Abstract
Summary
Adherence and persistence with osteoporosis medications are poor. We conducted a systematic literature review of interventions to improve adherence and persistence with osteoporosis medications. Seven studies met eligibility requirements and were included in the review. Few interventions were efficacious, and no clear trends regarding successful intervention techniques were identified. However, periodic follow-up interaction between patients and health professionals appeared to be beneficial.
Introduction
Adherence and persistence with pharmacologic therapy for osteoporosis are suboptimal. Our goal was to examine the design and efficacy of published interventions to improve adherence and persistence.
Methods
We searched medical literature databases for English-language papers published between January 1990 and July 2008. We selected papers that described interventions and provided results for control and intervention subjects. We assessed the design and methods of each study, including randomization, blinding, and reporting of drop-outs. We summarized the results and calculated effect sizes for each trial.
Results
Seven studies met eligibility requirements and were included in the review. Five of the seven studies provided adherence data. Of those five studies, three showed a statistically significant (p≤0.05) improvement in adherence by the intervention group, with effect sizes from 0.17 to 0.58. Five of the seven studies provided persistence data. Of those five, one reported statistically significant improvement in persistence by the intervention group, with an effect size of 0.36.
Conclusions
Few interventions were efficacious, and no clear trends regarding successful intervention techniques were identified in this small sample of studies. However, periodic follow-up interaction between patients and health professionals appeared to be beneficial.
Keywords: Adherence, Compliance, Intervention, Medication, Motivational interviewing, Osteoporosis, Persistence, Systematic review
Introduction
Osteoporosis primarily affects older adults and typically manifests as painful fractures that can limit independence. The National Osteoporosis Foundation estimates that 44 million Americans have osteoporosis or low bone mass (osteopenia) [1]. Without improvement in osteoporosis prevention and treatment, we can expect this disease to erode the quality of life for an increasing number of people as the population ages. Globally, the number of persons ≥60 years of age is predicted to increase from an estimated 673 million in 2005 to nearly 2 billion in 2050 [2]. Additionally, the rise in osteoporosis-related fractures is projected to be associated with an increase in direct medical costs from 17 billion dollars in 2005 to 25 billion dollars by 2025 [3].
Several effective pharmacologic therapies are available for the treatment of osteoporosis [4]. The method and frequency of administration of these therapies vary, accommodating different patient preferences. Currently, the most widely prescribed therapeutic agents are oral bisphosphonates [5]. Clinical trials have demonstrated that bisphosphonate therapy significantly reduces the risk of vertebral and nonvertebral fractures [6–10]. A meta-analysis of clinical trials reported that bisphosphonate therapy can reduce the risk of vertebral fractures by 40–50% and nonvertebral fractures by 20–40% [11].
While effective pharmacologic therapies for osteoporosis exist, adherence with these therapies is poor. A recent meta-analysis of observational studies reported that only 53% of the study population achieved a medication possession ratio (MPR) of ≥80% 6 months after initiating therapy, and only 43% achieved an MPR of ≥80% 7–12 months after initiating treatment [12]. In retrospective assessments, the MPR is the number of doses dispensed in relation to the dispensing period. Persistence, or the length of time a patient continues therapy, is similarly poor. It has been estimated that only 46% of new users of osteoporosis medications persist with therapy for a treatment period of 7–12 months [12]. These findings provide a strong rationale for developing interventions to improve adherence and persistence with osteoporosis medications.
Many interventions to improve medication adherence for conditions other than osteoporosis have been attempted. A 2008 systematic review by Haynes et al. assessed 81 interventions designed to improve adherence with medications during long-term treatments; they found that only 36 (44%) of the interventions led to improved adherence [13]. Furthermore, even the successful interventions typically produced only modest improvements in adherence and clinical outcomes. The majority of the successful interventions utilized a multifaceted approach involving patient counseling, supervision, and reminders. The interventions reviewed by Haynes et al. included chronic conditions such as diabetes, HIV, asthma, and hypertension. However, none of the included interventions addressed osteoporosis medication adherence.
