Stroke is the third-leading cause of death in the United States, Canada, Europe, and Japan. According to American Heart Association statistics, there are now 795 000 new strokes each year, resulting in 200 000 deaths, or 1 of every 16 deaths, per year in the United States.1 Ischemic stroke represents 80% of the total, and hemorrhagic stroke makes up the remainder. Stroke is the leading cause of adult disability in both North America and Medicare reimbursement for long-term adult care. The National Institutes of Health (NIH) estimate that stroke costs exceed $73 billion in US healthcare dollars per year.1 Improved treatments are needed to reduce the burden of human suffering and to lessen the financial burden on society.
Epidemiologically, there are 795 000 strokes per year in the United States,1 but this number does not represent the number of treatable strokes. The real number of ischemic strokes amenable to some form of endovascular revascularization intervention is probably a small subset of the total number. This disparity explains, at least in part, the relatively slow progress in stroke trial enrollment and scientific progress compared with the more rapid development of cardiac interventions. The number of acute strokes potentially requiring emergent intervention ranges from 58 000 to 120 000 per year, or 7% to 15% of the total number, because many acute ischemic strokes are due to hemorrhage, small-vessel occlusions (lacunar strokes), transient ischemic attacks, end-of-life strokes, and mild strokes that do not warrant the risk of an endovascular procedure.2 In most communities, only 1% to 7% of stroke victims arrive at hospital in time for stroke revascularization therapies. Even in communities with highly organized and active stroke programs, <10% of stroke victims receive immediate treatment.3 Even if such programs were instituted across the United States, ≈58 000 stroke victims (9% of 645 000 ischemic stroke patients) would receive treatment.2 The number potentially needing an endovascular intervention is less clear. Extensive brain injury is already present in many patients on admission. In comprehensive stroke centers following evidence-based guidelines for intervention, endovascular ischemic stroke procedures remain among the least common procedures that neurointerventionalists perform (on average, 8 procedures per year).4 The reason is that 20% of strokes are due to small-artery occlusions (lacunar strokes), and another 20% to large-artery occlusions causing severe strokes.
We have organized this review as follows. First, we discuss stroke imaging, because it emphasizes the essential problem in distinguishing ischemic penumbra, which is potentially recoverable cerebrum, from infarcted cerebrum, which is not recoverable. Second, we review intravascular fibrinolytic therapies, intravenous and intra-arterial fibrinolysis. Third, we describe mechanical revascularization therapies, including thromboembolectomy, suction thrombectomy, angioplasty and stent revascularization, and the new stent-retriever thrombectomy. Finally, we discuss the potential futility of some of these therapies without further scientific advancements.
Stroke Imaging
Neurological imaging has developed rapidly over the last 20 years, but stroke physiology is complex. Acutely injured but salvageable brain tissue is not clinically discernable from irrevocably infarcted tissue by physical examination. Most stroke patients will present with some core infarct that cannot be restored even by the most rapid intervention. When present, there is strong evidence that tissue with impaired blood flow surrounding the core infarct may be saved by early intervention. This so-called ischemic penumbra will progress to infarction without rapid revascularization. In contrast, an infarct without penumbra will not improve with intervention, and patients may actually be harmed by procedural revascularization causing hemorrhagic transformation. Modern imaging may provide information to draw this important distinction and to guide emergency triage.
The distinction between hemorrhagic and ischemic stroke is pivotal in the management of acute stroke. All major stroke trials have relied on the sensitivity and availability of the nonenhanced computed tomography (CT) brain scan primarily for the presence of acute hemorrhage to determine patient enrollment. Thus, most knowledge about the imaging manifestations of acute ischemic stroke and response to treatment is based on the simplest form of CT imaging. Ease of access, rapid scan time, and broad availability mean that CT remains the most commonly used form of acute stroke imaging and has the highest level of evidence.5 Therefore, nonenhanced CT brain scan remains the only cerebral imaging test required before intravenous administration of recombinant tissue-type plasminogen activator (rtPA).
Magnetic resonance imaging (MRI) has specific advantages and disadvantages compared with CT. Diffusion-weighted imaging has proven to be both sensitive and specific to the presence of early cerebral ischemia. Recent evidence suggests that MRI may also be as sensitive to the presence of acute hemorrhage as CT.6 Thus, MRI may be used as the primary imaging modality to assess patients with suspected acute stroke instead of CT.5 The disadvantages remain limited availability, longer scan times, and the incompatibility of the magnetic environment with iron-containing medical equipment and conductive bioprostheses (eg, cardiac pacers, internal defibrillators).
An optimal form of cerebral viability imaging remains elusive, and research is ongoing. The goal is to identify and measure the volume of brain tissue irrevocably damaged and tissue at risk for infarction that remains potentially salvageable. Now, CT angiography rivals the quality of diagnostic catheter angiography to assess for large cerebral artery occlusion, and CT perfusion imaging measures mean contrast transit time, relative cerebral blood volume, and relative blood flow. With contrast-enhanced MRI, perfusion-weighted imaging also permits determination of mean contrast transit time, relative cerebral blood volume, and relative cerebral blood flow. Comparison of perfusion data representing the ischemic penumbra with diffusion-weighted images representing the core infarct should allow physicians to make treatment decisions regarding the likely risks and benefits of intervention. In fact, some investigators have argued that use of the time interval from stroke onset to guide therapy should be abandoned in preference for imaging analysis of core infarct volume in relation to ischemic penumbra.7
The significance of perfusion imaging and its relevance to stroke pathophysiology are the subject of vigorous debate. The complexity and variability of cerebral circulation, including leptomeningeal, circle of Willis, and external carotid artery collaterals, mean that perfusion data remain difficult to apply to treatment of individual patients. Concerns about stroke application and misapplication persist. Analysis of diffusion-perfusion mismatch to guide stroke treatment shows benefit in some studies, and is the subject of the ongoing NIH-funded Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR-RESCUE) trial.8 Meanwhile, other studies have shown that perfusion imaging does not correlate well with the physiological parameters of cerebral ischemia it purportedly predicts,9 and diffusion-perfusion mismatch has not been shown to effectively differentiate between salvageable and unsalvageable tissue.10 For these and other reasons, the Advanced Neuroimaging for Acute Stroke Treatment group proposed the establishment of an Acute Stroke Imaging Consortium to facilitate the development and validation of advanced stroke imaging.11
The problem with the revascularization therapies that we discuss here is that infarcted cerebrum will not recover, yet tissue in the ischemic penumbra might recover if rescued in time. Unfortunately, that window of time is uncertain, and probably varies significantly from patient to patient. Great harm can occur if inappropriate revascularization results in hemorrhagic transformation of an ischemic infarct. The concern is that enthusiasm for the development of new treatment strategies has the potential to distort and exaggerate scientific research.12 It is important to evaluate and test the significance of stroke trial results rigorously so that there is not a movement to prematurely expand the indications and applications of treatment methods that may not yet be broadly effective.13 Meanwhile, stroke patients who might achieve good recovery despite presentation outside the confines of conventional treatment paradigms should not be overlooked. Their treatment should be the subject of additional carefully designed clinical trials.
