Abstract
Besides locomotion, organ protection, and calcium–phosphorus homeostasis, the three classical functions of the skeleton, bone remodeling affects energy metabolism through uncarboxylated osteocalcin, a recently discovered hormone secreted by osteoblasts. This review traces how energy metabolism affects osteoblasts through the central control of bone mass involving leptin, serotoninergic neurons, the hypothalamus, and the sympathetic nervous system. Next, the role of osteocalcin (insulin secretion, insulin sensitivity, and pancreas β-cell proliferation) in the regulation of energy metabolism is described. Then, the connections between insulin signaling on osteoblasts and the release of uncarboxylated osteocalcin during osteoclast bone resorption through osteoprotegerin are reported. Finally, the understanding of this new bone endocrinology will provide some insights into bone, kidney, and energy metabolism in patients with chronic kidney disease.
Keywords: blood glucose, bone, energy metabolism, fibroblast growth factor-23, leptin, serotonin, osteocalcin
The three classical functions of bone are locomotion, organ protection such as the skull, and calcium–phosphorus homeostasis. Locomotion has been critical for survival throughout evolution. Indeed, maintaining an excellent bone quality with good biomechanical properties is essential for locomotion and fracture prevention. To achieve this goal, bone tissue is constantly renewing itself through a physiological process called ‘bone remodeling', which repairs microdamage and participates in fracture healing. Bone remodeling involves specialized cells, starting with osteoclasts, which resorb preexisting bone. The osteoclasts then leave the lacunae, and osteoblasts deposit bone matrix that is secondarily mineralized to fill the lacunae. In healthy adults, bone remodeling is well balanced and can be seen as a true homeostatic function. With aging, major physiological functions including bone maintenance are altered. Osteoporosis, a major age-related disease, is characterized by unbalanced bone remodeling, low bone mass, and altered bone architecture, leading to increased susceptibility to fractures, reduced mobility, and altered quality of life. In chronic kidney diseases (CKD), bone health is also a major issue.1, 2 CKD-related mineral and bone disorder has been defined as the systemic disorder of mineral and bone metabolism because of CKD manifested by one or more of the following: (1) abnormalities of calcium, phosphorus, parathyroid hormone, or vitamin D metabolism; (2) abnormalities in bone turnover, mineralization, volume, linear growth, or strength; (3) and vascular or other soft tissue calcifications.1
Bone remodeling is an active process that requires a large energy input. This led to the hypothesis of Karsenty et al.3 that states that energy metabolism may regulate bone remodeling. This hypothesis was supported by the following clinical facts: obesity protects from osteoporosis, low body mass index increases fracture risk, and osteoporosis develops in patients with hypogonadism (for example, postmenopausal women, senior men, chemical castration). These observations suggest that appetite, reproduction, and bone might be regulated by a common hormonal system. From an endocrine viewpoint, if energy metabolism is able to regulate bone remodeling, there must be a feedback loop, and bone remodeling should affect energy metabolism. In the last few years, our understanding of these processes has developed greatly. This article will trace the course of development of our understanding of this new bone endocrinology.
ENERGY METABOLISM REGULATES BONE MASS: THE ROLES OF LEPTIN, SEROTONIN, AND SYMPATHETIC TONE
Association of leptin with bone mass
Leptin was the first focus of research into the link between energy metabolism and bone mass for several reasons: (1) leptin is an adipocyte hormone that regulates appetite (through inducing satiety and increasing energy expenditure) and reproduction through its receptor expressed in the hypothalamus and brainstem;4, 5 (2) leptin and bone remodeling emerged at the same time in evolution;6 and (3) leptin-deficient mice (ob/ob mice) have been observed to be obese and sterile. The known hypothalamic mediation of leptin's regulation of appetite and reproduction is of interest as most homeostatic functions are hypothalamically regulated, and bone remodeling may be considered as a homeostatic function. Analyses of bone from ob/ob mice showed that they have an increased bone mass.7 The bone mass phenotype of ob/ob mice can be rescued by intracerebroventricular (ICV) infusion of leptin, suggesting that leptin exerts an indirect influence on bone mass.7
Mechanism of leptin's influence on bone mass
Further investigations have explored the pathway of leptin's indirect control of bone mass. Clinically, human reflex sympathetic dystrophy is characterized by a rapid onset of osteoporosis in the affected region with labile vasomotor activity, trophic skin changes, pain, and swelling, because of dysregulated sympathetic tone. In some cases, β-blockers such as propranolol resolve reflex sympathetic dystrophy-associated symptoms and osteopenia.8 Outside the context of reflex sympathetic dystrophy, people receiving β-blockers experience 24–32% reductions in the risk of fractures, as shown in several large studies (Table 1).9, 10, 11, 12 Interestingly, leptin-deficient mice show a low sympathetic tone. Taken together, these observations suggest that leptin may exert its bone control through a neuronal pathway involving the sympathetic nervous system. This hypothesis has been tested in a number of in vivo studies.
