Abstract
Aortic sclerosis (ASc) represents the earliest stage of development of aortic valve thickening, and may eventually progress to aortic valve stenosis (AS). ASc is associated with intra-valvular inflammatory activation, and potentially with attenuation of the anti-inflammatory effect of nitric oxide (NO). We have shown that ASc occurs less frequently in obese individuals, in whom systemic inflammatory activity is generally increased. We explored these relationships further by stratifying a population of 253 ageing individuals according to BMI. Increasing BMI was associated with increased hs-CRP concentrations (r=0.43; p<0.001). However, presence/absence of ASc did not significantly modify this relationship. Furthermore, increasing BMI was independent of tissue responsiveness to NO, as measured via inhibition of platelet aggregation by the NO donor sodium nitroprusside. Therefore the association of low BMI with increased risk of ASc appears to interact neither with systemic inflammatory activation in such individuals, nor with any “paradoxical” occurrence of NO resistance.
Keywords: Aortic valve sclerosis, nitric oxide, BMI, inflammation
Introduction
Aortic stenosis (AS) is a result of a progressive increase in calcium deposition within the aortic valve, leading to increased stiffness and progressive narrowing of the valve. Whilst in the past the pathogenesis of AS was thought to be due to “wear and tear", it is now recognized that it is a complex process, involving inflammatory infiltration, endothelial disruption and dysfunction, fibrosis and calcification. Aortic sclerosis (ASc), the earliest stage of this process, is characterized by abnormal valve morphology in the absence of haemodynamically significant obstruction of valve orifice [1].
Even the ASc stage of the disease is associated with the considerable cardiovascular morbidity and mortality [2, 3]. Early studies identified a number of clinical factors associated with the presence of calcific aortic valve disease (not always distinguishing between stenosis and sclerosis). These include age, body mass index, smoking, hypertension, dyslipidaemia, diabetes and lipoprotein (a) levels [4-9]. In the more recent, and much larger, Cardiovascular Health Study [1] the risk factors independently associated with calcific aortic valve disease were age, male gender, lipoprotein (a) levels, height, hypertension, smoking and LDL levels, all of which remained significant when only ASc was considered. We have demonstrated that reduced platelet responsiveness to nitric oxide, advanced age and low BMI, but not conventional coronary risk factors, are independent correlates of presence of ASc in a randomly selected ageing Western population cohort [10].
Thus the investigations have been directed towards better understanding of pathogenesis of ASc, with the ultimate objective of developing strategies to retard its progression. Whilst a number of studies, mainly epidemiological, have examined the factors associated with the presence of ASc, very few have looked at the determinants of progression of ASc. Despite similarities in clinical factors associated with AS and atherogenesis, factors underlying progression are not identical. The putative risk factor that has attracted the most controversy is that of dyslipidemia. There is considerable evidence for the involvement (as distinct from pivotal importance) of cholesterol and other lipoproteins in pathogenesis of AS and ASc (reviewed by [11]), although not every study reported such an association [12-14]; yet none of the prospective, randomized, place-controlled trials to retard the progression of AS via cholesterol lowering have been successful [15-17].
Presence of inflammatory activation and infiltration has been demonstrated in AS/ASc lesions histologically [18, 19], yet the clinical relevance of the markers of inflammation has only been assessed in one prospective study to date. In a follow-up of the Cardiovascular Health Study, involving more than 5600 subjects, of whom 1610 had ASc and 94 had AS, the authors found that increasing age, male gender, African-American ethnicity and increases in low-density lipoprotein cholesterol levels were significant predictors of new development of AS and ASc (combined). Similarly, progression from ASc to AS was significantly associated with male gender, advancing age and African-American ethnicity. CRP levels were not associated with development or progression of calcific aortic valve disease [20].
Previous studies have widely reported the association between increasing BMI and systemic inflammatory activation (reviewed by [21]). Yet we have found a “paradoxical” association between low BMI and reduced risk of presence of ASc [10]. In fact this “paradoxical” relationship has also been documented as regards survival in patients with chronic kidney disease [22], where it is thought to be that malnourishment leads to even greater atherogenic risk than obesity.
