Abstract
Objective
To investigate how increased LDL levels interact with endothelial cells by using well-defined LDL preparations to limit experimental biases caused by heterogeneity of LDL preparations.
Methods
We pooled LDL from multiple subjects and prepared several types of LDL from a single source. Then we observed their effects on cultured endothelial cells with and without monocyte co-culture.
Results
Native and minimally oxidized LDL did not cause significant cell death under most circumstances, and did not up-regulate cellular adhesion molecule (CAM) expression. Native LDL did result in significant increases of MCP-1 release in five of eight subjects. However, extensively oxidized LDL caused a significant amount of cell death and dramatically decreased MCP-1 secretion. Minimally oxidized LDL elicited a mixed response pattern, with a great deal of variation among subjects. When endothelial cells were co-cultured with monocytes and treated with native LDL, significant up-regulation of CAMs was detected after 24 hours of exposure. Up-regulation was not seen in any treatment group that contained either native LDL or monocytes only, indicating a synergistic effect of LDL and monocytes on endothelial cells. Incubation of cultured monocytes with native LDL also resulted in TNF-α and IL-1β release in a dosage- and time-dependent manner. Neutralization of both TNF-α and IL-1β by 10 μg/mL polyclonal antibodies inhibited the up-regulation of CAMs.
Conclusion
Our results suggest that varying extents of oxidative modification of LDL lead to fundamentally different cytological effects and that native LDL exhibits greater endothelial activation capacity when it interactively cooperates with monocytes.
Keywords: LDL characterization, endothelial activation, oxidized LDL, co-culture, monocyte activation, large animal model
Introduction
Since its discovery 30 years ago, oxidative modification of LDL is considered an important mechanism in atherogenesis [1, 2]. Results from animal models and human subjects suggest that LDL and its derivatives are involved in endothelial dysfunction creating a procoagulatory and proinflammatory local environment, which is a key step in the genesis of both stable and unstable atherosclerosis [3-5]. However, the mechanisms by which LDL mediates these effects have been debated for decades; oxidized LDL preparations by individual investigators have shown numerous pro- and antiatherogenic properties [6]. While neither LDL particle concentration nor the fatty acid composition of the particles has been proven to promote atherosclerosis directly, a strong relationship between LDL and risk of coronary vascular disease has been well documented [7, 8].
The endothelium, a monolayer tissue lining on the inner surface of blood vessels, is the first target of circulating LDL [3, 9, 10]. However, assessment of the effect of LDL on endothelial cells has produced conflicting results. For example, while some have reported that LDL or oxidized LDL causes endothelial apoptosis [11, 12], others have claimed that these substances have growth-promoting effects [13, 14]. Because oxidative LDL preparations have neither been well defined nor thoroughly characterized, the heterogeneous nature of the preparations -due to their sources, methods of preparation and treatment, and conditions of storage and use - has been attributed to be responsible for the variable pathophysiological effects of LDL on the endothelium. In addition, there are few data demonstrating whether LDL damages the endothelium directly by itself or indirectly in concert with other cell types. To better understand LDL-mediated effects, we investigated both the effects of defined LDL preparations on endothelial cells and the potential involvement of mono-cytes in mediating the impact of LDL on endothelial cells.
This study was conducted using an Old World nonhuman primate, the baboon. There is increasing awareness that animal models play a critical role in providing relevant information about mechanistic questions [15]. Different animal models have provided insights into mechanisms of atherogenesis, but large animals, especially nonhuman primates, are better suited for translation to humans. This is particularly important in regard to investigations involving LDL because the oxidation of LDL is a complex process during which both the proteins and the lipids undergo oxidative changes and form complex products [6, 16, 17]. Since baboon and human LDL are remarkably similar [18], the baboon provides us a unique opportunity to address this question. At the Southwest National Primate Research Center, we are able to obtain a large and steady supply of blood and arteries that are from healthy baboons for experimental use and that give consistent and reproducible results. Our results have a direct impact on understanding the relationship of LDL to atherosclerosis in human beings.
