The sensory information from dental and craniofacial regions is first relayed in the spinal trigeminal nuclear complex (spinal trigeminal nucleus), which is further divided rostrocaudally into the subnuclei oralis (Vo), interpolaris (Vi) and caudalis (Vc) (Olszewski, 1950). It is widely accepted that nociceptive input from the cranial orofacial region is initially processed in the Vc (Sessle 2000) that exhibits considerable similarity with spinal dorsal horn and thus termed the medullary dorsal horn (MDH) (Gobel 1981). Despite apparent homology in nociceptive processing, recent observations have identified features of trigeminal pain processing in the MDH that are distinctly different from that of the spinal dorsal horn (Bereiter et al., 2000). Further, studies have pointed out increased excitability and sensitization of trigeminal pain pathways in non-laminar regions of the spinal trigeminal nuclear complex, particularly the trigeminal Vi/Vc transition zone, following injury and noxious stimulation of the dental and craniofacial region. Multiple lines of evidence suggest that the trigeminal Vi/Vc transition zone plays an important role in deep tissue pain processing, integrating nociceptive orofacial input, and the development of persistent orofacial pain.
I. Vi/Vc Neuronal Activation after Orofacial Injury
At the obex level, the ventral portion of the laminated Vc merges with the caudal Vi. Thus, rostral Vc with imperfectly laminated structures appears dorsally with Vi (ventral)atthe same coronalplane to form the trigeminal Vi/Vc transition zone (Fig. 1A). The dorsal portion of the Vi/Vc transition zone in the rat mainly involves the rostral end of the laminated Vc and the ventral Vi/Vc mainly includes the caudal Vi. Immunostaining with the anti-calcitonin gene-related peptide (CGRP) antibodies, the superficial laminae of the Vc can be clearly seen in the dorsal lateral portion of the Vi/Vc (Fig. 1A). The ventral pole of the Vi/Vc, the CGRP staining in the Vi appears less well organized, whereas the CGRP-like immunoreactivity (LI) is distributed across the full length of the superficial MDH (Fig. 1B). Somatotopy exists in the trigeminal transition zone. For example, the mandibular structures masseter muscle and temporomandibular joint (TMJ) are represented at the dorsomedial Vi/Vc (Klineberg, 1971; Nishimori et al., 1986; Capra, 1987; Takemura et al., 1987; Pfaller and Arvidsson, 1988; Shigenaga et al., 1988; Arvidsson and Raappana 1989); and corneal afferents terminate in the ventral Vi/Vc (Pozo and Cervero 1993; Hirata et al., 1999).
Fig. 1.
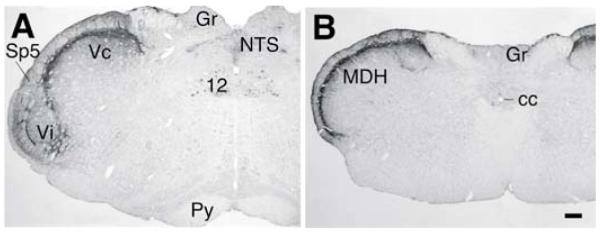
Digital photomicrograph illustrating the trigeminal Vi/Vc transition zone and laminated subnucleus caudalis (Vc). The sections in A and B were immunostained against anti-CGRP antibodies to illustrate the appearance of the Vi/Vc transition zone (A, about 0.4 mm rostral to the obex) and laminated Vc, or Medullary Dorsal Horn (MDH) (B, about 1.0 mm caudal to the obex). Note the the delineation of the Vi in the ventral Vi/Vc transition zone by calcitonin gene-related peptide staining (A). Scale bar = 0.2 mm. 12, hypoglossal nucleus; cc, central canal; Gr, gracile nucleus; NTS, Nucleus Tractus Solitarius; Py, pyramidal tract; Sp5, spinal trigeminal tract; Vi, subnucleus interpolaris of the spinal trigeminal complex. (Adapted from Wang et al., 2006, with permission from John Willey and Sons, License number: 2514340553127).
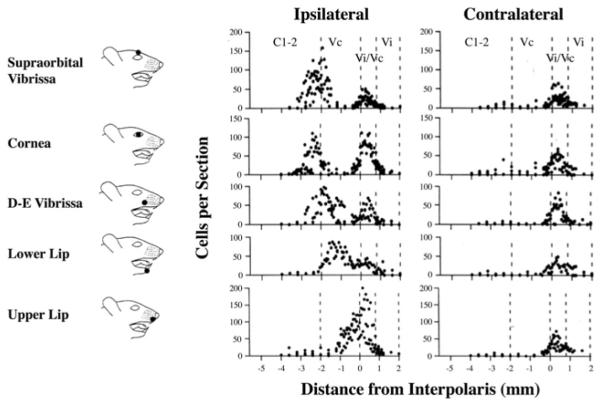
An interesting finding in recent years is that neuronal activation, as indicated by Fos protein expression, is consistently observed in the trigeminal Vi/Vc transition zone after a variety of noxious stimuli applied to the dental and craniofacial regions, including dural blood vessel and facial stimulation (Strassman and Vos, 1993, Strassman et al., 1994), corneal stimulation (Bereiter et al., 1994) and intravitreal capsaicin (Chang et al., 2010), noxious stimulation of the tooth (Coimbra and Coimbra, 1994), oral mucous membrane (Sugimoto et al., 1994) and the pulp (Chattipakorn et al., 2002, 2005; Oakden and Boissonade, 1998), chemical stimulation of the rat’s tongue (Carstens et al., 1995), mustard oil and adjuvant stimulation of the TMJ (Hathaway et al., 1995; Zhou et al. 1999), and masseter muscle inflammation (Imbe et al., 1999; Ro et al., 2003). These studies reveal an interesting pattern of trigeminal nociceptive neuronal activation (Fig. 2). First, stimulation-induced trigeminal Fos protein expression exhibits a bi-modal distribution, with one peak at the caudal Vc/C1,2 and a rostral peak at the Vi/Vc (Strassman and Vos, 1993). Second, while stimulus-induced Fos-LI in the Vc/C1,2, particularly in superficial laminae, is predominantly ipsilateral to the side of stimulation, Fos-LI in the Vi/Vc transition zone is often expressed bilaterally in the ventral Vi/Vc despite a unilateral injury.
Fig. 2.
Rostrocaudal distribution of Fos-immunoreactive neurons in the spinal trigeminal complex after orofacial stimulation. Cartoons on the right show site of stimulation. The number of Fos-positive cells is plotted against the distance from the subnucleus interpolaris. 0 ≈ obex level, positive = rostral, negative = caudal. Note bi-modal distribution of Fos-positive cells along the Vi/Vc and Vc/C1,2 ipsilateral to stimulation and one peak of Fos-positive cells at the Vi/Vc level contralateral to stimulation. (Adapted from Strassman and Vos, 1993, with permission from John Willey and Sons, License number: 2514340716050).