Table 1. Effect of β-blockers on fracture risk12.
| Study | Study design | Subjects | Fracture type | Hazard or odds ratio (95% CI) | Reference |
|---|---|---|---|---|---|
| SOF | Prospective cohort | 8412 (13.1% users) | Hip | 0.76 (0.58–0.99) | Reid et al.10 |
| GPRD | Retrospective case–control | 120,819 controls (17.6% users), 30,601 cases (16% users) | Any | 0.77 (0.72–0.83) | Schlienger et al.11 |
| Hip | 0.68 (0.52–0.89) | ||||
| Geelong Osteoporosis Study | Population-based case–control | 775 controls (14.5% users), 569 cases (10.4% users) | Any | 0.69 (0.49–0.96) | Pasco et al.9 |
Abbreviations: CI, confidence interval; GPRD, General Practice Research Database; SOF, Study of Osteoporotic Fractures.
The first key experiment was a parabiosis experiment in which one of two ob/ob mice with a surgically established common blood circulation received ICV leptin. Bone analysis of both animals showed that bone volume was corrected in the ICV leptin-treated mouse, but not in the related partner, suggesting that leptin's control of bone is exerted in a neuronal rather than an endocrine manner.13 Moreover, the treatment of ob/ob mice with isoproterenol, a β-agonist, does not affect appetite and body weight but does correct bone volume to normal levels, suggesting that leptin's effect on bone may involve β-adrenergic stimulation.13
Further leptin ICV experiments in ob/ob mice, using chemical ablation of specific nuclei in the hypothalamus, established that leptin separately inhibits appetite through the arcuate nucleus and bone mass through the ventromedial hypothalamus (VMH) nucleus. These experiments indicate that hypothalamic integrity is required in bone regulation. However, specific deletion of the hypothalamic leptin receptor (ObRb) does not inhibit ICV leptin correction,14 suggesting that leptin first acts in a different brain region to affect a neuromediator that signals secondarily to hypothalamic neurons to modulate bone mass and appetite.
Serotonin as a neuromediator for leptin activity
Serotonin was identified as a candidate neuromediator for leptin activity on the basis of the clinical observation that antiserotoninergic antidepressant drugs such as selective serotonin reuptake inhibitors increase patients' appetite and osteoporotic fracture risk,15 supporting a role for serotonin in appetite regulation and control of bone mass.16 Serotonin is distributed throughout the body: 95% circulating in blood and 5% in the brain as a neuromediator. Serotonin does not cross the blood–brain barrier.17, 18 In the brain, serotonin is formed from tryptophan by the brain-specific enzyme tryptophan hydroxylase 2 (Tph2), which is highly expressed in the brainstem but not in the hypothalamus. Tph2-null mutant mice with brain serotonin deficiency have high sympathetic tone and low bone mass because of high bone resorption and low bone formation.16
Neuronal connection between leptin and serotonin
Further experiments have explored neuronal connections between leptin and serotonin. Serotoninergic neurons (expressing Tph2) and leptin receptors are both located in the brainstem, but until recently it was not clear whether these serotoninergic neurons project on hypothalamic neurons (VMH) to regulate bone mass. Yadav et al.16 examined this aspect in four experiments. The first one used mice expressing a fluorescent protein in the axons of brainstem Tph2+ neurons through a Cre recombinase-mediated technology to show that these axons project to the VMH nucleus. The second experiment demonstrated histologically by anterograde and retrograde rhodamine dextran axon tracing that VMH neurons receive neuronal projections from Tph2 neurons in the brainstem. The third experiment demonstrated that VMH neurons express serotonin receptor (Htr2c) and that brainstem serotonin neurons express leptin receptor (ObRb). In the fourth experiment, Htr2c–/–-deficient mice have a low bone mass phenotype that is recapitulated by the double knockout strain (Htr2c+/–, Tph2+/–), genetically establishing the action of serotonin on bone mass in the hypothalamus.