Materials and methods
Isolation and oxidation of LDL
LDL was isolated from pooled plasma as described previously [19, 20]. Plasma was ultra-centrifuged at 105,000 xg for 24 h at 4°C. LDL in the density range of 1.019-1.063 g/mL was collected. Concentrations of isolated LDL were determined by total protein content based on the Lowry method (BCA, Sigma, St Louis, MO). The LDL was dialyzed against 1 liter of 0.01M PBS containing 0.1 mM EDTA; the dialysate was changed twice over 24 hours. A final concentration of 0.1 mM antioxidant butylated hydroxy-toluene (BHT) was added to the plasma that was used for obtaining native LDL. Before the oxidation experiment, LDL was applied to a Prepacked Econo-Pac Column (Bio-Rad, Hercules, CA) equilibrated with 0.01 M PBS to remove EDTA. The LDL preparation was then sterilized by passing through a 0.2-μM filter (Corning, Fisher Scientific, Pittsburg, PA). Extensively oxidized LDL was prepared by adding freshly prepared CuSO4 (Sigma, St Louis, MO) solution at the final concentration of 5 μM at 37 °C for 6 hours. Minimally oxidized LDL was obtained by incubation of freshly isolated LDL at 37°C for 6 hours without CuSO4. Native and oxidized LDL preparations were made from the same batch of pooled plasma for each independent experiment. The extent of LDL oxidation was determined by measuring thiobarbituric acid-reactive substances (TBARS, ZeptoMetrix, Buffalo, NY) and relative electrophoretic mobility on 2% aga-rose gel and staining the bands with oil red O. Relative electrophoretic mobility was defined as mobility of treated LDL divided by the mobility of untreated LDL.
Endothelial cell and monocyte isolation and culture
Primary endothelial cells were harvested from baboon femoral arteries using 0.1% collagenase and cultured as described before [19, 21]. Methods of monocyte isolation and culture were reported previously [32]. Briefly, peripheral blood mononuclear cells (PBMNCs) were obtained from buffy coats using a Ficoll gradient (Sigma, St Louis, MO). The supernatant was then centrifuged for 10 min at 1300 rpm to pellet cells. The PBMNCs were then re-suspended in 50 mL cell culture media. Purified PBMNCs were seeded in a 75 cm2 flask with no more than 200×106 cells, and incubated overnight at 37°C in 5% CO2. Non-adherent cells were then removed and discarded. Attached cells were rinsed gently and quickly with 10 mL PBS at room temperature. Cells were detached by adding 10 mL PBS/0.53 mM EDTA and incubated for 10 min at 37°C in a 5% CO2 incubator. The cells were then transferred into a 50 mL conical vial, and centrifuged for 10 min at 1300 rpm to obtain the pellet.
Cell growth and apoptotic measurement
To determine the extent of cell growth, cells grown in culture wells were dissolved in 0.1 N NaOH overnight, and total protein (an indicator related to cell number) was determined by the Lowry method (BCA, Sigma, St Louis, MO). Apoptotic rate was determined with an Annexin V-FITC Apoptotic Detection Kit (R&D Systems, Minneapolis, MN).
Identification and functional tests for isolated monocytes
Detached and isolated monocyte cultures were re-suspended and incubated with anti-CD14-FITC (Beckman Coulter, Brea, CA) and anti-CD36-FITC (StemCell Technologies, Vancouver, Canada) at dilutions recommended by the manufacturers. We performed flow cytometry with a CYAN Cytometer (DAKO, Carpinteria, CA) and determined the functionalities of isolated monocytes by measuring uptake of Dil-LDL (BTI, Stoughton, MA) and UEA-1 (Sigma, St. Louis, MO) using fluorescence microscopy as described previously [19, 21].
Monocyte activation by LDL
Cells were seeded at a density of 0.25×106/well on 12-well plates in 0.5 mL Macrophage Medium (Invitrogen, Carlsbad, CA). To observe LDL-mediated monocyte activation, we exposed cells to 50 and 100 μg/ml native LDL for 0, 0.5, 1, 4, 8, 12, and 24 hours in replicate wells. To confirm monocyte activation by LDL, we used 500 ng/mL LPS (Sigma, St Louis, MO) as a positive control.
Cytokine determination
We used ELISA kits from BD Biosciences (San Jose, CA) to measure TNF-α and IL-1β released from activated monocyte cultures. We determined MCP-1 with an ELISA kit (R&D Systems, Minneapolis, MN). These kits were tested to confirm cross-reactive to baboon samples (http://nhpreagents.bidmc.harvard.edu/NHP/).
Endothelial activation by LDL and monocyte co-culture
Endothelial cells were grown to 80 - 90% confluence before use. Media containing 2% FCS were replaced and the cells were maintained overnight. We then added native LDL, minimally oxidized LDL, and extensively oxidized LDL at indicated concentrations for 24 hours. In regard to co-culture, monocytes were seeded into the co-culture insert (8.0 μm pore size, Greiner bio-one, Germany) on top of the endothelial layer at a density of 0.5 - 1.0 × 106/well in the 12-well plates in 0.5 mL F-12K medium with 2% FCS.