Interventions to improve adherence and persistence with osteoporosis medications might face more significant obstacles than comparable interventions for other conditions. Although many patients recognize that osteoporosis is an important health concern, knowledge about the disease is poor—particularly with regard to prevention, treatment, and consequences [14, 15]. Furthermore, osteoporosis is asymptomatic until a fracture occurs and might be perceived as less serious than other chronic conditions such as HIV, diabetes, and asthma. Even if an osteoporotic fracture occurs, the individual might not connect the event with osteoporosis. In a survey of individuals who had experienced a fragility fracture, only 17% associated the fracture with osteoporosis [16].
We conducted a systematic review of interventions designed to improve adherence and persistence with osteoporosis medications. Our goal was to examine the types of interventions attempted, their efficacies, and trial methods.
Methods
Definition of terms
The definitions and measurements for adherence, compliance, and persistence are varied across publications. We use the definitions outlined by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [17]. ISPOR categorizes adherence and compliance as synonyms with the common definition of “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen” [17]. In this review, we use the term adherence (synonym: compliance) because it is most consistent with the nomenclature used by the authors of the included papers. An MPR is typically used to measure adherence. Persistence is defined as “the duration of time from initiation to discontinuation of therapy” [17]. Persistence can be measured as a continuous variable (days until medication discontinuation) or a dichotomous variable at the end of a study evaluation period (% of persistent and nonpersistent subjects).
Search strategy
We searched medical literature published between January 1990 and July 2008 using Medline, EMBASE, CINAHL, and Cochrane Systematic Review databases. We chose 1990 as the earliest publication date to coincide with the emergence of bisphosphonate therapy. In each database, we used the search term osteoporosis together with combinations of the following terms: adherence, persistence, compliance, treatment refusal, medication, and intervention. We extended our search by reviewing the bibliographies of relevant publications. In addition, we reviewed abstracts from the past 9 years of the American Society for Bone Mineral Research (ASBMR) annual meetings.
Study selection
We included papers that met the following criteria: the study described an intervention to improve adherence or persistence with osteoporosis medications; adherence or persistence measures were presented for both a control and intervention cohort; and an English-language version of the study was available. We did not restrict studies based on the authors’ definitions or measures of adherence or persistence.
Study evaluation
We developed a standardized data abstraction form, and two reviewers (TG and DHS) independently reviewed the included studies. Information abstracted from the studies included: design, setting, population demographics, intervention and control program features, data sources, and results. Discord between any results of the data abstraction was resolved by further review of the studies and discussion between the reviewers. The Jadad scale is a validated assessment tool used to assess the quality of clinical trials [18, 19]. Three reviewers (TG, MI, DHS) independently applied the Jadad scale to the included studies, and there was no disagreement regarding quality assessment.
We inspected the results of each study to determine if the intervention improved adherence or persistence with osteoporosis medications by a statistically significant margin. The definitions of medication adherence, persistence, and compliance were too heterogeneous to combine studies in a meta-analysis. However, we did calculate the effect sizes for each study using standard equations (see Appendix 1) [20].
Results
Study selection
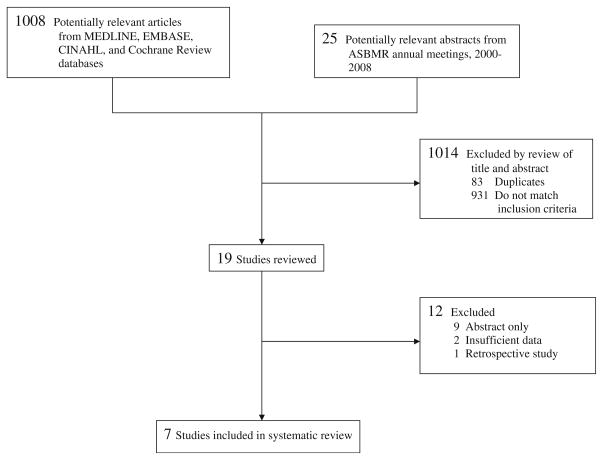
The study selection process is summarized in Fig. 1. Our initial database search yielded 1,008 publications, and a keyword search of past ASBMR annual meeting abstracts yielded 25 potentially relevant abstracts. We reviewed all titles and abstracts and subsequently excluded 1,014 studies that did not meet our inclusion criteria or were duplicates. We thoroughly reviewed the 19 remaining studies—eight published articles and eleven ASBMR abstracts. Two of the published articles were excluded because there were insufficient adherence data for the control arm [21, 22]. We sent requests for complete articles to the authors of the ASBMR abstracts and acquired two prepublication articles, of which one was found to match our inclusion criteria [23]. Thus, a total of seven studies were included in our systematic review [23–29].