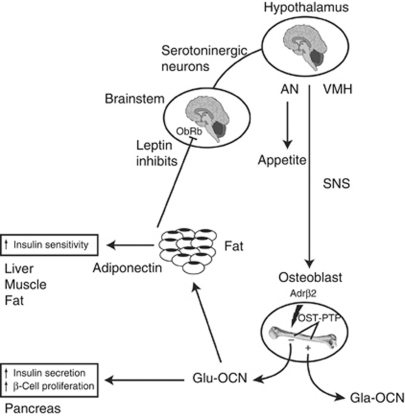
These experiments demonstrate that serotoninergic brainstem neurons (Tph2+) project axons to VMH neurons expressing serotonin receptor (Htr2c) and that serotonin is the signal link between the brainstem, where leptin acts on its receptor (ObRb), and the VMH nucleus in the hypothalamus. Taken together, these studies show that leptin inhibits bone formation through a serotonin central relay (brainstem to hypothalamus) and through hypothalamus-generated sympathetic tone (Figure 1). These key studies showed that fat (energy metabolism) controls bone mass, but did not address whether bone exerts a reciprocal influence on energy metabolism.
Figure 1.
Interactions between bone and energy metabolism. Leptin inhibits bone formation through a serotonin central relay (brainstem to hypothalamus) and through hypothalamus-generated sympathetic tone. In turn, osteoblasts secrete osteocalcin, a hormone regulating energy metabolism, through its stimulation on insulin secretion and sensitivity. Adrβ2, β2-adrenergic receptor; AN, arcuate nucleus; Gla-OCN, carboxylated osteocalcin; Glu-OCN, uncarboxylated osteocalcin; ObRb, leptin receptor; OST-PTP, osteotesticular protein tyrosine phosphatase; SNS, sympathetic nervous system; VMH, ventromedial hypothalamic nucleus. Adapted from Confavreux.22
THE ROLE OF OSTEOCALCIN IN REGULATION OF ENERGY METABOLISM BY THE SKELETON
Esp, an osteoblast-specific gene with a metabolic phenotype
Researchers have examined the role of bone in energy metabolism using a three-step research strategy: identification of osteoblast-specific genes; generation of global and osteoblast-specific knockout mutant mice for these genes; and finally studies of their energy metabolism phenotype.
The candidate gene Esp encodes osteotesticular protein tyrosine phosphatase, expressed in embryonic stem cells, Sertoli cells, and osteoblasts, but not in adipose tissue or pancreas. Its function was initially unknown in mice and humans. Esp-deficient mice (Esp–/–) have early postnatal lethality, which is not because of a skeletal defect but because of low blood glucose at birth. Esp–/– mice have lifelong low blood glucose and an improved glucose tolerance compared with wild-type mice.6 Interestingly, these characteristics reflect hyperinsulinemia, resulting from increased insulin secretion, increased size and number of pancreatic β-cells, and improved insulin sensitivity, with reduced visceral fat and increased adiponectin. Conversely, overexpressing Esp, specifically in osteoblasts, impairs glucose handling and induces a type 2 diabetes-like phenotype with reduced glucose tolerance because of reduced insulin secretion and increased peripheral insulin resistance. Thus, osteoblastic expression of Esp regulates pancreatic insulin production and adipose insulin sensitivity.6 These findings suggested that an osteoblast-specific tyrosine phosphatase deletion in mice improves insulin handling and reduces fat mass. However, osteotesticular protein tyrosine phosphatase is intracytoplasmic and therefore cannot act directly on distant cells to regulate energy metabolism. Therefore, the next question was to uncover which specific hormone is secreted by osteoblasts and acts on adipocytes and pancreatic β-cells.