Nitric oxide (NO) has been shown to exert a wide variety of physiologic effects in cardiovascular system and specifically related to aortic valve structure and function. NO has important anti-inflammatory and vasodilator properties; it limits calcification in cell culture of aortic valve fibroblasts [23] and impairment of NO responsiveness has been demonstrated in patients with AS and ASc [10, 24]. Impairment of platelet NO responsiveness in itself has been shown to be an independent marker of adverse cardiovascular events [25].
In the current investigation, we have therefore sought to resolve the paradox inherent in the inverse relationship between BMI and risk of ASc, given that increasing BMI is associated with systemic inflammatory activation, and yet with reduced prevalence of ASc [10]. We hypothesised that the BMI : hsCRP relationship would be attenuated or reversed in the presence of ASc. In view of the association of platelet NO resistance with prevalence of ASc [10], but also with obesity [26], we also evaluated possible dissociation of the BMI : platelet NO responsiveness relationship in ASc.
Materials and methods
Study population
The study cohort (n=253) represented a subset of the North Western Adelaide Health Study aged 51 to 77 years, who had not undergone aortic valve surgery. This cohort of ambulant but aging individuals was initially evaluated to identify risk factors for aortic valve calcification [10]. Subject characteristics are summarized in Table 1. All volunteers gave informed consent before the study. The study was approved by the Ethics of Human Research Committee of The Queen Elizabeth Hospital.
Table 1.
Patient characteristics: clinical data
| Characteristic | NoASc (AVBS score < 16 dB) (n=204) | ASc (AVBS score ≥ 16 dB) (n=49) | p value |
|---|---|---|---|
| Age (mean ± SD) (yrs) | 63 ± 6.0 | 64.9 ± 9 | 0.045 |
| BMI (kg/m2) | 28.5 ±5.1 | 26.7 ±4.2 | 0.019 |
| Gender (% male) | 42% | 49% | 0.372 |
| History of hypercholesterolemia | 61.8% | 53.1% | 0.565 |
| Statin therapy | 30.5% | 38.8% | 0.268 |
| Previous angina/MI | 11.9% | 20.4% | 0.114 |
| ACEI/AIIRB therapy | 34.8% | 25% | 0.193 |
| Hypertension | 44.1% | 32.7% | 0.146 |
| Family history of CVD | 51% | 57.1% | 0.439 |
| Smoking | 14.8% | 12.2% | 0.649 |
| Diabetes mellitus | 10.8% | 10.2% | 0.898 |
BMI - Body mass index; MI - Myocardial infarction; ACEI - Angiotensin converting enzyme inhibitor; AIIRB - Angiotensin II receptor blocker; CVD - Cardiovascular disease.
Doppler echocardiography
Complete transthoracic echocardiographic studies were performed in all subjects with a commercially available system (Vivid 5 [GE Vingmed, Horten, Norway], with a 2.5 MHz phased array probe). M-mode and 2-dimensional (2D) echo-cardiograms with Doppler analysis were obtained for all subjects. Mean and peak pressure gradients across the aortic valve were calculated with the modified Bernoulli equation, with continuous-wave Doppler recordings from the highest velocity available from any view. The aortic valve area was computed with the continuity equation with standard methods. Valve morphology was categorized on the basis of visual assessment, as previously described [27].
Ultrasound backscatter data analysis
Aortic valve backscatter values (AVBS) were obtained for all subjects with methods as previously published [28]. Briefly, 2D ultrasonic back-scatter images of the aortic valves were obtained from standard parasternal long-axis views over 3 cardiac cycles with a zoom of 8 cm. Three consecutive scans were acquired for each study subject. Backscatter values from the blood pool in the LV outflow tract and aortic root were used as reference values. Calibrated back-scatter values were obtained by subtracting the average blood pool value from the averaged backscatter values obtained from the aortic valves. Subjects with AVBS values ≥ 16dB were considered to have ASc in keeping with previously published data [28].