Fig. 1.
Flow of studies
Study characteristics
The general characteristics of the selected studies are summarized in Table 1. Although our database search included articles published since 1990, the publication dates of all included studies were between the years 2004 and 2007. Six of the seven included studies were randomized controlled trials [23–26, 28, 29], and one was a non-randomized historical control trial [27]. Study characteristics such as location, setting, and population size varied. Three studies were conducted exclusively in the USA [23, 27, 28], three exclusively in Europe [24, 26, 29], and one study had sites in 21 different countries [25]. The study settings included general practitioners’ offices and specialty clinics. Three studies involved a telephonic component whereby the patients received telephone calls at their residences [23, 27, 28]. The smallest study [29] enrolled fewer than 100 subjects, and the largest study [25] enrolled a total of more than 2,200 subjects. Unless otherwise noted, the number of participants in Table 1 represents new users of osteoporosis therapy.
Table 1.
Characteristics of selected studies designed to improve osteoporosis medication adherence
| Study | Design | Country | Setting | Study Population and Description | ||
|---|---|---|---|---|---|---|
| No. of participants | Description | |||||
| Shu et al, 2008 [23] | RCT | US | Physicians’ offices/Telephone/Mail | Control: | 46 | Usual care. |
| Intervention: | 80 | Patients received a letter with osteoporosis information and an automated phone call inviting them for a BMD test. Their physicians received osteoporosis education. | ||||
| Delmas et al, 2007 [25] | RCT | Multinational | Hospital-based and academic clinics | Control: | 1113 | Patients received Calcium, Vitamin D, and risedronate (5mg) with instructions for taking the medication. At weeks 13 and 25 patients received information about the importance of adherence with therapy. |
| Intervention: | 1189 | Same as control except patients also received feedback on their response to therapy based on BTM measurements at weeks 13 and 25. | ||||
| Cook et al, 2007 [27] | NRHCT | US | Clinic/Telephone | Control: | NR | National baseline data on osteoporosis medication adherence was used. |
| Intervention: | 188 | Patients received phone calls from nurse educators who provided counseling consistent with motivational interviewing principles. | ||||
| Cooper et al, 2006 [24] | RCT | UK | Physicians’ offices | Control: | 529 | Weekly alendronate tablet (70mg) prescribed. |
| Intervention: | 547 | Monthly ibandronate tablet (150mg) and a patient support program that included information about osteoporosis; monthly reminder phone calls from nurses who provided dosing instructions, osteoporosis information, and stressed the importance of adherence; and a newsletter at 3 months. | ||||
| Guilera et al, 2005 [26] | RCT | Spain | Physicians’ offices | Control: | 259a | Patients met with a physician (no leaflet provided). |
| Intervention: | 269a | Patients received a leaflet with information about osteoporosis and the importance of therapy adherence. The attending physician reviewed the leaflet with the patient. | ||||
| Schousboe et al, 2005 [28] | RCT | US | Clinic/Telephone | Control: | 31 | Patients received an osteoporosis informational brochure. |
| Intervention: | 37 | Patients received an osteoporosis informational brochure, BMD test, and four telephone consultations with a nurse educator. | ||||
| Clowes et al, 2004 [29] | RCT | UK | Osteoporosis clinic | Control: | 24 | Usual care. |
| Intervention: | Arm A: 24 Arm B: 25 |
Arm A: Patients met with nursing staff at 12, 24, 36 weeks and participated in pre-defined interviews consisting of open questions related to well-being and medication problems. Arm B: Same as Arm A except following each interview, patients were also presented with a graph showing their response to therapy based on BTM measurements. |
||||
RCT randomized controlled trial, NRHCT nonrandomized historical controlled trial, NR not reported
Includes chronic users of antiresorptive therapy
The types of interventions varied widely. In two studies, the intervention consisted of monitoring subjects’ bone turnover markers and providing feedback to the subjects based on their response to therapy [25, 29]. Five studies included a structured patient education component that was presented face-to-face or via brochures, letters, or telephone calls [23, 24, 26–28]. In addition to providing education about osteoporosis, one study provided extensive telephonic patient counseling in order to address individual barriers to medication adherence [27]. One study was unique in that the intervention did not only target osteoporosis patients but also provided their physicians with education related to osteoporosis diagnosis and treatment [23]. Finally, a single study compared adherence with a weekly alendronate dose versus a monthly dose of an ibandronate plus a patient support program [24].