Osteocalcin: a bone hormone regulating energy metabolism
Co-cultures of osteoblasts with adipocytes or osteoblasts with β-cells showed that an osteoblast-secreted factor regulates insulin and adiponectin expression. This factor, osteocalcin, shares several properties with other hormones: secretion, circulation in blood, and regulation. Indeed, osteocalcin is present in all vertebrates, is secreted by osteoblasts into the bloodstream and bone matrix, is specific to osteoblasts, and exists in carboxylated and uncarboxylated forms, suggesting a regulatory mechanism. Its function has been unknown until recently. However, we know that osteocalcin-deficient mice have a moderate late bone phenotype but an early development of obesity, even on a regular diet. The osteocalcin gene is located in a genomic region that confers risk of diabetes. Furthermore, the metabolic phenotype of osteocalcin-deficient mice is a mirror image of that of Esp-deficient mice. Osteocalcin-deficient mice have high blood glucose, hypoinsulinemia, few pancreatic β-cells, and reduced insulin sensitivity.6 In addition, treating wild-type mice with continuously infused uncarboxylated osteocalcin increases insulin secretion, improves glucose tolerance, and in higher doses also decreases fat mass.19
Osteocalcin has been shown to act partly through adiponectin, which is secreted by adipocytes in response to osteocalcin, promotes insulin sensitivity, and is reduced in obese or diabetic persons. Skeletally, adiponectin is correlated with decreasing bone mineral density and independently predicts low bone mass.5
In humans, epidemiological studies, such as the MrOS (Osteoporotic Fractures in Men) Sweden study in Swedish men20 or the First Nations Bone Health Study in Canadian women of aboriginal and European heritage,21 have found that osteocalcin levels are negatively correlated with fasting blood glucose in non-diabetic subjects.
In conclusion, osteocalcin is an osteoblast hormone regulating energy metabolism through its effects on insulin secretion and sensitivity. Osteoblastic osteocalcin secretion is partly controlled by the hypothalamus through sympathetic tone. Sympathetic tone is in turn controlled by leptin through serotoninergic signaling from the brainstem to the hypothalamus (Figure 1).22, 23
Osteoblast transcription factors involved in energy metabolism
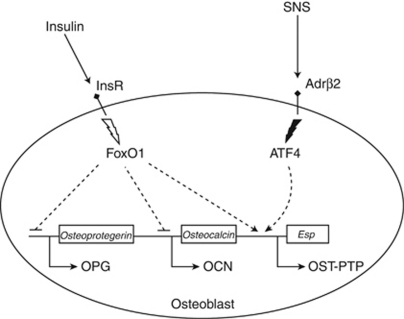
Osteoblasts are regulated by the osteoblast transcription factors, Osx and Runx2, which induce mesenchymal cells to differentiate into osteoblasts. Activating transcription factor 4 (ATF4) belongs to the cAMP-responsive element-binding protein transcription factor family, is regulated by sympathetic tone, and controls osteoblast functions such as bone formation, matrix mineralization, and receptor activator of nuclear factor κB ligand production. ATF4 also regulates osteoblast endocrine functions by inducing Esp and Ocn (the osteoblast endocrine genes). Indeed mice deficient in ATF4 show enhanced glucose tolerance and insulin sensitivity.24 The transcription factor FoxO1, which upregulates the enzymes of gluconeogenesis, is regulated by insulin.25 Deleting FoxO1 specifically in osteoblasts reduces Esp expression and increases osteocalcin, resulting in a metabolic phenotype protective against diabetes (Figure 2).26
Figure 2.