Biochemical measurements
Lipid profile, high-sensitivity C-reactive protein (hs-CRP), serum creatinine, calcium, phosphate, and 1,25 dihydroxy cholecalciferol (vitamin D levels) were measured by a 125I radioimmunoassay (Immunodiagnostic Systems Ltd., Bolden, United Kingdom). Creatinine clearance (CrCl) was calculated according to the Cockcroft-Gault equation and indexed for body surface area (BSA) with the Dubois and Dubois formula.
Statistical analyses
All data are expressed as mean ± SD unless otherwise stated. Normal distribution was tested for all continuous variables, and skewed data were normalized either by log or square root transformation. Comparisons between groups for normally distributed data were performed with nonpaired t tests and, comparisons for nonparametric data were made with the Mann-Whitney test. Correlations between transformed, continuous nonparametric data were made with linear regression. The impact of the presence or absence of ASc on the BMI : hs-CRP and BMI : platelet NO responsiveness relationships were tested by ANCOVA. All analyses were performed using GraphPad Prism 5 software (GraphPad, USA), and a p value of less than .05 was considered to be statistically significant.
Results
Subject characteristics
Baseline patient characteristics are shown in Table 1. There was a high proportion of obese subjects (31% of subjects had BMI ≥ 30), multiple coronary risk factors were frequently present, and there was extensive therapy with statins and angiotensin-converting enzyme inhibitor/angiotensin receptor blockers. ASc was present in 19% (n=49) of subjects based on AVBS scores. Subjects with ASc were generally older and leaner than those without ASc.
Biochemistry
Biochemical findings are summarized in Table 2. Plasma cholesterol concentrations were elevated beyond normal (> 5.5 mmol/l) in 26.4% of subjects at entry. In general, renal function was well-preserved: there were no patients on dialysis, with only 2 subjects with CrCl < 30 ml/ min/1.73 m2. Comparisons between subjects with and without ASc revealed that CrCl was significantly greater in subjects without ASc. Levels of hs-CRP were not significantly different for those with and without ASc.
Table 2.
Patient characteristics: baseline biochemical data (expressed as mean ± standard deviation)
| Parameter | No ASc (AVBS score < 16 dB) | ASc (AVBS score > 16 dB) | p value |
|---|---|---|---|
| hs-CRP (mmol/L) | 3.5 ±3.6 | 4 ±5.6 | 0.576 |
| % platelet responsiveness to SNP | 34.8 ±27.5 | 26.6 ± 25.4 | 0.084 |
| Total cholesterol (mmol/L) | 5±1 | 4.9 ±1 | 0.774 |
| LDL (mmol/L) | 2.9 ±0.9 | 2.8 ±0.7 | 0.549 |
| HDL (mmol/L) | 1.3 ±0.3 | 1.4 ± 0.4 | 0.126 |
| Calcium (mmol/L) | 2.2 ±0.1 | 2.2 ±0.1 | 0.7 |
| Phosphate (mmol/L) | 1±0.2 | 1±0.2 | 0.129 |
| CrCL (indexed for BSA) (ml/min/1.73m2) | 84.2 ±22.7 | 76 ±20 | 0.022 |
hs-CRP - high sensitivity C-reactive protein; SNP - sodium nitroprusside; LDL - low density lipoprotein; HDL - high density lipoprotein; CrCL - creatinine clearance.
BMI vs hs-CRP: impact of ASc
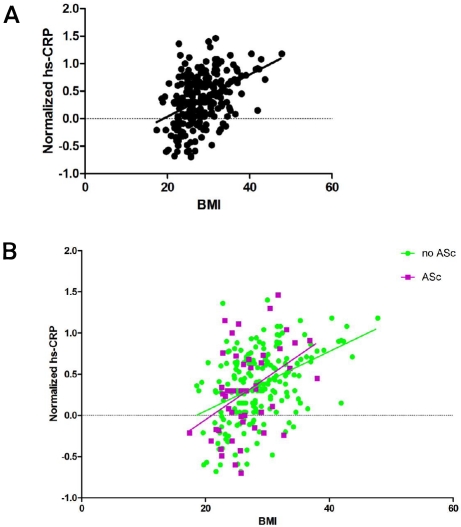
In the entire cohort there was a direct correlation between BMI and hs-CRP (Figure 1A; r=0.42; p<0.0001). When this relationship was considered separately for subjects with and without ASc (Figure 1B), the relationship was significant for each cohort [ASc: r=0.42, p=0.003; no ASc: r=0.43, p<0.0001], but the difference between gradients was not statistically significant (ANCOVA: p for interaction = 0.96).