Quality assessment
The results of the quality assessment are presented in Table 2. The Jadad quality assessment scale brings to attention three methodological attributes of clinical studies that are related to bias reduction [18, 19]. One point was awarded for each affirmative answer to the questions regarding randomization, double blinding, and description of dropouts. The possible scores on the scale range from a minimum of zero to a maximum of five points. None of the studies were described as double-blinded. All of the studies included adequate description of the withdrawals and dropouts. Six of the seven studies randomly assigned enrollees to control and intervention groups. The lone exception was Cook et al., who employed a nonrandomized historical control group based on unpublished medication adherence data [27].
Table 2.
Quality of reporting assessment using the Jadad scale
| Study | Study described as randomized? | Method of randomization described and appropriate? | Study described as double blind? | Method of double blinding described and appropriate? | Description of withdrawals and dropouts? | Score a (0–5 pts) |
|---|---|---|---|---|---|---|
| Shu et al, 2008 [23] | + | + | − | − | + | 3 pts |
| Delmas et al, 2007 [25] | + | + | − | − | + | 3 pts |
| Cook et al, 2007 [27] | − | − | − | − | + | 1 pt |
| Cooper et al, 2006 [24] | + | + | − | − | + | 3 pts |
| Guilera et al, 2005 [26] | + | + | − | − | + | 3 pts |
| Schousboe et al, 2005 [28] | + | + | − | − | + | 3 pts |
| Clowes et al, 2004 [29] | + | + | − | − | + | 3 pts |
+ Yes, − No
One point awarded for each affirmative item
Reported findings
The definitions used for the measured outcomes (adherence and persistence) and the statistical results of the studies are shown in Table 3. Five of the seven studies provided complete adherence data [23, 24, 26, 27, 29]. Of these, three studies showed a statistically significant (p≤0.05) improvement in adherence by the intervention group over the control group [24, 27, 29]. The effect sizes of these three studies ranged from 0.17 to 0.58. Cook et al., who provided the intervention group with a robust telephonic counseling program, reported that 69% of the intervention subjects were adherent versus 41% of the control group (effect size 0.58) [27]. Clowes et al. utilized two intervention arms [29]. Arm A subjects received attention from nurses while meeting for predefined interviews. Arm B subjects participated in the same interviews, but they were also provided with feedback based on their response to therapy. Adherence at 1 year was better in both intervention arms (arm A: 68%, effect size 0.53; arm B: 63%, effect size 0.42) compared to the control group (42%). Cooper et al. compared monthly ibandronate plus a patient support program to weekly alendronate without a support program [24]. At 6 months, 75% of the ibandronate users were adherent versus 68% of the alendronate users (effect size 0.17).
Table 3.