Osteoblast signaling pathways involved in energy metabolism regulation. Effects of insulin and sympathetic nervous system (SNS) on osteocalcin (OCN), osteotesticular protein tyrosine phosphatase (OST-PTP), and osteoprotegerin (OPG). Adrβ2, β2-adrenergic receptor; ATF4, activating transcription factor 4; InsR, insulin receptor. Adapted from Confavreux.22
Connection between osteocalcin and insulin signaling through bone remodeling
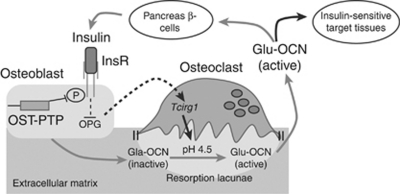
Until recently, experiments had not yet elucidated how the tyrosine phosphatase osteotesticular protein tyrosine phosphatase influences synthesis or activity of the hormone osteocalcin. Moreover, it was not established whether this process exists in humans as ESP in humans is a pseudogene. From an endocrine perspective, the fact that osteocalcin affects insulin raised the hypothesis that insulin should affect active osteocalcin in a feedback loop. A recent study by Ferron et al.27 provided a new key step in deciphering the interactions between bone and energy metabolism (Figure 3). Ferron et al. showed that insulin receptor (InsR) is expressed in osteoblasts and, interestingly, that mice lacking InsR specifically in osteoblasts have high blood glucose, low serum insulin, and altered glucose tolerance. Uncarboxylated osteocalcin was decreased in these mice. Karsenty's group27 established genetically that InsR in osteoblast and osteocalcin were in the same genetic pathway, as double-mutant heterozygous mice (InsRob+/–; Ocn+/–) have the same impaired glucose metabolic phenotype as osteocalcin-deficient mice (Ocn–/–). Next, they showed that insulin affects active osteocalcin in two ways: (1) insulin receptor is a substrate of the tyrosine phosphatase osteotesticular protein tyrosine phosphatase in mice and of protein tyrosine phosphatase 1B in humans; and (2) insulin receptor-deficient mice (InsRob–/–) have decreased bone resorption because of overexpression of osteoprotegerin, which blocks osteoclastogenesis and bone resorption. During bone resorption, the osteoclast vacuolar proton pump acidifies the sealed extracellular compartment, which decarboxylates osteocalcin into active uncarboxylated osteocalcin released into the bloodstream. This was established using osteoclast proton pump Tcirg1-deficient mice (Oc/Oc), which have an impaired glucose phenotype. In contrast, wild-type mice treated with receptor activator of nuclear factor κB ligand to obtain high bone resorption have an improved glucose tolerance.27
Figure 3.
Insulin and bone resorption affect circulating uncarboxylated osteocalcin (Glu-OCN). Insulin binds its receptor (InsR), which reduces osteoprotegerin (OPG) expression, thus enhancing osteoclast formation and activity. During bone resorption, acidification of the extracellular compartment decarboxylates inactive osteocalcin (Gla-OCN) to Glu-OCN. Glu-OCN is then released into the blood stream to affect target tissues. OST-PTP, osteotesticular protein tyrosine phosphatase. Adapted from Cell,27 with permission from Elsevier.
In summary, insulin signaling in osteoblasts improves glucose handling directly by increasing secretion of active uncarboxylated osteocalcin and indirectly by enhancing bone resorption, which releases uncarboxylated osteocalcin into the bloodstream; uncarboxylated osteocalcin then affects energy metabolism. Together, these findings raise many metabolic questions regarding the level of bone remodeling in diseases, and the effect of widely used antiresorptive therapies.
INSIGHTS INTO BONE, KIDNEY, AND ENERGY METABOLISM
The role of leptin in bone, kidney, and energy metabolism is still under investigation. Recently, it was shown that leptin administered to leptin-deficient mice stimulated fibroblast growth factor-23 synthesis and inhibited renal 1α-hydroxylase. Thus, leptin reduces serum calcium, serum phosphate, and active 1,25(OH)2D3 formation. Leptin replacement in ob/ob mice also significantly reduced renal NaPi-2a, NaPi-2c, and 1α-hydroxylase expression. These effects required the leptin receptor, as mice lacking leptin receptor (db/db) did not increase Fgf-23 or 1α-hydroxylase expression in response to leptin.28 Moreover, leptin appears to promote osteoblastic differentiation of vascular smooth muscle cells and thus may contribute to vascular calcification stimulated by hyperphosphatemia in CKD.29, 30
Some other bone–energy–kidney connections have also been suggested. In patients with CKD, serum adiponectin is inversely correlated with integral and cortical volumetric bone mineral density and cortical thickness, and serum osteocalcin is positively correlated with serum adiponectin.31 A randomized controlled trial is currently examining the effect of vitamin D supplementation on insulin resistance, serum adiponectin, serum osteocalcin, and multiple inflammatory markers in patients with CKD.32
In conclusion, the nascent field of bone–energy metabolism, a paradigm of integrative physiology, has only been made possible by the onset of mouse genetics.23 Future research is needed to decipher the mechanisms of the bone–energy endocrine axis and its relationship to other body systems. The transfer of discoveries made in mouse models into research in humans is in progress.