Intravenous Fibrinolysis
In any review of endovascular stroke therapy, there are important reasons to initiate the discussion with intravenous therapy for acute ischemic stroke. Intravenous fibrinolysis with rtPA remains the only Food and Drug Administration (FDA)–approved treatment (Level of Evidence 1, Class A recommendation) for stroke patients presenting within 3 hours after onset based on the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study (NINDS rt-PA).14 The European Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) registry enrolling 6483 patients confirmed the safety and efficacy of intravenous fibrinolysis3 (Table 1).15 Pooled analysis of 6 major trials showed that there could be additional benefit beyond the 3-hour limit despite increased risk. The European Cooperative Acute Stroke Study III (ECASS3) has now shown the safety and efficacy of intravenous thrombolysis with alteplase for ischemic stroke in selected patients treated 3 to 4.5 hours after stroke onset.16 Although many endovascular treatment protocols were originally designed with a 3-hour limitation for intravenous fibrinolysis in mind, the new 4.5-hour treatment horizon for selected patients should probably replace the 3-hour limit. Moreover, the latest AHA guidelines state that availability of endovascular treatment services should not supersede the use of intravenous fibrinolysis when indicated.17
Table 1.
Major Trials of Intravenous and Intra-Arterial Treatment for Acute Ischemic Stroke
| Major Stroke Treatment Trials
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NINDS rt-PA18 |
NINDS rt-PA Control18 |
SITS- MOST15 |
PROACT II rtPA19 |
PROACT-II Control19 |
MELT rtPA20 |
MELT Control20 |
IMS-121 | IMS-222 | CLOTBUST23 | EKOS 124 |
MERCI 225 |
MULTI- MERCI26 |
Penumbra Pivotal27 |
|
| Patients, n | 312 | 312 | 6483 | 121 | 59 | 57 | 57 | 80 | 81 | 63 | 14 | 141 | 164 | 125 |
| Age, y | 68 | 66 | 68 | 64 | 64 | 67 | 67 | 64 | 67 | 67 | 64 | 67 | 68 | 64 |
| Baseline NIHSS | 18 | 18 | 12 | 17 | 17 | 14 | 14 | 18 | 19 | 16 | 18 | 20 | 19 | 18 |
| Revascularization, % | 66 | 18 | 73 | 56 | 60 | 38 | 57 | 48 | 68 | 82 | ||||
| Symptomatic ICH, % | 1 | 7 | 1.7 | 10.9 | 2 | 9 | 2 | 6 | 10 | 4.8 | 14 | 7.8 | 9.8 | 11.2 |
| Asymptomatic ICH, % | 0.5 | 24 | 43 | 27.7 | 30.5 | 16.8 | ||||||||
| 90-d mortality, % | 24 | 21 | 11 | 25 | 27 | 5.3 | 3.5 | 16 | 16 | 15 | 36 | 43.5 | 34 | 32.8 |
| 90-d mRS ≤2 | 28 | 30 | 54 | 40 | 25 | 49.1 | 38.6 | 43 | 46 | 42 | 43 | 27.7 | 36 | 25 |
NINDS rt-PA indicates National Institute of Neurological Disorders Tissue Plasminogen Activator for Acute Ischemic Stroke Trial; SITS-MOST, Safe Implementation of Thrombolysis in Stroke-Monitoring Study; PROACT II, Prolyse in Acute Cerebral Thromboembolism trial; rtPA, recombinant tissue-type plasminogen activator; MELT, Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial; IMS, Interventional Management of Stroke; CLOTBUST, randomized trial of ultrasound-enhanced thrombolysis for acute ischemic stroke; EKOS, EKOS MicroLysUS infusion catheter study; MERCI, Mechanical Embolus Removal in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale; ICH, intracranial hemorrhage; and mRS, modified Rankin Scale.
Intra-Arterial Therapies
Endovascular therapy for patients with acute ischemic stroke is currently an area of intense investigation. An Accreditation Council for Graduate Medical Education–approved training pathway was developed to train physicians for endovascular treatment of cerebrovascular diseases, including ischemic stroke.28 Training criteria and standards of performance for endovascular stroke treatment are summarized in a consensus document.17,29 The American Stroke Association has given qualified endorsement of intra-arterial fibrinolysis, despite its lack of scientifically proven efficacy in >1 trial. Intra-arterial fibrinolysis has been studied in several randomized trials and numerous case series but proven according to FDA standards in only 1 trial using prourokinase, a drug no longer available.4 Thus, intra-arterial fibrinolysis has the status of community standard in many locales, but remains an off-label application of any available fibrinolytic agent requiring further experimental proof of safety and efficacy for on-label application. Similarly, 2 devices have been granted US FDA approval with an indication for mechanical stroke thrombectomy, but no thrombectomy device has yet been scientifically proven to improve patient outcomes.
It has been recognized for nearly 20 years that intravenous fibrinolysis is less likely to recanalize large cerebral artery occlusions. Intravenous fibrinolysis is time-consuming, and the action of fibrinolytic agents may take ≥2 hours.30 Occlusions in more proximal locations, including the intracranial internal carotid artery and proximal middle cerebral artery, are more resistant to intravenous delivery of lytic agents, with only 8% to 12% probability of early recanalization.31 In contrast, intra-arterial administration of fibrinolytic agents has been shown to significantly increase the chances of recanalization.4
Although intra-arterial fibrinolysis has been used for nearly 3 decades and has shown apparent benefit in case series, Prolyse in Acute Cerebral Thromboembolism II (PROACT II)4 is the only randomized trial to date on the safety and efficacy of intra-arterial fibrinolysis in acute ischemic stroke. In this study, 12 323 stroke patients were evaluated to identify 180 eligible patients with proximal middle cerebral artery occlusions (1.4% of screened patients). Patients were randomized to treatment with intra-arterial prourokinase with intravenous heparin versus intravenous heparin alone. More than 10 years later, 66% recanalization and 40% good neurological outcomes achieved in PROACT II remain the yardstick by which newer endovascular methods are judged. Although higher rates of recanalization have been accomplished in more recent trials, 40% favorable neurological outcomes and 25% mortality remain the standard for new devices and techniques even a decade later (Table 1).