Then we added 50 μg/ml or 100 μg/ml native LDL in 2% FCS medium for 24 hours; equal volume of PBS and 10 ng/mL TNF-α were added as vehicle and positive controls. Triplicate wells were evaluated for each treatment. Endothelial activation was determined by examining either cellular adhesion molecule expression or MCP-1 release, using established methods in our laboratory [19, 22]. The specific antibodies used to detect the cellular adhesion molecules included anti-human E-selectin conjugated with FITC (clone BBIG-E5; R&D Systems, Inc), anti-human ICAM-1 conjugated with APC (clone HA58; BD Biosciences, San Jose, Calif), and anti-human VCAM-1 conjugated PE (clone 5K267; US Biological, Swampscott, Mass). Flow cytometry was performed on a CYAN Cytometry (DAKO, Carpinteria, CA).
Roles of TNF-α and IL-1β in monocyte conditioned media on endothelial activation
To further confirm the contributions of TNF-α and IL-1β released from monocytes as a mechanism underlying endothelial activation, we added 100 μg/mL native LDL to monocyte cultures and collected conditioned media 24 hours later. We divided the conditioned media into two parts; we added 10 μg/mL goat anti-human TNF -α and 10 μg/mL anti-human IL-1β IgG to the neutralization groups (R&D Systems, Minneapolis, MN) and an equal amount of nonspecific goat IgG to the control groups. After incubation for 4 hours at 37°C in a humidified CO2 incubator, the control and neutralized groups were separately added to endothelial cell cultures and incubated for an additional 4 hours. Endothelial activation was monitored by expression of E-selectin, ICAM-1 and VCAM-1 using flow cytometry.
Statistical analysis
Data are expressed as the mean ± standard deviation of triplicate cell cultures. Statistical comparison of means was performed by two-tailed paired Student's t-test. The null hypothesis was rejected at p<0.05. All experiments were repeated at least three times.
Results
Characteristics of LDL preparations
We prepared native LDL, minimally oxidized LDL, and extensively oxidized LDL as described above. We used two parameters to distinguish these preparations, thiobarbituric acid-reactive substances (TBARS) and relative electrophoretic mobility (REM), respectively (Table 1 and Figure 1). Our results from at least three independent experiments showed that each of the three LDL preparations had distinct characteristics, indicating that LDL incubated under the specified conditions had undergone progressive oxidation. Native LDL had TBARS content ranging from zero to 2 nmol/mg protein, and extensively oxidized LDL had TBARS content from 160 to 200 nmol/mg protein. Extensively oxidized LDL had a higher electrophoretic mobility than minimally oxidized LDL and native LDL. Minimally oxidized LDL often showed a diffuse band of electrophoresis. Native LDL prepared using the current protocol retained maximally the chemical and physical features of freshly isolated LDL.
Table 1.
Characteristics of LDL preparations
| TBARS (nmol/mg protein)* | REM (centimeter)** | |
|---|---|---|
| Native LDL | 0 - 2 | 0 |
| Minimally oxidized LDL | 10 - 40 | 0.1-0.4 |
| Extensively oxidized LDL | 160 - 200 | > 1.0 |
Results represent the range of measurements from at least three independent experiments.
TBARS: thiobarbituric acid-reactive substances;
REM: relative electrophoretic mobility on 2% agarose gel relative to native LDL.
Figure 1.

Relative electrophoretic motilities of LDL with varying extents of oxidization. Panel A. Isolated LDL without treatment; Panel B. Native LDL; Panel C. LDL oxidized by 0.5 μM CUSO4 for 1 hour; Panel D. LDL oxidized by 0.5 μM CUSO4 for 3 hours; Panel E. LDL oxidized by 0.5 μM CUSO4 for 6 hours; Panel F. Minimally oxidized LDL.