Acknowledgments
This model was presented at the ‘50 Years of Discovery Following the Intact Nephron Hypothesis' symposium in Munich, Germany, 24–25 June 2010. This work was supported by grants from the Société Francaise de Rhumatologie, Association pour la Recherche sur le Cancer, Bettencourt-Schueller Foundation, and Philippe Foundation. The author is grateful to Gerard Karsenty and Patricia Ducy for their helpful discussion and teaching. The author meets all the International Council of Medical Journal Editors criteria and acknowledges the writing assistance of Kim Coleman Healy, PhD, and Andrew Cooper, PhD, of Envision Scientific Solutions. Publication of this supplement was supported by Genzyme Corporation.
CBC has received lecturer's fees from Genzyme Corporation.
Footnotes
TO CITE THIS ARTICLE: Confavreux CB. Bone: from a reservoir of minerals to a regulator of energy metabolism. Kidney Int 2011; 79 (Suppl 121): S14–S19.
References
- Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- Williams ME. Chronic kidney disease/bone and mineral metabolism: the imperfect storm. Semin Nephrol. 2009;29:97–104. doi: 10.1016/j.semnephrol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Dardeno TA, Chou SH, Moon HS, et al. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni P, Dozio E, Galliera E, et al. Molecular aspects of adipokine-bone interactions. Curr Mol Med. 2010;10:522–532. doi: 10.2174/1566524011009060522. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Visitsunthorn U, Prete P. Reflex sympathetic dystrophy of the lower extremity: a complication of herpes zoster with dramatic response to propranolol. West J Med. 1981;135:62–66. [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Sanders KM, et al. β-Adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:19–24. doi: 10.1359/JBMR.0301214. [DOI] [PubMed] [Google Scholar]
- Reid IR, Gamble GD, Grey AB, et al. β-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res. 2005;20:613–618. doi: 10.1359/JBMR.041202. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Kraenzlin ME, Jick SS, et al. Use of β-blockers and risk of fractures. JAMA. 2004;292:1326–1332. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- Turker S, Karatosun V, Gunal I. β-Blockers increase bone mineral density. Clin Orthop Relat Res. 2006;443:73–74. doi: 10.1097/01.blo.0000200242.52802.6d. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Richards JB, Papaioannou A, Adachi JD, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho R, Kapur S, Du L, et al. Relationship between central and peripheral serotonin 5-HT2A receptors: a positron emission tomography study in healthy individuals. Neurosci Lett. 1999;261:139–142. doi: 10.1016/s0304-3940(98)00998-7. [DOI] [PubMed] [Google Scholar]
- Mann JJ, McBride PA, Brown RP, et al. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry. 1992;49:442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- Ferron M, Hinoi E, Karsenty G, et al. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindblom JM, Ohlsson C, Ljunggren O, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785–791. doi: 10.1359/jbmr.081234. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Weiler HA, Lix LM, et al. Body composition and bone density in Canadian White and Aboriginal women: the First Nations Bone Health Study. Bone. 2008;42:990–995. doi: 10.1016/j.bone.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Confavreux CB. Interactions between bone tissue and energy metabolism. Joint Bone Spine. 2010;77:287–289. doi: 10.1016/j.jbspin.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310:21–29. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Hinoi E, Jung DY, et al. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119:2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- Rached MT, Kode A, Silva BC, et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120:357–368. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Maeda T, Kawane T, et al. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient ob/ob mice. J Bone Miner Res. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- McCullough PA, Agrawal V, Danielewicz E, et al. Accelerated atherosclerotic calcification and Mönckeberg's sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- McCullough PA, Chinnaiyan KM, Agrawal V, et al. Amplification of atherosclerotic calcification and Mönckeberg's sclerosis: a spectrum of the same disease process. Adv Chronic Kidney Dis. 2008;15:396–412. doi: 10.1053/j.ackd.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Bacchetta J, Boutroy S, Guebre-Egziabher F, et al. The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol Dial Transplant. 2009;24:3120–3125. doi: 10.1093/ndt/gfp262. [DOI] [PubMed] [Google Scholar]
- Petchey WG, Hickman IJ, Duncan E, et al. The role of 25-hydroxyvitamin D deficiency in promoting insulin resistance and inflammation in patients with chronic kidney disease: a randomised controlled trial. BMC Nephrol. 2009;10:41. doi: 10.1186/1471-2369-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]