The EKOS MicroLysUS infusion catheter (EKOS Corp, Bothell, WA) was designed to augment intra-arterial fibrinolysis by creating local convection currents at the site of occlusion using low-intensity, high-frequency ultrasound. This facilitates diffusion of the lytic agent into the thrombus, increasing its effective surface area of interaction. A phase I trial reported preliminary results in 14 patients with 60% recanalization at 60 minutes despite treatment of large clot burdens in some patients.32 The device was tested again in Interventional Management of Stroke (IMS) II33 with promising results (Table 1), and is an option for use in the NINDS rt-PA-funded IMS III trial,34 which is currently enrolling at 60 stroke centers worldwide.
The data on intra-arterial fibrinolysis for vertebrobasilar occlusion are limited. Mostly because neurological outcomes are abysmal without treatment, review of the literature generally supports potential benefit even as long as 18 to 24 hours after symptom onset.5 A meta-analysis of multiple case series comparing intravenous with intra-arterial fibrinolysis for acute vertebrobasilar stroke in 422 patients showed marginally better recanalization rates with intra-arterial therapy (65% versus 53%; P=0.5), good neurological outcomes in 22% to 24% of patients, but no clear difference in efficacy between the 2 modalities.35 Similar findings were demonstrated in 400 patients in the Basilar Artery International Cooperative Study (BASICS) trial.24
Lee et al31 performed a meta-analysis of randomized, controlled trials evaluating intra-arterial fibrinolysis for acute ischemic stroke. To date, PROACT II remains the only randomized, controlled trial of intra-arterial fibrinolysis to show a statistically significant clinical benefit.4 Four other trials demonstrated trends in favor of intra-arterial fibrinolysis, but were inadequately powered to demonstrate statistically significant improvement in neurological outcomes. For this reason, meta-analysis of combined data on 395 patients from 5 trials provides a strong indication of statistically significant good (odds ratio, 2.05; 95% confidence interval, 1.33 to 3.14; P=0.001) and excellent (odds ratio, 2.14; 95% confidence interval, 1.31 to 3.51; P=0.003) outcomes.31
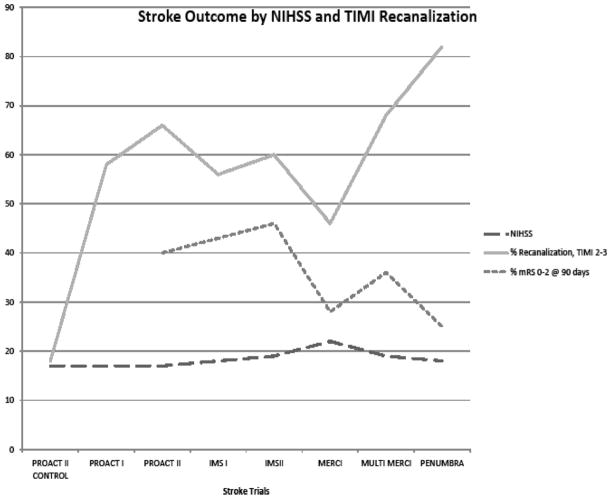
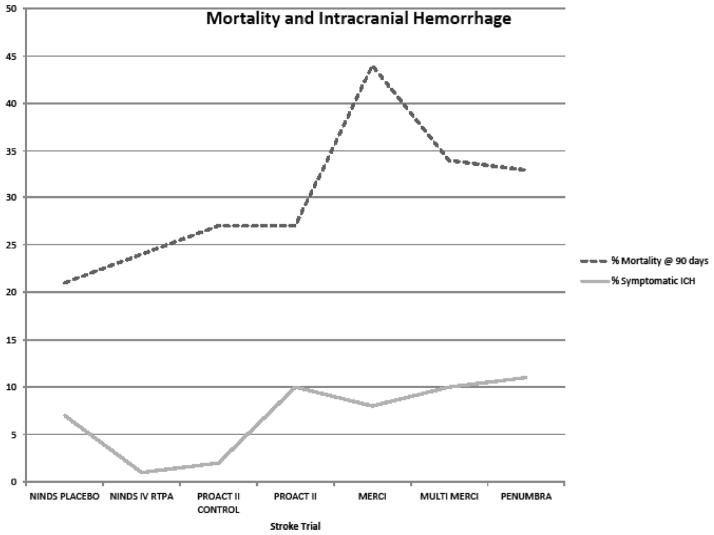
Lee et al31 noted an interesting discrepancy between recanalization of occluded cerebral arteries and clinical outcomes. Intra-arterial fibrinolysis was associated with an absolute increase in the rate of partial or complete vessel recanalization (46.8%) over placebo therapy. Yet, the rate of recanalization is 3 times higher than the absolute increase in good (14.8%) or excellent (13.0%) clinical outcomes. Although recanalization and reperfusion are presumably benefits of intra-arterial fibrinolysis,36 these authors speculate that the disparity is due to either limitations in the outcome measurement tools to adequately detect real clinical improvement in stroke victims or completion of stroke injury before revascularization and reperfusion.31 The gap between recanalization and clinical improvement becomes more pronounced during the evaluation of mechanical revascularization studies performed up to 8 hours after stroke onset (Table 1 and Figure 1). Ultimately, this issue must be addressed for endovascular stroke therapies to succeed. Patient selection for treatment still needs to be defined, which may be the role for advanced brain viability imaging.
Figure 1.
Higher rates of recanalization, approaching 90% in the Penumbra Pivotal Trial, do not correspond with similar rates of clinical improvement after treatment as measured by the modified Rankin scale (mRS) at 90 days. NIHSS indicates National Institutes of Health Scale; TIMI, Thrombolysis in Myocardial Infarction; PROACT II, Prolyse in Acute Cerebral Thromboembolism trial; IMS, Interventional Management of Stroke; and MERCI, Mechanical Embolus Removal in Cerebral Ischemia.
Mechanical Revascularization
Thromboembolectomy
Broadly speaking, spontaneous or therapeutic recanalization and reperfusion in acute ischemic stroke are associated with improved functional outcomes and reduced mortality in a meta-analysis of 53 studies.36 For patients who are ineligible for fibrinolytic therapy, those with large-artery occlusions and severe stroke disability, alternative methods of treatment are under investigation. Mechanical thrombectomy devices specifically for acute stroke intervention were first developed in the 1990s as engineering technologies for fabrication of microcatheters and guidewires specifically amenable to cerebral navigation improved. Mechanical devices offer theoretical advantages over pharmacological treatment, potentially including speed of recanalization, revascularization of large-artery occlusions, reduced risk of hemorrhage compared with lytics, and longer time window for use.