Figure 1.
A. Correlation of BMI with normalized plasma hs-CRP concentrations for the entire cohort (r=0.42; p<0.0001); B. ANCOVA: relationship between BMI and normalized plasma hs-CRP concentrations according to presence/absence of ASc (p=0.96)
BMI vs platelet NO responsiveness: impact of ASc
Surprisingly, there was no significant relationship between BMI and platelet responsiveness to NO in the entire population (Figure 2A). When subjects with and without ASc were considered separately (Figure 2B), there was a trend (ANCOVA: p=0.08) for subjects with ASc to have greater reductions in NO responsiveness with increasing BMI than those without ASc.
Figure 2.
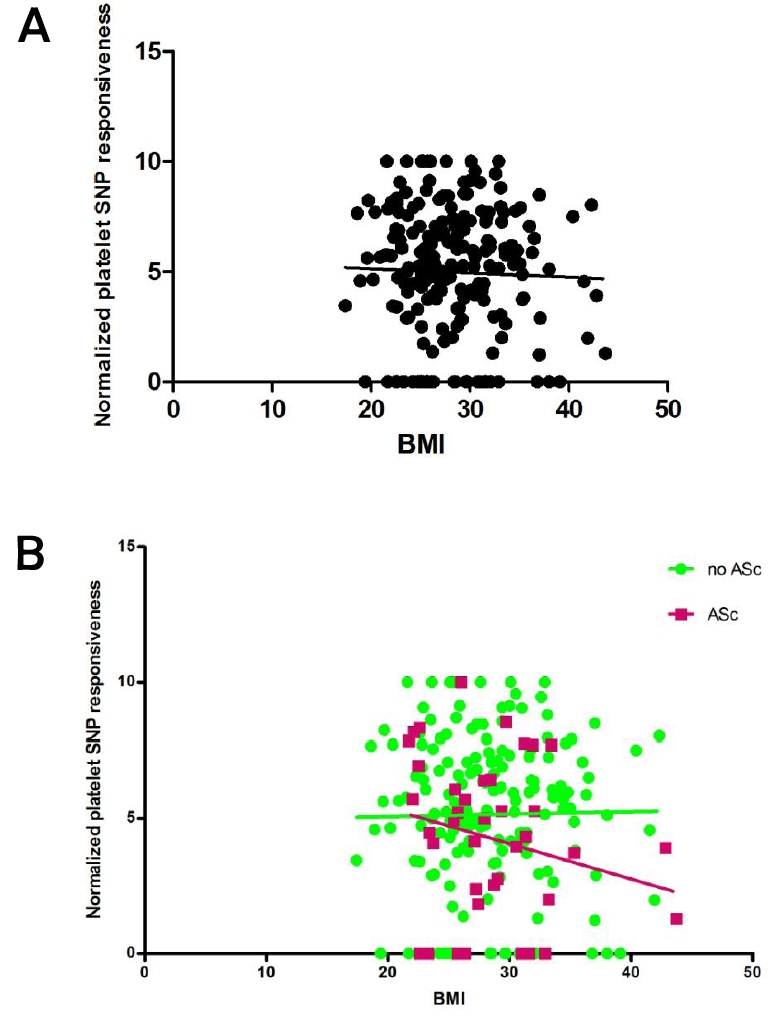
A. Correlation of BMI with normalized platelet SNP responsiveness for the entire cohort (p=0.6); B. ANCOVA: relationship between BMI and normalized platelet SNP responsiveness according to presence/absence of ASc (F= 3.1; p=0.08).
Discussion
This study demonstrates that the presence of ASc is not associated with significant perturbation of the BMI : hs-CRP relationship. Furthermore, in this population obesity was not significantly associated with platelet NO resistance, nor did a different relationship emerge in the presence of ASc.
The rationale for this investigation is the emerging evidence for inflammatory activation and impairment of NO signaling cascade in subjects with ASc as discussed below.