Effects of LDL preparations on cell growth, apoptosis, and activation
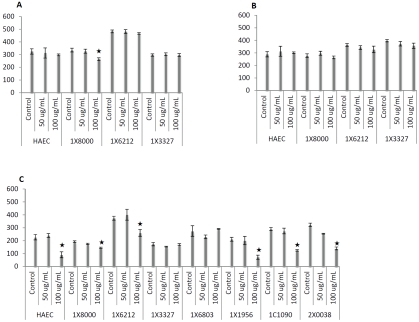
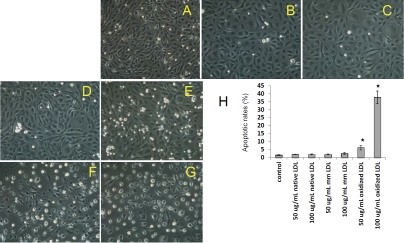
We investigated the effects of native, minimally oxidized, and extensively oxidized LDL on functional changes of baboon endothelial cells. Using total protein levels as an indicator of cell growth, we determined the cell mass after various treatments. After 24 hours of incubation, native LDL at a lower dosage (50 μg/mL) had no effect on cell growth, but a higher dosage of 100 μg/mL led to cell loss in selected samples (Figure 2A). Next, we investigated the effects of minimally oxidized LDL at concentrations of 50 and 100 μg/mL, and observed no significant changes in baboon endothelial cells or human arterial endothelial cells (HAEC) after 24 hours of treatment (Figure 2B). However, extensively oxidized LDL caused significant cell loss, although the degree of the response to extensively oxidized LDL varied among subjects tested. As shown in Figure 2C, five of seven baboons exhibited statistically significant reduction in cell mass after exposure to 100 μg/mL extensively oxidized LDL for 24 hours. Endothelial cells from five baboons (1×8000, 1×6212, 1×1956, 1×1090 and 2×0038) showed greater susceptibility to extensively oxidized LDL than the cells of the other two baboons (1×3327 and 1×6803), which exhibited minimal change after exposure. Observations of morphology under the microscope confirmed the detrimental effects of extensively oxidized LDL (Figure 3F, 3G), by comparison to native LDL (Figure 3B, 3C) and minimally oxidized LDL (Figure 3D, 3E). To determine the nature of cellular pathological changes, we examined their binding ability for Annexin V, an early indicator of apoptosis. We found that apoptotic rates were significantly elevated after exposure to extensively oxidized LDL (Figure 3H). Our results showed that extensively oxidized LDL caused significant endothelial cell death, whereas native or minimally oxidized LDL caused minimal damage within the observation period. Furthermore, we demonstrated that susceptibilities to LDL preparations varied among baboons to a significant extent, even when the same LDL was applied.
Figure 2.
Effects of three LDL preparations at 50 or 100 μg/mL on endothelial cell growth for 24 hours. Total protein content of lysates was used as an indicator of cell growth, using human aortic endothelial cells (HAEC) as a reference. LDL products at indicated dosages were added to endothelial cell cultures (as indicated by animal ID). Panel A shows that native LDL at 50 μg/mL did not affect growth of cells from any of the four baboons, but a significant decrease in total protein was observed in one culture (1×8000) at 100 μg/mL. Panel B shows that no significant differences in inhibition of cell growth were observed between control cultures and those incubated with minimally oxidized LDL at either concentration. Panel C shows five of seven subjects exhibited significant cell death after exposure to 100 μg/ mL oxidized LDL by comparison with their corresponding controls. * indicates p<0.05.
Figure 3.
Apoptotic effects of oxidized LDL on endothelial cells by morphological observations and Annexin V binding after 24 hours of treatment. Panel A. Control endothelial cells with PBS; Panel B. 50 μg/mL native LDL; Panel C. 100 μg/mL native LDL; Panel D. 50 μg/mL minimally oxidized LDL; Panel E. 100 μg/mL minimally oxidized LDL; Panel F. 50 μg/mL extensively oxidized LDL; Panel G.100 μg/mL extensively oxidized LDL. Panel H. Apoptotic rates of endothelial cells after treatment without and with LDL preparations. Original magnification at 100x. * indicates p<0.05, t-test.
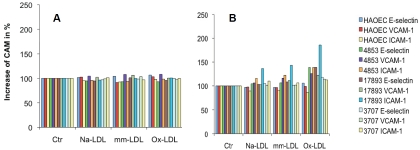
Next, we evaluated endothelial cell activation by the three LDL preparations through the determination of CAM expression and MCP-1 release. We exposed baboon endothelial cells to 100 μg/mL native, minimally oxidized and extensively oxidized LDL for 4 hours (Figure 4A) and for 24 hours (Figure 4B) to determine their respective effects on CAM expression. We did not see significantly increased CAM expression after 4 hours of treatment, but prolonged incubation over 24 hours induced moderate CAM expression in some subjects. The data also indicated a highly variable degree of susceptibility among baboons. However, the degrees of CAM expression induced by various LDL preparations were much lower than those induced in the same cells by TNF-α, as shown in Figure 7, indicating that they were not potent stimuli for endothelial CAM up-regulation. Then, we examined MCP-1 release into the culture supernatants after exposure to the three LDL preparations. As indicated in Table 2, exposure to native LDL, minimally oxidized LDL, and extensively oxidized LDL differentially affected MCP-1 release. In comparison to the control group, five of eight baboons had significantly increased MCP-1 release after treatment with 100 μg/mL native LDL and seven of eight baboons had significantly decreased MCP-1 release after treatment with 100 μg/mL extensively oxidized LDL. The effect of minimally oxidized LDL on MCP-1 release from endothelial cells was mixed. Three of the eight baboons exhibited significantly decreased levels, whereas one of the eight baboons exhibited a statistically significant increase. These data implied again that the extent of LDL oxidation had a tremendous impact on its biological consequences. Less oxidized LDL tends to maintain endothelial cells and to activate them to secrete chemokine MCP-1, but highly oxidized LDL causes cellular deterioration and death.
Figure 4.