Foreign-body retrieval devices for endovascular application were first reported in the 1980s. A broad variety of devices were used off-label for attempted stroke thromboembolectomy. Seven retrieval devices were evaluated for stroke applications in actual studies. Only 2 devices (Concentric MERCI and Penumbra Suction Thrombectomy System) to date have sought and received FDA approval as foreign-body retrievers with stroke indication. Procedural success is usually measured primarily by vessel recanalization. In fact, most stroke thrombectomy trials have focused on local recanalization of the occluded brain artery and defined procedural success by partial or complete recanalization only at the site of occlusion. Fragmentation, distal embolization, or relocation of the occlusive thrombus is usually not part of the primary outcome measure, which may partially explain limitations in clinical outcomes after treatment.37
The Mechanical Embolus Removal in Cerebral Ischemia (MERCI; Concentric Medical, Inc, Mountain View, CA) device was first to market, and probably remains the most widely distributed stroke retriever. A venture capital–funded endeavor, Concentric Medical, was the first to seek a stroke indication for its foreign-body retriever. By crossing the site of occlusion, this corkscrew-shaped device pulls the occlusive thromboembolus into an extracranial guide catheter under active suction. Successive generations of the MERCI device have been reported in 3 prospective, single-arm, nonrandomized, multicenter feasibility, and safety studies (Table 2). Patients enrolled in these studies were ineligible for intravenous fibrinolysis or had failed to respond to intravenous fibrinolysis. Enrolled patients were treated within 8 hours of stroke onset. On average, treatment began 4.3 hours after stroke onset. Recanalization, if achieved, occurred by 5.9 hours. Primary outcome measures included partial or complete recanalization of the occlusion (Thrombolysis in Myocardial [TIMI] grade 2 to 3) and safety. Secondary outcomes included clinical outcome (stroke, myocardial infarction, death) at 30 and 90 days.38 Multivariate analysis demonstrated that recanalization was associated with good outcome, whereas increased age and stroke severity were associated with poor outcome and death. Apparent limitations include operator learning curve, need to traverse the occluded artery to deploy the device distal to the occlusion, duration required to perform multiple passes with the device, clot fragmentation, and embolization.
Table 2.
Major Trials of Mechanical Devices for Treatment of Acute Ischemic Stroke
| Major Mechanical Thrombectomy Device Studies
|
||||
|---|---|---|---|---|
| MERCI Pilot | MERCI 1 | Multi-MERCI | PENUMBRA | |
| n | 28 | 151 | 164 | 125 |
| Age (mean), y | 68 | 67 | 68 | 64 |
| NIHSS | 22 | 20 | 19 | 18 |
| Time to treatment, h | ||||
| From presentation | 1.9 | |||
| From groin puncture | 2.5 | 4.3 | 4.3 | 4.3 |
| Recanalization (TIMI 2–3), % | ||||
| Device only | 43 | 46 | 57 | 82 |
| Adjuvant therapy | 64 | 69 | ||
| Complications, % | ||||
| Total | 1 | 13 | 9.8 | 13 |
| Clinically significant | 7.1 | 5.5 | 2.4 | |
| Hemorrhage, % | ||||
| Total | 43 | 7.8 | 9.8 | 28 |
| Clinically significant | 0 | 11.2 | ||
| Outcome (mRS 0–2) | ||||
| At 30 d | 20 | 23 | ||
| At 90 d | 28 | 36 | 25 | |
| Mortality, % | ||||
| At 30 d | 36 | 37 | 26 | |
| At 90 d | 43 | 34 | 33 | |
MERCI indicates Mechanical Embolus Removal in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale; TIMI, Thrombolysis in Myocardial Infarction; and mRS, modified Rankin Scale.
Suction Thrombectomy
The Penumbra stroke system (Penumbra, Inc, Alameda, CA) is increasingly popular and uses 2 types of devices to remove occlusive thromboembolus in acute ischemic stroke. An alternate strategy, the Penumbra devices act on the proximal face of the occlusion without traversing the occluded artery. An aspiration device is used to debulk and extract the clot. A second retriever device resembling a stent attached to a guidewire is used to remove resistant clot. Like the Concentric device, the time window for application remains 8 hours after stroke onset in patients who are ineligible for or failing intravenous fibrinolysis (Figure 2).
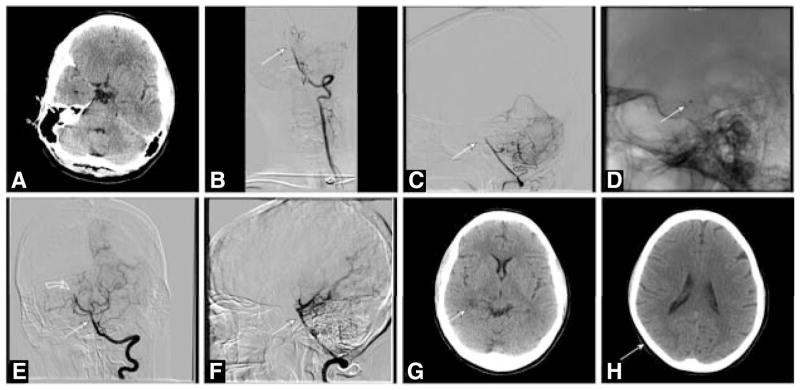
Figure 2.
A 44-year-old woman with atrial fibrillation admitted for complaint of dizziness progressing to coma and basilar artery occlusion not responsive to intravenous fibrinolysis with full-dose intravenous recombinant tissue-type plasminogen activator (rtPA; National Institutes of Health Stroke Scale score [NIHSS], 25). A, Nonenhanced computed tomography (CT) brain scan shows no infarction or hemorrhage at the time of rtPA administration 2.5 hours after stroke onset. B and C, Left vertebral arteriogram shows midbasilar artery occlusion (arrow). D, Lateral fluororadiography shows the 0.054-in Penumbra suction catheter near the dorsum clivus at the top of the basilar artery. E and F, Left vertebral arteriography shows recanalization of the basilar artery (straight arrows) with residual thromboembolic occlusion of the distal right posterior cerebral artery (curved arrow) within 6 hours of stroke onset. G and H, selected images from a nonenhanced CT brain scan 24 hours after acute stroke treatment shows only a small area of infarction in the distribution of the right posterior cerebral artery (arrows). After treatment, NIHSS measured 7. At 90 days, the patient experienced residual hemiparesis and diplopia (sixth cranial nerve palsy).