Definitions of measured outcomes and results
| Study | Measured Outcome(s) | Definition | Results | |||
|---|---|---|---|---|---|---|
| Control | Intervention | Effect Sizee | P Value | |||
| Shu et al, 2008 [23] | Adherence a | A medication possession ration (MPR) – the ratio of available medication to the total number of days in the follow-up period. | 76% | 89% | 0.24 | 0.28 |
| Persistence b | Days until discontinuation – where discontinuation is considered as at least 30 days without medication. | 79 days | 85 days | 0.19 | 0.16 | |
| Delmas et al, 2007 [25] | Persistence c | Time in days from first dose of risedronate until discontinuation of therapy. | 77% | 80% | 0.07 | 0.16 |
| Cook et al, 2007 [27] | Adherence d | Pharmacy fill records were used to measure adherence, but an explicit definition of adherence was not provided. | 41% | 69% | 0.58 | 0.009 |
| Cooper et al, 2006 [24] | Adherence d | Filling at least five of six prescriptions. | 73% | 80% | 0.17 | 0.008 |
| Persistence c | Number of days from study entry to date of the first failure to persist – where failure to persist is missing a month’s prescription or withdrawal from the study. | 39% | 57% | 0.36 | < 0.0001 | |
| Guilera et al, 2005 [26] | Adherence d | A subject self-reported measure based on the responses to a four question adherence questionnaire – the Morisky test. (No items failed = high adherence) | 47% | 53% | 0.10 | 0.38 |
| Schousboe et al, 2005 [28] | Persistence c | A subject self-reported measure. Subjects asked if they were taking the medication in a survey 12 months from enrollment. (Yes = persistent, No = non-persistent) | 71% | 65% | −0.13 | NS |
| Clowes et al, 2004 [29] | Adherence d | Ratio of tablets taken to tablets prescribed. (>75% = Adherent) | 42% | Arm A: 68% Arm B: 63% |
0.53 0.42 |
.05 .15 |
| Persistence c | Continuing to take medication for ≥ seven days prior to a 12 month study visit. | 67% | Arm A: 88% Arm B: 79% |
0.52 0.27 |
.06 .26 |
|
NS not statistically significant
Results reported as the median medication possession ratio for the study subpopulation new users of medication
Results reported as the median number of days until discontinuation of therapy for the study subpopulation new users of medication
Results reported as the percentage of subjects who were persistent throughout the study period
Results reported as the percentage of subjects who were adherent throughout the study period
Calculations for the effect sizes and general interpretation guidelines are described in Appendix 1
Five of the seven studies provided persistence data [23–25, 28, 29]. Only one of these studies reported a statistically significant improvement in persistence with therapy by the intervention group [24]. Cooper et al. reported that 57% of intervention subjects persisted with therapy [24]. While this percentage was suboptimal, it was significantly better than the 39% mark achieved in the control group (effect size 0.36). All other studies except Clowes et al. reported better persistence in the control group. Clowes et al. reported that 88% of intervention subjects who received only nurse attention (arm A) were persistent with therapy at 1 year, and 79% of subjects who received nurse attention plus response to therapy feedback (arm B) were persistent at 1 year [29]. Although these persistence results were better than the control group (67%), the differences were not statistically significant.
Discussion
If the rate of increase in the number of people at risk for osteoporosis outpaces efforts to improve prevention and treatment, then the physical, psychosocial, and economic burden of the disease will grow. While the interventions described in this systematic review represent important efforts towards improving adherence and persistence with osteoporosis medications and reducing the health and economic impact of the disease, their efficacies were limited. It is important to understand the strengths and limitations of these studies and to consider further steps that can improve future studies and, hopefully, improve adherence and persistence with osteoporosis medications.
The studies included in this review have several limitations. None of the studies were double-blinded trials. This limitation might have introduced elements of reporting bias, particularly in the studies that relied exclusively on self-reported adherence from the study subjects [26, 28]. Additionally, the ultimate measure of an effective osteoporosis medication adherence intervention is the reduction of osteoporotic fractures, yet only one study reported on this important clinical outcome [25]. Beyond measuring adherence and persistence, future studies should utilize longer, more detailed follow-up in order to measure fracture outcomes. Several of the interventions were limited in scope and dedicated minimal intervention time with the study subjects. Shu et al. focused intervention efforts on the physician and provided only mailed education material to the study patients [23]. Guilera et al. only provided an educational leaflet to study patients [26]. Delmas et al. enhanced care for the intervention group only by providing feedback based on their response to therapy [25]. It is notable that in both interventions which provided feedback based on response to therapy, such feedback did not appear to add value [25, 29].
That such one-dimensional strategies proved ineffective is consistent with the findings of the comprehensive medication adherence systematic review by Haynes et al., who reported that the most effective medication adherence interventions typically utilize multifaceted approaches [13]. This seems logical and particularly relevant to osteoporosis therapy because barriers to medication adherence vary widely among patients [30, 31]. Potential barriers to osteoporosis medication adherence and persistence include inconvenient dosing regimens, poor knowledge about the disease, concerns about side effects, low perceived risk of fracture, doubt regarding therapeutic benefits, and cost of the medications. As such, it is unrealistic to expect that a one-dimensional intervention such as education or feedback based on response to therapy could significantly improve adherence and persistence across a diverse population.