Expression of cellular adhesion molecules (CAMs) after treatment by LDL preparations. Panel A shows up-regulation of CAM by 100 μg/mL native LDL (Na-LDL), minimally oxidized LDL (mm-LDL), and extensively oxidized LDL (ox-LDL) for 4 hours. Panel B shows up-regulation of CAMs by 100 μg/mL native LDL (Na-LDL), minimally oxidized LDL (mm-LDL), and extensively oxidized LDL (ox-LDL) for 24 hours. Results are expressed as ratios in percentage of treated group versus the corresponding control (Ctr).
Figure 7.
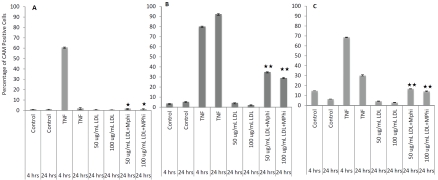
Expression of endothelial adhesion molecules induced by native LDL with or without co-culture with monocytes (MPhi). Panel A shows results for E-selectin, Panel B for ICAM-1, and Panel C for VCAM-1. P value indicates the comparison of cultures with 50 or 100 μg/mL LDL only vs. 50 or 100 μg/mL LDL with monocyte cultures. * indicates p<0.05; ** indicates p<0.001, t-test.
Table 2.
MCP-1 production in baboon endothelial cells after stimulation by 100 μg/mL native LDL, minimally oxidized LDL, and extensively oxidized LDL for 24 hours.
| MCP-1 Concentrations in Culture Medium (pg/ml) | ||||
|---|---|---|---|---|
| Animal ID | Control | Native LDL | Minimally oxidized LDL | Extensively oxidized LDL |
| 1X6803 | 1034 ±108 | 1842 ± 5 * | 1283 ±26 | 725 ± 33 |
| 8000 | 342 ± 33 | 1539 ± 25 * | 12 ± 15 * | 32 ± 29 * |
| 1X6212 | 1996 ±119 | 4000 ± 219 * | 505 ± 8 * | 185 ±107 * |
| 1X3327 | 508 ± 79 | 981±12 * | 400 ±119 | 98 ± 48 * |
| 16842 | 1868 ± 26 | 1932 ±77 | 1761 ±53 | 599 ± 31 * |
| 1C1090 | 1071 ±33 | 904 ±102 | 2372 ±44 * | 629 ± 51 * |
| 1X1956 | 1227 ± 61 | 1418 ±170 | 839 ±10 * | 483 ± 29 * |
| 2X0038 | 801 ± 19 | 1072 ± 52 * | 805 ± 56 | 462 ±13 * |
indicates p<0.05, t-test, treated vs. control.
Monocyte activation by LDL
Monocytes isolated according to our protocol were intact and functional; they attached to the culture flask, expressed CD14 on their surfaces, bound UEA-1, and up-took Dil-LDL (data not shown). To investigate whether LDL was able to activate monocytes, we incubated the cells with native LDL at 50 μg/mL and 100 μg/mL for 24 hours. Then we took supernatants at different time points and measured levels of TNF-α and IL-1β with ELISA kits. As a positive control, we used 500 ng/mL LPS, which is known to activate monocytes as evidenced by a variety of reactions including TNF-α and IL-1β production [23].
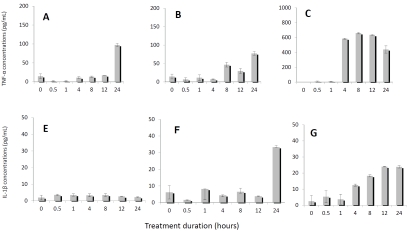
When baboon monocytes were incubated with 50 μg/mL and 100 μg/mL native LDL for 24 hours, the expression of TNF-α (Figure 5A, 5B) and IL-1β (Figure 5E, 5F) was increased in dosage- and time-dependent manners. These results clearly showed that LDL was able to activate monocytes to release proinflammatory cy-tokines, although native LDL was not as potent as LPS in stimulating monocytes at certain time points (Figure 5C, 5G). However, there were differences in the production of TNF-α and IL-1β under stimulation by LDL. TNF-α could be induced by both 50 and 100 μg/mL LDL concentration; but 50 μg/mL of LDL did not induce IL-1β production as effectively as 100 μg/mL LDL during 24 hours’ incubation, indicating that TNF -α is a more dominating cytokine in mediating LDL-induced reactions.
Figure 5.
Detection of TNF-α and IL-1β in supernatant of activated monocyte culture. Panels A-C show TNF-α concentrations after monocyte cultures were treated by 50 μg/mL native LDL (A), by 100 μg/mL native LDL (B), and by 500 ng/mL LPS (C) as a positive control. Panels E-G show IL-1β concentrations after monocyte cultures were treated with 50 μg/mL native LDL (E), with 100 μg/mL native LDL (F), and with 500 ng/mL LPS (G) as a positive control.