The Penumbra Pivotal Stroke Trial was a prospective, single-arm, multicenter study that showed a high rate of TIMI grade 2 to 3 recanalization in 81.6% of treated vessels. Yet, good clinical outcomes at 90 days (modified Rankin Scale [mRS] score, 0 to 2) still occurred in only 25% of patients. Both Penumbra devices received the CE mark in Europe; however, only the suction catheter system has received US FDA 510(k) approval.39 This study showed a significant improvement in the rate of local recanalization compared with previous studies using devices and fibrinolytic agents. Although recanalization correlated with improvements in patient outcome (>4 points on the NIH Stroke Scale [NIHSS] in 57.8%; mRS score, 0 to 2 in 25%), the relationship is not linear, and was less than expected. Efforts to address the disparity include questions about the adaptation and application of the TIMI scoring system for recanalization in cerebrovascular occlusive disease instead of cardiovascular disease.40 Limitations in clinical outcomes may reflect local recanalization with extensive distal embolization of the vascular tree, analogous to no reflow described in the cardiologic literature.
The relatively fewer good outcomes compared with prior reports using other methods such as intra-arterial fibrinolysis without mechanical interventions (Table 2) have led some to question the efficacy of mechanical methods without rigorous inquiry.41 The Agency for Healthcare Quality and Research, a division of the Department of Health and Human Services that advises the Center for Medicare and Medicaid Services, issued a technical brief on the current status of stroke therapy using neurothrombectomy devices. Originally available for consumer review on the Agency for Healthcare Quality and Research Web site (http://www.ahrq.gov/), this review has been published.42 The analysis concludes that there is a paucity of high-quality research, many unanswered questions, and a need for further research to determine the optimal device(s), their efficacy, and their safety. Although physicians may express reservations about the Agency for Health-care Quality and Research review because it potentially jeopardizes reimbursement for endovascular stroke therapy, few can argue the interpretation of the data and the concerns it raises.
Angioplasty and Stent Revascularization
Angioplasty is now commonly used in stroke revascularization, and is occasionally followed by stenting.43 It has been performed most commonly as a rescue procedure to displace thrombus or following early reocclusion after successful fibrinolysis. Angioplasty with stenting was originally described in acute stroke for underlying atherosclerotic stenosis after successful fibrinolysis. Stenting at the time of acute stroke has been used most frequently in Asia, where intracranial atherosclerotic disease is the most common cause of acute ischemic stroke. Recanalization rates with angioplasty are high with or without accompanying fibrinolysis, ranging from 60% to 90% and good outcomes (mRS score, 0 to 2 or NIHSS ≤ 4) between 36% and 74% in 2 small series.43,44
Stent revascularization in the cerebral circulation is an off-label application of these devices. For subacute cerebral ischemia resulting from symptomatic intracranial atherosclerotic disease, stent revascularization with the Wingspan device (Stryker/Target, Fremont, CA) is the subject of the ongoing NIH-funded Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis(SAMMPRIS) trial.45 In this study, stent angioplasty with the Wingspan device plus medical therapy was compared with medical therapy alone for the treatment of severe (≥70% stenosis) symptomatic intracranial atherosclerosis. Natural history data about intracranial stenosis treated with medical therapy46 appear similar to the risk of treatment with stent angioplasty in multicenter registries. Moreover, strong antithrombotic agents commonly used at the time of coronary stent revascularization with minimal risk of cerebral hemorrhage may result in unacceptable risk of hemorrhagic complications when applied to cerebral ischemic disease.47,48 Nevertheless, a number of authors have reported dramatic success using stents to treat acute ischemic stroke despite the foregoing concerns. No NIH-funded trial of cerebral stenting in acute ischemic stroke has been approved at this time (Figure 3).
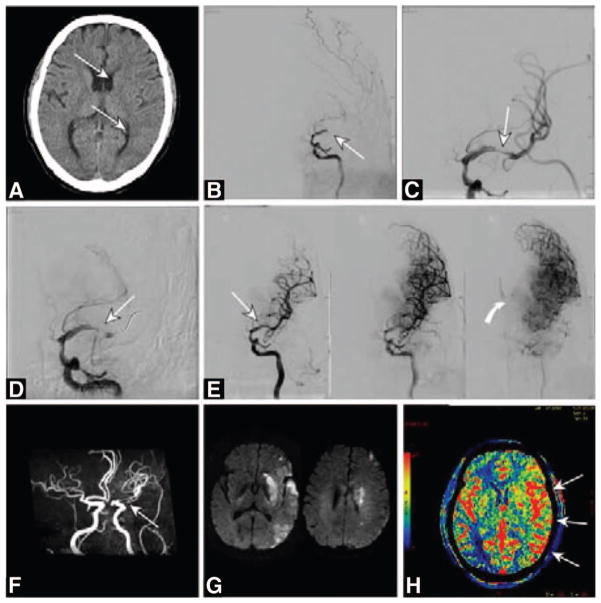
Figure 3.
A 67-year-old Hispanic man with acute right hemiplegia and global aphasia of 2-hour duration (National Institutes of Health Stroke Scale score, 23). A, Computed tomography brain scan showed early edema in basal ganglia structures and less than one third of the left middle cerebral artery distribution (arrows). The patient received full-dose intravenous recombinant tissue-type plasminogen activator (rtPA; 0.9 mg/kg). B, With no clinical improvement, he was triaged for emergency catheter arteriography. Left internal carotid arteriography in the frontal projection showed left middle cerebral artery occlusion (arrow). C, Mechanical thrombectomy was unsuccessful. Balloon angioplasty restored a thin channel of blood flow in the left middle cerebral artery (arrow). D, Repeat arteriography shows reocclusion despite systemic anticoagulation with rtPA (arrow). E, Stent angioplasty was performed with a 4.5×14-mm self-expanding nitinol stent (arrow). Successive angiographic images show reperfusion of the left middle cerebral artery with luxury perfusion (curved arrow) in the basal ganglia indicative of tissue injury and loss of autoregulation. F, Magnetic resonance (MR) angiography after the treatment procedure shows the patent vessel despite stent artifact. G, MR imaging with diffusion-weighted images shows restricted diffusion indicative of completed infarction in basal ganglia and scattered throughout the middle cerebral distribution. H, Computed tomography brain scan with perfusion-weighted imaging measuring relative cerebral blood flow shows increased perfusion throughout the left hemisphere after stent revascularization. At the time of discharge to rehabilitation, the patient had moderate hemiparesis (strength 4+/5) and resolving expressive aphasia.