ASc is associated with intra-valvular inflammatory activation
This includes evidence of local secretion of angiotensin II [29], presence of inflammatory infiltrates [18, 19], and identification of local formation of reactive oxygen species such as hydrogen peroxide [30]. Furthermore, we have demonstrated in an animal model that ASc/mild AS is associated with increased intra-valvular content of thoiredoxin interacting protein [31], a key regulator of inflammasome activation [32].
ASc is associated with impaired NO generation/ effect systemically
ASc is associated with endothelial dysfunction in humans [33]; in a rabbit model of ASc/mild AS there is also a relative increase in asymmetric dimethylarginine levels [31], a marker and mediator of endothelial dysfunction [34]. Furthermore, on multivariate analysis, presence of ASc is associated with impaired platelet NO responsiveness in humans [10]. Therefore it has been postulated that impaired NO generation/effect might (a) result from increased oxidative stress in ASc, (b) account for the increased risk of cardiovascular events in ASc patients [3]. However, ASc (and indeed AS) occurs predominantly in lean individuals, with a negative relationship on multivariate analysis between BMI and AVBS [10], a measure of valve echogenicity [28]. This relationship is puzzling, as increasing BMI is associated with systemic inflammatory activation, largely reflecting underlying changes in adipocytes [35]. In order to reconcile these superficially paradoxical findings, we hypothesized that the BMI : hs-CRP and BMI : platelet NO responsiveness relationships would be “reversed” in the presence of ASc.
In the case of hs-CRP, there was non-significant attenuation of the BMI : hs-CRP relationship in the presence of ASc (Figure 1B), such that these was no significant increase in hs-CRP with BMI increases in ASc subjects. It is possible that the lack of significant differences reflects Type II error. However, it is more likely that the result suggests that ASc per se is not associated with marked systemic inflammatory activation, at least as measured by hs-CRP.
The results with platelet NO responsiveness are surprising, in view of the previous data of Anfossi et al [26], who demonstrated NO resistance in an obese insulin-resistant cohort. While insulin responsiveness was not measured, there was no evidence of a relationship between BMI and platelet NO responsiveness overall. In subjects with ASc, there was a borderline significant (p=0.08) decline in platelet NO responsiveness with increasing BMI. Thus, there was no evidence to suggest that in subjects with ASc NO resistance might emerge in lean individuals. These data therefore suggest that the bases of platelet NO resistance and systemic inflammatory activation can be dissociated.
Conclusion
The inverse relationship between obesity and prevalence of ASc does not appear to be reflected in substantial alteration of obesity-related inflammatory activation or of BMI-selective disturbances of platelet NO responsiveness. Although ASc is clearly associated with intra-valvular inflammatory activation, those data suggest that it is not the product of a primary systemic process.
Acknowledgments
This work is supported in part by the grant from the National Health and Medical Research Council of Australia and Cardiovascular Lipid Research Grants (Australia). Dr. AL Sverdlov is a recipient of CardioVascular Lipid (CVL) research Grant. We would also like to thank the staff of the North Western Adelaide Health Study (NWAHS) for their help in patient recruitment.