The dual representation of orofacial nociceptive input in the spinal trigeminal complex is consistent with anatomical findings. It is known that sensory inputs from the orofacial region project to two regions of the spinal trigeminal complex, the Vc and caudal Vi (Arvidsson et al., 1992; Capra, and Dessem, 1992). The location of the terminal field in the caudal Vi is constant but the terminal field in the Vc shows somatopic differences. In general, primary afferent fibers from the perioral and perinasal regions, or the most anterior face (tongue, upper lip and snout), terminate most rostrally in the Vc, and fibers from progressively more posterior facial regions (lower lip, cornea, supraorbital vibrassae) terminate at successively lower levels (Shigenaga et al., 1986). Accordingly, primary afferent fibers that convey noxious stimuli from more caudal and lateral regions of the face have progressively more caudal terminations in reference to the obex. The fibers that innervate the circumoral zone terminate near the obex, whereas intermediate and peripheral zones terminate more caudally in the Vc. The anatomical relationship between the orofacial input and central termination explains the Fos expression pattern after noxious stimulation. For example, the terminal field for the lower lip is clearly caudal and separated from the Vi/Vc zone; so are the two distinct Fos peaks (Fig. 2). The primary terminal field for the upper lip is immediately caudal or slightly overlapped with the Vi/Vc zone (Arvidsson et al., 1992). Consistently, the caudal peak of Fos labeling is apparently missing after stimulation of the upper lip, as the Vi/Vc and caudal Fos peaks merge at the obex-Vi/Vc level (Fig. 2) (Strassman and Vos 1993).
What is the significance of neuronal activation at the Vi/Vc level? It has been shown that urethane anesthesia itself can induce Fos at the Vi/Vc, but not at the caudal Vc (Strassman and Vos, 1993). Methohexital (Brevital) anesthesia also induces Fos in the Vi/Vc (Zhou et al., 1999). However, the masseter inflammation-induced Fos in Vi/Vc cannot be explained by the effect of Brevital anesthesia alone (Imbe et al., 1999). Specific neuronal activation at the Vi/Vc transition zone after noxious stimulation, which correlates with behavioral hyperalgesia, suggests a previously unrecognized role of this region in trigeminal pain processing.
II. Functional Input and Output of the Vi/Vc Transition Zone
A. Peripheral Input
By comparing the masseteric inflammation induced Fos-LI to that induced by anesthesia and skin-cut over the masseter muscle, it was found that the bilateral periobex peak of Fos-LI was primarily a response to masseteric inflammation, while the skin-cut mainly induced the caudal ipsilateral peak of Fos-LI (Imbe et al., 1999; Ikeda et al., 2003). These findings suggest that the Vi/Vc transition zone plays an important role in the responses to deep tissue injury. This hypothesis is supported by recent studies discussed below.
Utilizing a double tracing protocol (Capra and Wax, 1989), we studied the central termination of masseter muscle afferents in the trigeminal transition zone, compared with that of the cutaneous afferents (Wang et al. 2006). Different neuronal tracers were injected either centrally (FluoroGold; ventral Vi/Vc or MDH) or peripherally (wheat germ agglutinin-conjugated horseradish peroxidase or cholera toxin B; masseter or overlying skin), in the same rat and the convergence of the tracers in the trigeminal ganglion was examined. A population of small- to medium-sized neurons was double-labeled after injections of the tracers into the masseter and Vi/Vc, masseter and MDH, or the skin and MDH. However, only a few double-labeled neurons were occasionally observed after injections of the tracers into the skin and Vi/Vc. These results indicate that while both masseter and cutaneous inputs project to the MDH, masseter afferents provide an additional input to the Vi/Vc. The ventral Vi/Vc also receives direct corneal input (Pozo and Cervero, 1993; Tashiro et al., 2010).
Behavioral pharmacology studies show that injection of an N-methyl-D-aspartate receptor antagonist, AP-5, into the Vi/Vc and MDH attenuated masseter inflammatory hyperalgesia. In contrast, hyperalgesia after inflammation of the skin overlying the masseter was attenuated by injection of AP-5 into the MDH but not Vi/Vc. Similar results were obtained by injecting glial inhibitors and IL-10, an anti-inflammatory cytokine, into the Vi/Vc or MDH (Shimizu et al. 2009b). These anatomical and behavioral observations support the view that there is differential involvement of trigeminal transition zone, and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia.
B. Visceral Input
The afferent projections to the ipsilateral dorsal Vi/Vc have been identified following HRP injection into the cervical vagus nerve (Gwyn et al., 1985). Electrical stimulation of the vagus nerve induces expression of Fos-LI in the Vi/Vc transition zone (Gieroba and Blessing, 1994; Yousfi-Malki and Puizillout, 1994). The vagotomy produces a decrease in the masseteric inflammation-induced Fos-LI in the Vi/Vc transition zone (Imbe et al., 1999). Taken together, a portion of the orofacial injury-induced Vi/Vc neuronal activation is likely a result of somatic-visceral integration that is mediated by vagal afferents to the brain.
C. Caudalis Input
The multiple lines of evidence indicate that neuronal activation in the transition zone, particularly the ventral pole, depends on input from the caudal Vc. Neurons in the caudal Vc project to rostral subnuclei of the spinal trigeminal nucleus (Ikeda et al., 1982; Lovick and Wolstencroft, 1983; Jacquin et al., 1990). The ascending pathway from caudal Vc modulates the activity of neurons in the more rostral subnuclei of the spinal trigeminal nucleus (Greenwood and Sessle, 1976; Scibetta and King 1969). Inhibition of caudal Vc affects Vi/Vc neuronal activity and evoked transmitter release (Bereiter et al., 2002; Hirata et al., 2003). Topical application of glutamate and morphine to the Vc/C1 region either facilitated or inhibited evoked activity of ~30% of corneal units tested in the Vi/Vc (Meng et al., 1998). Following injection of the retrograde tracer Fluorogold into the Vi/Vc transition zone, retrogradely labeled cells were observed in the Vc/C1,2 region (Sato et al., 2005). After lesions of the bilateral Vc, there is a selective reduction of inflammation-induced Fos-expressing neurons in the ventral Vi/Vc, but not in dorsal or intermediate Vi/Vc, or nucleus tractus solitarius (Sugiyo et al., 2005). These findings support that trigeminal transition zone activity, particularly the ventral Vi/Vc, is modulated by the activation of the caudal laminated Vc zone.