Stent Retriever Thrombectomy
To avoid the need for strong antithrombotic medications such as P2Y12 or glycoprotein IIb/IIIa inhibitors for cerebral stent implantation in acute thromboembolic stroke, a number of companies have developed hybrid devices combining the features of removable cerebral stents and clot retriever devices. For this reason, these devices are sometimes referred to as stent retrievers. Examples include the Solitaire (Covidien/eV3, Maple Grove, MN), now undergoing testing in the Solitaire FR With the Intention for Thrombectomy (SWIFT) trial49; Trevo (Concentric Medical Inc, Mountain View, CA), now undergoing testing in the Thrombectomy Revascularization of Large Vessel Occlusion in Acute Ischemic Stroke (TREVO) trial in the United States and Europe50; and pilot studies on a number of similar devices at earlier phases of development. Seifert et al51 reported on the use of the Solitaire device in conjunction with intravenous or intra-arterial fibrinolysis in 4 patients, achieving TIMI grade 2 to 3 recanalization in all patients and clinical improvement (mRS score, 1 at 30 days) in 50%. There was 1 death, and the other patient was lost to follow-up.51 Similarly, Castano et al52 reported pilot data using the Solitaire device in 18 patients for the treatment of acute ischemic stroke (mean NIHSS score, 19) within 8 hours of stroke onset and accomplished TIMI grade 2 to 3 recanalization in 90% without adjuvant therapy in 50 minutes on average and good outcomes (mRS score, 0 to 2 at 90 days) in 45%. Ten percent of patients experienced hemorrhages and 20% died within the follow-up period.52 Questions remain about the study designs used for FDA or CE device approval in which local recanalization is used as a surrogate marker for clinical recovery after stroke revascularization.
Futile Recanalization
Although recanalization rates in several studies correlate with favorable neurological outcome and functional recovery, the risk of death remains stable despite recanalization.53 Death occurs in 26% to 36% of treated patients across many studies (Figure 4). The most likely explanation is that the variability from patient to patient in the presence of good collateral cerebral circulation beyond an occlusion during an acute thromboembolic stroke may be the underlying cause. Collateral circulation to vessels beyond an acute occlusion may potentially sustain tissue viability until recanalization occurs. However, these collaterals are not universally present. Moreover, collateral channels may become overwhelmed when the site of occlusion is proximal because of the larger volume of ischemic brain tissue and prolonged time to revascularization. Baseline clinical conditions such as diabetes mellitus, hypertension, and patient age may directly or indirectly affect the quantity and quality of collateral vasculature. Predictors of futile recanalization have been independently associated with age >70 years, NIHSS score of 10 to 19, and NIHSS score >20. Although there was no clear effect of time to treatment across studies, this may be attributable to the heterogeneity of the studies analyzed (Figure 5). Therefore, careful patient selection for stroke intervention based on collateral circulation using some form of viability imaging remains a critical component to improve clinical outcomes, and is the reason that stroke intervention should remain a research protocol.53
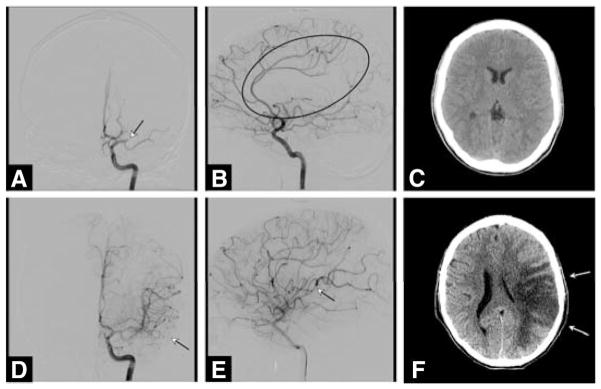
Figure 4.
A 13-year-old boy with complex cyanotic heart disease develops acute aphasia and right hemiplegia (National Institutes of Health Stroke Scale score, 26) 4 days after a fenestrated Fontan procedure. The patient was ineligible for intravenous fibrinolysis, and was taken immediately for endovascular treatment within an hour of stroke onset. A and B, Left internal carotid arteriography shows proximal occlusion of the left middle cerebral artery (arrow in image A; ellipsoid in image B). C, Computed tomography brain scan after rapid revascularization shows no evidence of stroke or hemorrhage (arrows in D and E). However, the patient did not improve clinically. F, Repeat computed tomography at 24 hours shows extensive infarction with hemorrhagic conversion. The family withdrew care, and the patient died.
Figure 5.
Mortality occurs in approximately one third of stroke patients and symptomatic intracranial hemorrhage (ICH) in ≈10% treated with endovascular procedures despite a trend toward higher rates of recanalization and a reduction in the use of fibrinolytic agents. NINDS rt-PA indicates National Institute of Neurological Disorders Tissue Plasminogen Activator for Acute Ischemic Stroke Trial; rtPA, recombinant tissue-type plasminogen activator; PROACT II, Prolyse in Acute Cerebral Thromboembolism trial; and MERCI, Mechanical Embolus Removal in Cerebral Ischemia trials.
For this reason, among others, investigators at the University of Houston (Houston, TX) and University of California Los Angeles have focused on identifying predictors of poor outcome after intra-arterial therapy. The goal was to develop a scoring system that could accurately stratify patients at high risk for futile recanalization. The University of Houston group developed a simple scoring system called the Houston Intra-Arterial Therapy (HIAT) score that was based on a retrospective analysis of 190 patients who underwent intra-arterial therapy at their center from 1998 to 2007. The HIAT score was then retrospectively applied to a different group of 175 patients treated with intra-arterial techniques between 1992 and 2007 at the University of California Los Angeles Medical Center. Treatment approaches were reportedly comparable at both centers. The HIAT score performed equally well in both cohorts. Furthermore, the maximal HIAT score showed an increase risk of intracranial hemorrhage, mortality, or poor outcome in nearly 100% of patients studied. Non–score-related predictors, including duration of intra-arterial therapy, were also associated with poorer outcome. (Difficult endovascular navigation or longer time to intervention could explain this observation.) Meanwhile, a large MRI perfusion-diffusion mismatch pattern possibly reflecting better tissue viability through collateral circulation correlated with better outcome.5,53
Houston Intra-Arterial Therapy, and other similar scoring systems, may become important in the triage of acute ischemic stroke patients. If validated in a prospective randomized, controlled trial, HIAT scoring could be useful in clinical decision making (ie, whether to proceed with aggressive and costly interventional treatment measures) and future trial design.
