, Datan E, Matassov D, Zakeri ZF. Lack of Bax prevents influenza A virus-induced apoptosis and causes diminished viral replication. J Virol. 2009;83:8233–8246. doi: 10.1128/JVI.02672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CW, Kehren JC, Dybdahl-Sissoko NR, Hinshaw VS. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol. 1996;70:663–666. doi: 10.1128/jvi.70.1.663-666.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhirnov OP, Klenk HD. Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling. Apoptosis. 2007;12:1419–1432. doi: 10.1007/s10495-007-0071-y. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, et al. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol. 2007;81:3058–3067. doi: 10.1128/JVI.02082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin E, Luke K, Jones J, Tumpey T, Konan K, Schultz-Cherry S. Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J Virol. 2005;79:8802–8811. doi: 10.1128/JVI.79.14.8802-8811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wang X, Guo L, Qiu Y, Li X, Yu H, et al. Influenza A virus induces p53 accumulation in a biphasic pattern. Biochem Biophys Res Commun. 2009;382:331–335. doi: 10.1016/j.bbrc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Ueda M, Daidoji T, Du A, Yang CS, Ibrahim MS, Ikuta K, et al. Highly pathogenic H5N1 avian influenza virus induces extracellular Ca2+ influx, leading to apoptosis in avian cells. J Virol. 2010;84:3068–3078. doi: 10.1128/JVI.01923-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172:1155–1170. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okada H, Itoh T, Tada T, Mase M, Nakamura K, et al. Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses. J Virol. 2009;83:7475–7486. doi: 10.1128/JVI.01434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- Barber MR, Aldridge JR, Jr, Webster RG, Magor KE. Association of RIG-I with innate immunity of ducks to influenza. Proc Natl Acad Sci USA. 2010;107:5913–5918. doi: 10.1073/pnas.1001755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.