References
- 1.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.Aronow WS, Ahn C, Shirani J, Kronzon I. Comparison of frequency of new coronary events in older subjects with and without valvular aortic sclerosis. _Am J Cardiol. 1999;83:599–600. doi: 10.1016/s0002-9149(98)00922-9. A598. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 4.Deutscher S, Rockette HE, Krishnaswami V. Diabetes and hypercholesterolemia among patients with calcific aortic stenosis. J Chronic Dis. 1984;37:407–415. doi: 10.1016/0021-9681(84)90108-5. [DOI] [PubMed] [Google Scholar]
- 5.Gotoh T, Kuroda T, Yamasawa M, Nishinaga M, Mitsuhashi T, Seino Y, Nagoh N, Kayaba K, Yamada S, Matsuo H, Hosoe M, Itoh Y, Kawai T, Igarashi M, Shimada K. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study) Am J Cardiol. 1995;76:928–932. doi: 10.1016/s0002-9149(99)80263-x. [DOI] [PubMed] [Google Scholar]
- 6.Hoagland PM, Cook EF, Flatley M, Walker C, Goldman L. Case-control analysis of risk factors for presence of aortic stenosis in adults (age 50 years or older) Am J Cardiol. 1985;55:744–747. doi: 10.1016/0002-9149(85)90149-3. [DOI] [PubMed] [Google Scholar]
- 7.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 8.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: atherosclerosis risk factors–a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 9.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 10.Ngo DT, Sverdlov AL, Willoughby SR, Nightingale AK, Chirkov YY, McNeil JJ, Horowitz JD. Determinants of occurrence of aortic sclerosis in an aging population. JACC Cardiovasc Imaging. 2009;2:919–927. doi: 10.1016/j.jcmg.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Rajamannan NM. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahler RC, Desser DR, Finkelhor RS, Brener SJ, Youssefi M. Factors leading to progression of valvular aortic stenosis. Am J Cardiol. 1999;84:1044–1048. doi: 10.1016/s0002-9149(99)00496-8. [DOI] [PubMed] [Google Scholar]
- 13.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 14.Wongpraparut N, Apiyasawat S, Crespo G, Yazdani K, Jacobs LE, Kotler MN. Determinants of progression of aortic stenosis in patients aged > or =40 years. Am J Cardiol. 2002;89:350–352. doi: 10.1016/s0002-9149(01)02241-x. [DOI] [PubMed] [Google Scholar]
- 15.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 16.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 17.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 19.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 20.Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, Otto CM, Griffin BP. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 21.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, Nasir K. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17:229–232. doi: 10.1111/j.0894-0959.2004.17311.x. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy JA, Hua X, Mishra K, Murphy GA, Rosenkranz AC, Horowitz JD. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur J Pharmacol. 2009;602:28–35. doi: 10.1016/j.ejphar.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Chirkov YY, Holmes AS, Willoughby SR, Stewart S, Horowitz JD. Association of aortic stenosis with platelet hyperaggregability and impaired responsiveness to nitric oxide. Am J Cardiol. 2002;90:551–554. doi: 10.1016/s0002-9149(02)02536-5. [DOI] [PubMed] [Google Scholar]
- 25.Willoughby SR, Stewart S, Holmes AS, Chirkov YY, Horowitz JD. Platelet nitric oxide responsiveness: a novel prognostic marker in acute coronary syndromes. Arterioscler Thromb Vasc Biol. 2005;25:2661–2666. doi: 10.1161/01.ATV.0000193622.77294.57. [DOI] [PubMed] [Google Scholar]
- 26.Anfossi G, Mularoni EM, Burzacca S, Ponziani MC, Massucco P, Mattiello L, Cavalot F, Trovati M. Platelet resistance to nitrates in obesity and obese NIDDM, and normal platelet sensitivity to both insulin and nitrates in lean NIDDM. Diabetes Care. 1998;21:121–126. doi: 10.2337/diacare.21.1.121. [DOI] [PubMed] [Google Scholar]
- 27.Cosmi JE, Kort S, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162:2345–2347. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 28.Ngo DT, Wuttke RD, Turner S, Marwick TH, Horowitz JD. Quantitative assessment of aortic sclerosis using ultrasonic backscatter. J Am Soc Echocardiogr. 2004;17:1123–1130. doi: 10.1016/j.echo.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien KD, Shavelle DM, Caulfield MT, McDonald TO, Olin-Lewis K, Otto CM, Probstfield JL. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 30.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo DT, Stafford I, Kelly DJ, Sverdlov AL, Wuttke RD, Weedon H, Nightingale AK, Rosenkranz AC, Smith MD, Chirkov YY, Kennedy JA, Horowitz JD. Vitamin D (2) supplementation induces the development of aortic stenosis in rabbits: interactions with endothelial function and thioredoxin-interacting protein. Eur J Pharmacol. 2008;590:290–296. doi: 10.1016/j.ejphar.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 32.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 33.Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini LA, Picano E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J Am Coll Cardiol. 2003;41:136–141. doi: 10.1016/s0735-1097(02)02622-0. [DOI] [PubMed] [Google Scholar]
- 34.Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 35.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]