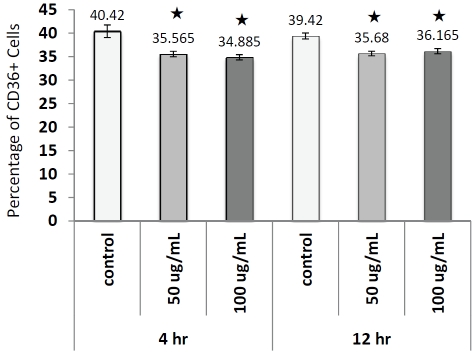
We also measured CD36, a natural receptor for native or oxidized LDL, on monocytes treated with different doses and durations of native LDL (Figure 6); significant decreases of CD36 levels were noticed after both 4 and 12 hours incubation. This decline of CD36 on the cell surface might be due to the internalization of this antigen when LDL binds to it. Overall, our results indicated that monocyte cultures were activated when they were cultured in media supplemented with native LDL.
Figure 6.
Detection of surface expression of CD36 on monocytes in culture in presence or absence 50 or 100 μg/mL LDL by flow cytometry. Numbers on top of bar represent average percentage of CD36+ cells for each treatment group. * indicates p<0.05, t-test.
Interactive effects of LDL and monocytes on endothelial cells
To determine whether LDL and monocytes interactively affect endothelial activation, we designed a co-culture system. We used a cell culture insert to keep endothelial cells and monocytes apart and added native LDL at 50 and 100 μg/mL concentrations for 24 hours in the presence or absence of monocytes. We found that native LDL, in the presence of monocytes in the co-culture system, synergistically activated endothelial cells. As indicated in Figure 7A, E-selectin levels were 0.7 ± 0.16% and 0.47 ± 0.17% when 50 μg/mL or 100 μg/mL native LDL was added. However, the expression levels increased significantly, to 1.72 ± 0.15% (t-test, p<0.05) and 1.30 ± 0.38% (t-test, p<0.05), respectively, when monocytes were included in the culture system. Similarly, ICAM-1 levels were increased by 4.25 ± 0.067% and 2.36 ± 1.95% when 50 μg/mL and 100 μg/mL native LDL were added. Their levels became 35.03 ± 3.01% (t-test, p<0.001) and 29.27 ± 7.08% (t-test, p< 0.001) when the endothelial cells were co-cultured with monocytes (Figure 7B). The same response pattern was observed for VCAM-1 (Figure 7C). These data indicate strongly that LDL maximally up-regulates endothelial adhesion molecules only when the endothelial cells are co-cultured with monocytes.
Neutralization studies
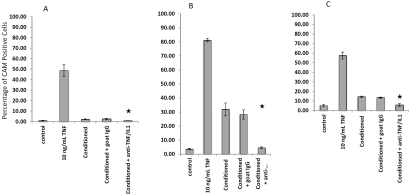
To further investigate the mechanism underlying monocyte activation of endothelial cells cultured in the presence of native LDL, we neutralized conditioned media by adding 10 μg/mL anti-humans TNF-α and 10 μg/mL anti-human IL-1β IgG for 4 hours at 37°C. After endothelial cell culture media were replaced by control media and neutralized media, we found that expression of E-selectin (Figure 8A), ICAM-1 (Figure 8B), and VCAM-1 (Figure 8C) was significantly inhibited by neutralized media as measured by flow cytometry. We conclude that TNF-α and IL-1β, released as a result of monocyte activation by LDL, were key players in mediating cascade reactions leading to endothelial activation.
Figure 8.
Inhibition of endothelial activation by conditioned media with or without neutralizing anti-TNF-α and anti-IL-1β antibodies. Conditioned media were harvested after 100 μg/mL native LDL had been incubated with monocyte cultures for 24 hours. Endothelial culture media were replaced by conditioned media with or without neutralizing antibodies at final concentrations at 10 μg/mL IgG, as indicated in the figure, for 4 hours. E-selectin (A), ICAM-1 (B), and VCAM-1 (C) values were determined by flow cytometry. * indicates p<0.05 for conditioned + goat IgG vs. conditioned + anti-TNF/IL1.
Discussion
Our current knowledge about the relationship between LDL and atherogenesis is quite restricted due to the lack of well-defined or characterized LDL products and the lack of standardized methods used to study this issue [3, 6, 24]. To overcome this shortcoming, we focused our investigation on three LDL preparations with distinct characteristics but from the same source, so that the influence of heterogeneous composition of LDL molecules would be limited.