D. Rostral Projections
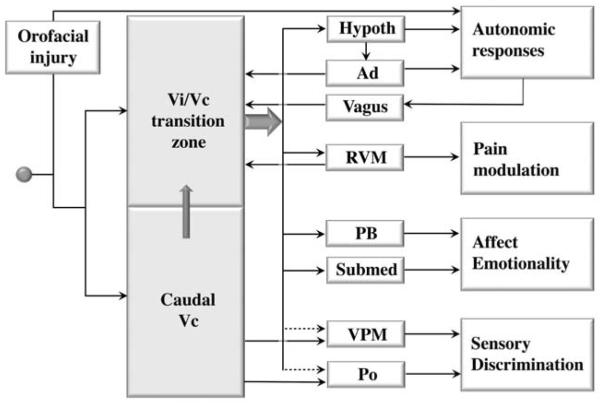
Neurons in the spinal trigeminal complex project to a variety of rostral brain structures related to somatosensory, as well as somatoautonomic and somatovisceral processing. It has been shown that neurons in the ventral portion of the Vi/Vc transition zone have a major projection to the nucleus submedius of the thalamus (Sm) (Yoshida et al., 1991). This Vi/Vc-Sm pathway is activated after orofacial injury. Through a combined Fluorogold retrograde tracing and Fos protein immunocytochemistry double labeling approach, rostrally projecting neurons that are activated after masseter inflammation can be identified (Ikeda et al., 2003). In the ventral portion of the Vi/Vc transition zone, about 40 percent of neurons exhibiting inflammation-induced Fos-LI project to the Sm. However, very few Fos-labeled neurons in the dorsal Vi/Vc project to the Sm. Anesthesia alone also induces Fos expression in ventral Vi/Vc neurons but these neurons do not project to Sm (Ikeda et al., 2003). About 20 percent of Fos-positive neurons in dorsal and ventral Vi/Vc project to the parabrachial nucleus and about 5 percent of Fos-positive neurons project to either the lateral hypothalamus or medial ventroposterior thalamic nucleus. The Sm plays a role in nociceptive processing, particularly related to aversive and emotional responses (Craig and Burton 1981). The parabrachial nucleus is involved in processing trigeminal nociceptive input and integrating emotional and autonomic responses (see Feil and Herbert 1995). The major projections from Vi/Vc are to the Sm and parabrachial nucleus, supporting the conclusion that this region plays a role in autonomic and hormonal functions and is emotionality related to persistent pain (Bereiter et al., 1996; Ikeda et al., 2003) (Fig. 3).
Fig. 3.
Summary of the role of the trigeminal Vi/Vc transition zone in persistent orofacial pain. Orofacial injury-related inputs not only activate caudal Vc neurons, but also reach the Vi/Vc transition zone. The Vi/Vc output accesses hypothalamus (Hypoth), rostral ventromedial medulla (RVM), parabrachial nucleus (PB), and nucleus submedius of the thalamus (Submed) to play a role in autonomic responses to injury, descending pain modulation and pain-related emotionality. The Vi/Vc transition zone also receives input from RVM and Vi/Vc neuronal activation is regulated by caudal Vc through internuclear connections, the adrenal cortex (Ad) through circulating glucocorticoids and vagal afferents. Also shown is the Vc output that is relayed through the thalamic medial ventrolateral nucleus (VPM) and posterior thalamic nucleus (PO) for discriminative pain. The Vi/Vc transition zone may play a minor role in discriminative pain (dashed arrows).
E. Reciprocal Interactions with the Brainstem Descending Circuitry
The rostral ventromedial medulla (RVM) is a key structure in descending pain modulation, which includes the midline nucleus raphe magnus and the adjacent reticular formation ventral to the gigantocellular reticular nucleus (Fields et al., 2006). In addition to inhibitory descending input, RVM facilitates neuropathic pain, secondary hyperalgesia and persistent pain (Porreca et al., 2002; Vanegas and Schaible, 2004; Ren and Dubner, 2008). The Vi/Vc transition zone has access to the RVM. Following the injection of Fluorogold into the RVM 7 days before injection of an inflammatory agent, Complete Freund’s Adjuvant (CFA), into the masseter muscle and perfusion of the rat at 2-hour post-inflammation, a population of neurons in the ventral Vi/Vc exhibited Fluorogold/Fos double staining, suggesting the connection between Vi/Vc and RVM and the activation of the Vi/Vc-RVM pathway after inflammation. This Vi/Vc-RVM projection pathway appears selective for the ventral portion of the Vi/Vc since no double-labeled neurons were found in the dorsal Vi/Vc or laminae I–IV of Vc (Sugiyo et al., 2005). Injection of an anterograde tracer, phaseolus vulgaris leucoagglutinin, into the RVM, resulted in labeling profiles overlapped with the region that showed Fluorogold/Fos double labeling in the Vi/Vc, suggesting that the connections between RVM and Vi/Vc are reciprocal. Excitotoxic lesions of the RVM or Vi/ Vc with ibotenic acid led to the elimination or attenuation of masseter hyperalgesia/allodynia developed after masseter inflammation (Sugiyo et al., 2005). Thus, there are reciprocal connections between the ventral Vi/Vc transition zone and RVM. The Vi/Vc-RVM pathway is activated after orofacial deep tissue injury and involved in descending facilitation of orofacial hyperalgesia.
III. Cellular and Chemical Mediators: Role of Neuron-Glia-Cytokine Interactions
Nerve signals arising from sites of tissue or nerve injury lead to long-term increases in excitability and plasticity in the central nervous system, often referred to as central sensitization. Central sensitization is brought about by a series of cellular events including neuronal depolarization, removal of the voltage-dependent magnesium block of the N-methyl-D-aspartate receptor (NMDAR); phosphorylation of NMDAR, alpha amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), and neurokinin (NK) 1 receptors and an increase in synaptic strength (Dubner and Ren, 2004). Ample evidence indicates that central sensitization underlies mechanisms of persistent pain (Woolf and Salter, 2000; Dubner and Ren, 2004).
There has been an increasing awareness of neuroimmune interactions and their role in the etiology of diseases including chronic pain (Ren and Dubner, 2010). The neuroimmune interactions are reciprocal, or bi-directional, and involve central glial cells, cytokines, and neurotransmitters and their receptors. The emerging literature strongly implicates a role for glia and proinflammatory cytokines in the genesis and maintenance of persistent or chronic pain (Watkins and Maier, 2005).