While multi-faceted interventions are considered more potent [13], in this small sample of studies, it is difficult to identify a clear trend as to which specific combination of intervention elements works best. It appears that the most efficacious interventions shared one important theme—interaction between study subjects and health care professionals. In each of the interventions with statistically significant improvement in adherence, the intervention subjects had periodic one-on-one follow-up with trained health care professionals, whereas the control subjects did not. The content and frequency of the interaction varied, but its presence appears to have been beneficial. Although nonrandomized in design, the intervention which demonstrated the greatest improvement in adherence employed an intervention whereby subjects participated in multiple counseling sessions with nurses [27]. The nurses were trained in a client-centered counseling style which holds promise for improving medication adherence—motivational interviewing [32]. This counseling style has been incorporated into several effective medication adherence interventions for chronic conditions including hypertension, HIV, and asthma [33–36]. Because reasons for non-adherence are multiple and personal, we believe the role of patient counseling is important and should be explored further in medication adherence interventions.
Our systematic review compiled evidence of the inconsistent manner in which the terms adherence and persistence are used in research literature. There was significant variance in how these terms were defined and measured among the seven papers included in this review (Table 3). This variance impeded comparison of the efficacies of the interventions across studies and precluded a meta-analysis of their results. Furthermore, it can be confusing to readers when identical terms have several different meanings across publications. For these reasons, we recommend that future studies adopt standard definitions, such as those put forth by ISPOR [17], and utilize a MPR for adherence (synonym: compliance) and a continuous or dichotomous variable for persistence based on the length of time from initiation to discontinuation of therapy.
Improving adherence and persistence with osteoporosis medications is a complex and challenging issue, and the health and economic consequences of non-adherence are serious. The emergence of intravenous medications with low-frequency dosing regimens, such as zoledronic acid, might improve adherence and persistence. However, it is unclear what the adherence with such regimens will be after the first year. Moreover, it is unlikely that these therapies will become the standard unless the costs of drug and administration are similar to the generic, oral formulations. Several intervention techniques described in this review may help to improve medication adherence, including meaningful follow-up interaction between patients and health care professionals, such as motivation interviewing. These techniques require further examination within the context of randomized trials. Such efforts might improve not only adherence to osteoporosis medications but also provide insight into medication adherence in general.
Acknowledgments
This review was supported by the National Institutes of Health (AR-47782). Dr. Shrank is supported by a career development award from the National Heart, Lung and Blood Institute (HL-090505). Dr. Brookhart is supported by a career development award from the National Institutes of Health (AG-027400).
Appendix 1. Calculations of Effect Size
For the studies (Delmas et al., Cook et al., Cooper et al., Guilera et al., Schousboe et al., Clowes et al.) for which we had the proportions (P) of the intervention and control populations who were adherent or persistent, the following calculation was used to determine effect size:
For the study (Shu et al.) for which we had the means (M) and standard deviations (σ) for adherence and persistence in the intervention and control populations, the following calculation was used to determine effect size:
As a general guideline, effect sizes ≅0.20 are considered to have negligible clinical importance; ≅0.50 moderate clinical importance; and ≅0.80 crucial clinical importance (see also Cohen 1987 [20]).
Footnotes
Conflicts of interest None.
Contributor Information
T. Gleeson, Division of Rheumatology, Immunology, and Allergy, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, PBB-B3, Boston, MA 02115, USA
M. D. Iversen, Division of Rheumatology, Immunology, and Allergy, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, PBB-B3, Boston, MA 02115, USA. MGH Institute of Health Professions, Boston, MA, USA
J. Avorn, Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
A. M. Brookhart, Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
J. N. Katz, Division of Rheumatology, Immunology, and Allergy, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, PBB-B3, Boston, MA 02115, USA. Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, MA, USA
E. Losina, Division of Rheumatology, Immunology, and Allergy, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, PBB-B3, Boston, MA 02115, USA. Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, MA, USA
F. May, Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
A. R. Patrick, Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
W. H. Shrank, Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
D. H. Solomon, Email: dsolomon@partners.org, Division of Rheumatology, Immunology, and Allergy, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, PBB-B3, Boston, MA 02115, USA. Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
References
- 1. [accessed 12/15/2008];National Osteoporosis Foundation, America’s bone health: the state of osteoporosis and low bone mass in our nation. 2008 Available at http://nof.org/advocacy/prevalence/index.htm.