Summary
The management of acute ischemic stroke is rapidly developing. Although acute ischemic stroke is a major cause of adult disability and death, the number of patients requiring emergency endovascular intervention remains unknown, but is a fraction of the overall stroke population. Public health initiatives endeavor to raise public awareness about acute stroke to improve triage for emergency treatment, and the medical community is working to develop stroke services at community and academic medical centers throughout the United States. There is an Accreditation Council for Graduate Medical Education–approved pathway for training in endovascular surgical neuroradiology, the specialty designed to train physicians specifically to treat cerebrovascular diseases.29 Primary and comprehensive stroke center designations have been defined,54 yet questions remain about the best delivery model. Telemedicine is available to help community medical centers cope with the complexity of stroke triage and treatment.55
Should comprehensive care be provided at every community center, or should patients with complex medical needs be triaged to major stroke centers with high-level surgical, intensive care, and endovascular capabilities? Although the answers to these and other questions about stroke care delivery remain unanswered owing to the paucity of empirical data, we are convinced that stroke care regionalization is crucial for delivery of high-quality comprehensive ischemic stroke treatment. A stroke team available 24 hours per day, 7 days per week requires specialty skills in stroke neurology, endovascular surgical neuroradiology, neurosurgery, neurointensive care, anesthesiology, nursing, and technical support for optimal success. Several physician groups with divergent training backgrounds (ie, interventional neuroradiology, neurosurgery, neurology, peripheral interventional radiology, and cardiology) lay claim to the treatment of stroke patients, particularly the endovascular or interventional methods. Few would challenge neurologists over the responsibility for emergency evaluation and triage of stroke victims for intravenous fibrinolysis, even though emergency physicians are most commonly the first to evaluate these patients. There are many unanswered questions about the role of imaging in defining best treatment. Perfusion imaging with CT or MRI appears to have relevance even though its role remains undefined and is the subject of ongoing research. Meanwhile, investigators are exploring new, and perhaps more specific, imaging methods with cerebral metabolic rate of oxygen and cellular acid-base imbalance. There are currently 6 ongoing trials of stroke intervention, many with proprietary technologies and private funding, competing for the same patient population as multicenter trials funded by the NIH. At the same time, much of the interventional stroke treatment currently occurs outside of trials in the community and academic settings without the collection of much-needed data. Market forces will certainly shape future stroke therapy, but it is unclear whether the current combination of private and public funding for these endeavors is the best method of development.
Footnotes
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Cloft HJ, Rabinstein A, Lanzino G, Kallmes DF. Intra-arterial stroke therapy: an assessment of demand and available work force. AJNR Am J Neuroradiol. 2009;30:453–458. doi: 10.3174/ajnr.A1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgenstern LB, Staub L, Chan W, Wein TH, Bartholomew LK, King M, Felberg RA, Burgin WS, Groff J, Hickenbottom SL, Saldin K, Demchuk AM, Kalra A, Dhingra A, Grotta JC. Improving delivery of acute stroke therapy: the TLL Temple Foundation Stroke Project. Stroke. 2002;33:160–166. doi: 10.1161/hs0102.101990. [DOI] [PubMed] [Google Scholar]
- 4.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial: Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 6.Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer A, Olkers P, Hirsch JG, Heiland S, Wilde P, Jansen O, Rother J, Hacke W, Sartor K. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35:502–506. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- 7.Yoo AJ, Barak ER, Copen WA, Kamalian S, Gharai LR, Pervez MA, Schwamm LH, Gonzalez RG, Schaefer PW. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41:1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 8.Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) US National Institutes of Health trial; [Accessed December 27, 2010]. http://clinicaltrials.gov/ct2/show/NCT00389467. [Google Scholar]
- 9.Heiss WD, Sobesky J. Comparison of PET and DW/PW-MRI in acute ischemic stroke. Keio J Med. 2008;57:125–131. doi: 10.2302/kjm.57.125. [DOI] [PubMed] [Google Scholar]
- 10.Cvoro V, Marshall I, Armitage PA, Bastin ME, Carpenter T, Rivers CS, Dennis MS, Wardlaw JM. MR diffusion and perfusion parameters: relationship to metabolites in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2010;81:185–191. doi: 10.1136/jnnp.2008.168393. [DOI] [PubMed] [Google Scholar]
- 11.Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, Davis S, Demaerschalk BM, Derdeyn CP, Donnan GA, Eastwood JD, Fiebach JB, Fisher M, Furie KL, Goldmakher GV, Hacke W, Kidwell CS, Kloska SP, Kohrmann M, Koroshetz W, Lee TY, Lees KR, Lev MH, Liebeskind DS, Ostergaard L, Powers WJ, Provenzale J, Schellinger P, Silbergleit R, Sorensen AG, Wardlaw J, Wu O, Warach S. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–1628. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 13.Palmer RF, Graham JW, Taylor B, Tatterson J. Construct validity in health behavior research: interpreting latent variable models involving self-report and objective measures. J Behav Med. 2002;25:525–550. doi: 10.1023/a:1020689316518. [DOI] [PubMed] [Google Scholar]
- 14.Tissue plasminogen activator for acute ischemic stroke: the National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 17.Meyers PM, Schumacher HC, Higashida RT, Barnwell SL, Creager MA, Gupta R, McDougall CG, Pandey DK, Sacks D, Wechsler LR. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association Council on Cardiovascular Radiology and Intervention, Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Interdisciplinary Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2009;119:2235–2249. doi: 10.1161/CIRCULATIONAHA.109.192217. [DOI] [PubMed] [Google Scholar]
- 18.Muir KW. Multicentre Acute Stroke Trial: Italy. Lancet. 1996;347:391. [PubMed] [Google Scholar]
- 19.Toth G, Albers GW. Use of MRI to estimate the therapeutic window in acute stroke: is perfusion-weighted imaging/diffusion-weighted imaging mismatch an EPITHET for salvageable ischemic brain tissue? Stroke. 2009;40:333–335. doi: 10.1161/STROKEAHA.108.525683. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, Miyamoto S, Sasaki M, Inoue T. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial (MELT) Japan. Stroke. 2007;38:2633–2639. doi: 10.1161/STROKEAHA.107.488551. [DOI] [PubMed] [Google Scholar]