We demonstrated in this study that LDL-mediated effects were largely dependent on the extent of LDL oxidation. Because we prepared LDL from a large pool of plasma and derived three types of LDL from the same pool, our results were not compromised by individual or batch-to-batch variation. After endothelial cells were incubated with three LDL preparations with varying extents of oxidation, they exhibited completely different pathological effects. Native LDL did not cause any detectable cell loss under most circumstances and it activated endothelial cells to secrete chemokines in some subjects; however, extensively oxidized LDL led to substantial cell death due to increased apoptosis. Minimally modified LDL exhibited a mixed response in regard to endothelial activation varying to a great extent among subjects. Our results clearly demonstrated, for the first time, a dramatically different effect of LDL on endothelial cells, depending on the degree of its oxidization, and thus explained in part why conflicting results have been reported [25-28].
In this study, we also explored the potential role that monocytes play in LDL-mediated endothelial cell activation, since immune factors are involved in vascular injury [29-31]. Because minimally oxidized LDL and extensively oxidized LDL may exert cytotoxic effects on endothelial cells, we focused on the effects of native LDL. To our surprise, we found that native LDL could become an extremely potent stimulus in a monocyte/endothelial cell co-culture system. As shown in Figure 7, native LDL did not induce significant expression of endothelial adhesion molecules in the absence of monocytes; however, when endothelial cells were exposed to native LDL together with monocytes, adhesion molecules including E-selectin, ICAM-1, and VCAM-1 were all significantly up-regulated. The co-culture system used in this research mimics the in vivo environment, suggesting that LDL and monocytes actively interact in the circulation. Our findings strongly imply it is the synergistic effect of native LDL and monocytes that generates profound consequences on pathological changes of the endothelium in vivo. Individual variation in susceptibility to atherogenesis may be due to interactions between LDL and monocytes, rather than to either LDL or immune cells alone, because the concentration of LDL has not been a consistent risk factor for cardiovascular disease, and risk factors interact in a non-linear manner [7, 8]. Furthermore, we studied the underlying mechanism and found that TNF-α and IL-1β, the dominant pro-inflammatory cytokines, were released in a dosage- and time-dependent manner when monocytes were activated by native LDL. When TNF-α and IL-1β in conditioned media were blocked by corresponding antibodies, endothelial activation was attenuated.
Another important observation was the dramatic difference among baboons in response to the LDL preparations. It has long been known that lipoproteins and other circulating factors account for approximately half of the variability in susceptibility to atherosclerosis, but the other biological risk factors for atherosclerosis have been elusive. Furthermore, while circulating levels of LDL cholesterol are highly correlated with extent and severity of atherosclerosis, there nevertheless is considerable variability in lesion development among people who have similar plasma LDL cholesterol levels. Our results suggest that genetic variation controlling endothelial and monocyte response to circulating LDL may have an important role in determining risk of atherosclerosis, and may account for a significant portion of the 50 percent of inter-individual variance in atherogenesis that is not explained by circulating risk factors.
Conclusion
In vitro studies using standardized LDL preparations indicate that the extent of oxidation determines the fate of endothelial cells. Circulating LDL may generate a profound pathological effect on the endothelium through the production of proinflammatory cytokines via activation of monocytes. To identify individuals at high risk for cardiovascular diseases, it is important to determine how risk factors interact to increase the likelihood of disease.
Acknowledgments
This work was supported by NIH grant P01 HL028972, NIH grants, P51RR013986, C06RR016228, and C06 RR017332 provided infrastructure support for this research.