The Vi/Vc transition zone contributes to central sensitization after orofacial injury. Sensitization of Vi/Vc neurons occurs after orofacial injury, which involves a cascade of cellular events including the activation of neurotransmitter receptors and neuron-glia-cytokine interactions. In response to orofacial tissue injury, glia in the Vi/Vc transition zone exhibit hyperactivity and inflammatory cytokines released, contributing to activity-dependent plasticity and hyperalgesia (Guo et al., 2007).
A. Vi/Vc Glial Response to Injury
Following peripheral injury, microglia and astrocytes show increased levels of activity or a hyperactive state, and are often referred to as “activated”. The specific expression of cellular markers is used to assess levels of glial activity. Among the most commonly used, glial fibrillary acidic protein (GFAP), an astrocytic intermediate cytoskeleton filament, is a marker of astrocytes and cluster of differentiation molecule 11b (CD11b), also known as Mac-1 or CR3, is the α-integrin marker of microglia. The monoclonal antibody OX-42 is commonly used to detect CD11b in brain microglia. Two calcium-binding peptides have been used as a functional marker of glia: S100B for astrocytes and Iba1 (ionized calcium-binding adapter molecule) for microglia.
Masseter inflammation induces glial hyperactivity in the Vi/Vc transition zone (Guo et al., 2007). Following injection of the inflammatory agent CFA into the masseter muscle, reactive astrocytes were clearly seen in the Vi/Vc transition zone, indicated by GFAP immunostaining. The activated astrocytes typically exhibited hypertrophy with thicker processes and larger and densely stained cell bodies. The increase in GFAP levels is seen as early as at 0.5-hours and lasts for about one week after inflammation. The activation of astrocytes after inflammation is associated with upregulation of connexin 43 (Cx43), an astrocytic gap junction protein. Double immunohistochemistry shows that the Cx43-LI co-localizes with GFAP, but not with CD11b, a marker of microglia, NeuN, a neuronal marker, or Cx36, a neuronal gap junction protein. The levels of CD11b are also upregulated by masseter inflammation. The glial hyperactivity induced by masseter inflammation in the Vi/Vc transition zone correlates with the development of hyperalgesia (Sugiyo et al., 2005; Watanabe et al., 2005).
B. Role of Inflammatory Cytokine IL-1beta
The hyperactivity of glia induced by masseter inflammation is accompanied by an increase in the inflammatory cytokine levels. Compared to the naive rats, the immunostaining for interleukin-1beta (IL-1beta a prototypic proinflammatory cytokine, is increased significantly in the Vi/Vc transition zone (Guo et al., 2007). Western blot shows the increase of IL-1beta in the Vi/Vc transition zone with a time course similar to that of astrocyte hypertrophy after masseter inflammation. Interestingly, IL-1beta is selectively induced in astrocytes, but not in microglia or neurons, in the ventral Vi/Vc transiton zone. The selective localization of IL-1beta in astrocytes has also been reported in other animal models, including bone cancer pain (Zhang et al., 2005), tissue and nerve injury (Kawasaki et al., 2008; Wei et al., 2008; Weyerbacher et al., 2010), and intracerebral hemorrhage (Wasserman et al., 2007). These findings suggest that astrocytes provide an alternative source of IL-1beta, in addition to the known release of IL-1beta from microglia after injury (Clark et al., 2006; Kawasaki et al., 2008). The CFA-induced increases in IL-1beta levels are reduced after the treatment with propentofylline, a glial inhibitor (Guo et al., 2007), suggesting that glial activation is upstream to cytokine induction in the Vi/Vc after inflammation.
The IL-1beta in the Vi/Vc plays a role in the development of orofacial hyper-algesia. The mechanical allodynia and hyperalgesia in the orofacial region can be assessed by applying a series of von Frey microfilaments to the skin. The response frequencies to a range of von Frey filament forces are determined. A stimulus-response frequency relationship is established and 50 perecnt effective force values are derived. The EF50 is defined as the force that induces 50 percent response frequency in rats. A significant reduction of EF50 vs. the baseline level indicates mechanical allodynia and hyperalgesia (Guo et al., 2004). When an IL-1 receptor antagonist (IL-1ra) was administered intrathecally via osmotic pumps through a cannula implanted at the level of the obex prior to the induction of masseter inflammation, the behavioral allodynia and hyperalgesia were significantly attenuated (Guo et al., 2007). Direct injection of IL-1beta into the ventral Vi/Vc transition zone produces mechanical allodynia and hyperalgesia (Shimizu et al.,2009a).
C. Glia, Cytokine and NMDA Receptor Activation
The Vi/Vc glial hyperactivity and inflammatory cytokine release facilitates central sensitization through interactions with the NMDAR. NMDAR phosphorylation is widely accepted as an indication of synaptic plasticity and correlates with the time course of persistent pain (Guo et al., 2002, 2004; Brenner et al., 2004). IL-1beta facilitates NMDAR phosphorylation in an ex vivo medullary slice preparation (Guo et al., 2007). Incubation of IL-1beta in the medullary slices induced a significant and dose-dependent increase in phospho-ser896-NR1 (P-NR1) levels in the Vi/Vc transition zone. In contrast, the application of TNF-alpha, the other prototypic inflammatory cytokine, does not affect P-NR1 levels at the dose tested (Guo et al., 2007). Microinjection of IL-1beta into the ventral Vi/Vc transition zone in vivo also produced an increase in P-ser896-NR1 levels that is blocked by IL-1ra (Guo et al., 2007).
The IL-1beta-induced NR1 phosphorylation is blocked by chelerythrine, a PKC inhibitor, confirming that PKC is involved in this effect. 2APB, a membrane permeable IP3 receptor antagonist also blocks IL-1beta-induced NR1 phosphorylation. The NMDAR channel blocker MK-801 does not affect the IL-1beta-induced increase in P-ser896-NR1 (Guo et al., 2007). The involvement of the PKC inhibitor in IL-1beta-facilitated P-NR1 suggests that a key link between IL-1beta and subsequent activation of NMDAR involves phospholipases PLA2 or PLC, since PKC is a downstream effector of arachidonic acid and diacylglycerol. This hypothesis has been tested. The PLC inhibitor U73122 and PLA2 inhibitor AACOCF3 blocked the effect of IL-1beta on NMDAR phosphorylation in Vi/Vc. It is well known that IL-1R signaling leads to transcriptional regulation of cellular function (Martin and Wesche, 2002). These findings show that the effect of IL-1beta on NMDAR phosphorylation is attributable to the post-translational regulation through IL-1R signaling; and that the intermediate pathway involves PLC, PLA2 and subsequent PKC activation and intracellular Ca2+ release.