- 2.United Nations, D.o.E.a.S.A. Population and Division, World Population Prospects: The 2006 Revision, Highlights, Working Paper No. ESA/P/WP.202. 2007. [Google Scholar]
- 3.Burge R, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Cole Z, Dennison E, Cooper C. Update on the treatment of post-menopausal osteoporosis. Br Med Bull. 2008;86:129–143. doi: 10.1093/bmb/ldn017. [DOI] [PubMed] [Google Scholar]
- 5.Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2(4):211–219. doi: 10.1038/ncpendmet0121. quiz following 238. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. Jama. 1998;280 (24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348 (9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 8.Harris ST, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Jama. 1999;282 (14):1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 9.Reginster J, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 10.Chesnut IC, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 11.Cranney A, et al. Meta-analyses of therapies for postmenopausal osteoporosis IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23(4):570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 12.Kothawala P, et al. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82(12):1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 13.Haynes RB, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Cadarette SM, et al. Psychometric properties of the “Osteoporosis and You” questionnaire: osteoporosis knowledge deficits among older community-dwelling women. Osteoporos Int. 2007;18(7):981–989. doi: 10.1007/s00198-007-0326-z. [DOI] [PubMed] [Google Scholar]
- 15.Werner P. Knowledge about osteoporosis: assessment, correlates and outcomes. Osteoporos Int. 2005;16(2):115–127. doi: 10.1007/s00198-004-1750-y. [DOI] [PubMed] [Google Scholar]
- 16.Giangregorio L, et al. Do patients perceive a link between a fragility fracture and osteoporosis? BMC Musculoskelet Disord. 2008;9:38. doi: 10.1186/1471-2474-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer JA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, et al. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73. doi: 10.1016/0197-2456(94)00031-w. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioral sciences. L Erlbaum Associates; Hillsdale, NJ: 1987. [Google Scholar]
- 21.Cuddihy MT, et al. A prospective clinical practice intervention to improve osteoporosis management following distal forearm fracture. Osteoporos Int. 2004;15(9):695–700. doi: 10.1007/s00198-004-1597-2. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar SR, et al. Persistence, reproducibility, and cost-effectiveness of an intervention to improve the quality of osteoporosis care after a fracture of the wrist: results of a controlled trial. Osteoporos Int. 2007;18(3):261–270. doi: 10.1007/s00198-006-0248-1. [DOI] [PubMed] [Google Scholar]
- 23.Shu ASM, Polinski J, Jan S, Patel M, Truppo C, Breiner C, Chen Y, Weiss T, Solomon DH. Adherence to Osteoporosis Medications After Patient and Physician Education: A Post-Hoc Analysis of a Randomized Controlled Trial. American Journal of Managed Care. 2009 (in press) [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper A, Drake J, Brankin E. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60 (8):896–905. doi: 10.1111/j.1742-1241.2006.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmas PD, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–1304. doi: 10.1210/jc.2006-1526. [DOI] [PubMed] [Google Scholar]
- 26.Guilera M, et al. Does an educational leaflet improve self-reported adherence to therapy in osteoporosis? The OPTIMA study. Osteoporos Int. 2006;17(5):664–671. doi: 10.1007/s00198-005-0031-8. [DOI] [PubMed] [Google Scholar]
- 27.Cook PF, Emiliozzi S, McCabe MM. Telephone counseling to improve osteoporosis treatment adherence: an effectiveness study in community practice settings. Am J Med Qual. 2007;22(6):445–456. doi: 10.1177/1062860607307990. [DOI] [PubMed] [Google Scholar]
- 28.Schousboe JT, et al. Education and phone follow-up in postmenopausal women at risk for osteoporosis: effects on calcium intake, exercise frequency, and medication use. Dis Manag Health Outcomes. 2005;13(6):395–404. [Google Scholar]
- 29.Clowes JA, Peel NFA, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–1123. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 30.Rossini M, et al. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int. 2006;17(6):914–921. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 31.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20(4):764–771. doi: 10.1016/s0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 32.Rollnick S, Miller W. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 33.DiIorio C, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–283. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golin CE, et al. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmaling KB, et al. A randomised controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psychol Med Settings. 2001;8(3):167–172. [Google Scholar]
- 36.Ogedegbe G, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]