References
- 1.Pritchard KA, Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;(Suppl 1):328–331. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 5.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and patho-physiology. Antioxid Redox Signal. 2010;13:39–75. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipopro-tein. Methods Mol Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lada AT, Rudel LL. Associations of low density lipoprotein particle composition with athero-genicity. Curr Opin Lipidol. 2004;15:19–24. doi: 10.1097/00041433-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:305–311. doi: 10.1097/MOL.0b013e32833b7756. [DOI] [PubMed] [Google Scholar]
- 9.Hazen SL, Chisolm GM. Oxidized phosphatidyl-cholines: pattern recognition ligands for multiple pathways of the innate immune response. Proc Natl Acad Sci USA. 2002;99:12515–12517. doi: 10.1073/pnas.212532799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009;73:595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- 11.Napoli C. Oxidation of LDL, atherogenesis, and apoptosis. Ann N Y Acad Sci. 2003;1010:698–709. doi: 10.1196/annals.1299.127. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi M, Okazaki H, Ogata Y, Takeuchi K, Ikeda U, Shimada K. Lysophosphatidylcholine induces apoptosis in human endothelial cells through a p38-mitogen-activated protein kinase -dependent mechanism. Atherosclerosis. 2002;161:387–394. doi: 10.1016/s0021-9150(01)00674-8. [DOI] [PubMed] [Google Scholar]
- 13.Zettler ME, Prociuk MA, Austria JA, Massaeli H, Zhong G, Pierce GN. OxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am J Physiol Heart Circ Physiol. 2003;284:H644–53. doi: 10.1152/ajpheart.00494.2001. [DOI] [PubMed] [Google Scholar]
- 14.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185:219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Vilahur G, Padro T, Badimon L. Atherosclerosis and thrombosis: insights from large animal models. J Biomed Biotechnol. 2011;2011:907575. doi: 10.1155/2011/907575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barter PJ, Gooden JM, Rajaram OV. Species differences in the activity of a serum triglyceride transferring factor. Atherosclerosis. 1979;33:165–169. doi: 10.1016/0021-9150(79)90113-8. [DOI] [PubMed] [Google Scholar]
- 17.Groener JE, Bax W, Stuani C, Pagani F. Difference in substrate specificity between human and mouse lysosomal acid lipase: low affinity for cholesteryl ester in mouse lysosomal acid lipase. Biochim Biophys Acta. 2000;1487:155–162. doi: 10.1016/s1388-1981(00)00091-3. [DOI] [PubMed] [Google Scholar]
- 18.Chapman MJ, Goldstein S. Comparison of the serum low density lipoprotein and of its apopro-tein in the pig, rhesus monkey and baboon with that in man. Atherosclerosis. 1976;25:267–291. doi: 10.1016/0021-9150(76)90033-2. [DOI] [PubMed] [Google Scholar]
- 19.Shi Q, Vandeberg JF, Jett C, Rice K, Leland MM, Talley L, Kushwaha RS, Rainwater DL, Vandeberg JL, Wang XL. Arterial endothelial dysfunction in baboons fed a high-cholesterol, high-fat diet. Am J Clin Nutr. 2005;82:751–759. doi: 10.1093/ajcn/82.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel RP, Darley-Usmar VM. Molecular mechanisms of the copper dependent oxidation of low -density lipoprotein. Free Radic Res. 1999;30:1–9. doi: 10.1080/10715769900300011. [DOI] [PubMed] [Google Scholar]
- 21.Shi Q, Wang J, Wang XL, VandeBerg JL. Comparative analysis of vascular endothelial cell activation by TNF-α and LPS in humans and baboons. Cell Biochem Biophys. 2004;40:289–303. doi: 10.1385/CBB:40:3:289. [DOI] [PubMed] [Google Scholar]
- 22.Rainwater DL, Shi Q, Mahaney MC, Hodara V, Vandeberg JL, Wang XL. Genetic regulation of endothelial inflammatory responses in baboons. Arterioscler Thromb Vasc Biol. 2010;30:1628–1633. doi: 10.1161/ATVBAHA.110.205740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amar S, Oyaisu K, Li L, Van Dyke T. Moesin: a potential LPS receptor on human monocytes. J Endotoxin Res. 2001;7:281–286. [PubMed] [Google Scholar]
- 24.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 25.Adams MR, Kinlay S, Blake GJ, Orford JL, Ganz P, Selwyn AP. Atherogenic lipids and endothelial dysfunction: mechanisms in the genesis of ischemic syndromes. Annu Rev Med. 2000;51:149–167. doi: 10.1146/annurev.med.51.1.149. [DOI] [PubMed] [Google Scholar]
- 26.Batt KV, Avella M, Moore EH, Jackson B, Suckling KE, Botham KM. Differential effects of low-density lipoprotein and chylomicron remnants on lipid accumulation in human macrophages. Exp Biol Med (Maywood) 2004;229:528–537. doi: 10.1177/153537020422900611. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen T, Evensen SA, Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979;39:361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- 28.Coffey MD, Cole RA, Colles SM, Chisolm GM. In vitro cell injury by oxidized low density lipoprotein involves lipid hydroperoxide-induced formation of alkoxyl, lipid, and peroxyl radicals. J Clin Invest. 1995;96:1866–1873. doi: 10.1172/JCI118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimayuga PC, Chyu KY, Cercek B. Immune responses regulating the response to vascular injury. Curr Opin Lipidol. 2010;21:416–421. doi: 10.1097/MOL.0b013e32833cacbe. [DOI] [PubMed] [Google Scholar]
- 30.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkind MS. Impact of innate inflammation in population studies. Ann N Y Acad Sci. 2010;1207:97–106. doi: 10.1111/j.1749-6632.2010.05736.x. [DOI] [PubMed] [Google Scholar]
- 32.Shi Q, Cox LA, Glenn J, Tejero ME, Hondara V, Vandeberg JL, Wang XL. Molecular pathways mediating differential responses to lipopolysac-charide between human and baboon arterial endothelial cells. Clin Exp Pharmacol Physiol. 2010;37:178–84. doi: 10.1111/j.1440-1681.2009.05260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]