Direct administration of IL-1beta into the ventral trigeminal transition zone produced orofacial hyperalgesia that lasted for hours (Shimizu et al., 2009a). Pretreatment with glial inhibitors does not block IL-1beta-induced hyperalgesia even with relatively high doses that have been shown to be effective in attenuating hyperalgesia after injury (Wei et al., 2008; Shimizu et al., 2009b). Although the glial inhibitor/modulator propentofylline do not block IL-1beta-induced hyperalgesia, propentofylline was able to attenuate hyperalgesia after masseter inflammation (Shimizu et al., unpublished observations). Likewise, fluorocitrate is unable to block IL-1beta-induced NMDA receptor phosphorylation in the Vi/Vc transition zone in vitro, but attenuated masseter inflammatory hyperalgesia (Guo et al., 2007). Thus, IL-1beta-induced hyperalgesia appears to be downstream to glial activity and involves activation of NMDAR. Direct application of IL-1beta bypasses glial cells, to produce neuronal hyperexcitability and hyperalgesia.
The NMDAR plays a major role in central sensitization and pain hypersensitivity, including orofacial hyperalgesia (Chiang et al., 1997; Bereiter and Bereiter, 2000; Guo et al., 2007; Wang et al., 2009). Injection of the NMDAR antagonist into the Vi/Vc transition zone attenuates masseter inflammatory hyperalgesia (Wang et al., 2006). The IL-1beta signaling facilitates NMDAR activity in neurons (Viviani et al., 2003; Yang et al., 2005; Guo et al., 2007; Zhang et al., 2008). IL-1beta exaggerates NMDA and glutamate-evoked hippocampal neuron death in the rat (Ma et al., 2002-2003). Consistently, IL-1R colocalizes with the NMDAR NR1 subunit in Vi/Vc neurons (Guo et al., 2007). Thus, IL-1R signaling may selectively regulate NMDAR function and increase synaptic strength via post-translational phosphorylation and contribute to inflammation-induced pain hypersensitivity. Collective evidence suggests that glial activation, inflammatory cytokine release and NMDAR activation are sequential events in the nervous system responses to injury, and that IL-1beta and its interaction with NMDA receptors plays a critical role in the central mechanisms of hyperalgesia.
IV. Functional Significance of Vi/Vc in Trigeminal Pain Processing
Noxious stimulation of the different dental and craniofacial regions induces bimodal neuronal activation in the spinal trigeminal complex. While the caudal peak of neuronal activity varies rostrocaudally and mediolaterally in the Vc according to somatotopy, the rostral peak is consistently located at the obex or the trigeminal transition zone level (Strassman and Vos, 1993). Further, anesthetic alone induces Fos protein expression and increased phosphorylation of the extracellular signal-regulated kinase in the Vi/Vc transition zone (Imbe et al., 1999; Shoda et al., 2009). These findings suggest that neuronal activation in the ventral Vi/Vc transition zone lacks somatotopy, although there are selective primary afferent inputs to the Vi/Vc transition zone (Klineberg, 1971; Shigenaga et al., 1988; Pozo and Cervero 1993; Zhou et al., 1999; Hirata et al., 1999; Imbe et al., 1999; Wang et al., 2006). Evidence suggests that the Vi/Vc response, particularly the ventral Vi/Vc, to injury is related to the integration of somatosensory and visceral/autonomic functions, and the engagement of descending pain modulation, but less concerned with somatotopically organized pain behavior, that is mainly mediated by laminated Vc (Fig. 3).
A. Somatovisceral Somatoautonomic Integration
It is suggested that anesthetics-induced Fos expression in the brainstem nuclei could be explained at least in part by its cardiovascular effects, since they induce a decrease in the arterial blood pressure and heart rate (Rocha and Herbert, 1997). The administration of nitroglycerin, a vasodilator, also induces hypotension and bilateral Fos-LI in the ventral portion of Vi/Vc (Tassorelli and Joseph, 1995; Bereiter et al. 1994) show that mustard oil stimulation of the cornea induces Fos-LI in a group of neurons located in the ventral Vi/Vc and that local anesthesia of this area greatly attenuated the mustard oil-induced adrenal and autonomic responses. Vi/Vc neuronal activation is also related to injury-produced environmental stress and visceral input. Adrenalectomy and vagotomy selectively reduced Fos expression at the Vi/Vc transition zone with little effect on the caudal Fos peak (Imbe et al., 1999), suggesting that Vi/Vc neuronal activation depends on the integrity of the adrenal cortex and vagus nerve. Thus, in addition to somatotopically organized nociceptive responses, orofacial injury is also coupled to somatovisceral and somatoautonomic activity that contributes to central neural activation mediated through the Vi/Vc.
B. Descending Modulation
Trigeminal neurons have connections with neurons in the nucleus raphe magnus, the major component of the RVM (Beitz et al., 1983; Basbaum et al., 1986). The spinal trigeminal nucleus receives serotonergic and enkephalinergic projections from the nucleus raphe magnus (Beitz, 1982; Beitz et al., 1987). Pain modulating neurons in nucleus raphe magnus also have terminals in the spinal trigeminal nucleus (Mason and Fields, 1989), which may modulate trigeminal nociceptive transmission (Chiang et al., 1994).
The Vi/Vc neurons also relay nociceptive input from the orofacial deep tissues to RVM neurons (Sugiyo et al., 2005). Inflammation-activated neurons in the Vi/Vc transition zone project to the RVM and there is RVM neuronal activation after TMJ inflammation (Zhou et al., 1999). An anterograde tracing study suggests that RVM neurons project to the area of the ventral trigeminal transition zone, where activated RVM projecting neurons are localized (Sugiyo et al., 2005). This suggests reciprocal neural pathways between these two regions. These results further emphasize the importance of Vi/Vc neurons in engaging descending pain modulation.
Behavioral studies confirm the functional significance of the RVM-Vi/Vc connections (Sugiyo et al., 2005; Shimizu et al., 2009a). Unilateral CFA-induced inflammation of the masseter muscle produces mechanical allodynia and hyper-algesia in the orofacial region overlapping the masseter muscle. Excitotoxic lesions of the RVM or Vi/Vc transition zone lead to significant attenuation of behavioral hyperalgesia after masseter inflammation, indicating that modulatory inputs from the RVM enhance the hyperalgesia/allodynia, found after masseter inflammation. These results are consistent with the view that hyper-algesia in animal models of inflammatory and neuropathic pain are closely linked to the activation of descending pathways (Porreca et al., 2002; Ren and Dubner, 2008; Vanegas and Schaible, 2004). Masseter inflammation-induced hyperalgesia is maintained by descending facilitatory drive that is recruited by the Vi/Vc-RVM circuitry, at least partially.