- 21.Yasaka M, O’Keefe GJ, Chambers BR, Davis SM, Infeld B, O’Malley H, Baird AE, Hirano T, Donnan GA. Streptokinase in acute stroke: effect on reperfusion and recanalization: Australian Streptokinase Trial Study Group. Neurology. 1998;50:626–632. doi: 10.1212/wnl.50.3.626. [DOI] [PubMed] [Google Scholar]
- 22.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS Study: a randomized controlled trial: Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 23.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 24.Schonewille WJ, Wijman CA, Michel P, Algra A, Kappelle LJ. The Basilar Artery International Cooperation Study (BASICS) Int J Stroke. 2007;2:220–223. doi: 10.1111/j.1747-4949.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 25.Arnold M, Schroth G, Nedeltchev K, Loher T, Remonda L, Stepper F, Sturzenegger M, Mattle HP. Intra-arterial thrombolysis in 100 patients with acute stroke due to middle cerebral artery occlusion. Stroke. 2002;33:1828–1833. doi: 10.1161/01.str.0000020713.89227.b7. [DOI] [PubMed] [Google Scholar]
- 26.Arnold M, Nedeltchev K, Mattle HP, Loher TJ, Stepper F, Schroth G, Brekenfeld C, Sturzenegger M, Remonda L. Intra-arterial thrombolysis in 24 consecutive patients with internal carotid artery T occlusions. J Neurol Neurosurg Psychiatry. 2003;74:739–742. doi: 10.1136/jnnp.74.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 28.ACGME Program Requirements for Graduate Medical Education in Endovascular Surgical Neuroradiology. Accreditation council for graduate medical education website; 2008. [Accessed October 18, 2010]. http://www.acgme.org/acWebsite/downloads/RRC_progReq/182_endovascular_neuroradiology_01012008_u06102008.pdf. [Google Scholar]
- 29.Meyers PM, Schumacher HC, Alexander MJ, Derdeyn CP, Furlan AJ, Higashida RT, Moran CJ, Tarr RW, Heck DV, Hirsch JA, Jensen ME, Linfante I, McDougall CG, Nesbit GM, Rasmussen PA, Tomsick TA, Wechsler LR, Wilson JA, Zaidat OO. Performance and training standards for endovascular ischemic stroke treatment. J Neurosurg. 2010;113:149–152. doi: 10.3171/2009.12.jns091813. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke. 2004;35:2493–2498. doi: 10.1161/01.STR.0000143728.45516.c6. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Hong KS, Saver JL. Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke. 2010;41:932–937. doi: 10.1161/STROKEAHA.109.574335. [DOI] [PubMed] [Google Scholar]
- 32.Mahon BR, Nesbit GM, Barnwell SL, Clark W, Marotta TR, Weill A, Teal PA, Qureshi AI. North American Clinical Experience with the EKOS MicroLysUS Infusion Catheter for the Treatment of Embolic Stroke. AJNR Am J Neuroradiol. 2003;24:534–538. [PMC free article] [PubMed] [Google Scholar]
- 33.The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 34.Khatri P, Hill MD, Palesch YY, Spilker J, Jauch EC, Carrozzella JA, Demchuk AM, Martin R, Mauldin P, Dillon C, Ryckborst KJ, Janis S, Tomsick TA, Broderick JP. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008;3:130–137. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke. 2006;37:922–928. doi: 10.1161/01.STR.0000202582.29510.6b. [DOI] [PubMed] [Google Scholar]
- 36.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 37.Nogueira RG, Schwamm LH, Hirsch JA. Endovascular approaches to acute stroke, part 1: drugs, devices, and data. AJNR Am J Neuroradiol. 2009;30:649–661. doi: 10.3174/ajnr.A1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 39.The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 40.Saver JL, Liebeskind DS, Nogueira RG, Jahan R. Need to clarify Thrombolysis In Myocardial Ischemia (TIMI) scale scoring method in the Penumbra Pivotal Stroke Trial. Stroke. 2010;41:e115–e116. doi: 10.1161/STROKEAHA.109.566406. [DOI] [PubMed] [Google Scholar]
- 41.Ciccone A, Valvassori L, Gasparotti R, Scomazzoni F, Ballabio E, Sterzi R. Debunking 7 myths that hamper the realization of randomized controlled trials on intra-arterial thrombolysis for acute ischemic stroke. Stroke. 2007;38:2191–2195. doi: 10.1161/STROKEAHA.106.465567. [DOI] [PubMed] [Google Scholar]
- 42.Baker WL, Colby JA, Tongbram V, Talati R, Silverman IE, White M, Kluger J, Coleman CI. Neurothrombectomy devices for the treatment of acute ischemic stroke: state of the evidence. Ann Intern Med. 2011;154:243–252. doi: 10.7326/0003-4819-154-4-201102150-00306. [DOI] [PubMed] [Google Scholar]
- 43.Nakano S, Iseda T, Yoneyama T, Kawano H, Wakisaka S. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke. 2002;33:2872–2876. doi: 10.1161/01.str.0000038985.26269.f2. [DOI] [PubMed] [Google Scholar]
- 44.Qureshi AI, Siddiqui AM, Suri MF, Kim SH, Ali Z, Yahia AM, Lopes DK, Boulos AS, Ringer AJ, Saad M, Guterman LR, Hopkins LN. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery. 2002;51:1319–1329. doi: 10.1097/00006123-200211000-00040. [DOI] [PubMed] [Google Scholar]
- 45.SAMMPRIS: Stenting vs. Aggressive Medical Management for preventing Recurrent Stroke in Intracranial Stenosis. US National Institutes of Health trial; [Accessed October 14, 2010]. http://clinicaltrials.gov/ct2/show/NCT00576693. [Google Scholar]
- 46.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 47.AbESTT-II. [Accessed August 17, 2005];Abciximab in Emergent Stroke Treatment Trial-II. http://www.strokecenter.org/trials/trialDetail.aspx?tid=568.
- 48.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 49. [Accessed October 15, 2010];Solitaire FR with the Intention for Thrombectomy (SWIFT) http://clinicaltrials.gov/ct2/show/NCT01054560.
- 50. [Accessed October 15, 2010];Thrombectomy Revascularization of Large Vessel Occlusion in Acute Ischemic Stroke (TREVO) http://clinicaltrials.gov/ct2/show/NCT01088672.
- 51.Seifert M, Ahlbrecht A, Dohmen C, Spuentrup E, Moeller-Hartmann W. Combined interventional stroke therapy using intracranial stent and local intraarterial thrombolysis (LIT) Neuroradiology. 2010;53:273–282. doi: 10.1007/s00234-010-0719-0. [DOI] [PubMed] [Google Scholar]
- 52.Castano C, Dorado L, Guerrero C, Millan M, Gomis M, Perez de la Ossa N, Castellanos M, Garcia MR, Domenech S, Davalos A. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke. 2010;41:1836–1840. doi: 10.1161/STROKEAHA.110.584904. [DOI] [PubMed] [Google Scholar]
- 53.Hussein HM, Georgiadis AL, Vazquez G, Miley JT, Memon MZ, Mohammad YM, Christoforidis GA, Tariq N, Qureshi AI. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. 2010;31:454–458. doi: 10.3174/ajnr.A2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, Koroshetz W, Marler JR, Booss J, Zorowitz RD, Croft JB, Magnis E, Mulligan D, Jagoda A, O’Connor R, Cawley CM, Connors JJ, Rose-DeRenzy JA, Emr M, Warren M, Walker MD. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]
- 55.Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, Handschu R, Jauch EC, Knight WA, Levine SR, Mayberg M, Meyer BC, Meyers PM, Skalabrin E, Wechsler LR. A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2009;40:2616–2634. doi: 10.1161/STROKEAHA.109.192360. [DOI] [PubMed] [Google Scholar]