C. Deep vs. Cutaneous Injury
Deep orofacial tissue inflammation produces stronger central neuronal activation in the spinal trigeminal complex, including the Vi/Vc region than does cutaneous inflammation (Zhou et al., 1999; Imbe et al., 2001). Compared to cutaneous CFA injection, the injection of CFA into the TMJ produces a significantly stronger inflammation associated with greater Fos expression. It has been shown that mustard oil injections into the TMJ versus cutaneous tissues produces greater activation of masticatory muscles (Yu et al., 1993) than the cutaneous mustard oil injections and that TMJ inflammation resulted in more widespread excitation of medullary dorsal horn neurons than perioral cutaneous inflammation (Iwata et al., 1999).
There is also a differential involvement of the Vi/Vc transition zone in deep vs. cutaneous orofacial hyperalgesia. Injection of the NMDAR antagonist AP-5, anti-inflammatory cytokine IL-10 or glial inhibitors fluorocitrate and minocycline into the ventral Vi/Vc transition zone only attenuates hyperalgesia after masseter inflammation, without an effect on hyperalgesia associated with cutaneous injury (Wang et al., 2006; Shimizu et al., 2009b). In contrast, both masseter and cutaneous hyperalgesia are attenuated after the injection of these agents into the caudal Vc. Western blot analysis shows a selective enhancement of NMDAR phosphorylation and GFAP upregulation in the Vi/Vc transition zone after masseter, but not cutaneous inflammation (Shimizu et al., 2009b). These observations indicate that, in addition to Vc, deep orofacial input engages Vi/Vc neurons in developing central sensitization and hyperalgesia. Noxious cutaneous or superficial input has access to the Vi/Vc (Strassman and Vos, 1993; Zhou et al., 1999). The development of cutaneous hyperalgesia depends on caudal Vc and does not require coordination by Vi/Vc neurons (Shimizu et al., 2009b; Chang et al., 2010). Thus, while both deep and cutaneous orofacial nociceptive inputs are processed in the laminated Vc, the ventral pole of Vi/Vc is involved in the coordination of sensorimotor functions of the trigeminal system associated with the response to and recuperation from deep tissue injury. This response includes roles in nociceptive hyperexcitability involving glia and NMDAR, somatovisceral and somatoautomic integration, and descending pain modulation (also see Dubner and Ren, 2006).
Acknowledgments
The authors’ work is supported by US National Institutes of Health grants DE11964, NS060735 and NS059028.
References
- Arvidsson J, Pfaller K, Gmeiner S. The ganglionic origins and central projections of primary sensory neurons innervating the upper and lower lips in the rat Somatosens. Mot. Res. 1992;9:199–209. doi: 10.3109/08990229209144771. [DOI] [PubMed] [Google Scholar]
- Arvidsson J, Raappana P. An HRP study of the central projections from primary sensory neurons innervating the rat masseter muscle. Brain Res. 1989;480:111–118. doi: 10.1016/0006-8993(89)91573-4. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Ralston DD, Ralston HJ., 3rd Bulbospinal projections in the primate: a light and electron microscopic study of a pain modulating system. J. Comp. Neurol. 1986;250:311–323. doi: 10.1002/cne.902500305. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The nuclei of origin of brainstem serotonergic projections to the rodent spinal trigeminal nucleus. Neurosci. Lett. 1982;32:223–228. doi: 10.1016/0304-3940(82)90297-x. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Clements JR, Ecklund LJ, Mullett MM. The nuclei of origin of brainstem enkephalin and cholecystokinin projections to the spinal trigeminal nucleus of the rat. Neuroscience. 1987;20:409–425. doi: 10.1016/0306-4522(87)90101-1. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Wells WE, Shepard RD. The location of brainstem neurons which project bilaterally to the spinal trigeminal nuclei as demonstrated by the double fluorescent retrograde tracer technique. Brain Res. 1983;258:305–312. doi: 10.1016/0006-8993(83)91156-3. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF. Morphine and NMDA receptor antagonism reduce c-Fos expression in spinal trigeminal nucleus produced by acute injury to the TMJ region. Pain. 2000;85:65–77. doi: 10.1016/s0304-3959(99)00246-8. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF, Hathaway CB. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity in central trigeminal neurons and blocks select endocrine and autonomic responses to corneal stimulation in the rat. Pain. 1996;64:179–189. doi: 10.1016/0304-3959(95)00095-X. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hathaway CB, Benetti AP. Caudal portions of the spinal trigeminal complex are necessary for autonomic responses and display Fos-like immunoreactivity after corneal stimulation in the cat. Brain Res. 1994;657:73–82. doi: 10.1016/0006-8993(94)90955-5. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Shen S, Benetti AP. Sex differences in amino acid release from rostral trigeminal subnucleus caudalis after acute injury to the TMJ region. Pain. 2002;98:89–99. doi: 10.1016/s0304-3959(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur. J. Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Capra NF. Localization and central projections of primary afferent neurons that innervate the temporomandibular joint in cats. Somatosens. Res. 1987;4:201–213. doi: 10.3109/07367228709144607. [DOI] [PubMed] [Google Scholar]
- Capra NF, Dessem D. Central connections of trigeminal primary afferent neurons: topographical and functional considerations. Crit. Rev. Oral. Biol. Med. 1992;4:1–52. doi: 10.1177/10454411920040010101. [DOI] [PubMed] [Google Scholar]
- Capra NF, Wax TD. Distribution and central projections of primary afferent neurons that innervate the masseter muscle and mandibular periodontium: a double-label study. J. Comp. Neurol. 1989;279:341–352. doi: 10.1002/cne.902790302. [DOI] [PubMed] [Google Scholar]
- Carstens E, Saxe I, Ralph R. Brainstem neurons expressing c-Fos immunoreactivity following irritant chemical stimulation of the rat’s tongue. Neuroscience. 1995;69:939–953. doi: 10.1016/0306-4522(95)00297-v. [DOI] [PubMed] [Google Scholar]
- Chang Z, Okamoto K, Tashiro A, Bereiter DA. Ultraviolet irradiation of the eye and Fos-positive neurons induced in trigeminal brainstem after intravitreal or ocular surface transient receptor potential vanilloid 1 activation. Neuroscience. 2010;170:678–685. doi: 10.1016/j.neuroscience.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, Sigurdsson A, Light AR, Narhi M, Maixner W. Trigeminal c-Fos expression and behavioral responses to pulpal inflammation in ferrets. Pain. 2002;99:61–69. doi: 10.1016/s0304-3959(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Chattipakorn S, Chattipakorn N, Light AR, Narhi M, Maixner W. Comparison of Fos expression within the ferret’s spinal trigeminal nuclear complex evoked by electrical or noxious-thermal pulpal stimulation. J. Pain. 2005;6:569–580. doi: 10.1016/j.jpain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Hu JW, Sessle BJ. Parabrachial area and nucleus raphe magnus-induced modulation of nociceptive and non nociceptive trigeminal subnucleus caudalis neurons activated by cutaneous or deep inputs. J. Neurophysiol. 1994;71:2430–2445. doi: 10.1152/jn.1994.71.6.2430. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Hu JW, Sessle BJ. NMDA receptor involvement in neuroplastic changes induced by neonatal capsaicin treatment in trigeminal nociceptive neurons. J. Neurophysiol. 1997;78:2799–2803. doi: 10.1152/jn.1997.78.5.2799. [DOI] [PubMed] [Google Scholar]
- Clark AK, D’Aquisto F, Gentry C, Marchand F, McMahon SB, Malcangio M. Rapid co-release of interleukin 1beta and caspase 1 in spinal cord inflammation. J. Neurochem. 2006;99:868–880. doi: 10.1111/j.1471-4159.2006.04126.x. [DOI] [PubMed] [Google Scholar]
- Coimbra F, Coimbra A. Dental noxious input reaches the subnucleus caudalis of the trigeminal complex in the rat, as shown by c-Fos expression upon thermal or mechanical stimulation. Neurosci. Lett. 1994;173:201–204. doi: 10.1016/0304-3940(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Craig AD, Jr., Burton H. Spinal and medullary lamina I projection to nucleus. submedius in medial thalamus: a possible pain center. J. Neurophysiol. 1981;45:443–466. doi: 10.1152/jn.1981.45.3.443. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J. Orofacial. Pain. 2004;18:299–305. [PubMed] [Google Scholar]
- Dubner R, Ren K. Pathophysiology of Persistent Orofacial Pain. In: Laskin DM, Greene CS, Hylander WL, editors. Temporomandibular Disorders: An Evidence-Based Approach to Diagnosis and Treatment. Quintessence Publishing Co, Inc; Chicago: 2006. pp. 89–97. [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J. Comp. Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5th edit Elsevier; Churchill Livingstone: 2006. pp. 125–142. [Google Scholar]
- Gieroba ZJ, Blessing WW. Fos-containing neurons in medulla and pons after unilateral stimulation of the afferent abdominal vagus in conscious rabbits. Neuroscience. 1994;59:851–858. doi: 10.1016/0306-4522(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Gobel S, Hockfield S, Ruda MA. Anatomical similarities between medullary and spinal dorsal horns. In: Kawamura Y, Dubner R, editors. Oral-Facial Sensory and Motor Functions. Quintessence; Tokyo: 1981. pp. 211–223. [Google Scholar]
- Greenwood LF, Sessle BJ. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: II. Role of trigeminal nucleus caudalis in modulating responses to innocuous and noxious stimuli. Brain Res. 1976;117:227–238. doi: 10.1016/0006-8993(76)90732-0. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J. Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J. Neurosci. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwyn DG, Leslie RA, Hopkins DA. Observations on the afferent and efferent organization of the vagus nerve and the innervation of the stomach in the squirrel monkey. J. Comp. Neurol. 1985;239:163–175. doi: 10.1002/cne.902390204. [DOI] [PubMed] [Google Scholar]
- Hathaway CB, Hu JW, Bereiter DA. Distribution of Fos-like immunoreactivity in the caudal brainstem of the rat following noxious chemical stimulation of the temporomandibular joint. J. Comp. Neurol. 1995;356:444–456. doi: 10.1002/cne.903560311. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J. Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Bereiter DA. GABA (A) receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J. Neurophysiol. 2003;90:2837–2849. doi: 10.1152/jn.00544.2003. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Matsushita M, Tanami T. Termination and cells of origin of the ascending intranuclear fibers in the spinal trigeminal nucleus of the cat A study with the horseradish peroxidase technique. Neurosci. Lett. 1982;31:215–220. doi: 10.1016/0304-3940(82)90022-2. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Terayama R, Jue SS, Sugiyo S, Dubner R, Ren K. Differential rostral projection of caudal brainstem neurons receiving trigeminal input after masseter inflammation. J. Comp. Neurol. 2003;465:220–233. doi: 10.1002/cne.10836. [DOI] [PubMed] [Google Scholar]
- Imbe H, Dubner R, Ren K. Masseter inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845:165–175. doi: 10.1016/s0006-8993(99)01913-7. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation: Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238–247. doi: 10.1159/000047887. [DOI] [PubMed] [Google Scholar]
- Iwata K, Tsuboi Y, Tashiro A, Imai T, Sumino R, Dubner R, Ren K. Enhancement and depression of medullary dorsal horn neuronal activity after noxious and non-noxious stimulation of the face in rats with persistent temporomandibular joint and cutaneous inflammation. J. Neurophysiol. 1999;82:1244–1253. doi: 10.1152/jn.1999.82.3.1244. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Chiaia NL, Haring JH, Rhoades RW. Intersubnuclear connections within the rat trigeminal brainstem complex. Somatosens. Mot. Res. 1990;7:399–420. doi: 10.3109/08990229009144716. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klineberg I. Structure and function of temporomandibular joint innervation. Ann. R. Coll. Surg. Engl. 1971;49:268–288. [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Wolstencroft JH. Projections from brain stem nuclei to the spinal trigeminal nucleus in the cat. Neuroscience. 1983;9:411–420. doi: 10.1016/0306-4522(83)90303-2. [DOI] [PubMed] [Google Scholar]
- Ma XC, Gottschall PE, Chen LT, Wiranowska M, Phelps CP. Role and mechanisms of interleukin-1 in the modulation of neurotoxicity. Neuroimmunomodulation. 2002;10:199–207. doi: 10.1159/000068322. [DOI] [PubMed] [Google Scholar]
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta. 2002;1592:265–280. doi: 10.1016/s0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- Mason P, Fields HL. Axonal trajectories and terminations of on- and off-cells in the cat lower brainstem. J. Comp. Neurol. 1989;288:185–207. doi: 10.1002/cne.902880202. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Differential effects of morphine on corneal-responsive neurons in rostral versus caudal regions of spinal trigeminal nucleus in the rat. J. Neurophysiol. 1998;79:2593–2602. doi: 10.1152/jn.1998.79.5.2593. [DOI] [PubMed] [Google Scholar]
- Nishimori T, Sera M, Suemune S, Yoshida A, Tsuru K, Tsuiki Y, Akisaka T, Okamoto T, Dateoka Y, Shigenaga Y. The distribution of muscle primary afferents from the masseter nerve to the trigeminal sensory nuclei. Brain Res. 1986;372:375–381. doi: 10.1016/0006-8993(86)91148-0. [DOI] [PubMed] [Google Scholar]
- Oakden EL, Boissonade FM. Fos expression in the ferret trigeminal nuclear complex following tooth pulp stimulation. Neuroscience. 1998;84:1197–1208. doi: 10.1016/s0306-4522(97)00550-2. [DOI] [PubMed] [Google Scholar]
- Olszewski J. On the anatomical and functional organization of the spinal trigeminal nucleus. J. Comp. Neurol. 1950;92:401–413. doi: 10.1002/cne.900920305. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J. Comp. Neurol. 1988;268:91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- Pozo MA, Cervero F. Neurons in the rat spinal trigeminal complex driven by corneal nociceptors: receptive-field properties and effects of noxious stimulation of the cornea. J. Neurophysiol. 1993;70:2370–2378. doi: 10.1152/jn.1993.70.6.2370. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending control mechanisms. In: Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, editors. The Senses: A Comprehensive Reference, Vol. 5, Pain. Academic Press; San Diego: 2008. pp. 723–762. [Google Scholar]
- Ren K, Dubner R. Nat. Med. 2010. Interactions between the immune and nervous systems in pain. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY, Harriott A, Crouse U, Capra NF. Innocuous jaw movements increase c-Fos expression in trigeminal sensory nuclei produced by masseter muscle inflammation. Pain. 2003;104:539–548. doi: 10.1016/S0304-3959(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Rocha MJ, Herbert H. Effects of anesthetics on Fos protein expression in autonomic brain nuclei related to cardiovascular regulation. Neuropharmacology. 1997;36:1779–1781. doi: 10.1016/s0028-3908(97)00143-3. [DOI] [PubMed] [Google Scholar]
- Sato T, Kitagawa J, Ren K, Tanaka H, Tanabe A, Watanabe T, Mitsuhashi Y, Iwata K. Activation of trigeminal intranuclear pathway in rats with temporomandibular joint inflammation. J. Oral. Sci. 2005;47:65–69. doi: 10.2334/josnusd.47.65. [DOI] [PubMed] [Google Scholar]
- Scibetta CJ, King RB. Hyperpolarizing influence of trigeminal nucleus caudalis on primary afferent preterminals in trigeminal nucleus oralis. J. Neurophysiol. 1969;32:229–238. doi: 10.1152/jn.1969.32.2.229. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit.Rev.OralBiol. Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Chen IC, Suemune S, Nishimori T, Nasution ID, Yoshida A, Sato H, Okamoto T, Sera M, Hosoi M. Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J. Comp. Neurol. 1986;243:388–408. doi: 10.1002/cne.902430309. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J. Comp. Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Chai B, LaGraize SC, Wei F, Dubner R, Ren K. Microinjection of IL-1beta into the trigeminal transition zone produces NMDA receptor-dependent orofacial hyper-algesia in rats. The Open Pain Journal. 2009a;2:76–83. doi: 10.2174/1876386300902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol. Pain. 2009b;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda E, Kitagawa J, Suzuki I, Nitta-Kubota I, Miyamoto M, Tsuboi Y, Kondo M, Masuda Y, Oi Y, Ren K, Iwata K. Increased Phosphorylation of Extracellular Signal-Regulated Kinase in Trigeminal Nociceptive Neurons Following Propofol Administration in Rats. J. Pain. 2009 b;10:573–585. doi: 10.1016/j.jpain.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Mineta Y, Vos BP. Distribution of Fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J. Neurosci. 1994;14:3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Vos BP. Somatotopic and laminar organization of Fos-like immunore-activity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J. Comp. Neurol. 1993;331:495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Hara T, Shirai H, Abe T, Ichikawa H, Sato T. c-Fos induction in the subnucleus cudalis following noxious mechanical stimulation of the oral mucous membrane. Exp. Neurol. 1994;129:251–256. doi: 10.1006/exnr.1994.1167. [DOI] [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J. Comp. Neurol. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Sakai A. Topographic organization of central terminal region of different sensory branches of the rat mandibular nerve. Exp. Neurol. 1987;96:540–557. doi: 10.1016/0014-4886(87)90217-2. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Chang Z, Bereiter DA. Behavioral and neurophysio-logical correlates of nociception in an animal model of photokeratitis. Neuroscience. 2010;169:455–462. doi: 10.1016/j.neuroscience.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995;682:167–181. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res. Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wei F, Dubner R, Ren K. Selective distribution and function of primary afferent nociceptive inputs from deep muscle tissue to the brainstem trigeminal transition zone. J. Comp. Neurol. 2006;498:390–402. doi: 10.1002/cne.21062. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Mao J, Sung B, Mao J. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain. 2009;141:97–103. doi: 10.1016/j.pain.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–154. doi: 10.1016/j.brainres.2007.08.058. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Guo W, Zou S, Sugiyo S, Dubner R, Ren K. Antibody array analysis of peripheral and blood cytokine levels in rats after masseter inflammation. Neurosci. Lett. 2005;382:128–133. doi: 10.1016/j.neulet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J. Intern. Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J. Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148:237–246. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu ZW, Wen L, Qiao HF, Zhou WX, Zhang YX. Interleukin-1beta enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Res. 2005;1034:172–179. doi: 10.1016/j.brainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Yoshida AK, Dostrovsky JO, Sessle BJ, Chiang CY. Trigeminal projections to the nucleus submedius of the thalamus in the rat. J. Comp. Neurol. 1991;307:609–625. doi: 10.1002/cne.903070408. [DOI] [PubMed] [Google Scholar]
- Yousfi-Malki M, Puizillout JJ. Induction of Fos-like protein in neurons of the medulla oblongata after electrical stimulation of the vagus nerve in anesthetized rabbit. Brain Res. 1994;635:317–322. doi: 10.1016/0006-8993(94)91454-0. [DOI] [PubMed] [Google Scholar]
- Yu XM, Sessle BJ, Hu JW. Differential effects of cutaneous and deep application of inflammatory irritant on mechanoreceptive field properties of trigeminal brain stem nociceptive neurons. J. Neurophysiol. 1993;70:1704–1707. doi: 10.1152/jn.1993.70.4.1704. [DOI] [PubMed] [Google Scholar]
- Zhang R, Yamada J, Hayashi Y, Wu Z, Koyama S, Nakanishi H. (200). Inhibition of NMDA-induced outward currents by interleukin-1beta in hippocampal neurons. Biochem Biophys Res Commun. 2008;372:816–820. doi: 10.1016/j.bbrc.2008.05.128. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zhou QQ, Imbe H, Dubner R, Ren K. Persistent trigeminal Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J. Comp. Neurol. 1999;412:276–291. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]